Nb and Mo Influencing the High-Temperature Wear Behavior of HVOF-Sprayed High-Entropy Alloy Coatings
Abstract
1. Introduction
2. Materials and Methods
3. Results
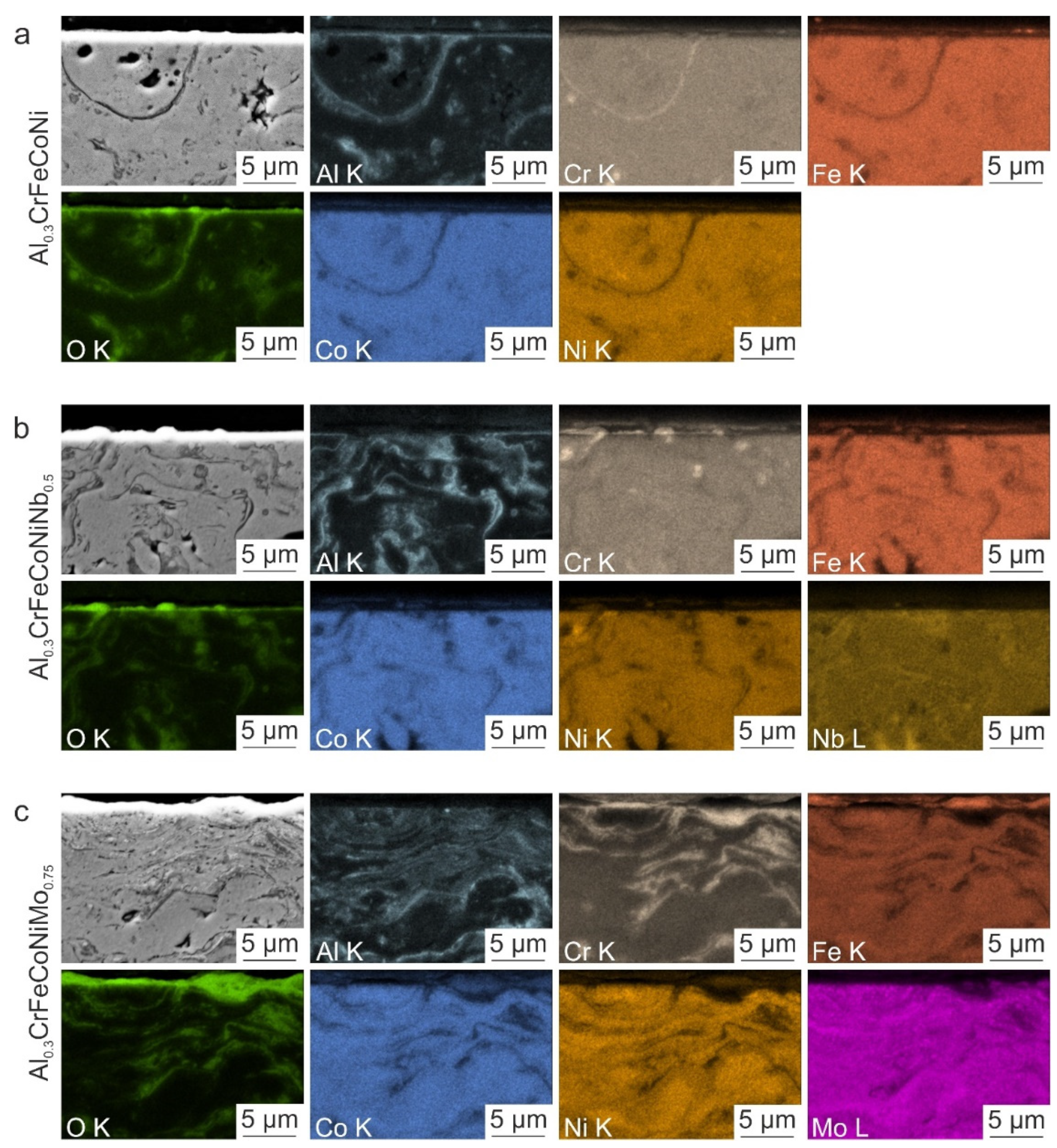
3.1. Characterization of the Initial Microstructure
3.2. High-Temperature Wear Tests
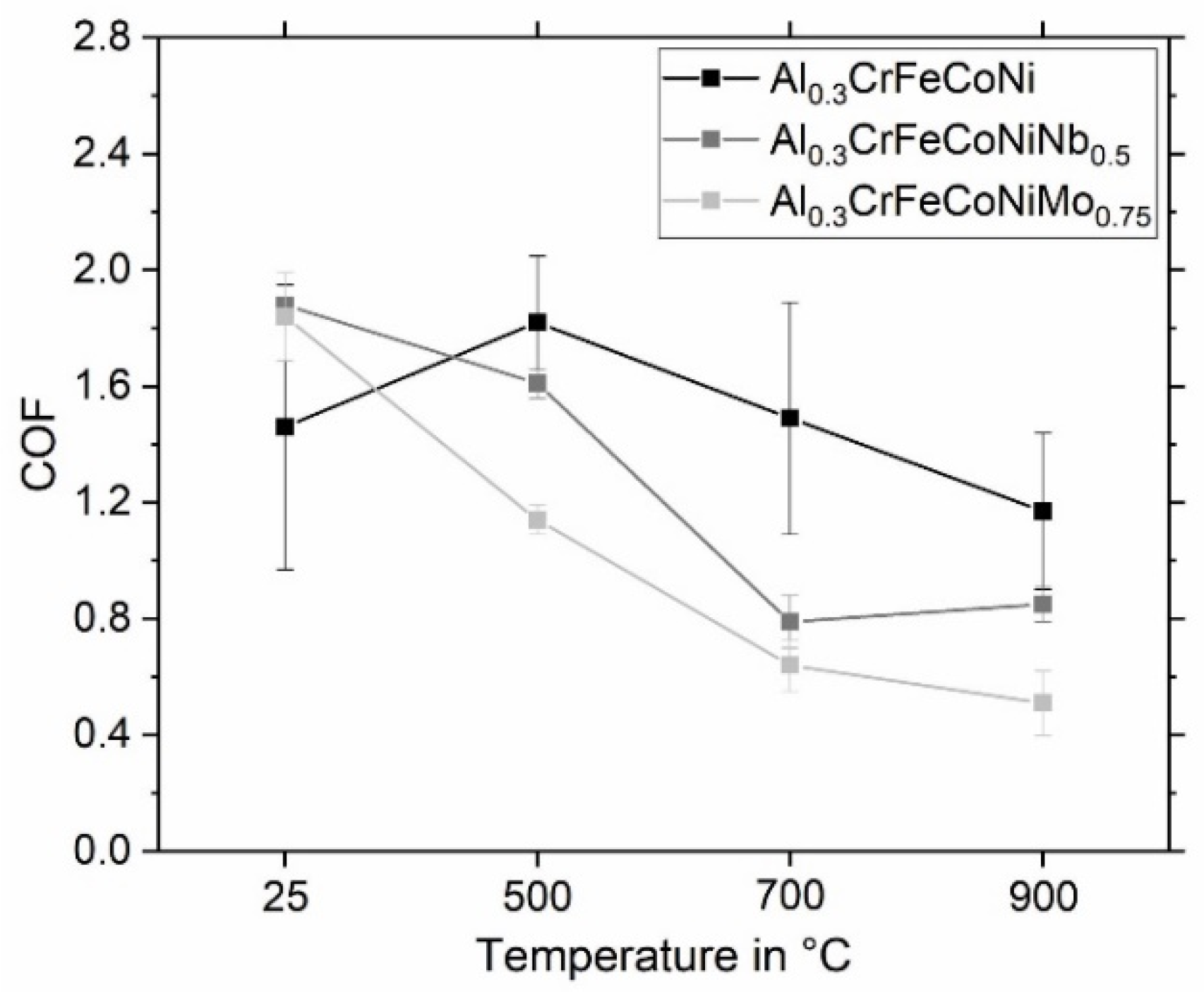
3.2.1. Frictional Behavior
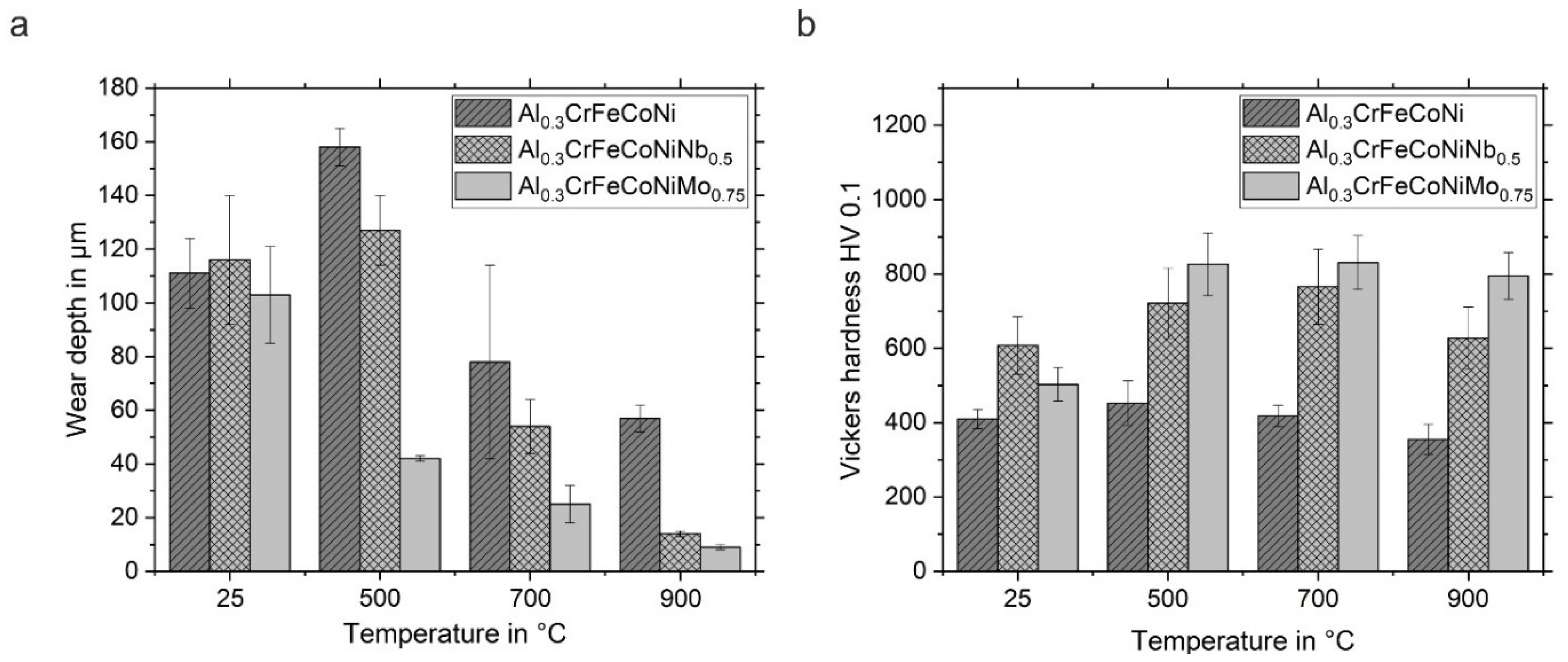
3.2.2. Wear Depth and Vickers Hardness after High-Temperature Wear Tests
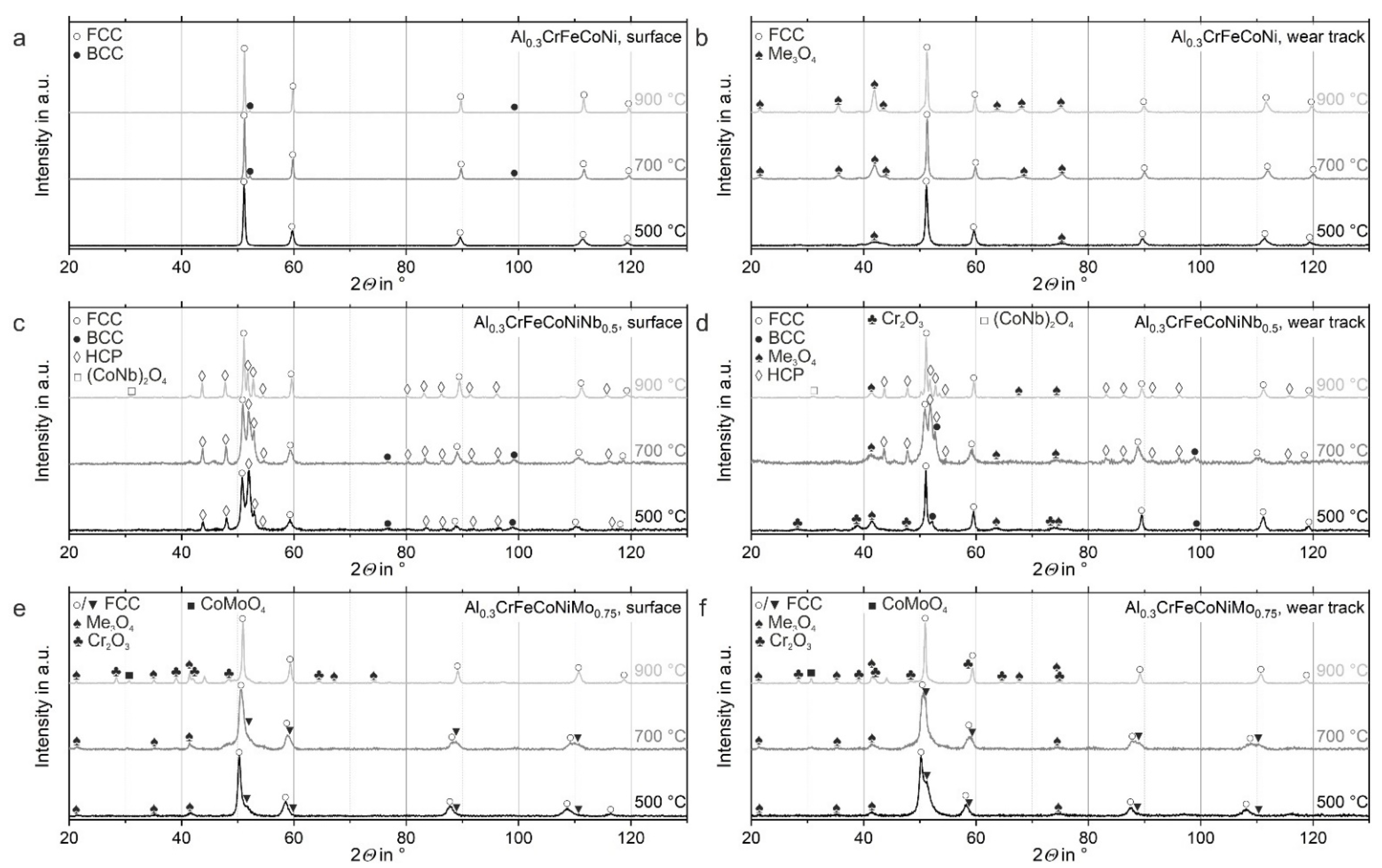
3.2.3. XRD Investigations after High-Temperature Wear Tests
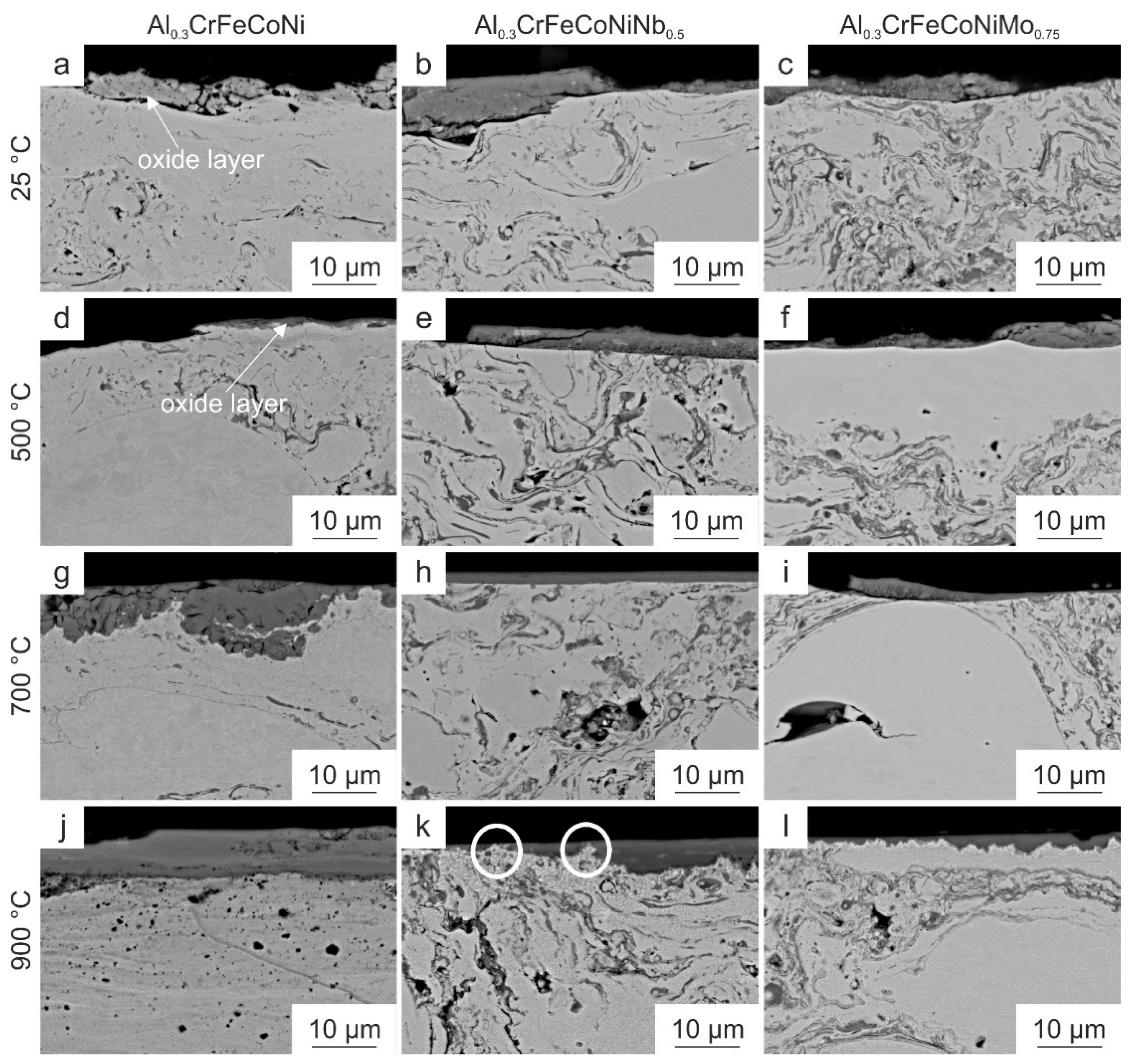
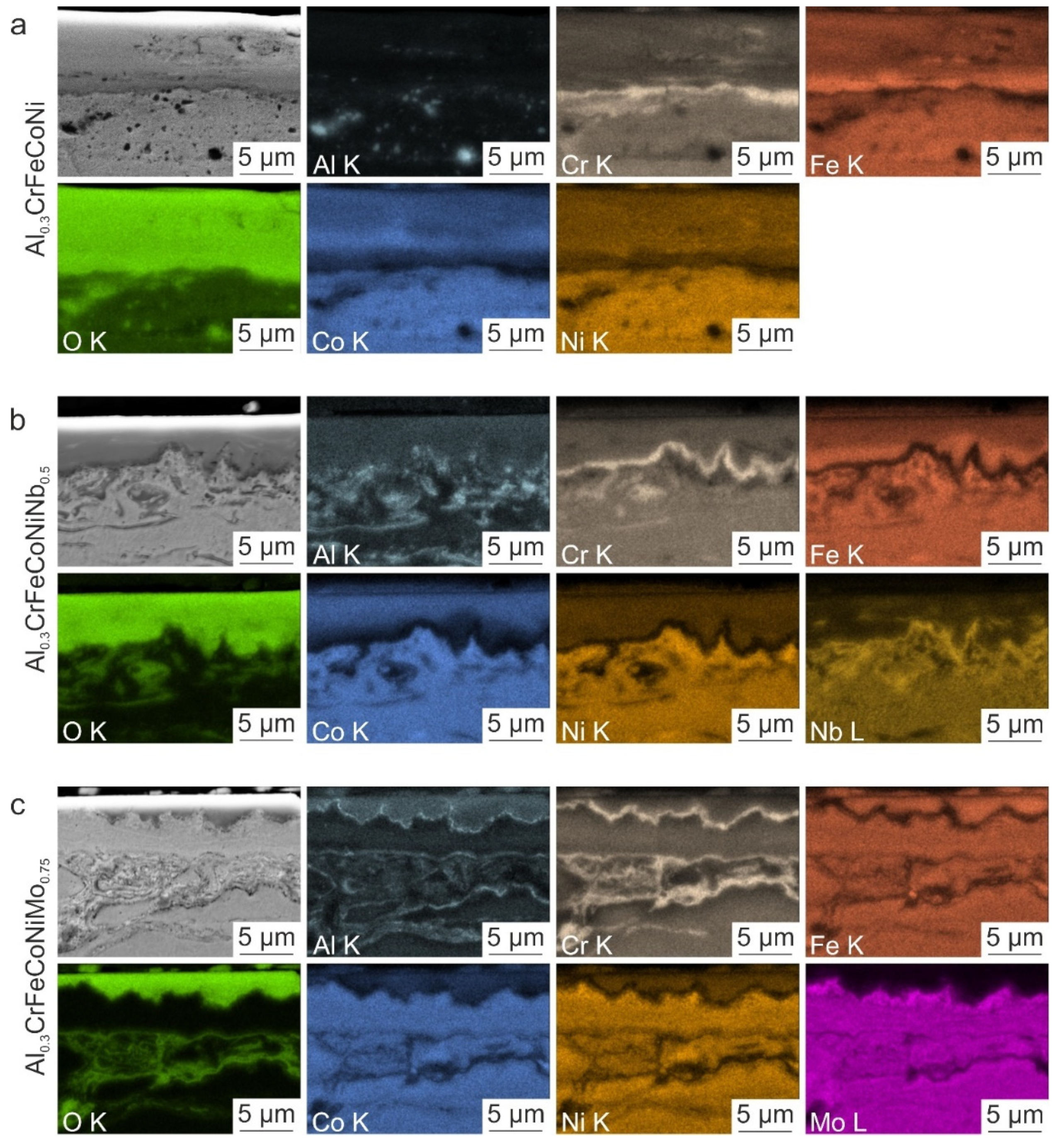
3.3. Microstructural Characterization of the Wear Tracks
4. Conclusions
- Adding Nb and Mo to Al0.3CrFeCoNi increases the hardness due to the hard phases formed next to the FCC phase (HCP in Al0.3CrFeCoNiNb0.5 and BCC as well as Me3O4 in Al0.3CrFeCoNiMo0.75)
- The COF decreases with increasing temperature due to the formation of an oxide layer on the surface after high-temperature wear tests, as the friction combination changes from ceramic on metal to ceramic on ceramic
- The wear depth decreases with increasing temperature as the wear mechanisms change from pronounced abrasion, delamination and adhesion to oxidative wear
- The lowest wear depths were observed for the Nb- and Mo-containing HVOF-sprayed coatings due to the formation of thin and strongly adherent oxide layers
- The strong adhesion is attributed to the formation of a convoluted interface between the oxide layer and the Nb- and Mo-containing HVOF-sprayed coating
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303+274. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Alaneme, K.K.; Bodunrin, M.O.; Oke, S.R. Processing, alloy composition and phase transition effect on the mechanical and corrosion properties of high entropy alloys: A review. J. Mater. Res. Technol. 2016, 5, 384–393. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Praveen, S.; Kim, H.S. High-Entropy Alloys: Potential Candidates for High-Temperature Applications—An Overview. Adv. Eng. Mater. 2018, 20, 1700645. [Google Scholar] [CrossRef]
- Mishra, R.S.; Haridas, R.S.; Agrawal, P. High entropy alloys—Tunability of deformation mechanisms through integration of compositional and microstructural domains. Mater. Sci. Eng. A 2021, 812, 141085. [Google Scholar] [CrossRef]
- Wu, L.; Guo, X.; Zhang, J. Abrasive resistant coatings—A review. Lubricants 2014, 2, 66–89. [Google Scholar] [CrossRef]
- Thorpe, M.L.; Richter, H.J. A pragmatic analysis and comparison of HVOF processes. J. Therm. Spray Technol. 1992, 1, 161–170. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Meng, X.; Xie, Y. A Review on High Entropy Alloys Coatings: Fabrication Processes and Property Assessment. Adv. Eng. Mater. 2019, 21, 1900343. [Google Scholar] [CrossRef]
- Löbel, M.; Lindner, T.; Mehner, T.; Lampke, T. Microstructure and wear resistance of AlCoCrFeNiTi high-entropy alloy coatings produced by HVOF. Coatings 2017, 7, 144. [Google Scholar] [CrossRef]
- Löbel, M.; Lindner, T.; Hunger, R.; Berger, R.; Lampke, T. Precipitation hardening of the HVOF sprayed single-phase high-entropy alloy CrFeCoNi. Coatings 2020, 10, 701. [Google Scholar] [CrossRef]
- Löbel, M.; Lindner, T.; Lampke, T. High-temperature wear behaviour of AlCoCrFeNiTi0.5 coatings produced by HVOF. Surf. Coat. Technol. 2020, 403, 126379. [Google Scholar] [CrossRef]
- Löbel, M.; Lindner, T.; Clauß, S.; Pippig, R.; Dietrich, D.; Lampke, T. Microstructure and Wear Behavior of the High-Velocity-Oxygen-Fuel Sprayed and Spark Plasma Sintered High-Entropy Alloy AlCrFeCoNi. Adv. Eng. Mater. 2021, 23, 2001253. [Google Scholar] [CrossRef]
- Löbel, M.; Lindner, T.; Grimm, M.; Rymer, L.M.; Lampke, T. Influence of Aluminum and Molybdenum on the Microstructure and Corrosion Behavior of Thermally Sprayed High-Entropy Alloy Coatings. J. Therm. Spray Technol. 2021, 25, 2074–2083. [Google Scholar] [CrossRef]
- Löbel, M.; Lindner, T.; Mehner, T.; Rymer, L.M.; Björklund, S.; Joshi, S.; Lampke, T. Microstructure and Corrosion Properties of AlCrFeCoNi High-Entropy Alloy Coatings Prepared by HVAF and HVOF. J. Therm. Spray Technol. 2022, 31, 247–255. [Google Scholar] [CrossRef]
- Wu, J.M.; Lin, S.J.; Yeh, J.W.; Chen, S.K.; Huang, Y.S.; Chen, H.C. Adhesive wear behavior of AlxCoCrCuFeNi high-entropy alloys as a function of aluminum content. Wear 2006, 261, 513–519. [Google Scholar] [CrossRef]
- Huo, W.Y.; Shi, H.F.; Ren, X.; Zhang, J.Y. Microstructure and wear behavior of cocrfemnnbni high-entropy alloy coating by TIG cladding. Adv. Mater. Sci. Eng. 2015, 2015, 647351. [Google Scholar] [CrossRef]
- Jiang, H.; Jiang, L.; Qiao, D.; Lu, Y.; Wang, T.; Cao, Z.; Li, T. Effect of Niobium on Microstructure and Properties of the CoCrFeNbxNi High Entropy Alloys. J. Mater. Sci. Technol. 2017, 33, 712–717. [Google Scholar] [CrossRef]
- Jiang, H.; Han, K.; Li, D.; Cao, Z. Synthesis and characterization of AlCoCrFeNiNbx high-entropy alloy coatings by laser cladding. Crystals 2019, 9, 56. [Google Scholar] [CrossRef]
- Löbel, M.; Lindner, T.; Lampke, T. Enhanced wear behaviour of spark plasma sintered AlCoCrFeNiTi high-entropy alloy composites. Materials 2018, 11, 2225. [Google Scholar] [CrossRef]
- Löbel, M.; Lindner, T.; Mehner, T.; Lampke, T. Influence of titanium on microstructure, phase formation and wear behaviour of AlCoCrFeNiTix high-entropy alloy. Entropy 2018, 20, 505. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; He, F.; Qiao, Z.; Wang, Z.; Liu, W.; Yang, J. Effects of temperature and microstructure on the triblogical properties of CoCrFeNiNbx eutectic high entropy alloys. J. Alloys Compd. 2019, 775, 1376–1385. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, Z.; Wu, G.; Tong, X.; Xiong, Y.; Cheng, X.; Wang, X.; Yamaguchi, T. Microstructure and high-temperature properties of laser cladded AlCoCrFeNiTi0.5 high-entropy coating on Ti 6Al-4V alloy. Surf. Coat. Technol. 2021, 418, 127243. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, X.; Wang, W.; Liu, B.; Lv, Y.; Yang, W.; Xu, D.; Liu, Y. A review on fundamental of high entropy alloys with promising high–temperature properties. J. Alloys Compd. 2018, 760, 15–30. [Google Scholar] [CrossRef]
- Saboktakin Rizi, M.; Minouei, H.; Lee, B.J.; Toroghinejad, M.R.; Hong, S.I. Effects of carbon and molybdenum on the nanostructural evolution and strength/ductility trade-off in Fe40Mn40Co10Cr10 high-entropy alloys. J. Alloys Compd. 2022, 911, 165108. [Google Scholar] [CrossRef]
- Du, L.M.; Lan, L.W.; Zhu, S.; Yang, H.J.; Shi, X.H.; Liaw, P.K.; Qiao, J.W. Effects of temperature on the tribological behavior of Al0.25CoCrFeNi high-entropy alloy. J. Mater. Sci. Technol. 2019, 35, 917–925. [Google Scholar] [CrossRef]
- Joseph, J.; Haghdadi, N.; Shamlaye, K.; Hodgson, P.; Barnett, M.; Fabijanic, D. The sliding wear behaviour of CoCrFeMnNi and AlxCoCrFeNi high entropy alloys at elevated temperatures. Wear 2019, 428–429, 32–44. [Google Scholar] [CrossRef]
- Joseph, J.; Haghdadi, N.; Annasamy, M.; Kada, S.; Hodgson, P.D.; Barnett, M.R.; Fabijanic, D.M. On the enhanced wear resistance of CoCrFeMnNi high entropy alloy at intermediate temperature. Scr. Mater. 2020, 186, 331–335. [Google Scholar] [CrossRef]
- Löbel, M.; Lindner, T.; Pippig, R.; Lampke, T. High-temperature wear behaviour of spark plasma sintered AlCoCrFeNiTi0.5 high-entropy alloy. Entropy 2019, 21, 582. [Google Scholar] [CrossRef]
- Cui, Y.; Shen, J.; Manladan, S.M.; Geng, K.; Hu, S. Wear resistance of FeCoCrNiMnAlx high-entropy alloy coatings at high temperature. Appl. Surf. Sci. 2020, 512, 145736. [Google Scholar] [CrossRef]
- Erdoğan, A.; Gök, M.S.; Zeytin, S. Analysis of the high-temperature dry sliding behavior of CoCrFeNiTi0.5Alx high-entropy alloys. Friction 2020, 8, 198–207. [Google Scholar] [CrossRef]
- Hong, S.; Li, J.; Zhao, P.; Xu, Y.; Li, W. Evolution in Wear and High-Temperature Oxidation Resistance of Laser-Clad AlxMoNbTa Refractory High-Entropy Alloys Coatings with Al Addition Content. Coatings 2022, 12, 121. [Google Scholar] [CrossRef]
- Ghadami, F.; Davoudabadi, M.A.; Ghadami, S. Cyclic Oxidation Properties of the Nanocrystalline AlCrFeCoNi High-Entropy Alloy Coatings Applied by the Atmospheric Plasma Spraying Technique. Coatings 2022, 12, 372. [Google Scholar] [CrossRef]
- Wu, M.; Chen, K.; Xu, Z.; Li, D.Y. Effect of Ti addition on the sliding wear behavior of AlCrFeCoNi high-entropy alloy. Wear 2020, 462, 203493. [Google Scholar] [CrossRef]
- Bao, Y.; Guo, L.; Zhong, C.; Song, Q.; Yang, K.; Jiang, Y.; Wang, Z. Microstructure and Wear Resistance of Fe4CoCrNiB0.2Mox (x=0, 0.5, 1) High Entropy Alloys. J. Wuhan Univ. Technol. Sci. Ed. 2022, 37, 261–269. [Google Scholar] [CrossRef]
- Zhu, J.M.; Fu, H.M.; Zhang, H.F.; Wang, A.M.; Li, H.; Hu, Z.Q. Microstructures and compressive properties of multicomponent AlCoCrFeNiMox alloys. Mater. Sci. Eng. A 2010, 527, 6975–6979. [Google Scholar] [CrossRef]
- Preuß, B.; Lindner, T.; Uhlig, T.; Wagner, G.; Lampke, L. Niobium and Molybdenum as Alloying Constituents in Al0.3CoCrFeNi to Develop Eutectic High-Entropy Alloys for HVOF Spraying. J. Therm. Spray Technol. 2022, 1–10. [Google Scholar] [CrossRef]
- Davis, J.R. Handbook of Thermal Spray Technology; ASM International: Almere, The Netherlands, 2004. [Google Scholar]
- Tejero-Martin, D.; Rezvani Rad, M.; McDonald, A.; Hussain, T. Beyond Traditional Coatings: A Review on Thermal-Sprayed Functional and Smart Coatings; Springer: Berlin/Heidelberg, Germany, 2019; Volume 28. [Google Scholar]
- Sidhu, T.S.; Prakash, S.; Agrawal, R.D. State of the art of HVOF coating investigations—A review. Mar. Technol. Soc. J. 2005, 39, 53–64. [Google Scholar] [CrossRef]
- Robinson, V.N.E. Imaging with backscattered electrons in a scanning electron microscope. Scanning 1980, 3, 15–26. [Google Scholar] [CrossRef]
- del Moricca, M.; Varma, S.K. High temperature oxidation characteristics of Nb-10W-XCr alloys. J. Alloys Compd. 2010, 489, 195–201. [Google Scholar] [CrossRef]
- Gatzen, C.; Smokovych, I.; Scheffler, M.; Krüger, M. Oxidation-Resistant Environmental Barrier Coatings for Mo-Based Alloys: A Review. Adv. Eng. Mater. 2021, 23, 2001016. [Google Scholar] [CrossRef]
- Shun, T.T.; Du, Y.C. Microstructure and tensile behaviors of FCC Al0.3CoCrFeNi high entropy alloy. J. Alloys Compd. 2009, 479, 157–160. [Google Scholar] [CrossRef]
- Wang, W.R.; Wang, W.L.; Wang, S.C.; Tsai, Y.C.; Lai, C.H.; Yeh, J.W. Effects of Al addition on the microstructure and mechanical property of AlxCoCrFeNi high-entropy alloys. Intermetallics 2012, 26, 44–51. [Google Scholar] [CrossRef]
- He, F.; Wang, Z.; Shang, X.; Leng, C.; Li, J.; Wang, J. Stability of lamellar structures in CoCrFeNiNbx eutectic high entropy alloys at elevated temperatures. Mater. Des. 2016, 104, 259–264. [Google Scholar] [CrossRef]
- Liu, W.H.; He, J.Y.; Huang, H.L.; Wang, H.; Lu, Z.P.; Liu, C.T. Effects of Nb additions on the microstructure and mechanical property of CoCrFeNi high-entropy alloys. Intermetallics 2015, 60, 1–8. [Google Scholar] [CrossRef]
- Cordero, B.; Gómez, V.; Platero-Prats, A.E.; Revés, M.; Echeverría, J.; Cremades, E.; Barragán, F.; Alvarez, S. Covalent radii revisited. J. Chem. Soc. Dalt. Trans. 2008, 21, 2832–2838. [Google Scholar] [CrossRef]
- Inal, K.; Neale, K.W.; Aboutajeddine, A. Forming limit comparisons for FCC and BCC sheets. Int. J. Plast. 2005, 21, 1255–1266. [Google Scholar] [CrossRef]
- Liang, H.; Yao, H.; Qiao, D.; Nie, S.; Lu, Y.; Deng, D.; Cao, Z.; Wang, T. Microstructures and Wear Resistance of AlCrFeNi2W0.2Nbx High-Entropy Alloy Coatings Prepared by Laser Cladding. J. Therm. Spray Technol. 2019, 28, 1318–1329. [Google Scholar] [CrossRef]
- Ungár, T. Microstructural parameters from X-ray diffraction peak broadening. Scr. Mater. 2004, 51, 777–781. [Google Scholar] [CrossRef]
- Yang, J.F.; Jiang, Y.; Hardell, J.; Prakash, B.; Fang, Q.F. Influence of service temperature on tribological characteristics of self-lubricant coatings: A review. Front. Mater. Sci. 2013, 7, 28–39. [Google Scholar] [CrossRef]
- Motallebzadeh, A.; Atar, E.; Cimenoglu, H. Sliding wear characteristics of molybdenum containing Stellite 12 coating at elevated temperatures. Tribol. Int. 2015, 91, 40–47. [Google Scholar] [CrossRef]
- Patel, P.; Alidokht, S.A.; Sharifi, N.; Roy, A.; Harrington, K.; Stoyanov, P.; Chromik, R.R.; Moreau, C. Microstructural and Tribological Behavior of Thermal Spray CrMnFeCoNi High Entropy Alloy Coatings. J. Therm. Spray Technol. 2022, 31, 1285–1301. [Google Scholar] [CrossRef]
- Sommer, K.; Heinz, R.; Schöfer, J. Verschleiß Metallischer Werkstoffe; Springer Vieweg: Wiesbaden, Germany, 2018. [Google Scholar]
- Rynio, C.; Hattendorf, H.; Klöwer, J.; Eggeler, G. The evolution of tribolayers during high temperature sliding wear. Wear 2014, 315, 1–10. [Google Scholar] [CrossRef]
- Tian, L.H.; Xiong, W.; Liu, C.; Lu, S.; Fu, M. Microstructure and Wear Behavior of Atmospheric Plasma-Sprayed AlCoCrFeNiTi High-Entropy Alloy Coating. J. Mater. Eng. Perform. 2016, 25, 5513–5521. [Google Scholar] [CrossRef]
- Holcomb, G.R.; Tylczak, J.; Carney, C. Oxidation of CoCrFeMnNi High Entropy Alloys. JOM 2015, 67, 2326–2339. [Google Scholar] [CrossRef]
- Minouei, H.; Kheradmandfard, M.; Saboktakin Rizi, M.; Jalaly, M.; Kim, D.E.; Hong, S.I. Formation mechanism of high-entropy spinel thin film and its mechanical and magnetic properties: Linking high-entropy alloy to high-entropy ceramic. Appl. Surf. Sci. 2022, 576, 873. [Google Scholar] [CrossRef]
- Hou, P.Y. Oxidation of Metals and Alloys. In Shreir’s Corrosion, 1st ed.; Cottis, B., Ed.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 1, pp. 195–239. [Google Scholar]
- Cui, W.; Li, W.; Chen, W.-T.; Liou, F. Laser Metal Deposition of an AlCoCrFeNiTi0.5 High-Entropy Alloy Coating on a Ti6Al4V Substrate: Microstructure and Oxidation Behavior. Crystals 2020, 10, 638. [Google Scholar] [CrossRef]
- Gorr, B.; Mueller, F.; Christ, H.J.; Mueller, T.; Chen, H.; Kauffmann, A.; Heilmaier, M. High temperature oxidation behavior of an equimolar refractory metal-based alloy 20Nb-20Mo-20Cr-20Ti-20Al with and without Si addition. J. Alloys Compd. 2016, 688, 468–477. [Google Scholar] [CrossRef]





| Material | Al | Cr | Fe | Co | Ni | Nb | Mo |
|---|---|---|---|---|---|---|---|
| Al0.3CrFeCoNi | 8.8 | 23.1 | 22.9 | 22.5 | 22.7 | - | - |
| Al0.3CrFeCoNiNb0.5 | 7.8 | 20.5 | 20.4 | 19.9 | 20.5 | 11.1 | - |
| Al0.3CrFeCoNiMo0.75 | 7.6 | 19.7 | 20.3 | 19.7 | 20.1 | - | 12.7 |
| O2 | Kerosene | λ | Carrier Gas (Ar) Pressure | Nozzle Length | Powder Feed Rate | Spraying Distance | Surface Speed | Spray Path Offset | Coating Layers |
|---|---|---|---|---|---|---|---|---|---|
| (L/min) | (L/h) | (bar) | (mm) | (g/min) | (mm) | (m/min) | (mm) | ||
| 900 | 24 | 1.1 | 8 | 150 | 2 × 40 | 350 | 60 | 5 | 24 |
| Reciprocating Wear Test | |
|---|---|
| Force (N) | 26 |
| Frequency (Hz) | 40 |
| Test duration (s) | 900 |
| Amplitude (mm) | 0.5 |
| Temperatures (°C) | 25, 500, 700, 900 |
| Material | Al | Cr | Fe | Co | Ni | Nb | Mo |
|---|---|---|---|---|---|---|---|
| Al0.3CrFeCoNi | 9.2 | 22.9 | 22.8 | 22.3 | 22.9 | - | - |
| Al0.3CrFeCoNiNb0.5 | 8.0 | 20.5 | 20.3 | 19.8 | 20.0 | 11.4 | - |
| Al0.3CrFeCoNiMo0.75 | 7.9 | 19.9 | 20.4 | 19.9 | 20.4 | - | 11.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rymer, L.-M.; Lindner, T.; Lampke, T. Nb and Mo Influencing the High-Temperature Wear Behavior of HVOF-Sprayed High-Entropy Alloy Coatings. Coatings 2023, 13, 9. https://doi.org/10.3390/coatings13010009
Rymer L-M, Lindner T, Lampke T. Nb and Mo Influencing the High-Temperature Wear Behavior of HVOF-Sprayed High-Entropy Alloy Coatings. Coatings. 2023; 13(1):9. https://doi.org/10.3390/coatings13010009
Chicago/Turabian StyleRymer, Lisa-Marie, Thomas Lindner, and Thomas Lampke. 2023. "Nb and Mo Influencing the High-Temperature Wear Behavior of HVOF-Sprayed High-Entropy Alloy Coatings" Coatings 13, no. 1: 9. https://doi.org/10.3390/coatings13010009
APA StyleRymer, L.-M., Lindner, T., & Lampke, T. (2023). Nb and Mo Influencing the High-Temperature Wear Behavior of HVOF-Sprayed High-Entropy Alloy Coatings. Coatings, 13(1), 9. https://doi.org/10.3390/coatings13010009







