In Situ Fabrication of Polydeoxyribonucleotide-Impregnated Hydroxyapatite onto a Magnesium Surface
Abstract
:1. Introduction
2. Materials and Methods
2.1. PDRN-Impregnated HA Coating
2.2. Characterization of PDRN-Impregnated HA Layer on Mg
2.3. Drug Release Test
2.4. Effect of PDRN on In Vitro Cellular Proliferation
2.5. Statistical Analysis
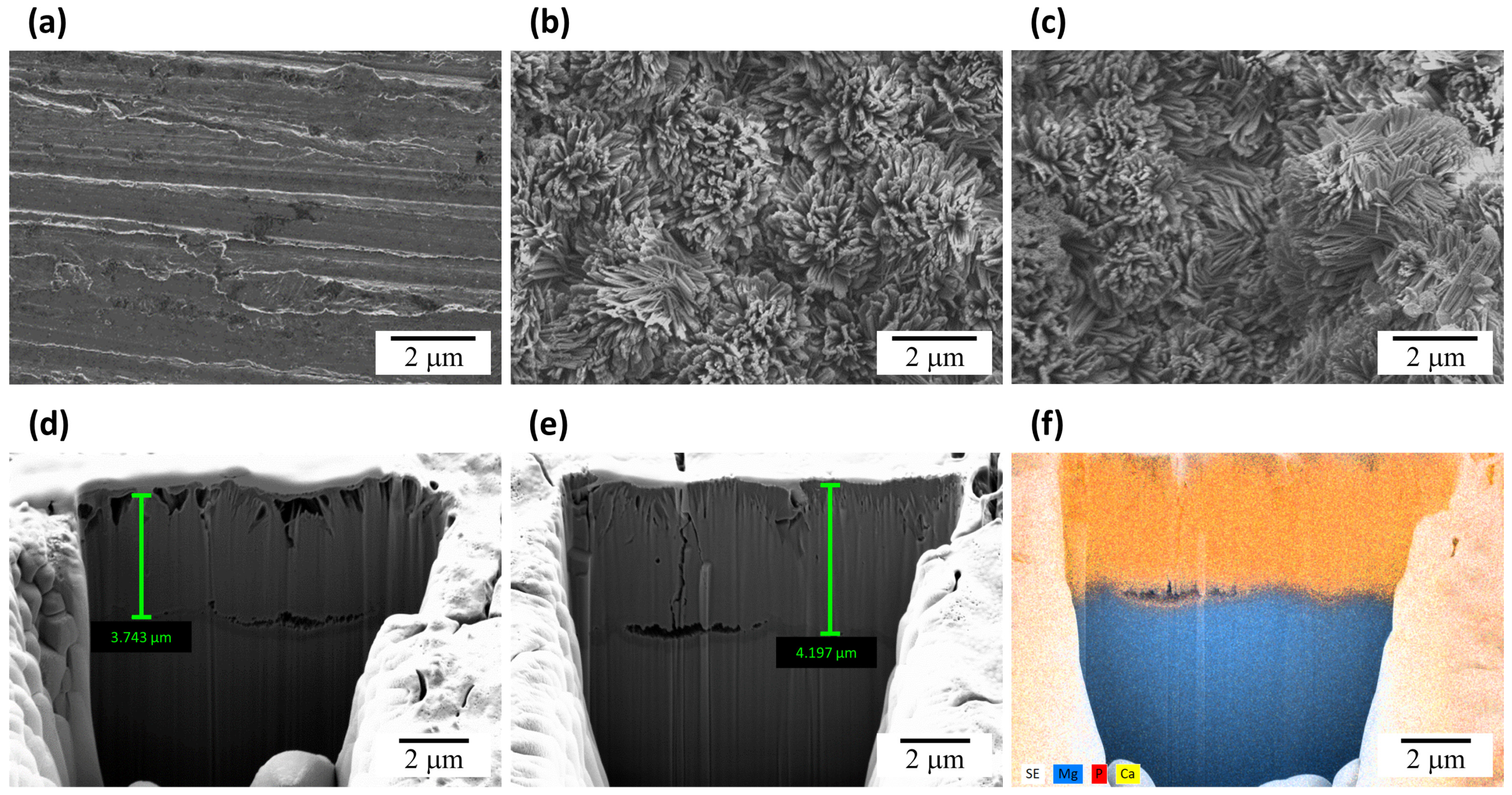
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Kumar, K.; Gill, R.S.; Batra, U. Challenges and opportunities for biodegradable magnesium alloy implants. Mater. Technol. 2017, 33, 153–172. [Google Scholar] [CrossRef]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective. Biomaterials 2017, 112, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty Banerjee, P.; Al-Saadi, S.; Choudhary, L.; Harandi, S.E.; Singh, R. Magnesium implants: Prospects and challenges. Materials 2019, 12, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, G. Control of biodegradation of biocompatable magnesium alloys. Corr. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Waizy, H.; Seitz, J.M.; Reifenrath, J.; Weizbauer, A.; Bach, F.W.; Meyer-Lindenberg, A.; Denkena, B.; Windhagen, H. Biodegradable magnesium implants for orthopedic applications. J. Mater. Sci. 2012, 48, 39–50. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, J.K.; Hopkins, C.; Chow, D.H.; Qin, L. Biodegradable magnesium-based implants in orthopedics—A general review and perspectives. Adv. Sci. 2020, 7, 1902443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, X.N.; Zhou, W.R.; Zheng, Y.F.; Cheng, Y.; Wei, S.C.; Zhong, S.P.; Xi, T.F.; Chen, L.J. Corrosion fatigue behaviors of two biomedical Mg alloys—AZ91D and WE43—In simulated body fluid. Acta Biomater. 2010, 6, 4605–4613. [Google Scholar] [CrossRef] [PubMed]
- Cheon, K.H.; Park, C.; Kang, M.H.; Kang, I.G.; Lee, M.K.; Lee, H.; Kim, H.E.; Jung, H.D.; Jang, T.S. Construction of tantalum/poly(ether imide) coatings on magnesium implants with both corrosion protection and osseointegration properties. Bioact. Mater. 2021, 6, 1189–1200. [Google Scholar] [CrossRef]
- Kirkland, N.T.; Birbilis, N.; Staiger, M.P. Assessing the corrosion of biodegradable magnesium implants: A critical review of current methodologies and their limitations. Acta Biomater. 2012, 8, 925–936. [Google Scholar] [CrossRef]
- Byun, S.H.; Lim, H.K.; Kim, S.M.; Lee, S.M.; Kim, H.E.; Lee, J.H. The bioresorption and guided bone regeneration of absorbable hydroxyapatite-coated magnesium mesh. J. Craniofac. Surg. 2017, 28, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.H.; Lee, H.; Jang, T.S.; Seong, Y.J.; Kim, H.E.; Koh, Y.H.; Song, J.H.; Jung, H.D. Biomimetic porous Mg with tunable mechanical properties and biodegradation rates for bone regeneration. Acta Biomater. 2019, 84, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Su, Y.; Gu, R.; Zhang, Z.; Li, G.; Lian, J.; Ren, L. Enhanced corrosion resistance and biocompatibility of biodegradable magnesium alloy modified by calcium phosphate/collagen coating. Surf. Coat. Technol. 2020, 401, 126318. [Google Scholar] [CrossRef]
- Kang, I.G.; Kim, J.; Kim, J.; Park, S.; Kim, H.E.; Han, C.M. PLLA Membrane with embedded hydroxyapatite patterns for improved bioactivity and efficient delivery of growth factor. Macromol. Biosci. 2020, 20, e2000136. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Kang, M.H.; Kim, H.E.; Lim, H.K.; Byun, S.H.; Lee, J.H.; Lee, S.M. Innovative micro-textured hydroxyapatite and poly(l-lactic)-acid polymer composite film as a flexible, corrosion resistant, biocompatible, and bioactive coating for Mg implants. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 81, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Huh, C.K.; Lee, J.H.; Kim, K.W.; Kim, M.Y. Histologic study of bone-forming capacity on polydeoxyribonucleotide combined with demineralized dentin matrix. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 7. [Google Scholar] [CrossRef] [Green Version]
- Koo, Y.; Yun, Y. Effects of polydeoxyribonucleotides (PDRN) on wound healing: Electric cell-substrate impedance sensing (ECIS). Mater. Sci. Eng. C 2016, 69, 554–560. [Google Scholar] [CrossRef]
- Buffoli, B.; Favero, G.; Borsani, E.; Boninsegna, R.; Sancassani, G.; Labanca, M.; Rezzani, R.; Nocini, P.F.; Albanese, M.; Rodella, L.F. Sodium-DNA for bone tissue regeneration: An experimental study in rat calvaria. BioMed. Res. Int. 2017, 2017, 7320953. [Google Scholar] [CrossRef] [Green Version]
- Veronesi, F.; Dallari, D.; Sabbioni, G.; Carubbi, C.; Martini, L.; Fini, M. Polydeoxyribonucleotides (PDRNs) from skin to musculoskeletal tissue regeneration via adenosine A2A receptor involvement. J. Cell Physiol. 2017, 232, 2299–2307. [Google Scholar] [CrossRef]
- Lim, H.K.; Kwon, Y.J.; Hong, S.J.; Choi, H.G.; Chung, S.M.; Yang, B.E.; Lee, J.H.; Byun, S.H. Bone regeneration in ceramic scaffolds with variable concentrations of PDRN and rhBMP-2. Sci. Rep. 2021, 11, 11470. [Google Scholar] [CrossRef]
- Guizzardi, S.; Martini, D.; Bacchelli, B.; Valdatta, L.; Thione, A.; Scamoni, S.; Uggeri, J.; Ruggeri, A. Effects of heat deproteinate bone and polynucleotides on bone regeneration: An experimental study on rat. Micron 2007, 38, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Squadrito, F.; Bitto, A.; Irrera, N.; Pizzino, G.; Pallio, G.; Minutoli, L.; Altavilla, D. Pharmacological activity and clinical use of PDRN. Front. Pharmacol. 2017, 8, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raposio, E.; Guida, C.; Coradeghini, R.; Scanarotti, C.; Parodi, A.; Baldelli, I.; Fiocca, R.; Santi, P.L. In vitro polydeoxyribonucleotide effects on human pre-adipocytes. Cell Prolif. 2008, 41, 739–754. [Google Scholar] [CrossRef] [PubMed]
- Jelić, D.; Papović, S.; Vraneš, M.; Gadžurić, S.; Berto, S.; Alladio, E.; Gajic, D.; Janković, B. Thermo-analytical and compatibility study with mechanistic explanation of degradation kinetics of ambroxol hydrochloride tablets under non-isothermal conditions. Pharmaceutics 2021, 13, 1910. [Google Scholar] [CrossRef]
- Tomozawa, M.; Hiromoto, S. Growth mechanism of hydroxyapatite-coatings formed on pure magnesium and corrosion behavior of the coated magnesium. Appl. Surf. Sci. 2011, 257, 8253–8257. [Google Scholar] [CrossRef]
- Guillen-Romero, L.D.; Oropeza-Guzman, M.T.; Lopez-Maldonado, E.A.; Iglesias, A.L.; Paz-Gonzalez, J.A.; Ng, T. Serena-Gomez, E.; Villarreal-Gomez, L.J. Synthetic hydroxyapatite and its use in bioactive coatings. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800018817463. [Google Scholar]
- Shin, D.Y.; Park, J.U.; Choi, M.H.; Kim, S.; Kim, H.E.; Jeong, S.H. Polydeoxyribonucleotide-delivering therapeutic hydrogel for diabetic wound healing. Sci. Rep. 2020, 10, 16811. [Google Scholar] [CrossRef]
- Guizzardi, S.; Galli, C.; Govoni, P.; Boratto, R.; Cattarini, G.; Martini, D.; Belletti, S.; Scandroglio, R. Polydeoxyribonucleotide (PDRN) promotes human osteoblast proliferation: A new proposal for bone tissue repair. Life Sci. 2003, 73, 1973–1983. [Google Scholar] [CrossRef]
- Nakamura, E.I.; Uezono, Y.; Narusawa, K.I.; Shibuya, I.; Oishi, Y.; Tanaka, M.; Yanagihara, N.; Nakamura, T.; Izumi, F. ATP activates DNA synthesis by acting on P2X receptors in human osteoblast-like MG-63 cells. Am. J. Physiol. Cell Physiol. 2000, 279, C510–C519. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-Y.; Kang, I.-G.; Han, C.-M. In Situ Fabrication of Polydeoxyribonucleotide-Impregnated Hydroxyapatite onto a Magnesium Surface. Coatings 2023, 13, 72. https://doi.org/10.3390/coatings13010072
Kim J-Y, Kang I-G, Han C-M. In Situ Fabrication of Polydeoxyribonucleotide-Impregnated Hydroxyapatite onto a Magnesium Surface. Coatings. 2023; 13(1):72. https://doi.org/10.3390/coatings13010072
Chicago/Turabian StyleKim, Jin-Young, In-Gu Kang, and Cheol-Min Han. 2023. "In Situ Fabrication of Polydeoxyribonucleotide-Impregnated Hydroxyapatite onto a Magnesium Surface" Coatings 13, no. 1: 72. https://doi.org/10.3390/coatings13010072




