Influence of CO2 Curing on the Alkali-Activated Compound Mineral Admixtures’ Corrosion Resistance to NaCl Dry–Wet Alternations
Abstract
:1. Introduction
2. Experimental Arrangement
2.1. Raw Materials
2.2. Specimen Preparation
2.3. Measurements
2.3.1. Macro-Performance
2.3.2. Microscopic Performance
3. Results and Discussions
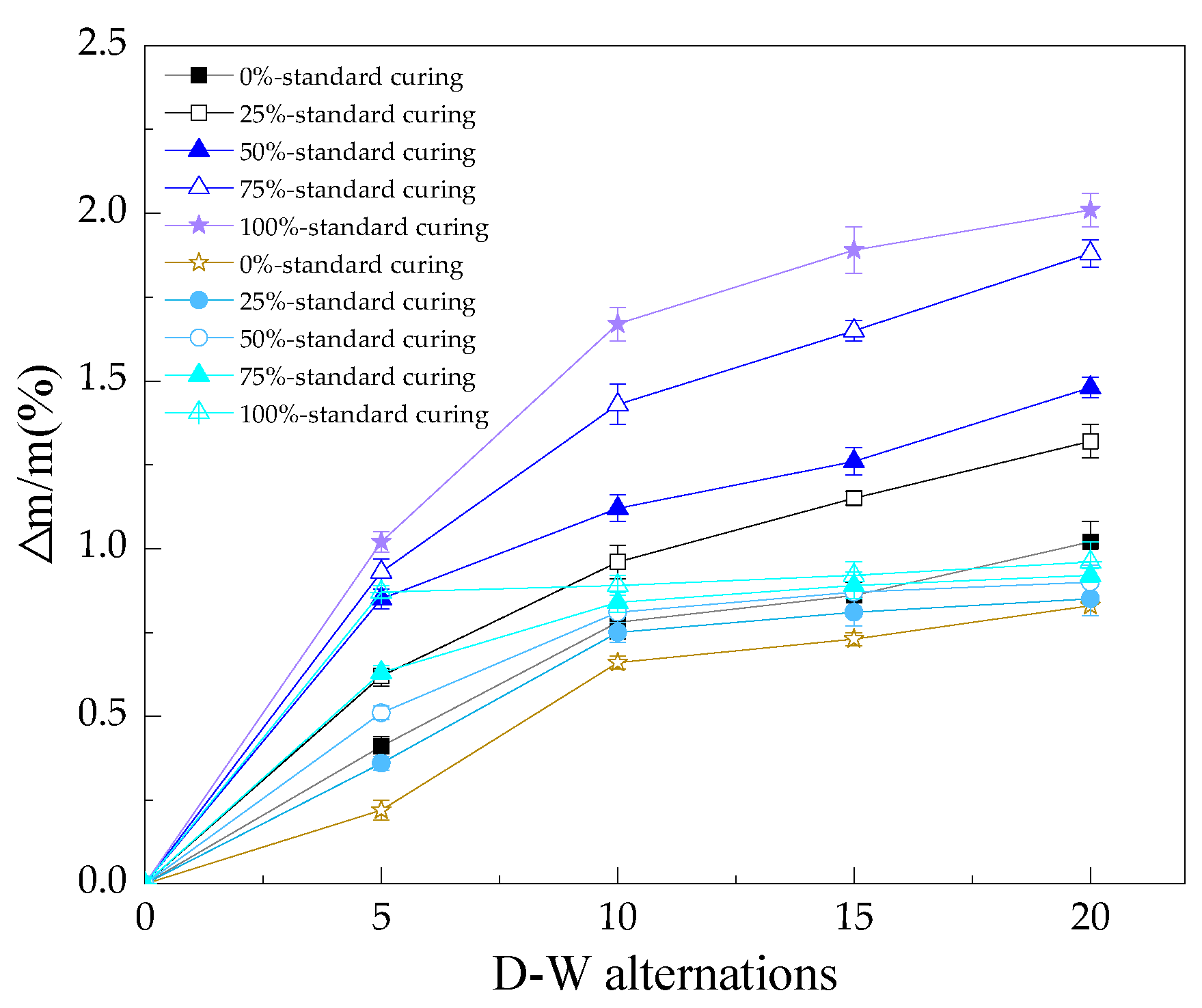
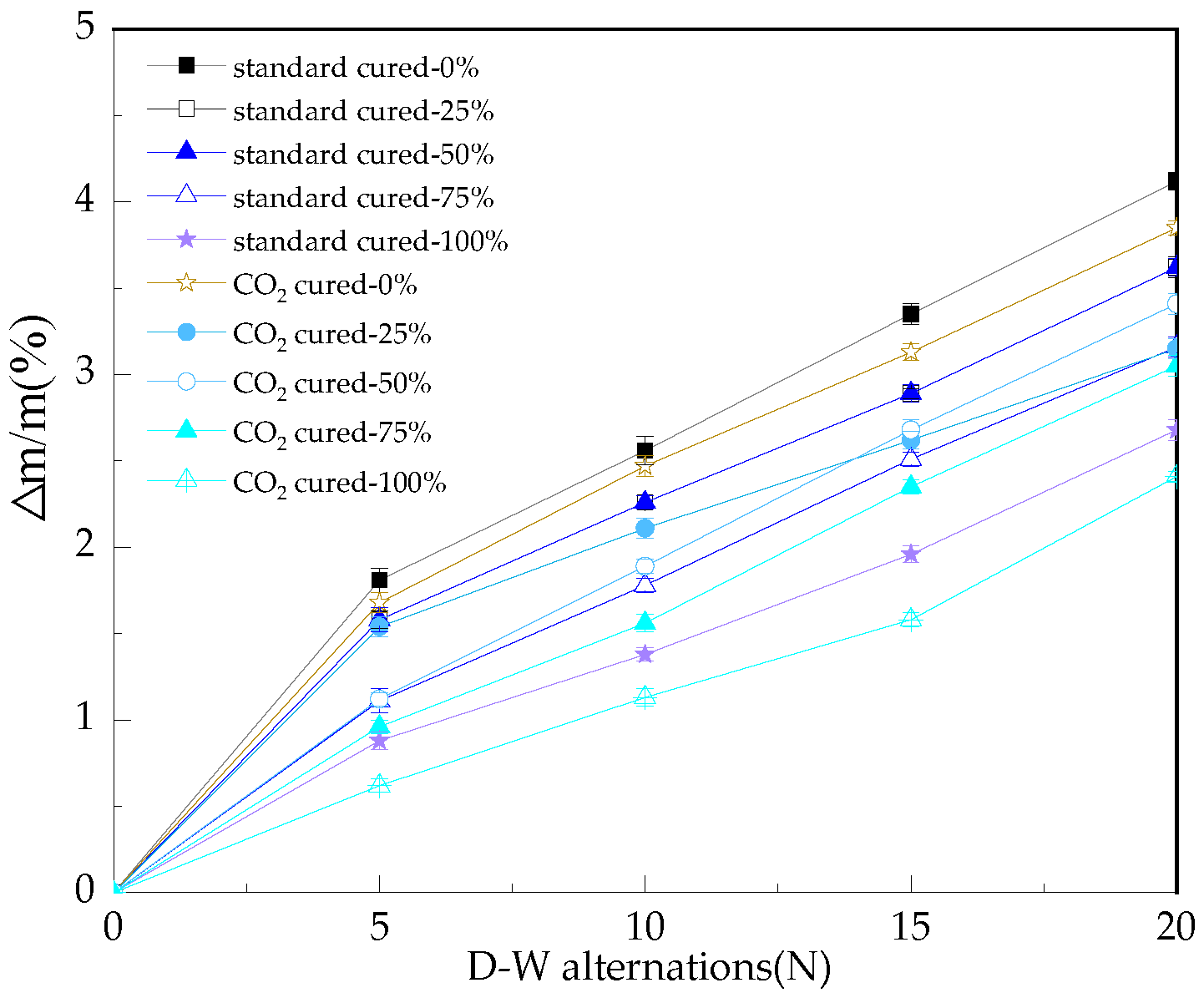
3.1. The Mass Loss Rate of the Reinforced Alkali-Activated Mineral Admixtures
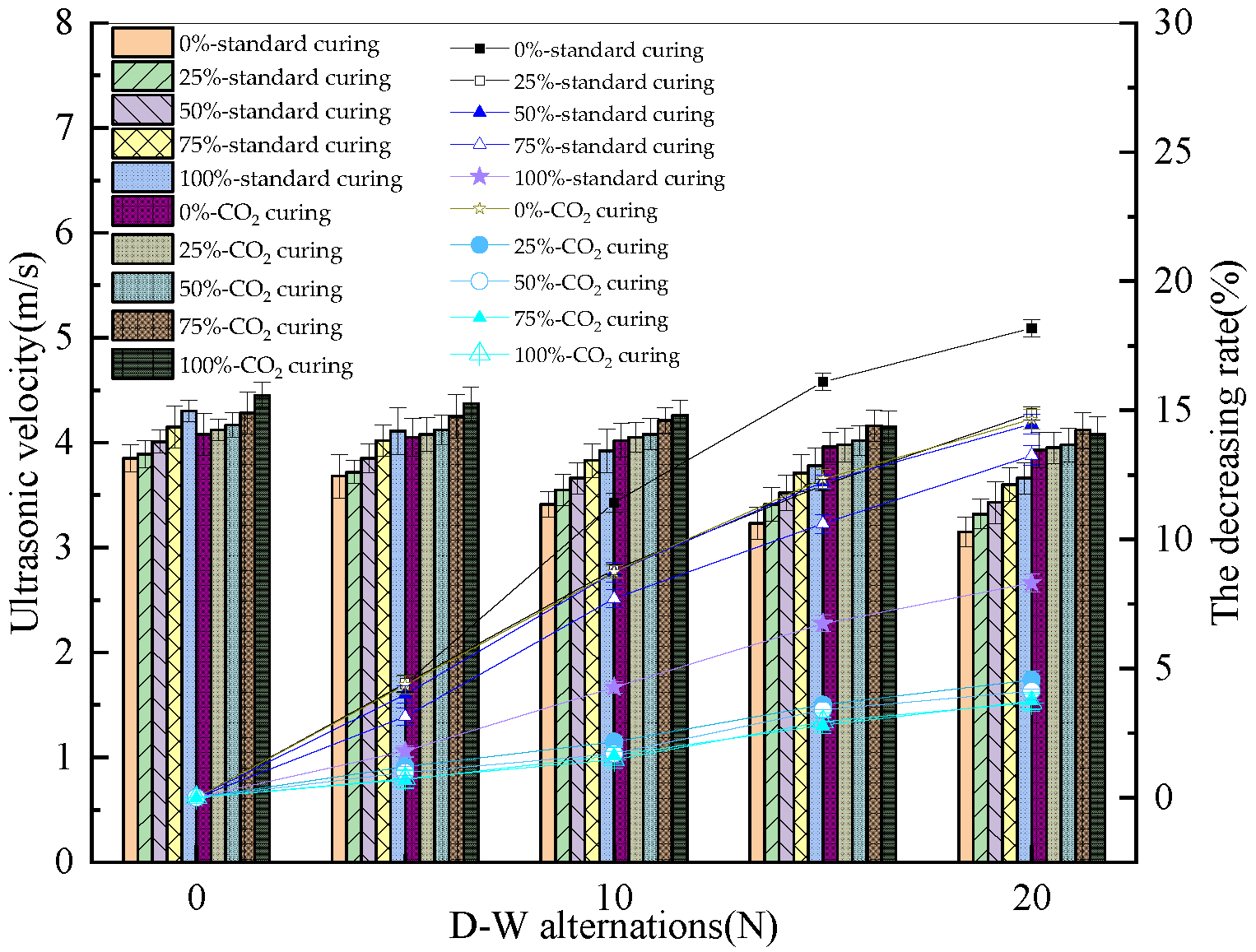
3.2. The Ultrasonic Velocity of the Reinforced Alkali-Activated Mineral Admixtures
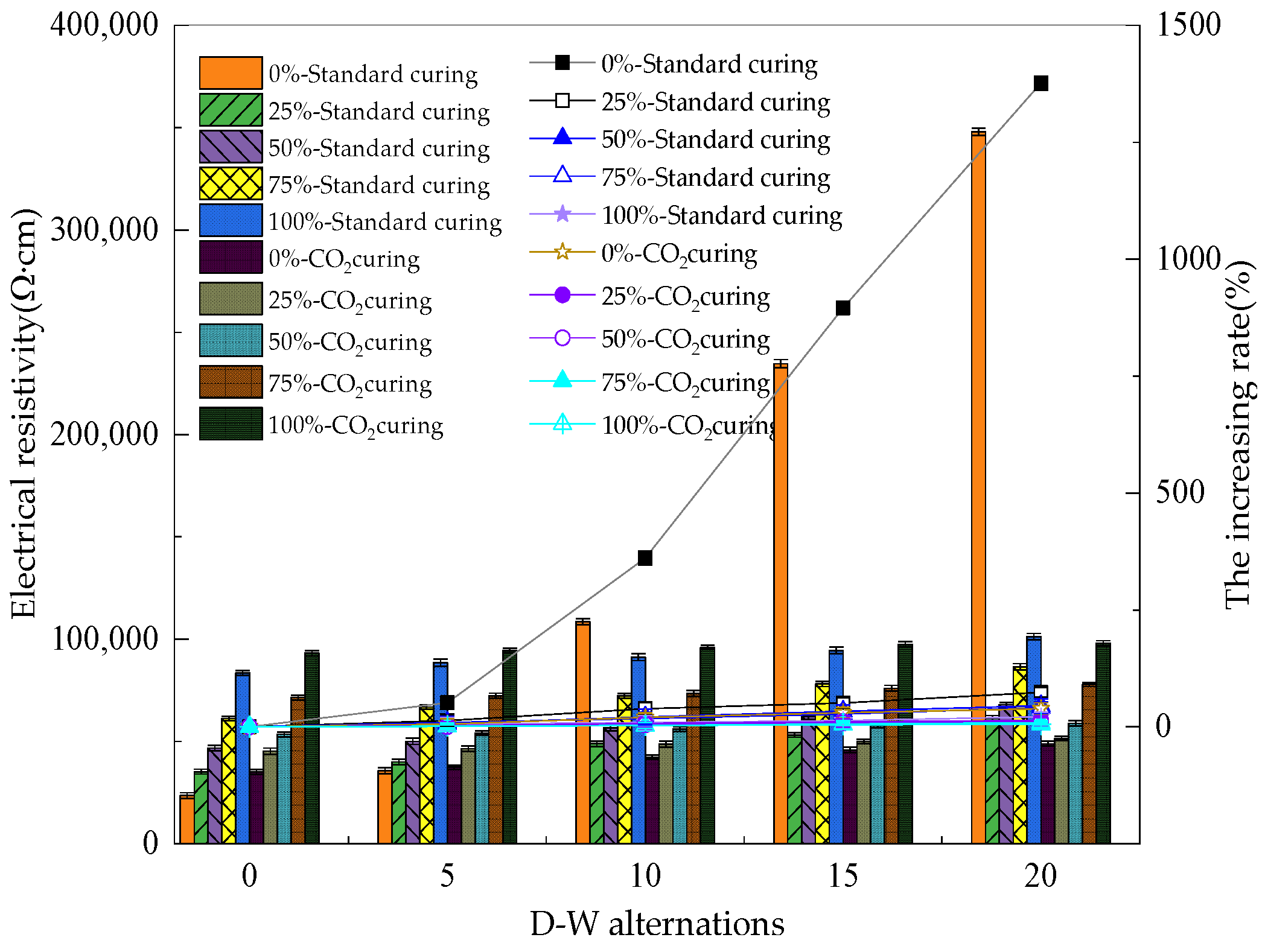
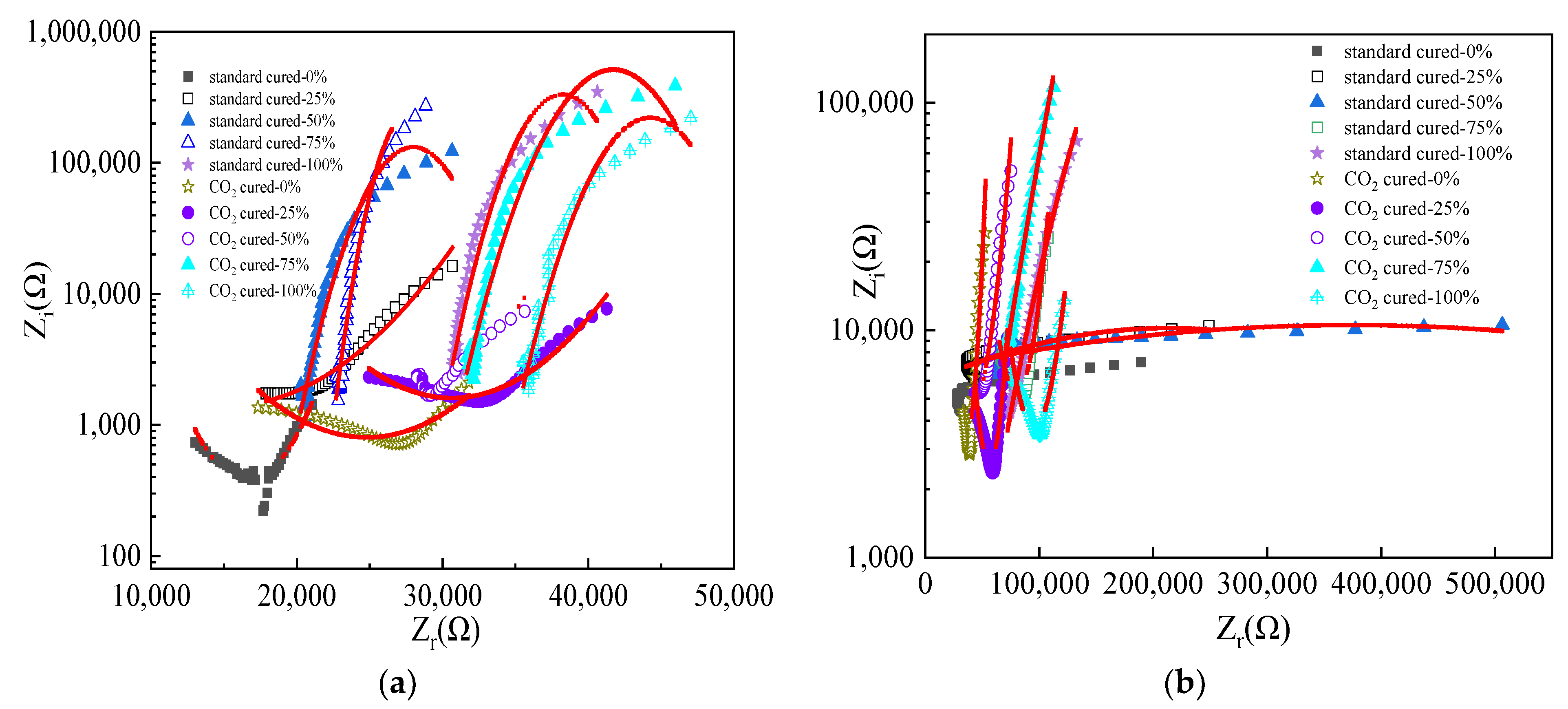
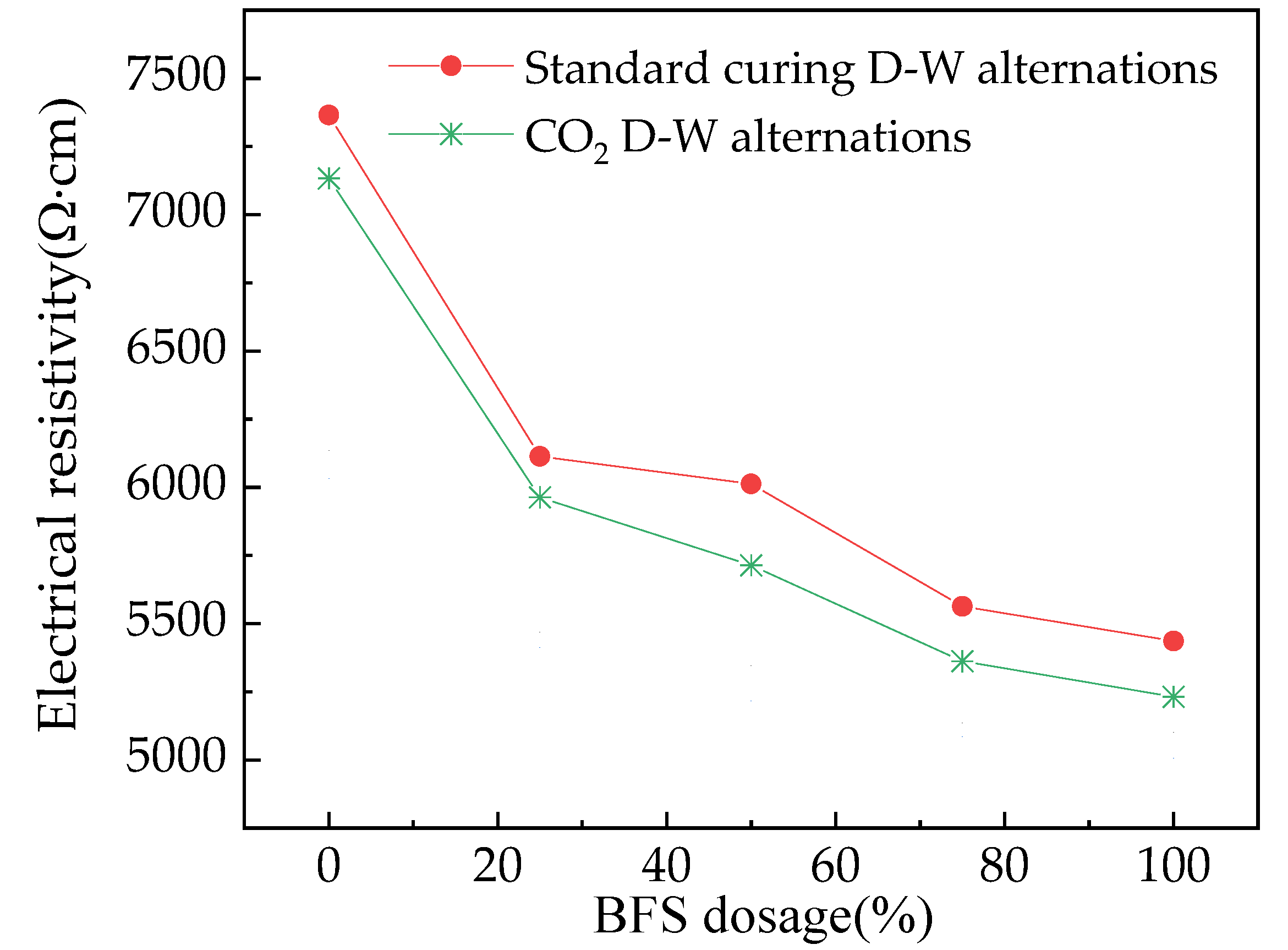
3.3. The Electrical Parameters of Reinforced Alkali-Activated Mineral Admixtures
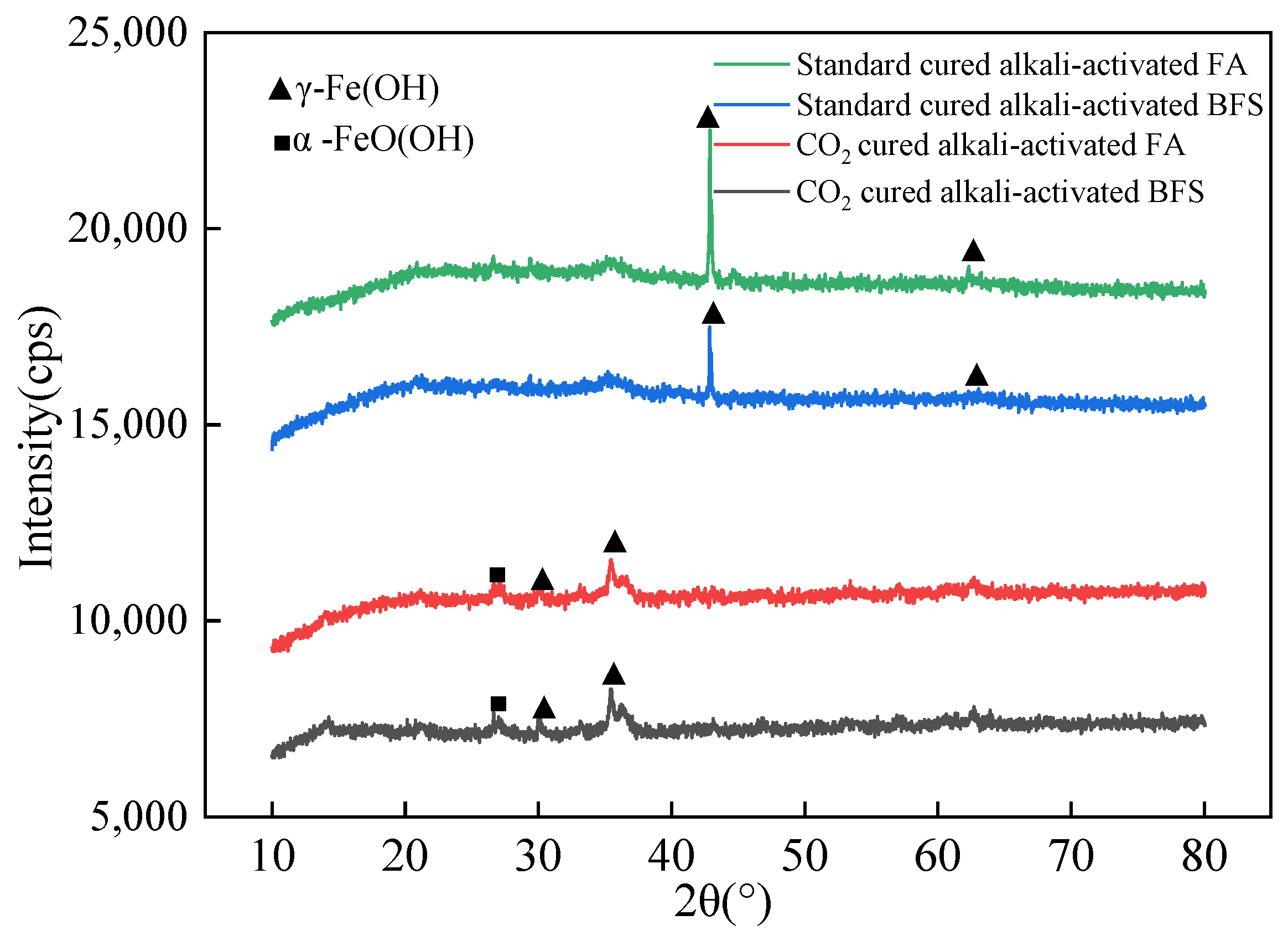
3.4. The Micro-Structure Research Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, J. Laboratory characterization of controlled low-strength material and its application to construction of flexible pipe drainage system. Health Manpow. Manag. 2000, 19, 30–59. [Google Scholar]
- Haselbach, L.; Valavala, S.; Montes, F. Permeability predictions for sand clogged Portland cement pervious concrete pavement systems. J. Environ. Manag. 2005, 81, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Gao, X.; Zhang, Q. Evaluating performance of concrete pavement joint repair using different materials to reduce reflective cracking in asphalt concrete overlay. Road Mater. Pavement Des. 2014, 15, 966–976. [Google Scholar] [CrossRef]
- Witoon, T.; Lapkeatseree, V.; Numpilai, T.; Cheng, C.; Limtrakul, J. CO2 hydrogenation to light olefins over mixed Fe-Co-K-Al oxides catalysts prepared via precipitation and reduction methods. Chem. Eng. J. 2022, 428, 131389. [Google Scholar] [CrossRef]
- Witoon, T.; Numpilai, T.; Nijpanich, S.; Chanlek, N.; Kidkhunthod, P.; Cheng, C.; Ng, K.; Vo, D.N.; Ittisanronnachai, S.; Wattanakit, C.; et al. Enhanced CO2 hydrogenation to higher alcohols over K-Co promoted In2O3 catalysts. Chem. Eng. J. 2022, 431, 133211. [Google Scholar] [CrossRef]
- Liska, M.; Jin, F.; Yi, Y.; Al-Tabbaa, A. Mechanism of reactive magnesia-ground granulated blastfurnace slag (GGBS) soil stabilization. Can. Geotech. J. 2016, 53, 773–782. [Google Scholar]
- Li, Y.; Li, X.; Tan, Y. Effect of aging on fatigue performance of cement emulsified asphalt repair material. Constr. Build. Mater. 2021, 292, 123417. [Google Scholar] [CrossRef]
- Chen, X.; Wang, S.; Zhou, Y.; Cheeseman, C.; Bi, W.; Zhang, T. Improved low-carbon magnesium oxysulfate cement pastes containing boric acid and citric acid. Cem. Concr. Compos. 2022, 134, 104813. [Google Scholar] [CrossRef]
- Liu, M.; Tan, H.; He, X. Effects of nano-SiO2 on early strength and microstructure of steam-cured high volume flfly ash cement system. Constr. Build. Mater. 2019, 194, 350–359. [Google Scholar] [CrossRef]
- Celikten, S.; Sarldemir, M.; Deneme, I.O. Mechanical and microstructural properties of alkali-activated slag and slag+ fly ash mortars exposed to high temperature. Constr. Build. Mater. 2019, 217, 50–61. [Google Scholar] [CrossRef]
- Huang, G.; Wang, H.; Shi, F. Coupling Effect of Salt Freeze-Thaw Cycles and Carbonation on the Mechanical Performance of Quick Hardening Sulphoaluminate Cement-Based Reactive Powder Concrete with Basalt Fibers. Coatings 2021, 11, 1142. [Google Scholar] [CrossRef]
- Li, S.; Feng, Y.; Yang, J.; Cerny, R. Expansion mechanism and properties of magnesium oxide expansive hydraulic cement for engineering applications. Adv. Mater. Sci. Eng. 2021, 2021, 5542072. [Google Scholar] [CrossRef]
- Li, S.; Yang, J.; Zhang, P. Hydration and hardening properties of reactive magnesia and Portland cement composite. Constr. Build. Mater. 2022, 327, 126779. [Google Scholar] [CrossRef]
- Ozata, S.; Akturk, B.; Yuzer, N. Utilization of waste Cappadocia earth as a natural pozzolan in alkali activation: A parametric study. Constr. Build. Mater. 2022, 329, 127192. [Google Scholar] [CrossRef]
- Yang, J.; Huang, J.; Su, Y.; He, X.; Tan, H.; Yang, W.; Strnadel, B. Eco-friendly treatment of low-calcium coal fly ash for high pozzolanic reactivity: A step towards waste utilization in sustainable building material. J. Clean. Prod. 2019, 238, 117962. [Google Scholar] [CrossRef]
- Matějková, P.; Matějka, V.; Sabovčík, T.; Gryžbon, L.; Vlček, J. Alkali Activation of Ground Granulated Blast Furnace Slag and Low Calcium Fly Ash Using “One-Part” Approach. J. Sustain. Metall. 2022, 8, 511–521. [Google Scholar] [CrossRef]
- Supriya, J.; Raut, A. Performance parameter analysis of magnesia based cement products-a review. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1197, 012078. [Google Scholar] [CrossRef]
- Glukhovsky, V.D.; Rostovskaja, G.S.; Rumyna, G.V. High strength slag—Alkaline cements. In Proceedings of the 7th International Congress on the Chemistry of Cement, Paris, France, 11–13 July 1980. [Google Scholar]
- Alomayri, T.; Adesina, A.; Das, S. Influence of amorphous raw rice husk ash as precursor and curing condition on the performance of alkali activated concrete. Case Stud. Constr. Mater. 2021, 15, e00777. [Google Scholar] [CrossRef]
- Alrefaei, Y.; Dai, J. Effects of delayed addition of polycarboxylate ether on one-part alkali-activated fly ash/slag pastes: Adsorption, reaction kinetics, and rheology. Constr. Build. Mater. 2022, 323, 126611. [Google Scholar] [CrossRef]
- Feng, S.; Zhu, J.; Wang, R.; Qu, Z.; Song, L.; Wang, H. The Influence of CaO and MgO on the Mechanical Properties of Alkali-Activated Blast Furnace Slag Powder. Materials 2022, 15, 6128. [Google Scholar] [CrossRef]
- Xian, X.; Zhang, D.; Lin, H.; Shao, Y. Ambient pressure carbonation curing of reinforced concrete for CO2 utilization and corrosion resistance. J. CO2 Util. 2022, 56, 101861. [Google Scholar] [CrossRef]
- Ju, Y.; Zhao, J.; Wang, D.; Song, Y. Experimental study on flexural behaviour of reinforced reactive powder concrete pole. Constr. Build. Mater. 2021, 312, 125399. [Google Scholar] [CrossRef]
- Chi, M. Effects of dosage of alkali-activated solution and curing conditions on the properties and durability of alkali-activated concrete. Constr. Build. Mater. 2012, 35, 240–245. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, Z.; Zhu, Y.; Tian, L. Durability of alkali-activated fly ash concrete: Chloride penetration in pastes and mortars. Constr. Build. Mater. 2014, 65, 51–59. [Google Scholar] [CrossRef]
- Wang, H.; Jin, K.; Zhang, A.; Zhang, L.; Han, Y.; Liu, J.; Shi, F.; Feng, L. External erosion of sodium chloride on the degradation of self-sensing and mechanical properties of aligned stainless steel fiber reinforced reactive powder concrete. Constr. Build. Mater. 2021, 287, 123028. [Google Scholar] [CrossRef]
- AL-Ameeri, A.S.; ImranRafiq, M.; Tsioulou, O.; Rybdylova, O. Impact of climate change on the carbonation in concrete due to carbon dioxide ingress: Experimental investigation and modelling. J. Build. Eng. 2021, 44, 102594. [Google Scholar] [CrossRef]
- Qin, L.; Gao, X. Properties of coal gangue-Portland cement mixture with carbonation. Fuel 2019, 245, 1–12. [Google Scholar] [CrossRef]
- Kamal, N.L.M.; Itam, Z.; Sivaganese, Y.; Beddu, S. Carbon dioxide sequestration in concrete and its effects on concrete compressive strength. Mater. Today Proc. 2020, 31, A18–A21. [Google Scholar] [CrossRef]
- Adesina, A. Recent advances in the concrete industry to reduce its carbon dioxide emissions. Environ. Chall. 2020, 1, 100004. [Google Scholar] [CrossRef]
- Zhu, J.; Qu, Z.; Liang, S.; Li, B.; Du, T.; Wang, H. The macro-scopic and microscopic properties of cement paste with carbon dioxide curing. Materials 2022, 15, 1578. [Google Scholar] [CrossRef]
- Ashraf, W.; Olek, J. Carbonation behavior of hydraulic and non-hydraulic calcium silicates: Potential of utilizing low-lime calcium silicates in cement-based materials. J. Mater. Sci. 2016, 51, 6173–6191. [Google Scholar] [CrossRef]
- GB/T1345-2005; The Test Sieving Method for Fineness of Cement. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2005.
- Wang, H.; Shi, F.; Shen, J.; Zhang, A.; Zhang, L.; Huang, H.; Liu, J.; Jin, K.; Feng, L.; Tang, Z. Research on the self-sensing and mechanical properties of aligned stainless steel fiber reinforced reactive powder concrete. Cem. Concr. Compos. 2021, 119, 104001. [Google Scholar] [CrossRef]
- Mei, K.; He, Z.; Yi, B.; Lin, X.; Wang, J.; Wang, H.; Liu, J. Study on electrochemical characteristics of reinforced concrete corrosion under the action of carbonation and chloride. Case Stud. Constr. Mater. 2022, 17, e01351. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, A.; Wang, Q.; Han, Y.; Tang, Z. Corrosion resistance of wollastonite modified magnesium phosphate cement paste exposed to freeze-thaw cycles and acid-base corrosion. Case Stud. Constr. Mater. 2020, 13, e00421. [Google Scholar] [CrossRef]
- Zhang, B.; Tan, H.; Shen, W.; Xu, G.; Ma, B.; Ji, X. Nano-silica and silica fume modifified cement mortar used as Surface Protection Material to enhance the impermeability. Cem. Concr. Compos. 2018, 92, 7–17. [Google Scholar] [CrossRef]
- Jin, F.; Gu, K.; Al-Tabbaa, A. Strength and hydration properties of reactive MgO-activated ground granulated blastfurnace slag paste. Cem. Concr. Compos. 2015, 57, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Lei, L.; Wang, Q.; Xu, S.; Wang, N.; Zheng, X. Fabrication of superhydrophobic concrete used in marine environment with anti-corrosion and stable mechanical properties. Constr. Build. Mater. 2020, 251, 118946. [Google Scholar] [CrossRef]
- Qin, L.; Gao, X.; Chen, T. Influence of mineral admixtures on carbonation curing of cement paste. Constr. Build. Mater. 2019, 212, 653–662. [Google Scholar] [CrossRef]
- Cao, H.; Liang, Z.; Peng, X.; Cai, X.; Wang, K.; Wang, H.; Lyu, Z. Research of Carbon Dioxide Curing on the Properties of Reactive Powder Concrete with Assembly Unit of Sulphoaluminate Cement and Ordinary Portland Cement. Coatings 2022, 12, 209. [Google Scholar] [CrossRef]
- Liang, J.; Zhu, H.; Chen, L.; Qinglin, X.; Ying, G. Rebar corrosion investigation in rubberaggregate concrete via the chloride electro-accelerated test. Materials 2019, 12, 862. [Google Scholar] [CrossRef] [Green Version]
- Cui, L.; Wang, H. Research on the Mechanical Strengths and the Following Corrosion Resistance of Inner Steel Bars of RPC with Rice Husk Ash and Waste Fly Ash. Coatings 2021, 11, 1480. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, A.; Zhang, L.; Liu, J.; Han, Y.; Wang, J. Research on the Influence of Carbonation on the Content and State of Chloride Ions and the Following Corrosion Resistance of Steel Bars in Cement Paste. Coatings 2020, 10, 1071. [Google Scholar] [CrossRef]
- Zhang, X.W.; Liu, Y.Y.; Li, C.Q.; Tian, L.; Yuan, F.; Zheng, S.L.; Sun, Z.M. Fast and lasting electron transfer between γ-FeOOH and g-C3N4/kaolinite containing N vacancies for enhanced visible-light-assisted peroxymonosulfate activation. Chem. Eng. J. 2022, 429, 132374. [Google Scholar] [CrossRef]
- Jadhav, A.S.; Mokhtar, A.A.; Sunit, K.S.; Ahmed, S.A.; Amit, B.; Vadali, V.S.S.S.; Nitin, K.L. γ-FeOOH and γ-FeOOH decorated multi-layer graphene: Potential materials for selenium(VI) removal from water. J. Water Process Eng. 2020, 37, 101396. [Google Scholar] [CrossRef]
- Chen, G.; Ma, C.G.; Fu, Z.H.; Wang, J.; Dai, P.B. Significantly enhancing fracture toughness of epoxy composite with promising γ-FeOOH@Fe2O3 hybrid nanoparticles by magnetic field assistanc. Nano Mater. Sci. 2022, 4, 139–150. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, A.; Zhang, L.; Liu, J.; Han, Y.; Shu, H.; Wang, J. Study on the influence of compound rust inhibitor on corrosion of steel bars in chloride concrete by electrical parameters. Constr. Build. Mater. 2020, 262, 120763. [Google Scholar] [CrossRef]







| Types | 0.3 μm | 0.6 μm | 1 μm | 4 μm | 8 μm | 64 μm | 360 μm |
|---|---|---|---|---|---|---|---|
| BFS | 0.03 | 0.1 | 3.5 | 19.6 | 35.0 | 97.9 | 100 |
| FA | 12.3 | 41.7 | 66.2 | 100 | 100 | 100 | 100 |
| Types | SiO2 | Al2O3 | FexOy | MgO | CaO | SO3 | K2O | Na2O | Ti2O | LI |
|---|---|---|---|---|---|---|---|---|---|---|
| BFS | 34.1 | 14.7 | 0.2 | 9.7 | 35.9 | 0.2 | 3.5 | — | — | — |
| FA | 55.00 | 29.58 | 6.00 | 0.62 | 4.50 | 0.11 | 1.26 | 2.13 | 0.06 | 0.74 |
| Specimens | FA | BFS | Alkali Equivalent | W/B | Modulus | Na2O·nSiO2 | NaOH |
|---|---|---|---|---|---|---|---|
| A1 | 300 | 0 | 3% | 0.3 | 1 | 29.83 | 6.69 |
| A2 | 240 | 60 | 3% | 0.3 | 1 | 29.83 | 6.69 |
| A3 | 180 | 120 | 3% | 0.3 | 1 | 29.83 | 6.69 |
| A4 | 120 | 180 | 3% | 0.3 | 1 | 29.83 | 6.69 |
| A5 | 60 | 240 | 3% | 0.3 | 1 | 29.83 | 6.69 |
| A6 | 0 | 300 | 3% | 0.3 | 1 | 29.83 | 6.69 |
| Specimens | A1 | A2 | A3 | A4 | A5 |
|---|---|---|---|---|---|
| Standard curing—5 alternations | 3.15 | 2.89 | 2.46 | 2.33 | 0.31 |
| CO2 curing—5 alternations | 3.41 | 3.23 | 2.87 | 2.68 | 0.46 |
| Standard curing—10 alternations | 2.71 | 2.53 | 2.14 | 1.98 | 0.24 |
| CO2 curing—10 alternations | 3.79 | 3.56 | 3.24 | 3.11 | 0.59 |
| Standard curing—20 alternations | 4.12 | 3.98 | 3.66 | 3.27 | 0.89 |
| CO2 curing—20 alternations | 4.08 | 3.79 | 3.60 | 2.96 | 0.67 |
| Specimens | Chi-Square |
|---|---|
| Standard cured-0%-Before NaCl D–W alternation | 0.012 |
| Standard cured-25%-Before NaCl D–W alternation | 0.014 |
| Standard cured-50%-Before NaCl D–W alternation | 0.008 |
| Standard cured-75%-Before NaCl D–W alternation | 0.0056 |
| Standard cured-100%-Before NaCl D–W alternation | 0.013 |
| CO2-cured-0%-Before NaCl D–W alternation | 0.0097 |
| CO2-cured-25%-Before NaCl D–W alternation | 0.012 |
| CO2-cured-50%-Before NaCl D–W alternation | 0.011 |
| CO2-cured-75%-Before NaCl D–W alternation | 0.0089 |
| CO2-cured-100%-Before NaCl D–W alternation | 0.0091 |
| Standard cured-0%-After NaCl D–W alternation | 0.0099 |
| Standard cured-25%-After NaCl D–W alternation | 0.012 |
| Standard cured-50%-After NaCl D–W alternation | 0.01 |
| Standard cured-75%-After NaCl D–W alternation | 0.0087 |
| Standard cured-100%-After NaCl D–W alternation | 0.0065 |
| CO2-cured-0%-After NaCl D–W alternation | 0.0079 |
| CO2-cured-25%-After NaCl D–W alternation | 0.0088 |
| CO2-cured-50%-After NaCl D–W alternation | 0.0092 |
| CO2-cured-75%-After NaCl D–W alternation | 0.0097 |
| CO2-cured-100%-After NaCl D–W alternation | 0.0045 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Cheng, W.; Xu, H.; Cai, Z.; Yin, M.; Shi, F. Influence of CO2 Curing on the Alkali-Activated Compound Mineral Admixtures’ Corrosion Resistance to NaCl Dry–Wet Alternations. Coatings 2023, 13, 67. https://doi.org/10.3390/coatings13010067
Sun H, Cheng W, Xu H, Cai Z, Yin M, Shi F. Influence of CO2 Curing on the Alkali-Activated Compound Mineral Admixtures’ Corrosion Resistance to NaCl Dry–Wet Alternations. Coatings. 2023; 13(1):67. https://doi.org/10.3390/coatings13010067
Chicago/Turabian StyleSun, Houchao, Wenjie Cheng, Haoxin Xu, Zhangjie Cai, Minggan Yin, and Feiting Shi. 2023. "Influence of CO2 Curing on the Alkali-Activated Compound Mineral Admixtures’ Corrosion Resistance to NaCl Dry–Wet Alternations" Coatings 13, no. 1: 67. https://doi.org/10.3390/coatings13010067





