The Effect of Processing Parameters on the Mechanical Properties of Calcia-Stabilized Zirconia (CSZ) for Dental Use
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
- (1)
- Mechanical alloying of larger-sized commercial powders (ZrO2 and CaO) was successfully carried out in a cryogenic environment and micron-sized powders were reduced to nanosized grains.
- (2)
- SEM, XRD, and EDS studies on the powders prepared were carried out to validate the presence of nanograins by calculating size using Scherrer’s formula and also validated that powders retained the cubic structure at room temperature.
- (3)
- Density is measured for various sintered samples and found as above 90% theoretical density for samples of all conditions.
- (4)
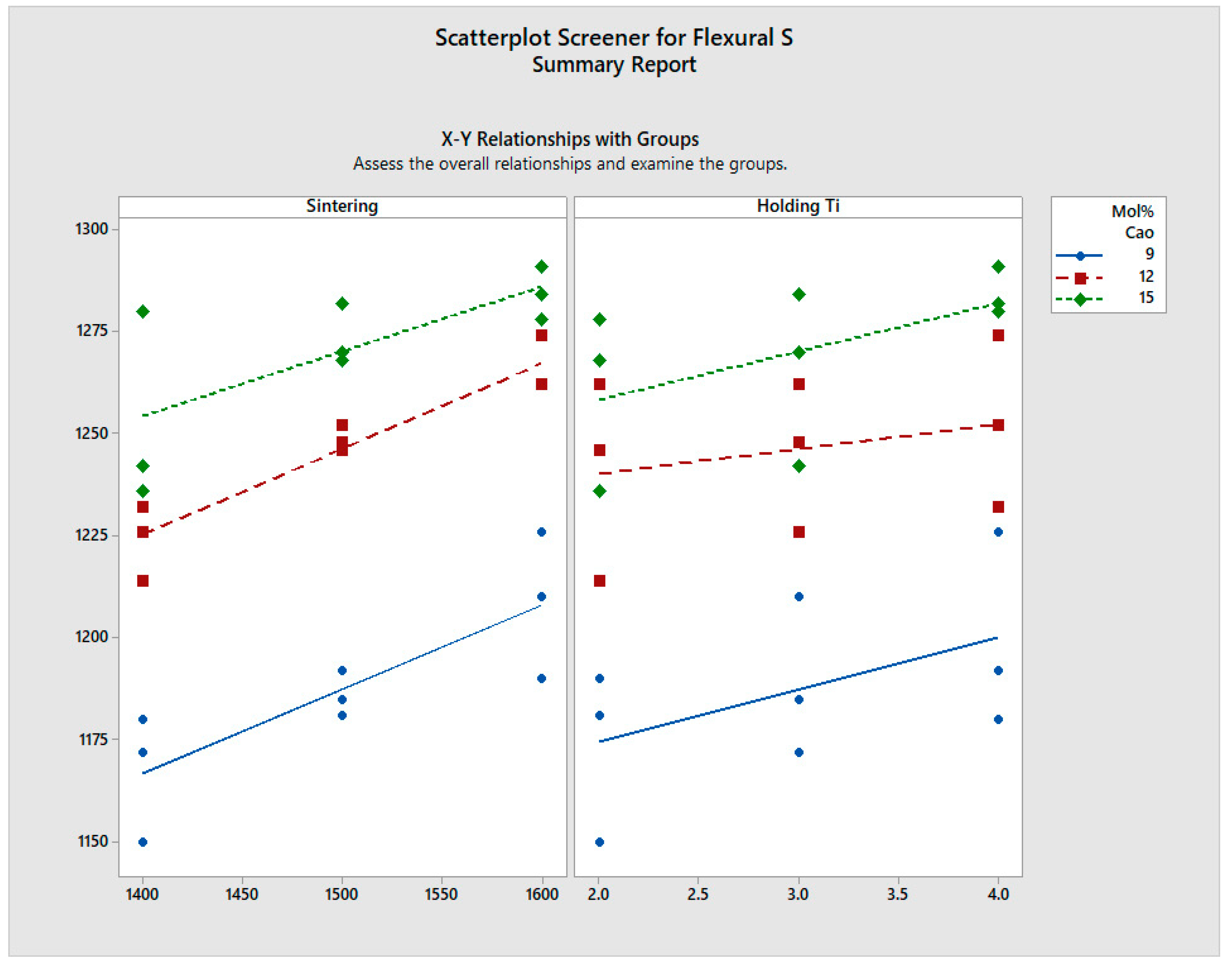
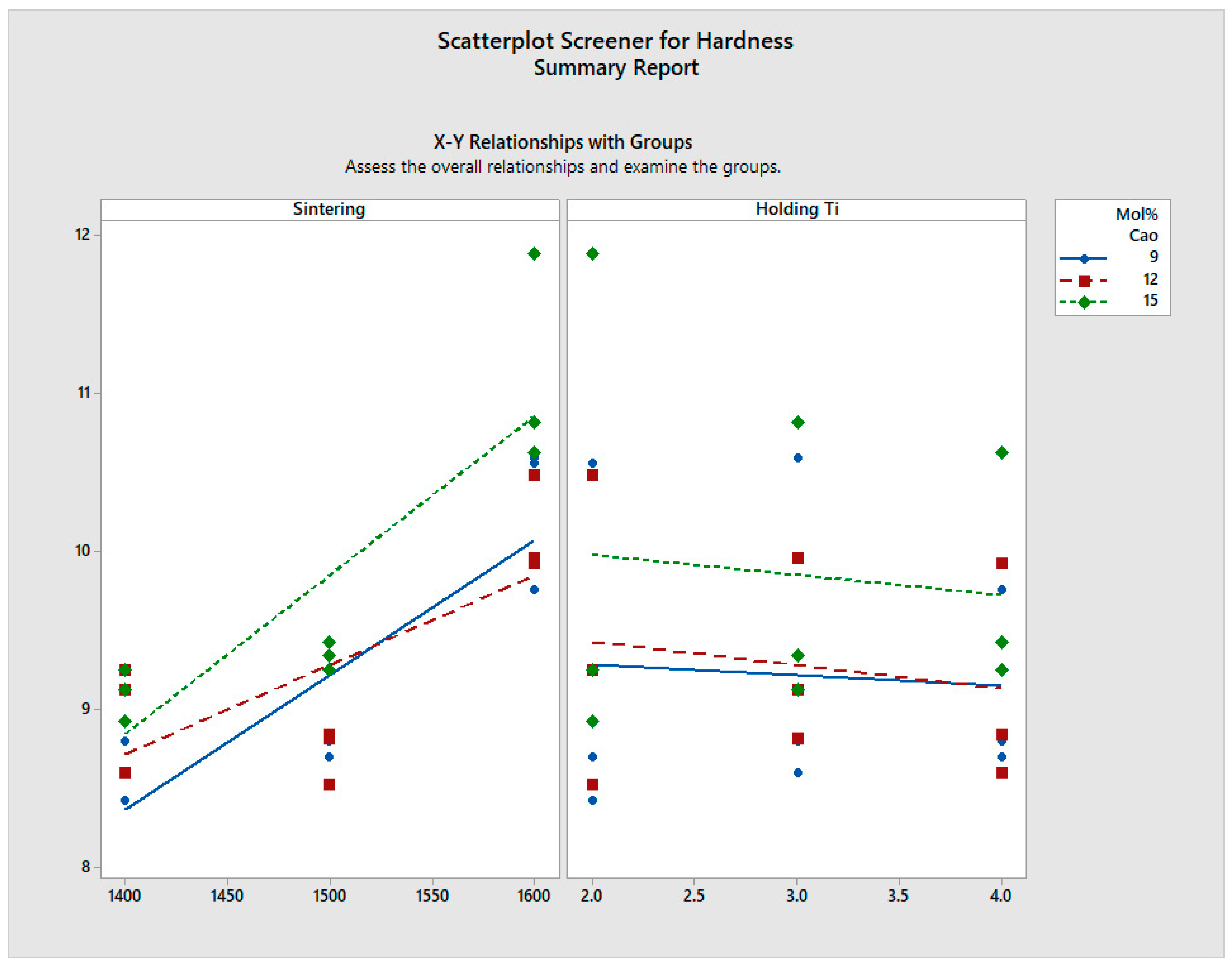
- Vickers hardness of 11.88 GPawas found for the 15 mol% CaO-stabilised zirconia sintered at 1600 °C and 2 h. Flexural strength varied between 1.1 GPa and 1.3 GPa for all the samples, showing an increase in strength with an increase in sintering temperature. A very marginal increase in flexural strength is noted with increased dwell time.
- (5)
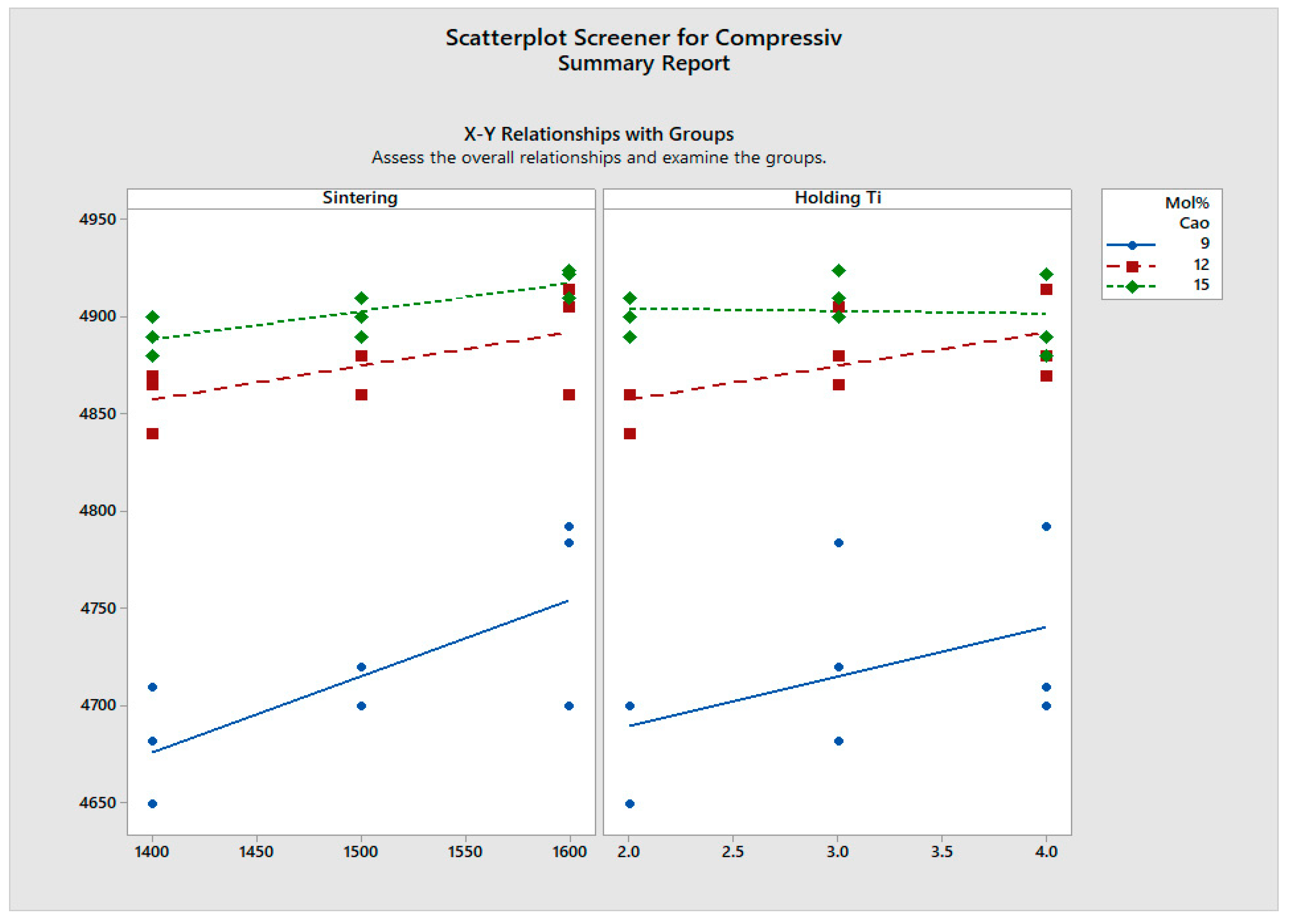
- Compressive strength also reflects similar trend as flexural strength.
- (6)
- From the literature, it is known that sintering temperatures beyond 1600 °C and longer dwell times are not suitable for improving mechanical properties, as samples may get burnt out and become brittle.
- (7)
- Mechanical properties achieved by cryo-milling are comparable to the already in use materials, such as ZTA or ceria-doped zirconia. Low-cost CSZ obtained by cryo-milling can be utilised as an alternative, cheaper dental material.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Afrashtehfar, K.I.; Del Fabbro, M. Clinical performance of zirconia implants: A meta-review. J. Prosthet. Dent. 2020, 123, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Kannan, M.; Singh, S.; Prasad, R.R. Synthetic Methods to Obtain Calcia-Stabilized Zirconia Powders—A Review. Adv. Mech. Eng. 2021, 405–416. [Google Scholar] [CrossRef]
- Ming, W.Y.; Guo, X.D.; Xu, Y.J.; Zhang, G.J.; Jiang, Z.W.; Li, Y.Z.; Li, X.K. Progress in non-traditional machining of amorphous alloys. Ceram. Int. 2022, 49, 1585–1604. [Google Scholar] [CrossRef]
- Raigrodski, A.J. Contemporary materials and technologies for all-ceramic fixed partial dentures: A review of the literature. J. Prosthet. Dent. 2004, 92, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Denry, I.; Kelly, J.R. State of the art of zirconia for dental applications. Dent. Mater. 2008, 24, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Piconi, C.; Maccauro, G. Zirconia as a ceramic biomaterial. Biomaterials 1999, 20, 1–25. [Google Scholar] [CrossRef]
- Christel, P.; Meunier, A.; Heller, M.; Torre, J.; Peille, C. Mechanical properties and short-term in vivo evaluation of yttrium-oxide-partially-stabilized zirconia. J. Biomed. Mater. Res. 1989, 23, 45–61. [Google Scholar] [CrossRef]
- Guazzato, M.; Albakry, M.; Ringer, S.P.; Swain, M.V. Strength, fracture toughness and microstructure of a selection of all-ceramic materials. Part II. Zirconia-based dental ceramics. Dent. Mater. 2004, 20, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Jin, P.; Wang, Y.; Pei, G.; Wu, J.; Wang, Z. X-ray diffraction analysis of the yttria stabilized zirconia powder by mechanical alloying and sintering. Ceram. Int. 2020, 46, 9691–9697. [Google Scholar] [CrossRef]
- Hannink, R.H.; Kelly, P.M.; Muddle, B.C. Transformation toughening in zirconia-containing ceramics. J. Am. Ceram. Soc. 2000, 83, 461–487. [Google Scholar] [CrossRef]
- Daou, E.E. The Zirconia Ceramic: Strengths and Weaknesses. Open Dent. J. 2014, 8, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Kohorst, P.; Borchers, L.; Strempel, J.; Stiesch, M.; Hassel, T.; Bach, F.-W.; Hübsch, C. Low-temperature degradation of different zirconia ceramics for dental applications. Acta Biomater. 2012, 8, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.; Sinha, N.; Basu, B. Microstructure, mechanical and tribological properties of microwave sintered calcia-doped zirconia for biomedical applications. Ceram. Int. 2008, 34, 1509–1520. [Google Scholar] [CrossRef]
- Drazin, J.W.; Castro, R.H. Phase stability in calcia-doped zirconia nanocrystals. J. Am. Ceram. Soc. 2016, 99, 1778–1785. [Google Scholar] [CrossRef]
- Hussein, A.I.; Mat, A.N.C.; Wahab, N.A.A.A.; Rahman, I.A.; Husein, A.; Ab-Ghani, Z. Synthesis and Properties of Novel Calcia-Stabilized Zirconia (Ca-SZ) with Nano Calcium Oxide Derived from Cockle Shells and Commercial Source for Dental Application. Appl. Sci. 2020, 10, 5751. [Google Scholar] [CrossRef]
- Kurapova, O.Y.; Glumov, O.; Pivovarov, M.; Golubev, S.; Konakov, V. Structure and conductivity of calcia stabilized zirconia ceramics, manufactured from freeze-dried nanopowder. Rev. Adv. Mater. Sci. 2017, 52, 134–141. [Google Scholar]
- Chen, C.; Qiang, S.; Li, J.; Zhang, L. Sintering and Phase Transformation of 7 wt% Calcia-stabilized Zirconia Ceramics. J. Mater. Sci. Ed. 2009, 24, 304–307. [Google Scholar]
- Turon-Vinas, M.; Zhang, F.; Vleugels, J.; Anglada, M. Effect of calcia co-doping on ceria-stabilized zirconia. J. Eur. Ceram. Soc. 2018, 38, 2621–2631. [Google Scholar] [CrossRef] [Green Version]
- Stawarczyk, B.; Özcan, M.; Hallmann, L.; Ender, A.; Mehl, A.; Hämmerlet, C.H. The effect of zirconia sintering temperature on flexural strength, grain size, and contrast ratio. Clin. Oral Investig. 2012, 17, 269–274. [Google Scholar] [CrossRef] [Green Version]
- Ersoy, N.M.; Aydoğdu, H.M.; Değirmenci, B.Ü.; Çökük, N.; Sevimay, M. The effects of sintering temperature and duration on the flexural strength and grain size of zirconia. Acta Biomater. Odontol. Scand. 2015, 1, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Wang, C.-A. Effect of sintering temperature on compressive strength of porous yttria-stabilized zirconia ceramics. Ceram. Int. 2010, 36, 1697–1701. [Google Scholar] [CrossRef]
- Bruni, Y.; Booth, F.; Garrido, L.; Aglietti, E. Densification and mechanical properties of ZrO2–CaAl4O7 composites obtained by reaction sintering. Int. J. Mater. Res. (Former. Z. Metallkd.) 2016, 107, 851–859. [Google Scholar] [CrossRef]
- Tanaka, H.; Maeda, T.; Narikiyo, H.; Morimoto, T. Mechanical properties of partially stabilized zirconia for dental applications. J. Asian Ceram. Soc. 2019, 7, 460–468. [Google Scholar] [CrossRef]
- Fischer, J.; Stawarczyk, B.; Hammerle, C. Flexural strength of veneering ceramics for zirconia. J. Dent. 2008, 36, 316–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renshaw, C.E.; Murdza, A.; Schulson, E.M. Experimental Verification of the Isotropic Onset of Percolation in 3D Crack Networks in Polycrystalline Materials with Implications for the Applicability of Percolation Theory to Crustal Rocks. J. Geophys. Res. Solid Earth 2021, 126, e2021JB023092. [Google Scholar] [CrossRef]



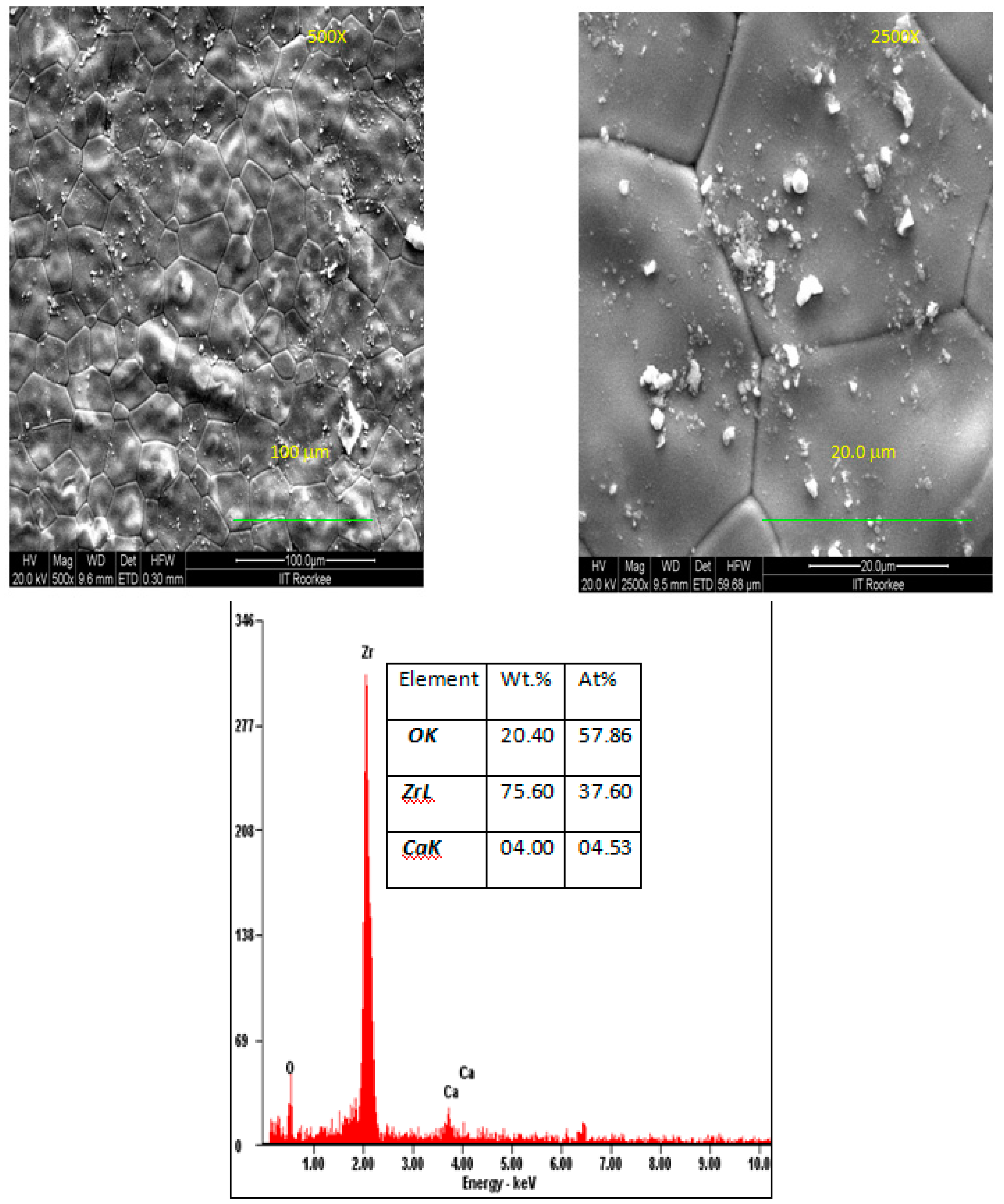
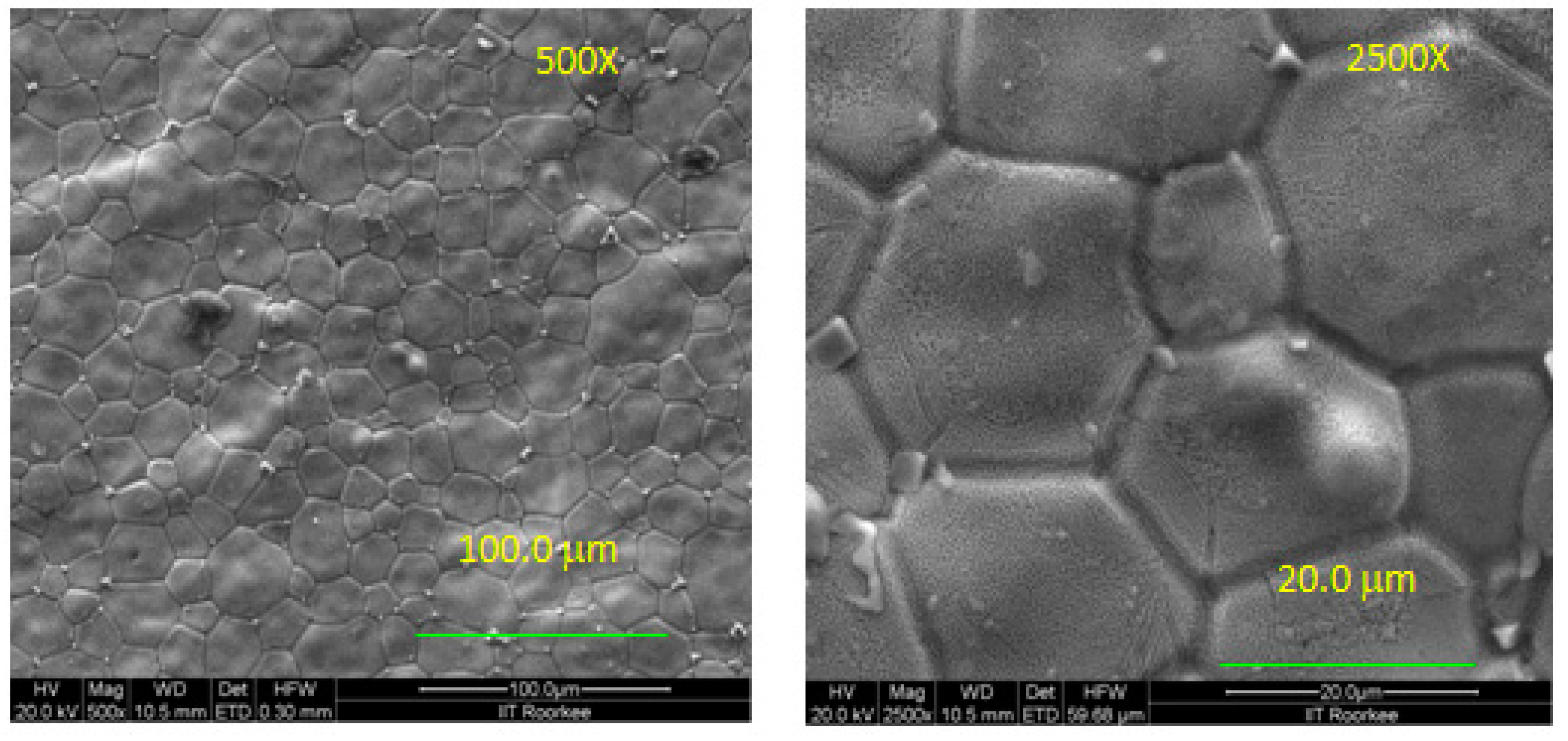

| 9 Mol% | 12 Mol% | 15 Mol% | ||||
|---|---|---|---|---|---|---|
| Element | Wt.% | At% | Wt.% | At% | Wt.% | At% |
| O K | 31.28 | 71.14 | 31.28 | 71.14 | 31.28 | 71.14 |
| Zr L | 65.88 | 26.28 | 65.88 | 26.28 | 65.88 | 26.28 |
| Ca K | 02.85 | 02.58 | 02.85 | 02.58 | 02.85 | 02.58 |
| CaO μm | ZrO2 μm | 9 Mol% CaO nm | 12 Mol% CaO nm | 15 Mol% CaO nm |
|---|---|---|---|---|
| 10 | 5 | 21.07 | 21.69 | 22.15 |
| Mol % | Sintering Temperature (°C) | Density | % Change | |
|---|---|---|---|---|
| Before Sintering (g/cm3) | After Sintering (g/cm3) | |||
| 9 | 1400 | 3.55 | 5.21 | 46 |
| 1500 | 3.55 | 5.23 | 47 | |
| 1600 | 3.55 | 5.28 | 48.5 | |
| 12 | 1400 | 3.51 | 5.11 | 45 |
| 1500 | 3.51 | 5.18 | 47.5 | |
| 1600 | 3.51 | 5.26 | 49.5 | |
| 15 | 1400 | 3.68 | 5.19 | 41 |
| 1500 | 3.68 | 5.26 | 43 | |
| 1600 | 3.68 | 5.29 | 43.5 | |
| Mol % | Dwell Time (h) | Density | % Change | |
|---|---|---|---|---|
| Before Sintering (g/cm3) | After Sintering (g/cm3) | |||
| 9 | 2 | 3.55 | 5.21 | 46 |
| 3 | 3.55 | 5.21 | 46 | |
| 4 | 3.55 | 5.22 | 47 | |
| 12 | 2 | 3.51 | 5.11 | 45 |
| 3 | 3.51 | 5.11 | 45 | |
| 4 | 3.51 | 5.12 | 45.5 | |
| 15 | 2 | 3.68 | 5.17 | 40 |
| 3 | 3.68 | 5.18 | 40.5 | |
| 4 | 3.68 | 5.19 | 41 | |
| S. No. | Mol% CaO | Sintering Temp (°C) | Holding Time (h) | Hardness (GPa) | Flexural Strength (MPa) | Compressive Strength (MPa) | |
|---|---|---|---|---|---|---|---|
| 1 | 9% | A | 1400 | 2 | 8.42 | 1150 | 4650 |
| 2 | 1500 | 2 | 8.7 | 1181 | 4700 | ||
| 3 | 1600 | 2 | 10.56 | 1190 | 4700 | ||
| 4 | B | 1500 | 2 | 8.7 | 1180 | 4700 | |
| 5 | 1500 | 3 | 8.8 | 1185 | 4720 | ||
| 6 | 1500 | 4 | 8.7 | 1192 | 4700 | ||
| 10 | 12% | A | 1400 | 2 | 9.25 | 1214 | 4840 |
| 11 | 1500 | 2 | 8.52 | 1246 | 4860 | ||
| 12 | 1600 | 2 | 10.48 | 1262 | 4860 | ||
| 13 | B | 1500 | 2 | 8.52 | 1246 | 4860 | |
| 14 | 1500 | 3 | 8.81 | 1248 | 4880 | ||
| 15 | 1500 | 4 | 8.84 | 1252 | 4880 | ||
| 19 | 15% | A | 1400 | 2 | 8.92 | 1236 | 4890 |
| 20 | 1500 | 2 | 9.25 | 1268 | 4900 | ||
| 21 | 1600 | 2 | 11.88 | 1278 | 4910 | ||
| 22 | B | 1500 | 2 | 9.25 | 1268 | 4900 | |
| 23 | 1500 | 3 | 9.34 | 1270 | 4910 | ||
| 24 | 1500 | 4 | 9.42 | 1282 | 4890 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kannan, M.; Singh, S.; Prasad, R.R. The Effect of Processing Parameters on the Mechanical Properties of Calcia-Stabilized Zirconia (CSZ) for Dental Use. Coatings 2023, 13, 75. https://doi.org/10.3390/coatings13010075
Kannan M, Singh S, Prasad RR. The Effect of Processing Parameters on the Mechanical Properties of Calcia-Stabilized Zirconia (CSZ) for Dental Use. Coatings. 2023; 13(1):75. https://doi.org/10.3390/coatings13010075
Chicago/Turabian StyleKannan, M., Satyendra Singh, and Raja Ram Prasad. 2023. "The Effect of Processing Parameters on the Mechanical Properties of Calcia-Stabilized Zirconia (CSZ) for Dental Use" Coatings 13, no. 1: 75. https://doi.org/10.3390/coatings13010075





