Evaluation in Real Conditions of New Anticorrosive Formulations Based on Polyphenols from Natural Sources and Encapsulated Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Encapsulation of Zinc Oxide Nanoparticles
2.2. Formulation and Application of Primer-Anticorrosive Coating and Top Coat
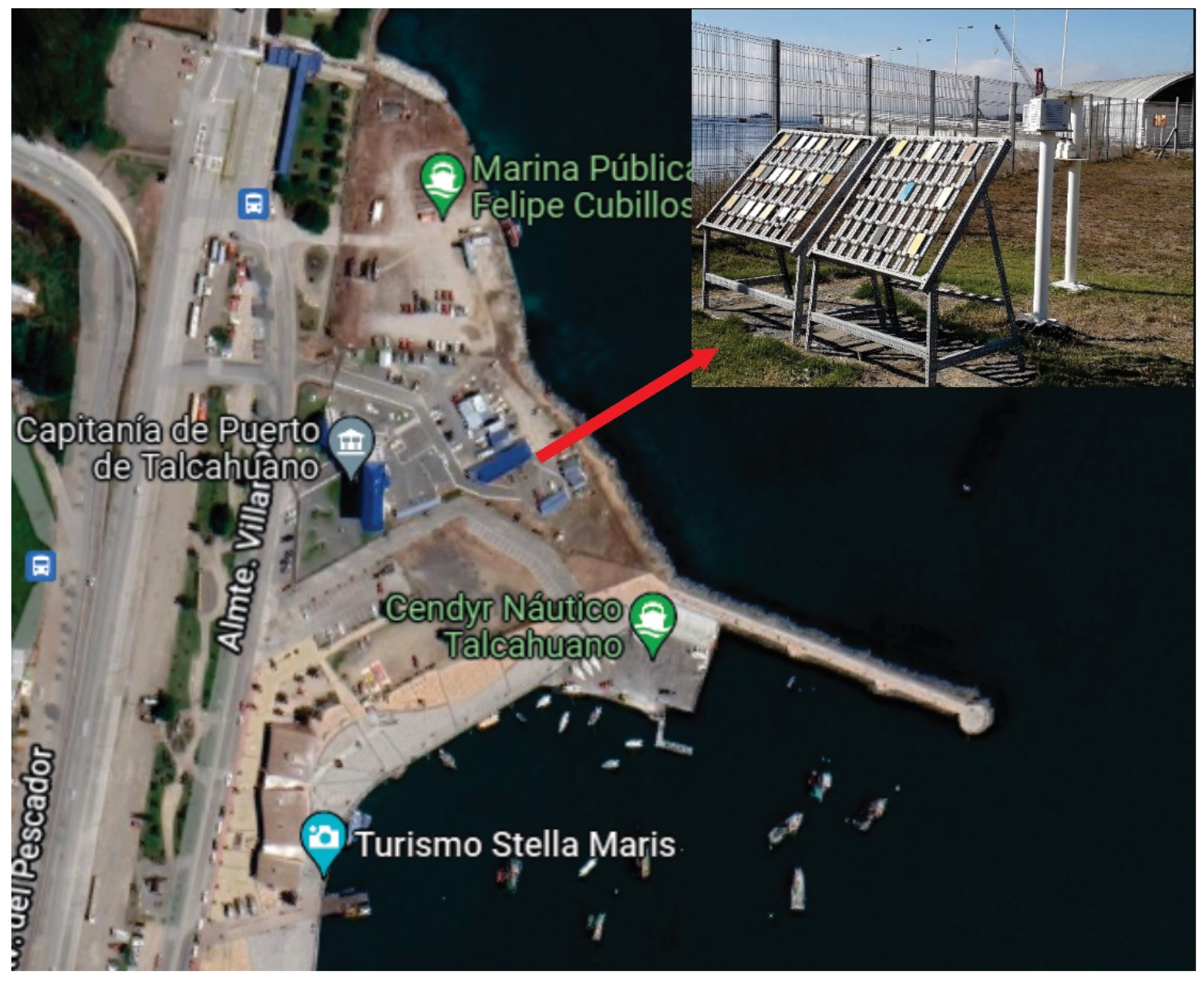
2.3. Meteorological and Meteorochemical Characterization of the Monitoring Station
2.4. Evaluation of Functional Properties in Accelerated Tests
2.4.1. Film Properties
2.4.2. Mechanical Properties
2.4.3. Resistance to Corrosion
3. Results and Discussions
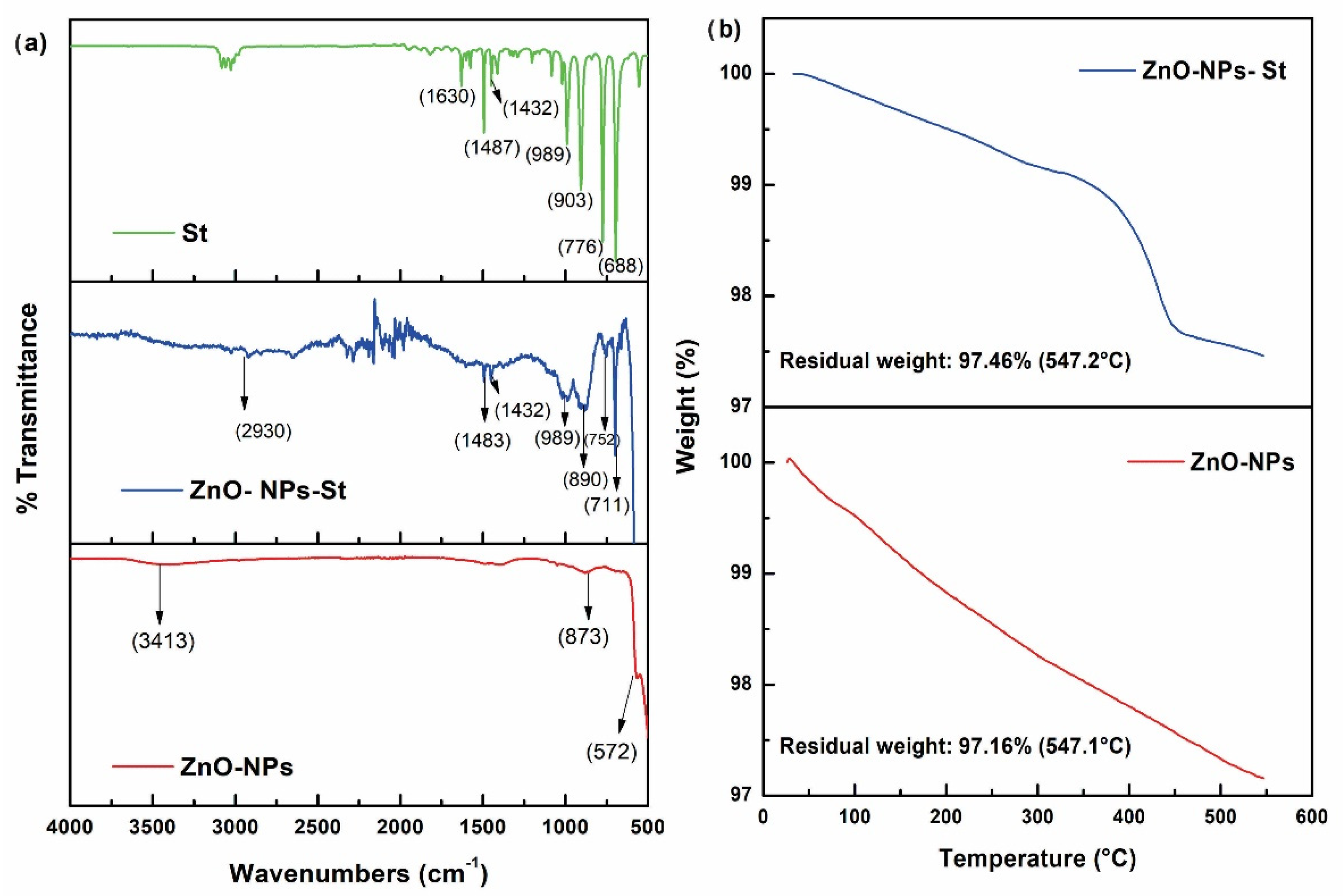
3.1. Synthesis, Functionalization, and Encapsulation of ZnO Nanoparticles
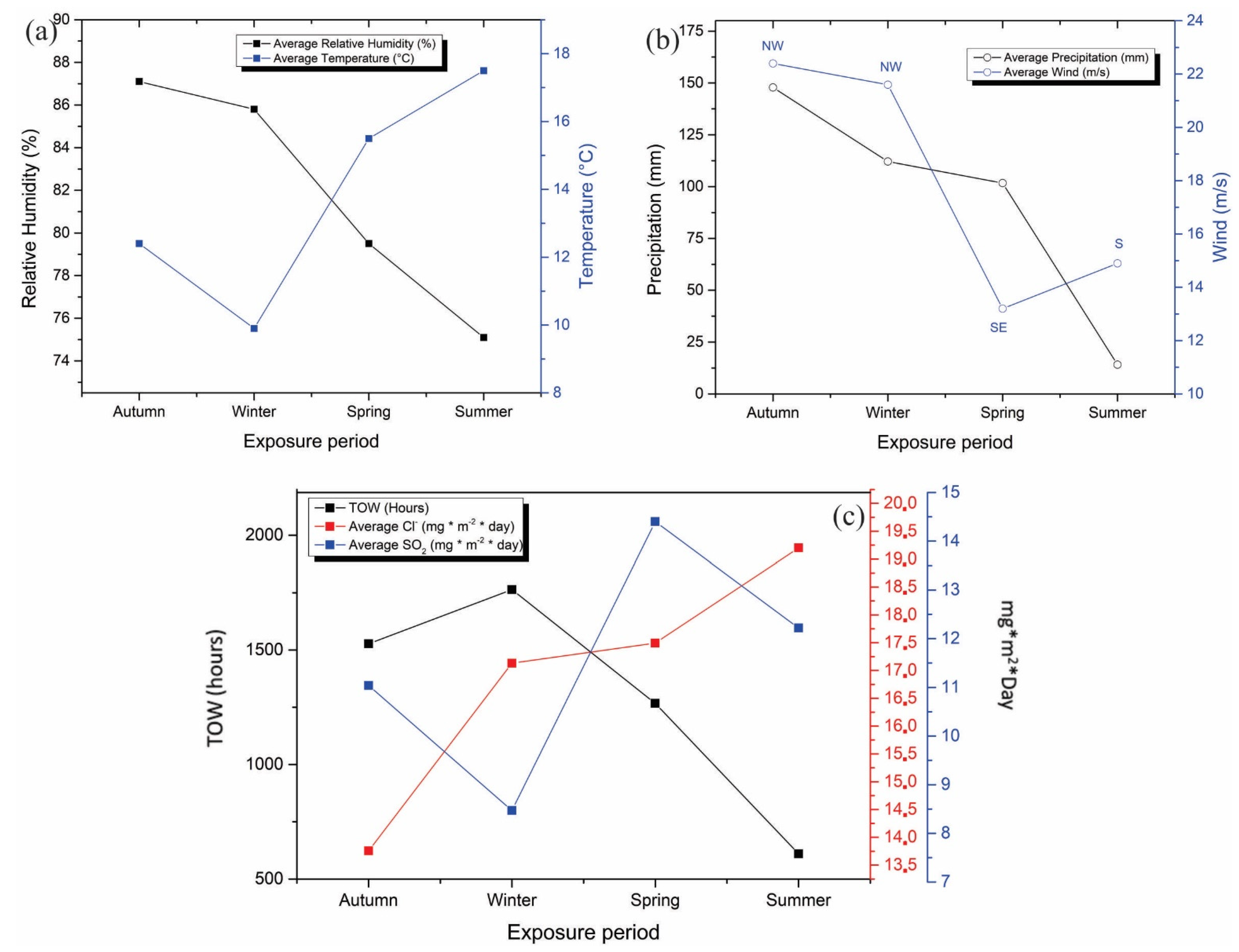
3.2. Meteorological and Meteorochemical Characterization of the Monitoring Station
3.3. Evaluation of the Functional Properties of the Coating in the Accelerated Corrosion Chamber and in the Field
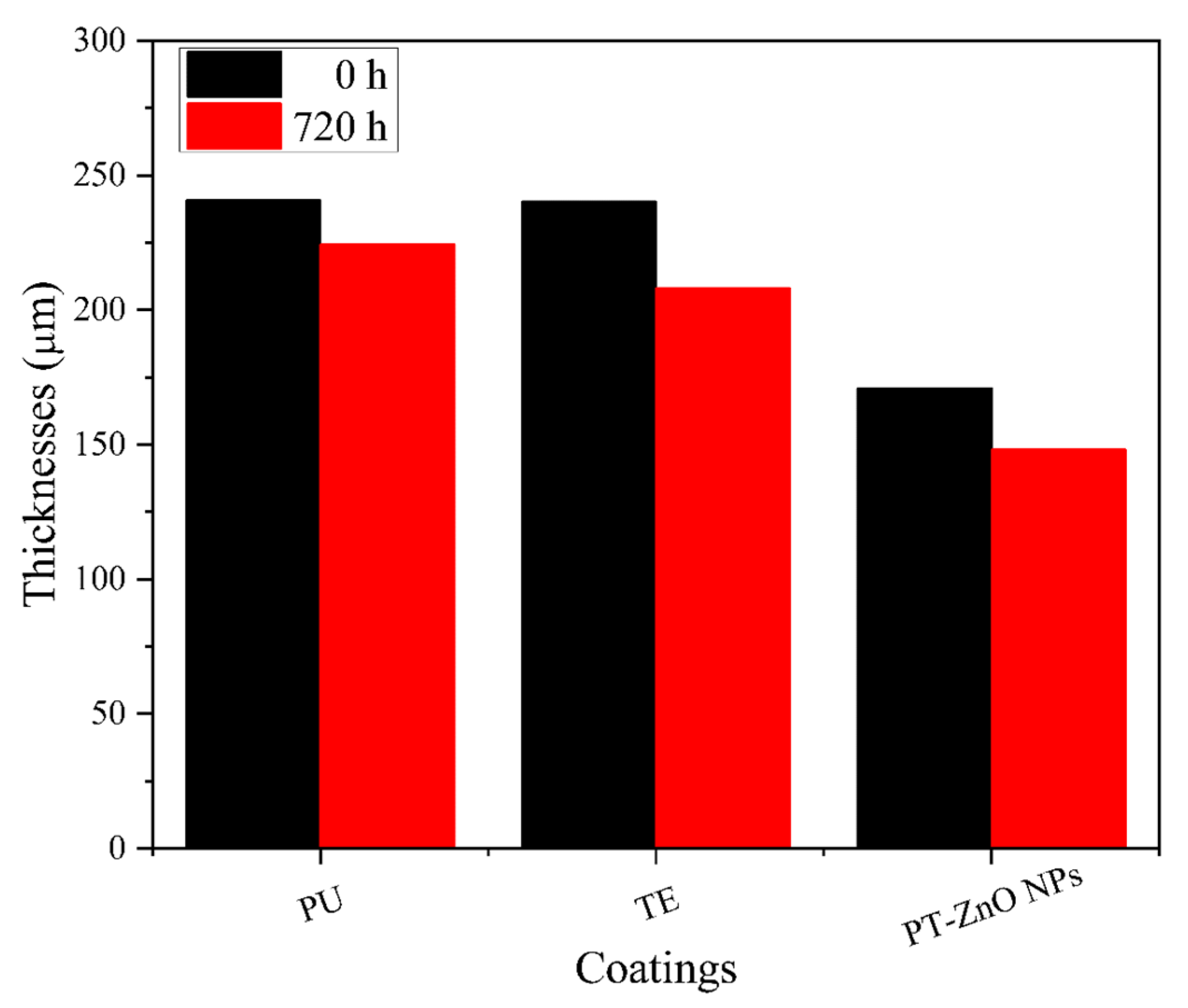
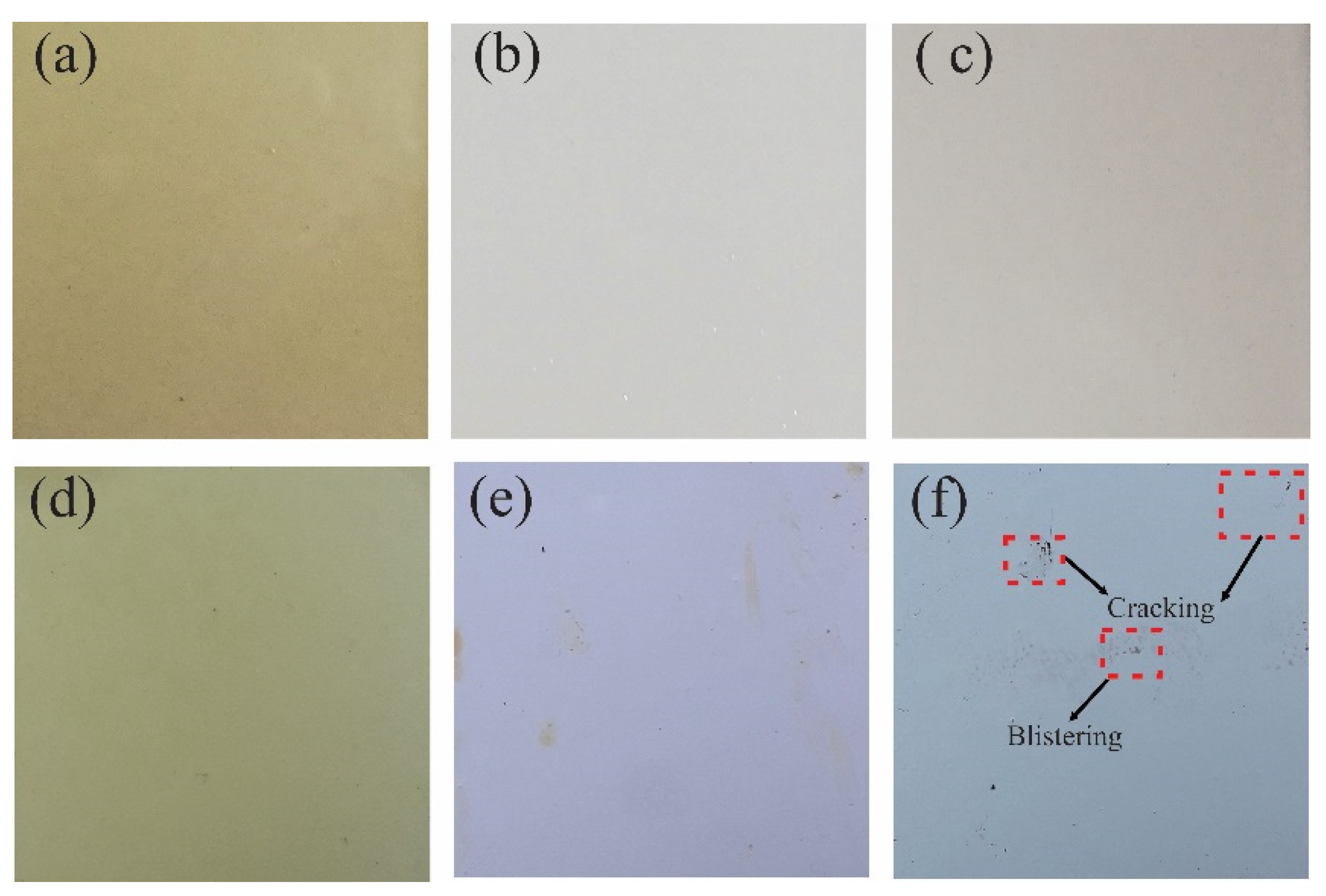
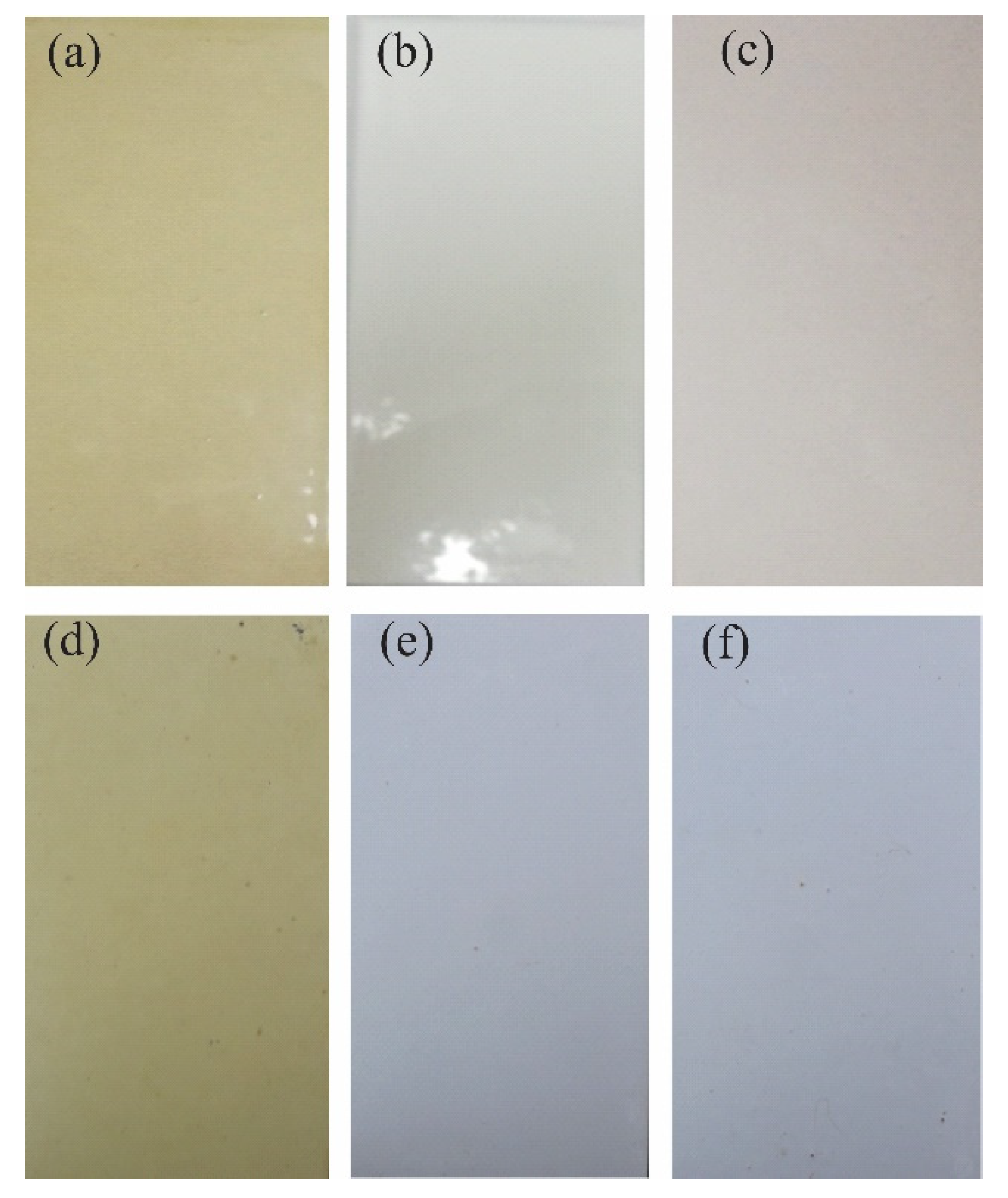
3.3.1. Film Properties
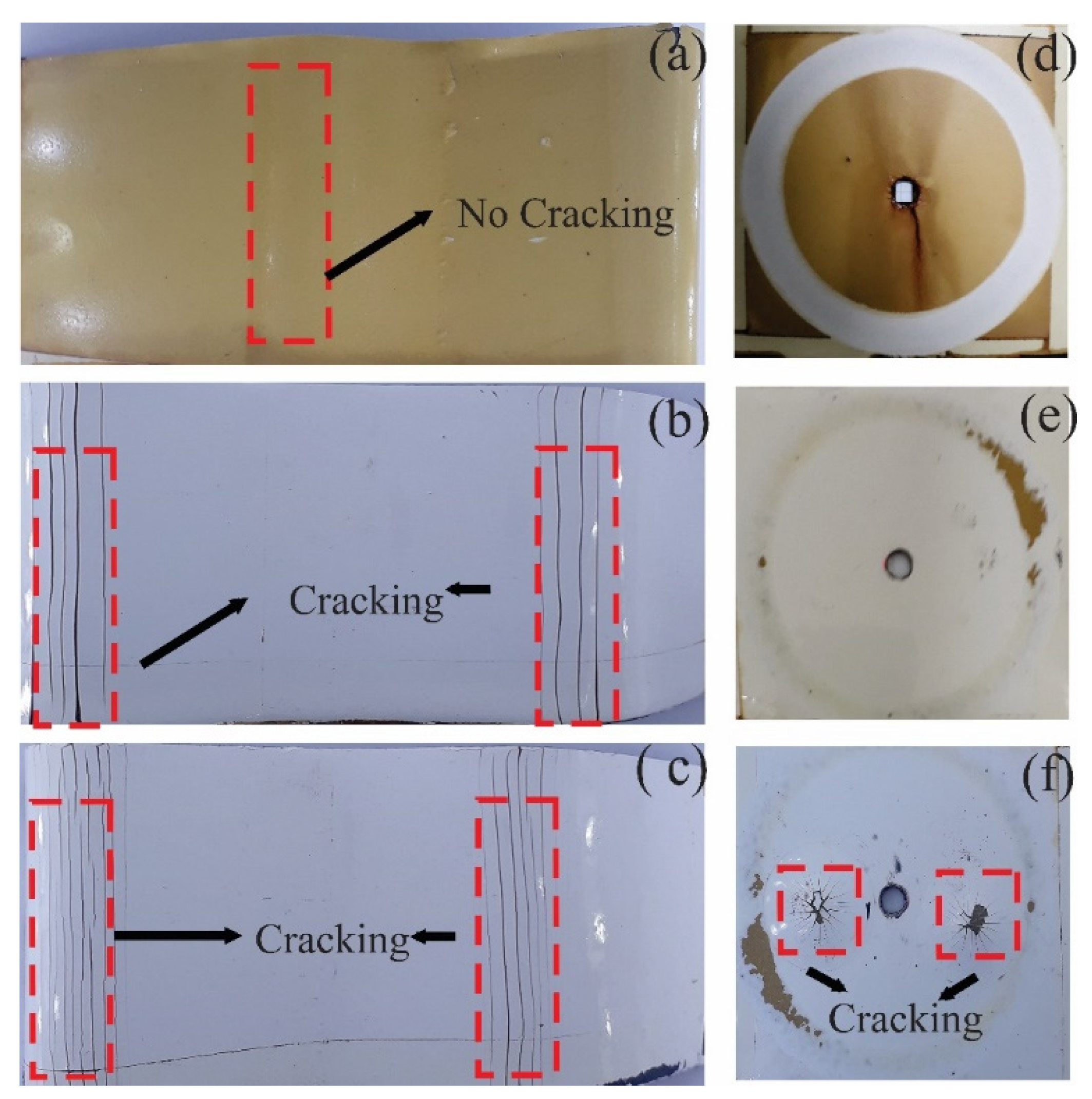
3.3.2. Mechanical Properties
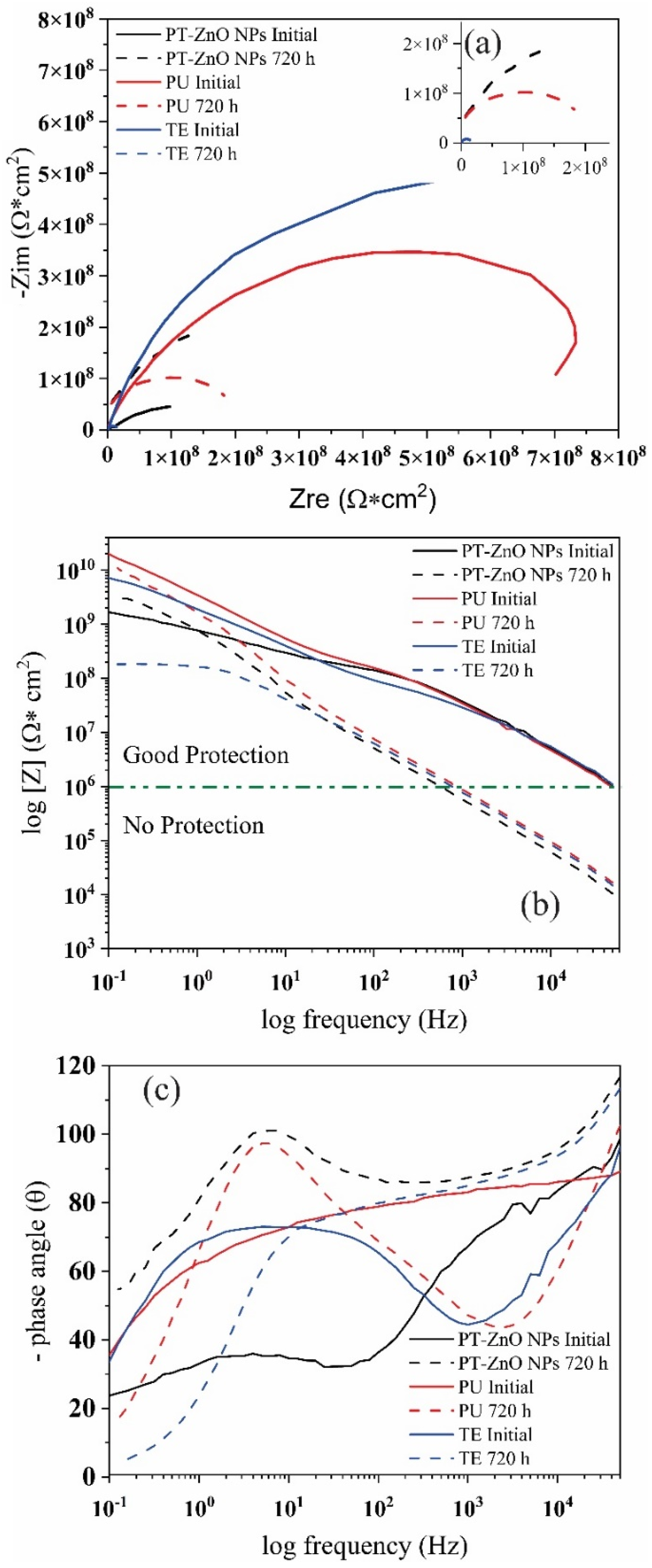
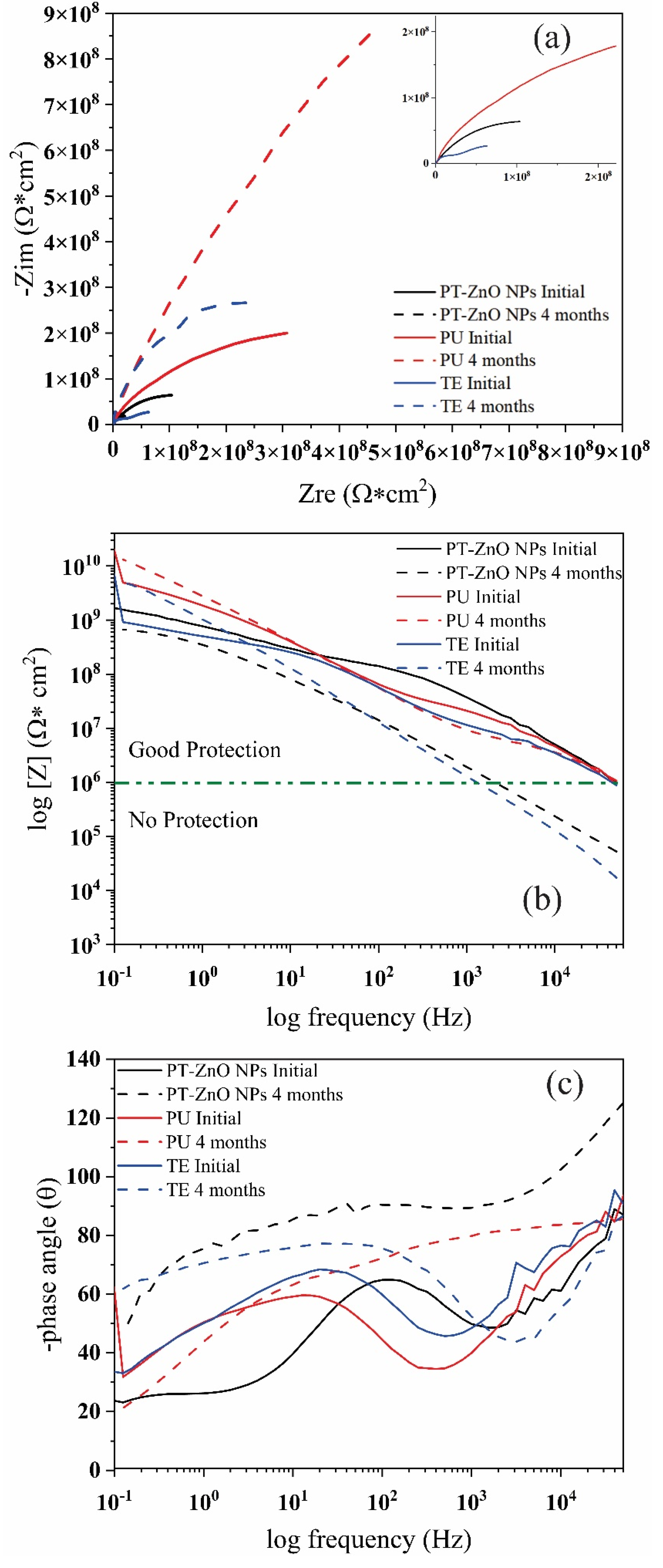
3.3.3. Evaluation of Anticorrosive Properties
Coating Exposed to Prohesion Tests (Exposure to a Weathering Chamber and Salt Spray)
Coating Exposed in the Field
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soares, C.G.; Garbatov, Y.; Zayed, A.; Wang, G. Influence of environmental factors on corrosion of ship structures in marine atmosphere. Corros. Sci. 2009, 51, 2014–2026. [Google Scholar] [CrossRef]
- Morcillo, M.; Alcántara, J.; Díaz, I.; Chico, B.; Simancas, J.; De la Fuente, D. Marine atmospheric corrosion of carbon steels. Rev. Metal. 2015, 51, e045. [Google Scholar] [CrossRef]
- De la Fuente, D.; Díaz, I.; Simancas, J.; Chico, B.; Morcillo, M. Long-term atmospheric corrosion of mild steel. Corros. Sci. 2011, 53, 604–617. [Google Scholar] [CrossRef]
- Morcillo, M.; Chico, B.; Díaz, I.; Cano, H.; de la Fuente, D. Atmospheric corrosion data of weathering steels. A review. Corros. Sci. 2013, 77, 6–24. [Google Scholar] [CrossRef]
- Palraj, S.; Selvaraj, M.; Maruthan, K.; Natesan, M. Kinetics of atmospheric corrosion of mild steel in marine and rural environments. J. Mar. Sci. Appl. 2015, 14, 105–112. [Google Scholar] [CrossRef]
- Vera, R.; Delgado, D.; Rosales, B.M. Effect of atmospheric pollutants on the corrosion of high power electrical conductors—Part 2. Pure copper. Corros. Sci. 2007, 49, 2329–2350. [Google Scholar] [CrossRef]
- De la Fuente, D.; Díaz, I.; Alcántara, J.; Chico, B.; Simancas, J.; Llorente, I.; García-Delgado, A.; Jiménez, J.A.; Adeva, P.; Morcillo, M. Corrosion mechanisms of mild steel in chloride-rich atmospheres. Mater. Corros. 2016, 67, 227–238. [Google Scholar] [CrossRef]
- Amalvy, J.I.; Aznar, A.C.; Pardini, O.R.; Guzmán, G.A. Waterborne Anticorrosive Systems for Steel Protection—Part 1: Formulation and Testing. Corrosion 2002, 58, 871–880. [Google Scholar] [CrossRef]
- Montemor, M.F. Functional and smart coatings for corrosion protection: A review of recent advances. Surf. Coat. Technol. 2014, 258, 17–37. [Google Scholar] [CrossRef]
- Riaz, U.; Nwaoha, C.; Ashraf, S.M. Recent advances in corrosion protective composite coatings based on conducting polymers and natural resource derived polymers. Prog. Org. Coat. 2014, 77, 743–756. [Google Scholar] [CrossRef]
- Akram, D.; Sharmin, E.; Ahmad, S. Synthesis, characterization and corrosion protective properties of boron-modified polyurethane from natural polyol. Prog. Org. Coat. 2008, 63, 25–32. [Google Scholar] [CrossRef]
- Rani, B.E.A.; Basu, B.B.J. Green Inhibitors for Corrosion Protection of Metals and Alloys: An Overview. Int. J. Corros. 2012, 2012, 380217. [Google Scholar] [CrossRef]
- Twite, R.L.; Bierwagen, G.P. Review of alternatives to chromate for corrosion protection of aluminum aerospace alloys. Prog. Org. Coat. 1998, 33, 91–100. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Zheludkevich, M.L.; Yasakau, K.A.; Montemor, M.F.; Ferreira, M.G.S. High effective organic corrosion inhibitors for 2024 aluminium alloy. Electrochim. Acta 2007, 52, 7231–7247. [Google Scholar] [CrossRef]
- Eugene, U.; O’Donnell, P.S.; Ifeoma, V.J.; Feyisayo, V.A. The effect of corrosion inhibitors on stainless steels and aluminium alloys: A review. African J. Pure Appl. Chem. 2016, 10, 23–32. [Google Scholar] [CrossRef]
- Mukherjee, A.; Joshi, M.; Misra, S.C.; Ramesh, U.S. Antifouling paint schemes for green SHIPS. Ocean Eng. 2019, 173, 227–234. [Google Scholar] [CrossRef]
- Montoya, L.F.; Contreras, D.; Jaramillo, A.F.; Carrasco, C.; Fernández, K.; Schwederski, B.; Rojas, D.; Melendrez, M.F. Study of anticorrosive coatings based on high and low molecular weight polyphenols extracted from the Pine radiata bark. Prog. Org. Coat. 2019, 127, 100–109. [Google Scholar] [CrossRef]
- LeBozec, N.; Thierry, D.; Le Calvé, P.; Favennec, C.; Pautasso, J.-P.; Hubert, C. Performance of marine and offshore paint systems: Correlation of accelerated corrosion tests and field exposure on operating ships. Mater. Corros. 2015, 66, 215–225. [Google Scholar] [CrossRef]
- Jaramillo, A.F.; Montoya, L.F.; Prabhakar, J.M.; Sanhueza, J.P.; Fernández, K.; Rohwerder, M.; Rojas, D.; Montalba, C.; Melendrez, M.F. Formulation of a multifunctional coating based on polyphenols extracted from the Pine radiata bark and functionalized zinc oxide nanoparticles: Evaluation of hydrophobic and anticorrosive properties. Prog. Org. Coat. 2019, 135, 191–204. [Google Scholar] [CrossRef]
- Selvakumar, N.; Jeyasubramanian, K.; Sharmila, R. Smart coating for corrosion protection by adopting nano particles. Prog. Org. Coat. 2012, 74, 461–469. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Attar, M.M. Studying the corrosion resistance and hydrolytic degradation of an epoxy coating containing ZnO nanoparticles. Mater. Chem. Phys. 2011, 130, 1208–1219. [Google Scholar] [CrossRef]
- Noor Idora, M.S.; Ferry, M.; Wan Nik, W.B.; Jasnizat, S. Evaluation of tannin from Rhizophora apiculata as natural antifouling agents in epoxy paint for marine application. Prog. Org. Coat. 2015, 81, 125–131. [Google Scholar] [CrossRef]
- Mansour, E.M.E.; Abdel-Gaber, A.M.; Abd-El Nabey, B.A.; Khalil, N.; Khamis, E.; Tadros, A.; Aglan, H.; Ludwick, A. Developing and Testing of a New Anticorrosive Coating Containing Algae as a Natural Inhibitor for Preventing Marine Corrosion of Steel. Corrosion 2003, 59, 242–249. [Google Scholar] [CrossRef]
- Rosales, B.M.; Di, A.R.; De Rincón, O.; Rincón, A.; Elsner, C.I.; Marchisio, B. An evaluation of coil coating formulations in marine environments. Prog. Org. Coat. 2004, 50, 105–114. [Google Scholar] [CrossRef]
- Elsner, C.I.; Cavalcanti, E.; Ferraz, O.; Di Sarli, A.R. Evaluation of the surface treatment effect on the anticorrosive performance of paint systems on steel. Prog. Org. Coat. 2003, 48, 50–62. [Google Scholar] [CrossRef]
- Schmidt, D.P.; Shaw, B.A.; Sikora, E.; Shaw, W.W.; Laliberte, L.H. Corrosion protection assessment of sacrificial coating systems as a function of exposure time in a marine environment. Prog. Org. Coat. 2006, 57, 352–364. [Google Scholar] [CrossRef]
- Medina, M.C.; Rojas, D.; Flores, P.; Peréz-Tijerina, E.; Meléndrez, M.F. Effect of ZnO nanoparticles obtained by arc discharge on thermo-mechanical properties of matrix thermoset nanocomposites. J. Appl. Polym. Sci. 2016, 133, 1–8. [Google Scholar] [CrossRef]
- Jaramillo, A.F.; Baez-Cruz, R.; Montoya, L.F.; Medinam, C.; Pérez-Tijerina, E.; Salazar, F.; Rojas, D.; Melendrez, M.F. Estimation of the surface interaction mechanism of ZnO nanoparticles modified with organosilane groups by Raman Spectroscopy. Ceram. Int. 2017, 43, 11838–11847. [Google Scholar] [CrossRef]
- Solis-Pomar, F.; Jaramillo, A.; Lopez-Villareal, J.; Medina, C.; Rojas, D.; Mera, A.C.; Meléndrez, M.F.; Pérez-Tijerina, E. Rapid synthesis and photocatalytic activity of ZnO nanowires obtained through microwave-assisted thermal decomposition. Ceram. Int. 2016, 42, 18045–18052. [Google Scholar] [CrossRef]
- Mostafaei, A.; Zolriasatein, A. Synthesis and characterization of conducting polyaniline nanocomposites containing ZnO nanorods. Prog. Nat. Sci. Mater. Int. 2012, 22, 273–280. [Google Scholar] [CrossRef]
- Martínez, C.; Briones, F.; Villarroel, M.; Vera, R. Effect of atmospheric corrosion on the mechanical properties of SAE 1020 structural steel. Materials 2018, 11, 591. [Google Scholar] [CrossRef]
- Valverde, B.; Vera, R.; Henríquez, N.; Olave, E. Evaluation of atmospheric galvanic aluminum corrosion in electrical transmission towers at different sites in the Valparaíso region, Chile. Wire-on-Bolt Test (CLIMAT). Mater. Corros. 2021, 72, 1607–1619. [Google Scholar] [CrossRef]
- Vera, R.; Araya, R.; Garín, C.; Ossandón, S.; Rojas, P. Study on the effect of atmospheric corrosion on mechanical properties with impact test: Carbon steel and Galvanized steel. Mater. Corros. 2019, 70, 1151–1161. [Google Scholar] [CrossRef]
- De la Fuente, D.; Alcántara, J.; Chico, B.; Díaz, I.; Jiménez, J.A.; Morcillo, M. Characterisation of rust surfaces formed on mild steel exposed to marine atmospheres using XRD and SEM/Micro-Raman techniques. Corros. Sci. 2016, 110, 253–264. [Google Scholar] [CrossRef]
- Ismail, E.A.; Motawie, A.M.; Sadek, E.M. Synthesis and characterization of polyurethane coatings based on soybean oil–polyester polyols. Egypt. J. Pet. 2011, 20, 1–8. [Google Scholar] [CrossRef]
- Sharmin, E.; Ashraf, S.M.; Ahmad, S. Synthesis, characterization, antibacterial and corrosion protective properties of epoxies, epoxy-polyols and epoxy-polyurethane coatings from linseed and Pongamia glabra seed oils. Int. J. Biol. Macromol. 2007, 40, 407–422. [Google Scholar] [CrossRef]
- Nnaji, N.J.N.; Okoye, C.O.B.; Obi-Egbedi, N.O.; Ezeokonkwo, M.A.; Ani, J.U. Spectroscopic characterization of red onion skin tannin and it’s use as alternative aluminium corrosion inhibitor in hydrochloric acid solutions. Int. J. Electrochem. Sci. 2013, 8, 1735–1758. [Google Scholar]
- Velayutham, T.S.; Majid, W.H.A.; Ahmad, A.B.; Kang, G.Y.; Gan, S.N. Synthesis and characterization of polyurethane coatings derived from polyols synthesized with glycerol, phthalic anhydride and oleic acid. Prog. Org. Coat. 2009, 66, 367–371. [Google Scholar] [CrossRef]
- Yu, D.; Wang, J.; Tian, J.; Xu, X.; Dai, J.; Wang, X. Preparation and characterization of TiO2/ZnO composite coating on carbon steel surface and its anticorrosive behavior in seawater. Compos. Part B Eng. 2013, 46, 135–144. [Google Scholar] [CrossRef]
- Olad, A.; Nosrati, R. Preparation and corrosion resistance of nanostructured PVC/ZnO-polyaniline hybrid coating. Prog. Org. Coat. 2013, 76, 113–118. [Google Scholar] [CrossRef]
- Palraj, S.; Selvaraj, M.; Maruthan, K.; Rajagopal, G. Corrosion and wear resistance behavior of nano-silica epoxy composite coatings. Prog. Org. Coat. 2015, 81, 132–139. [Google Scholar] [CrossRef]
- Sørensen, P.A.; Kiil, S.; Dam-Johansen, K.; Weinell, C.E. Anticorrosive coatings: A review. J. Coat. Technol. Res. 2009, 6, 135–176. [Google Scholar] [CrossRef]
- Mostafaei, A.; Nasirpouri, F. Epoxy/polyaniline–ZnO nanorods hybrid nanocomposite coatings: Synthesis, characterization and corrosion protection performance of conducting paints. Prog. Org. Coat. 2014, 77, 146–159. [Google Scholar] [CrossRef]
- Matamala, G.; Smeltzer, W.; Droguett, G. Use of Tannin Anticorrosive Reaction Primer to Improve Traditional Coating Systems. Corrosion 1994, 50, 270–275. [Google Scholar] [CrossRef]
- Bakhshandeh, E.; Jannesari, A.; Ranjbar, Z.; Sobhani, S.; Saeb, M.R. Anti-corrosion hybrid coatings based on epoxy–silica nano-composites: Toward relationship between the morphology and EIS data. Prog. Org. Coat. 2014, 77, 1169–1183. [Google Scholar] [CrossRef]
- Vivier, V.; Orazem, M.E. Impedance Analysis of Electrochemical Systems. Chem. Rev. 2022, 122, 11131–11168. [Google Scholar] [CrossRef]
- Yu, F.; Dai, X.; Beebe, T.; Hsiai, T. Electrochemical impedance spectroscopy to characterize inflammatory atherosclerotic plaques. Biosens. Bioelectron. 2011, 30, 165–173. [Google Scholar] [CrossRef]
- Bastidas, D.M. Interpretation of impedance data for porous electrodes and diffusion processes. Corrosion 2007, 63, 515–521. [Google Scholar] [CrossRef]
- Lasia, A. Electrochemical Impedance Spectroscopy and its Applications. Mod. Asp. Electrochem. 1999, 32, 143–248. [Google Scholar] [CrossRef]
- Park, S.-M.; Yoo, J.-S.; Chang, B.-Y.; Ahn, E.-S. Novel instrumentation in electrochemical impedance spectroscopy and a full description of an electrochemical system. Pure Appl. Chem. 2006, 78, 1069–1080. [Google Scholar] [CrossRef]
- Akbarinezhad, E.; Bahremandi, M.; Faridi, H.R.; Rezaei, F. Another approach for ranking and evaluating organic paint coatings via electrochemical impedance spectroscopy. Corros. Sci. 2009, 51, 356–363. [Google Scholar] [CrossRef]
- Mansfeld, F.; Kendig, M.W.; Tsai, S. Evaluation of Corrosion Behavior of Coated Metals with AC Impedance Measurements. Corrosion 1982, 38, 478–485. [Google Scholar] [CrossRef]
- O’Donoghue, M.; Garrett, R.; Datta, V.; Roberts, P.; Aben, T. EIS: Testing coatings for rapid immersion service. Mater. Perform. 2003, 28, 36–41. [Google Scholar]
- Walter, G.W. The application of impedance spectroscopy to study the uptake of sodium chloride solution in painted metals. Corros. Sci. 1991, 32, 1041–1058. [Google Scholar] [CrossRef]
- Alizadeh Razin, A.; Ramezanzadeh, B.; Yari, H. Detecting and estimating the extent of automotive coating delamination and damage indexes after stone chipping using electrochemical impedance spectroscopy. Prog. Org. Coat. 2016, 92, 95–109. [Google Scholar] [CrossRef]



| Primer Components | Percentages (%) | Components of Top Coat | Percentages (%) |
|---|---|---|---|
| Resin | 35.22 | Poliol 112 | 30.11 |
| Tannins | 0.1–5 | BYK 333 | 0.44 |
| Titanium Dioxide | 10.62 | BYK 425 | 12.00 |
| Calcium Kaolin | 15.60 | Additives (BYK 333, Purmol 333, Antiterra) | 0.94 |
| Mica Muscovite | 17.80 | ZnO-NPs | 3.00 |
| Additives | 0.42 | Thinner | 38.40 |
| ZnO-NPs | 3.00 | Crosslinking | 30.11 |
| Crosslinking | 8.8 | - | - |
| Thinner | Adjustment up to 100% | - | - |
| Accelerated Tests | Cycle A (Salt Fog Chamber) | Cycle B (Weathering Chamber) |
|---|---|---|
| Conditions of each test | 1 h salt spray at 25 °C | 4 h UV exposure, lamps UVA 340, 60 °C |
| 1 h drying at 35 °C | 4 h condensation at T 50 °C |
| Coatings | Failure | |
|---|---|---|
| Time (h) | 0 | 720 |
| PT-ZnO NPs | Cohesive | Adhesive Primer/Substrate |
| PU | Adhesive | Cohesive/Primer |
| TE | Adhesive | Adhesive Primer/top |
| Flexibility | ||||||
|---|---|---|---|---|---|---|
| Time (h) | 0 | 720 | ||||
| Coatings | Diameter | Diameter | ||||
| 10″ | 5″ | 2″ | 10″ | 5″ | 2″ | |
| PT-ZnO NPs | Pass | Pass | Pass | Pass | Pass | Fail |
| PU | Pass | Pass | Fail | Fail | - | - |
| TE | Pass | Fail | Fail | Fail | - | - |
| Coatings | Time (h) | Z [Ω/cm2] |
|---|---|---|
| PT-ZnO NPs | 0 | 2.36 × 109 |
| 720 | 3.00 × 109 | |
| PU | 0 | 6.40 × 1010 |
| 720 | 1.04 × 1010 | |
| TE | 0 | 8.98 × 109 |
| 720 | 1.82 × 108 |
| Coatings | Time (Months) | Z [Ω/cm2] |
|---|---|---|
| PT-ZnO NPs | 0 | 3.51 × 109 |
| 4 | 6.70 × 108 | |
| PU | 0 | 5.50 × 1010 |
| 4 | 1.30 × 1010 | |
| TE | 0 | 6.70 × 109 |
| 4 | 5.19 × 108 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez, J.; Díaz-Gómez, A.; Montoya, L.F.; Samhitha, S.S.; Rojas, D.; Oñate, Á.; Jaramillo, A.F.; Melendrez, M.F. Evaluation in Real Conditions of New Anticorrosive Formulations Based on Polyphenols from Natural Sources and Encapsulated Nanoparticles. Coatings 2023, 13, 8. https://doi.org/10.3390/coatings13010008
Ramírez J, Díaz-Gómez A, Montoya LF, Samhitha SS, Rojas D, Oñate Á, Jaramillo AF, Melendrez MF. Evaluation in Real Conditions of New Anticorrosive Formulations Based on Polyphenols from Natural Sources and Encapsulated Nanoparticles. Coatings. 2023; 13(1):8. https://doi.org/10.3390/coatings13010008
Chicago/Turabian StyleRamírez, Jesús, Andrés Díaz-Gómez, Luis Felipe Montoya, Saireddy Shiva Samhitha, David Rojas, Ángelo Oñate, Andrés Felipe Jaramillo, and Manuel Francisco Melendrez. 2023. "Evaluation in Real Conditions of New Anticorrosive Formulations Based on Polyphenols from Natural Sources and Encapsulated Nanoparticles" Coatings 13, no. 1: 8. https://doi.org/10.3390/coatings13010008






