Effect of Metakaolin on the Water Resistance of Magnesium Phosphate Cement Mortar
Abstract
:1. Introduction
2. Test Content
2.1. Raw Materials and Proportions
2.1.1. Reburned Magnesium Oxide Powder
2.1.2. Ammonium Dihydrogen Phosphate
2.1.3. Retarder
2.1.4. Metakaolin
2.1.5. Aggregate
2.1.6. Water
2.1.7. Mix Proportion
2.2. Manufacture of Test Samples
2.3. Test Methods
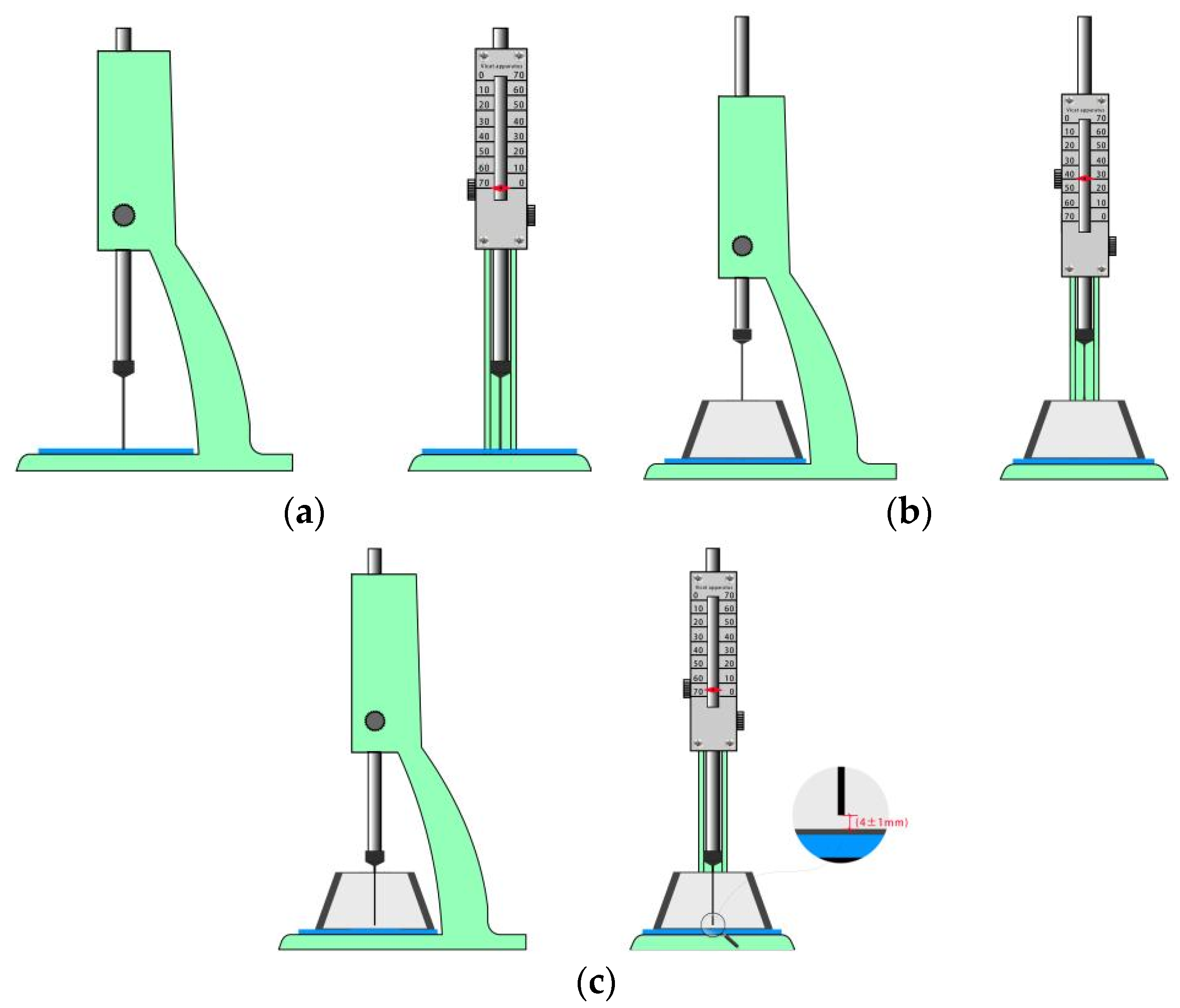
2.3.1. Setting Time Test
2.3.2. Fluidity Test
2.3.3. Curing Conditions
2.3.4. Compressive Strength Test
2.3.5. Water Resistance Test
2.3.6. Microcosmic Testing
3. Results and Discussion
3.1. Analysis of Setting Time and Fluidity
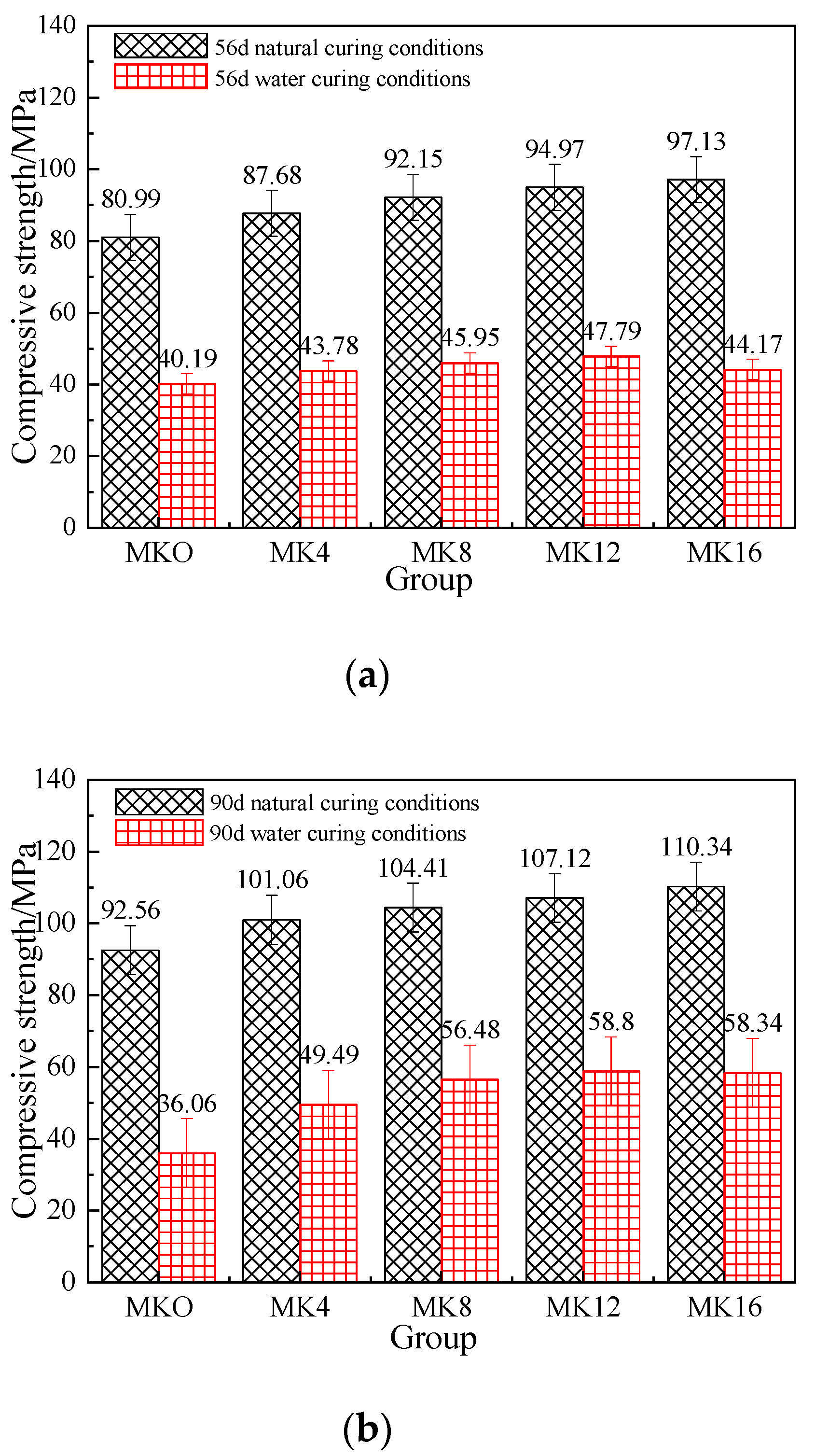
3.2. Compressive Strength Analysis
3.3. Water Resistance Analysis
3.3.1. Effect of Metakaolin on the Mechanical Strength of Magnesium Phosphate Cement Mortar
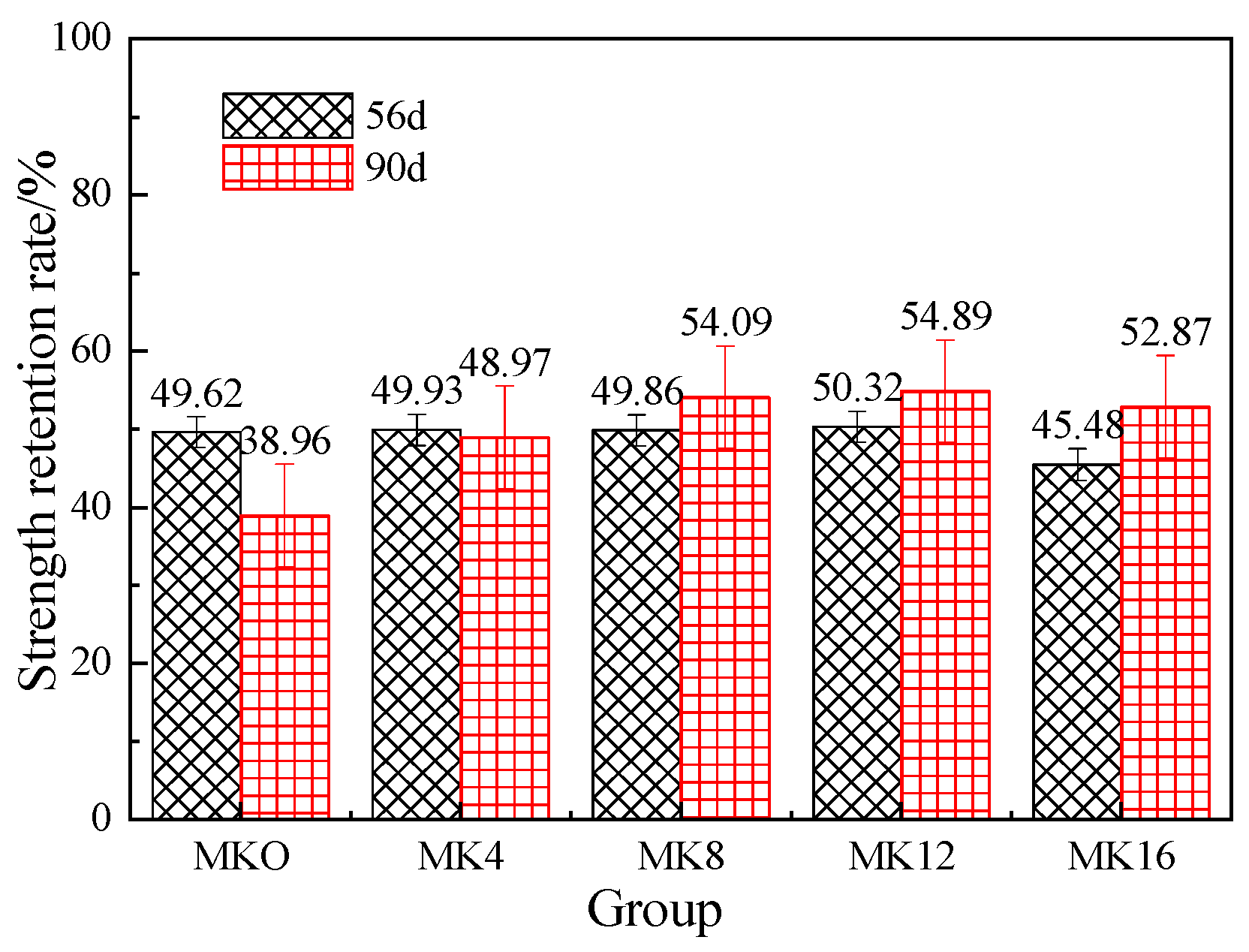
3.3.2. Effect of Metakaolin on the Strength Retention of Magnesium Phosphate Mortar
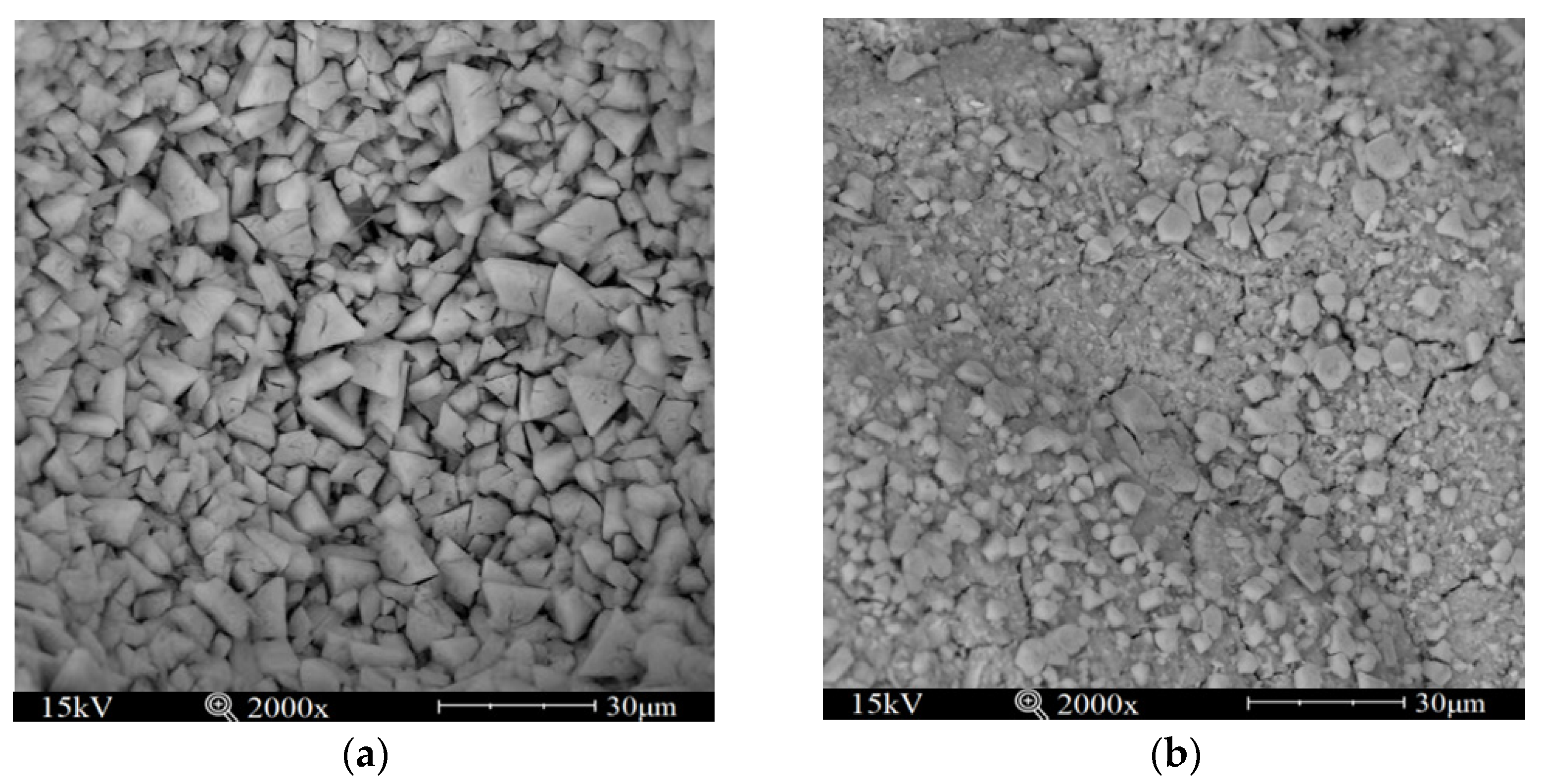
3.4. Microanalysis
3.5. Mechanism Analysis of MK Modified MPC
3.5.1. Retarding Mechanism
3.5.2. Hydration and Hardening Mechanism
4. Conclusions
- (1)
- Setting time: within a certain range, with an increase in metakaolin content, the setting time of MPC mortar gradually decreases.
- (2)
- Fluidity: with an increase in metakaolin content, the effect on fluidity is not significant when the metakaolin content is low (≤8%); when the dosage is larger (>8%), it will lead to a decrease in the fluidity of MPC. When the content of metakaolin is 12% and 16%, the corresponding fluidity is 190 mm and 163 mm, respectively. The fluidity decreases rapidly with the increase in metakaolin content. Compared with the basic group MK0, the fluidity of the MK12 and MK16 groups, respectively, decreased by 6.9% and 20.1%. In the MK16 group, the decrease in fluidity was particularly significant.
- (3)
- Compressive strength: (a) Metakaolin can effectively improve the compressive strength of magnesium phosphate mortar, and its compressive strength gradually increases with age. (b) At the same age, with the increase in metakaolin content, the overall strength of magnesium phosphate cement mortar shows an increasing trend. In terms of 1d strength, the mortar strength of the MK12 and MK16 groups showed a decreasing trend compared to the other groups. After 7 days of aging, with the increase in metakaolin content, the mortar strength of MPC gradually increased. This indicates that metakaolin is beneficial for the long-term strength of MPC mortar. (c) Compared to the other groups, the MK12 group had the highest strength after 1 h, with a regular growth rate with age and high long-term strength. Overall, a 12% metakaolin content was found to be optimal.
- (4)
- Water resistance: (a) With an increase in age, the strength retention rate of magnesium phosphate cement mortar showed a reverse shrinkage. (b) The water resistance of mortar specimens at the same age showed a trend of first increasing and then decreasing with the increase in metakaolin content and reached the highest value when the content was 12%. The retention rate of 56 d strength was 50.32%, and the retention rate of 90 d strength was 54.89%. The long-term water resistance effect was obvious. (c) Metakaolin can improve the pore structure of magnesium phosphate cement mortar and has a significant effect on improving the water resistance of MPC. The increase in the strength retention rate is a result of both chemical and physical actions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fang, Q.; Xiao, B.F.; Chen, Y.; Ding, Z. Review on research progress of mineral admixtures in magnesium phosphate cement. Concrete 2021, 67–72. [Google Scholar]
- Qin, G.X.; Jiao, B.X. Review of magnesium phosphate cement performance. J. Chin. Ceram. Soc. 2019, 38, 1075–1079+1085. [Google Scholar]
- Liu, J.X.; Li, Z.Y.; Zhang, M.L.; Hai, R. Research progress on magnesium phosphate cement modified by mineral admixtures. Inorg. Salt Ind. 2022, 54, 18–23. [Google Scholar]
- Wang, Y.M.; Lv, Y.; Liu, Z.L.; Jiang, W.G. Research progress of magnesium phosphate cement. Sci. Technol. Inf. 2019, 17, 113–116. [Google Scholar]
- Tan, H.; Zhang, X.; Guo, Y.; Ma, B.; Jian, S.; He, X.; Zhi, Z.; Liu, X. Improvement in fluidity loss of magnesia phosphate cement by incorporating polycarboxylate su-perplasticizer. Constr. Build. Mater. 2018, 165, 887–897. [Google Scholar] [CrossRef]
- Chang, Y.; Shi, C.J.; Yang, N.; Yang, J. Research progresses on durability of magnesium phosphate cement based aterials. J. Chin. Ceram. Soc. 2014, 42, 486–493. [Google Scholar]
- Qin, J.; Qian, J.; You, C.; Fan, Y.; Li, Z.; Wang, H. Bond behavior and interfacial micro-characteristics of magnesium phosphate cement onto old concrete substrate. Constr. Build. Mater. 2018, 167, 166–176. [Google Scholar] [CrossRef]
- Li, C.M.; Wang, P.M.; Wang, A.; Yin, A.H. Effect of admixtures on properties of magnesium phosphate cement and the mechanism. Concrete 2015, 115–117+125. [Google Scholar]
- Fang, Y.; Cui, P.; Ding, Z.; Zhu, J.X. Properties of a magnesium phosphate cement-based fire-retardant coating containing glass fiber or glass fiber powder. Constr. Build. Mater. 2018, 162, 553–560. [Google Scholar] [CrossRef]
- Wang, L.; Iris, K.M.; Tsang, D.C.; Yu, K.; Li, S.; Poon, C.S.; Dai, J.G. Upcycling wood waste into fibre-reinforced magnesium phosphate cement particleboards. Constr. Build. Mater. 2018, 159, 54–63. [Google Scholar] [CrossRef]
- Fan, Y.R. Study on Bond Properties of Magnesium Phosphate Cement Based Materials. Ph.D. Thesis, Chongqing University, Chongqing, China, 2016. [Google Scholar]
- Lv, Z.L. Study on the Effect of Mineral Admixture on the Performance of Magnesium Phosphate Cement. Master’s Thesis, Chang’an University, Xi’an, China, 2018. [Google Scholar]
- Cai, S.M. Application of magnesium phosphate cement in civil engineering. Guangdong Build. Mater. 2019, 35, 23–26. [Google Scholar]
- Zhang, Q.Z. Research status of hydration mechanism of magnesium phosphate cement. New Build. Mater. 2018, 45, 63–66. [Google Scholar]
- Zhang, S.Y.; Shi, H.S.; Huang, S.W.; Zhang, P. Dehydration characteristics of struvite-K pertaining to magnesium potassium phosphate cement system in non-isothermal condition. J. Therm. Anal. Calorim. 2013, 111, 35–40. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, X.T.; Huang, J.; Luo, Z.Q.; Fu, Y.Z. Research status of mineral admixtures on properties and mechanism of magnesium phosphate cement. J. Process Eng. 2021, 21, 629–638. [Google Scholar]
- Yao, Y.; Wang, Z.D.; Wang, L. Durability of concrete under combined mechanical load and environmental actions: A review. J. Sustain. Cem.-Based Mater. 2012, 1, 2–15. [Google Scholar] [CrossRef]
- Wu, C.Y.; Chen, C.; Zhang, H.F.; Tan, Y.; Yu, H. Preparation of magnesium oxysulfate cement using magnesium-rich byproducts from the production of lithium carbonate from salt lakes. Constr. Build. Mater. 2018, 172, 597–607. [Google Scholar] [CrossRef]
- Mo, L.W.; Lyu, L.M.; Deng, M.; Qian, J. Influence of fly ash and metakaolin on the microstructure and compressive strength of magnesium potassium phosphate cement paste. Cem. Concr. Res. 2018, 111, 116–129. [Google Scholar] [CrossRef]
- Xu, B.W.; Lothenbach, B.; Ma, H.Y. Properties of Fly Ash Blended Magnesium Potassium Phosphate Mortars: Effect of the Ratio Between Fly Ash and Magnesia. Cem. Concr. Compos. 2018, 90, 169–177. [Google Scholar] [CrossRef]
- Zheng, D.D.; Ji, T.; Wang, C.Q.; Sun, C.J.; Lin, X.J.; Hossain KM, A. Effect of the combination of fly ash and silica fume on water resistance of magnesium potassium phosphate cement. Constr. Build. Mater. 2016, 106, 415–421. [Google Scholar] [CrossRef]
- Gardner, L.J.; Bernal, S.A.; Walling, S.A.; Corkhill, C.L.; Provis, J.L.; Hyatt, N.C. Characterization of magnesium potassium phosphate cements blended with fly ash and ground granulated blast furnace slag. Cem. Concr. Res. 2015, 74, 78–87. [Google Scholar] [CrossRef]
- Liao, W.Y.; Ma, H.Y.; Sun, H.F.; Huang, Y.; Wang, Y. Potential large-volume beneficial use of low-grade fly ash in magnesia phosphate cement based materials. Fuel 2017, 209, 490–497. [Google Scholar] [CrossRef]
- Wang, S. Study on the Bonding Performance of Magnesium Phosphate Cement Based Materials in Water Environment. Master’s Thesis, Zhengzhou University, Zhengzhou, China, 2022. [Google Scholar]
- Zhao, J.T.; Li, X.G.; Zhang, Y.; Liu, H.; Yin, X. Effects of fly ash on magnesium phosphate cement. J. Chin. Ceram. Soc. 2018, 37, 695–700. [Google Scholar]
- Ghahari, S.A.; Mohammadi, A.; Ramezanianpour, A.A. Performance assessment of natural pozzolan roller compacted concrete pavements. Case Stud. Constr. Mater. 2017, 7, 82–90. [Google Scholar] [CrossRef]
- Li, D.X.; Li, P.X.; Feng, C.H. Research on water resistance of magnesium phosphate cement. J. Build. Mater. 2009, 12, 505–510. [Google Scholar]
- Gao, M.; Liu, N.; Chen, B. Effect of silica fume on properties of magnesium phosphate cement mortar. J. Build. Mater. 2020, 23, 29–34. [Google Scholar]
- Shi, Y.W.; Chen, B. Magnesium phosphate cement modified by metakaolin. J. Chin. Ceram. Soc. 2018, 46, 1111–1116. [Google Scholar]
- Lu, X.; Chen, B. Experimental study of magnesium phosphate cements modified by metakaolin. Constr. Build. Mater. 2016, 123, 719–726. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, Q.; Yang, J.M.; Wang, G. Effect of aluminous silica mineral admixture on physical and mechanical properties of potassium magnesium phosphate cement mortar. New Build. Mater. 2019, 46, 122–126. [Google Scholar]
- Fu, X.Y.; Yang, J.M.; Shan, C.M.; Tao, T. Effect of metakaolin and fly ash on bond properties between MKPC paste and concrete. Bull. Chin. Ceram. Soc. 2019, 38, 2242–2249. [Google Scholar]
- Fu, C.H. Influence of Mineral Powder on Properties of Magnesium Phosphate Cement Mortar. Master’s Thesis, Chang’an University, Xi’an, China, 2022. [Google Scholar]
- Hao, K.; Liu, R.Q.; Zhang, Y.; Yang, Y. Study on the effect of admixture on the microstructure and water resistance of magnesium phosphate cement. J. Shenyang Univ. Sci. Technol. 2022, 41, 34–40+45. [Google Scholar]
- Bai, W.L.; Li, M.; Xie, Q. Experimental study on the preparation and mechanical properties of modified magnesium phosphate cement. Ind. Constr. 2021, 51, 161–166+203. [Google Scholar]
- Lin, L.D. Study on the Stability of Magnesium Phosphate Cement Cured in Water and its Influencing Factors. Master’s Thesis, University of Chongqing, Chongqing, China, 2022. [Google Scholar]
- Shilar, F.A.; Ganachari, S.V.; Patil, V.B.; Nisar, K.S. Evaluation of structural performances of metakaolin based geopolymer concrete. J. Mater. Res. Technol. 2022, 20, 3208–3228. [Google Scholar] [CrossRef]
- Coelho, T.P.P.; Bezerra, B.P.; Verza, J.R.; Luz, A.P.; Morelli, M.R. Physico-mechanical properties of metakaolin and diatomite-based geopolymers. Mater. Lett. 2023, 349, 134784. [Google Scholar] [CrossRef]
- Liu, R.; Wang, W.; Qi, D.; Yang, Y. Static and dynamic mechanical properties of magnesium phosphate cement modified by metakaolin after high-temperature treatment. Constr. Build. Mater. 2023, 392, 131933. [Google Scholar]
- Singh, S.B.; Munjal, P. Bond strength and compressive stress strain characteristics of brick masonry. J. Build. Eng. 2017, 9, 10–16. [Google Scholar] [CrossRef]
- Lo, K.-W.; Lin, K.-L.; Cheng, T.-W.; Chang, Y.-M.; Lan, J.-Y. Effect of nano-SiO2 on the alkali-activated characteristics of spent catalyst metakaolin-based geopolymers. Constr. Build. Mater. 2017, 143, 455–463. [Google Scholar] [CrossRef]
- Chithambaram, S.J.; Kumar, S.; Prasad, M.M.; Adak, D. Effect of parameters on the compressive strength of fly ash based geopolymer concrete. Struct. Concr. 2018, 19, 1202–1209. [Google Scholar] [CrossRef]
- GB/T 1346-2011; Test Methods for Water Requirement of Normal Consistency, Setting Time, and Soundness of Portland Cements. Standards Press of China: Beijing, China, 2011.
- GB/T 2419-2005; Test Method for Fluidity of Cement Mortar. Standards Press of China: Beijing, China, 2005.
- GB/T 17671-1999; Method of Testing Cements-Determination of Strength. Standards Press of China: Beijing, China, 1999.
- Lv, Z.L.; Guan, B.W.; Wang, L.F.; Xu, A.H.; Chen, H.X. Effect of the metakaolin on early hydration process of magnesium phosphate cement. Bull. Sci. Technol. 2018, 34, 126–130. [Google Scholar]
- Qin, Z.; Zhou, S.; Ma, C.; Long, G.; Xie, Y.; Chen, B. Roles of metakaolin in magnesium phosphate cement: Effect of the replacement ratio of magnesia by metakaolin with different particle sizes. Constr. Build. Mater. 2019, 227, 116675. [Google Scholar] [CrossRef]
- Liu, N.; Chen, B. Experimental research on magnesium phosphate cements containing alumina. Constr. Build. Mater. 2016, 121, 354–360. [Google Scholar] [CrossRef]
- Rao, M.J.; Lin, Y.Q.; Yang, H.Q.; Li, J.Z.; Shi, Y. Effect of phosphorous slag on microstructure and hydration mechanism of cement-based materials. J. Comput. Theor. Nanosci. 2016, 13, 4764–4770. [Google Scholar] [CrossRef]
- Shi, Y.W.; Chen, B.; Ahmad, M.R. Effects of alumina as an effective constituent of metakaolin on properties of magnesium phosphate cements. J. Mater. Civ. Eng. 2019, 31, 04019147. [Google Scholar] [CrossRef]
- Ahmad, M.R.; Chen, B. Experimental investigation on the volume stability of magnesium phosphate cement with different types of mineral admixtures. Constr. Build. Mater. 2018, 190, 466–478. [Google Scholar] [CrossRef]
- Qin, Z.; Ma, C.; Zheng, Z.; Long, G.; Chen, B. Effects of metakaolin on properties and microstructure of magnesium phosphate cement. Constr. Build. Mater. 2020, 234, 117353. [Google Scholar] [CrossRef]
- Tan, Y.S.; Yu, H.F.; Li, Y.; Bi, W.; Yao, X. The effect of slag on the properties of magnesium potassium phosphate cement. Constr. Build. Mater. 2016, 126, 313–320. [Google Scholar] [CrossRef]
- Xu, B.; Ma, H.; Shao, H.; Li, Z.; Lothenbach, B. Influence of fly ash on compressive strength and micro-characteristics of magnesium potassium phosphate cement mortars. Cem. Concr. Res. 2017, 99, 86–94. [Google Scholar] [CrossRef]
- Sun, C.J.; Lin, X.J.; Ji, T. Influence of the ratio of KH2PO4 to MgO, ratio of water to bind, content change of borax on water resistance of magnesium-potassium phosphate cement. J. Fuzhou Univ. Nat. Sci. Ed. 2016, 44, 856–862. [Google Scholar]
- Rouzic, M.L.; Chaussadent, T.; Platret, G.; Stefan, L. Mechanisms of kstruvite formation in magnesium phosphate cements. Cem. Concr. Res. 2017, 91, 117–122. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, Z.; Chen, B. Influence of low-grade bauxite on the properties of magnesium phosphate cement. Constr. Build. Mater. 2020, 242, 118052. [Google Scholar] [CrossRef]
- Sugama, T.; Kukacka, L.E. Magnesium monophosphate cements derived from diammonium phosphate solutions. Cem. Concr. Res. 1983, 13, 407–416. [Google Scholar] [CrossRef]



| Materials | MgO | SiO2 | Al2O3 | Fe2O3 | CaO | Others |
|---|---|---|---|---|---|---|
| M | 95.0 | 2.1 | 0.4 | 0.4 | 1.7 | 0.4 |
| Materials | SiO2 | Al2O3 | Fe2O3 | CaO | Others |
|---|---|---|---|---|---|
| MK | 51.3 | 45.9 | 0.5 | 0.3 | 2.0 |
| Group Number | Mix Ratio (%) | Mass Ratio | STP/M (%) | B/M (%) | ||
|---|---|---|---|---|---|---|
| (M + P + B + STP) | MK | P/M | S/M | |||
| MK0 | 100 | 0 | 1/3.3 | 1/1.1 | 4.7 | 2.2 |
| MK4 | 100 | 4 | 1/3.3 | 1/1.1 | 4.7 | 2.2 |
| MK8 | 100 | 8 | 1/3.3 | 1/1.1 | 4.7 | 2.2 |
| MK12 | 100 | 12 | 1/3.3 | 1/1.1 | 4.7 | 2.2 |
| MK16 | 100 | 16 | 1/3.3 | 1/1.1 | 4.7 | 2.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, B.; Jiao, Y.; Cao, R.; Zhai, J.; Zhang, Q. Effect of Metakaolin on the Water Resistance of Magnesium Phosphate Cement Mortar. Coatings 2023, 13, 1664. https://doi.org/10.3390/coatings13101664
Wu B, Jiao Y, Cao R, Zhai J, Zhang Q. Effect of Metakaolin on the Water Resistance of Magnesium Phosphate Cement Mortar. Coatings. 2023; 13(10):1664. https://doi.org/10.3390/coatings13101664
Chicago/Turabian StyleWu, Bin, Yuxin Jiao, Ruidong Cao, Jianming Zhai, and Qiang Zhang. 2023. "Effect of Metakaolin on the Water Resistance of Magnesium Phosphate Cement Mortar" Coatings 13, no. 10: 1664. https://doi.org/10.3390/coatings13101664






