Abstract
Since bentonite can absorb neutrons and gamma rays without sacrificing structural integrity, it is frequently used as the main shielding material in many nuclear installations. Recently, there has been a trend toward enhancing the shielding qualities of bentonite by adding various chemicals. However, the majority of the added materials either require particular handling procedures or pose health risks. The availability of environmentally friendly additives would be wonderful. The addition of barite to bentonite composites greatly raises the density of the specimens. Additionally, the performance of bentonite–barite composites as radiation shielding materials is improved by adding various amounts of bulk and nano Bi2O3 as a filler (6%, 13%, and 20%). Energy dispersive X-ray analysis (EDX) was used to determine the chemical makeup of the produced specimens. The scanning electron microscopy (SEM) pictures showed the samples’ cross-sections’ porosity and homogeneity. 241Am, 133Ba, 137Cs, and 60Co are radioactive sources that emit energies of 59.53, 80.99, 356.01, 661.66, 1173.23, and 1332.5 keV, respectively, and the NaI (Tl) scintillation detector was used in this investigation. The area under the peak of the observed energy spectra was measured using the Genie 2000 program in both the specimen’s absence and presence. The coefficients for linear and mass attenuation were calculated. To determine the theoretical mass attenuation coefficients, the XCOM program was utilized and then compared to the corresponding experimental values. Various radiation shielding parameters dependent on the linear attenuation coefficient were computed for each studied composite. These parameters include the mean free path (MFP), half value layer (HVL), and tenth value layer (TVL). Also, the Zeff and the EABF were determined for each specimen. According to the findings, bismuth oxide was added to bentonite–barite composites to reduce the transmitted flux through the specimens, which increased the LAC of the bentonite–barite composites. Furthermore, adding nanosized bismuth oxide particles increased the sample’s density and improved the material’s shielding properties. At a photon energy of 0.356 keV, the relative deviation (∆%) between the experimental nano- and micro values for Bi2O3 (20 wt%) was 12.1974, confirming that the nanoparticles increase attenuation efficiency.
1. Introduction
Gamma rays are a form of electromagnetic radiation that find extensive applications across various industries, including medicine, agriculture, industry, dentistry, and scientific research. Gamma photons have the remarkable capacity to penetrate deeply, travel at light speed, and possess no charge or mass. These unique characteristics pose significant challenges when it comes to defending against gamma rays. The utilization of gamma sources in hospitals and research facilities for diagnostic purposes has granted individuals access to potentially hazardous areas. Consequently, it becomes imperative to develop a suitable and effective shielding substance to safeguard against gamma rays. The effectiveness of shielding materials depends on several factors, including the thickness, type of medium, attenuation performance, and the presence of high atomic number (z) materials [1,2,3,4].
In order to create a shield that effectively mitigates gamma radiation, it is crucial to conduct comprehensive studies on the interaction between gamma rays and matter. These studies will provide valuable insights into the mechanisms through which gamma radiation interacts with different materials, enabling the development of robust shielding solutions [5]. Gamma radiation has been reduced by the use of lead because lead is a high-Z element. However, lead is poisonous, heavy, and chemically unstable. Due to these drawbacks, scientists are actively seeking lead-free materials that are not only non-toxic but also possess strong gamma-ray attenuation properties [6]. Bismuth oxide (Bi2O3) was chosen for this study due to its higher atomic number and non-toxic nature. Additionally, it is considered a high-density material that is suitable for radiation shielding. Bismuth oxide has been successfully applied to a wide range of materials, including glass, rubber, and building materials [7,8].
In recent years, there has been a significant focus on the capability of bentonite composites to block radiation, which was driven by the expanding nuclear sector worldwide. Bentonite composites are increasingly being utilized in nuclear technology and science, particularly for protection against gamma and X-rays. As a result of extensive research efforts, numerous papers on the usage of bentonite composites as radiation shields have been published globally [9]. This has positioned bentonite composites as a desirable technique for creating mixtures that can effectively attenuate high-energy gamma rays. Gallala et al. [10] have been dedicated to increasing the gamma-ray shielding capability of bentonite with aggregate additives of different mixtures of cement–barite–bismuth as bulk or nano materials. High-performance concrete, including barite aggregate and bismuth powder, increased the shielding properties of the concrete against gamma rays. Ouda et al. [11] show that concrete’s aggregate is crucial in influencing the material’s mechanical characteristics by using the coarse aggregates of barite, magnetite, goethite, and serpentine, which significantly affect its shielding properties. As a result, mixing magnetite as a fine aggregate with high-performance concrete improves its ability to shield γ-rays. Mcconn et al. [12] investigated that the attenuation of gamma rays is influenced by the additives of bentonite, the density, the thickness of the specimen, and the gamma-ray energy. Sallem et al. [13] demonstrated that ball-milled calcinated bentonite has a higher gamma-ray attenuation capacity than ordinary calcinated bentonite. Al-Ghamdi et al. [14] deduced that pure bentonite ceramic, granite, and marble all exhibit less radiation shielding compared to bentonite ceramic with CdO and Bi2O3. The densities of barite and bismuth are equal to 4.5 g/cm3 and 9.75 g/cm3, respectively, which are considered to be high densities. So, bismuth and barite additives are excellent absorbent materials for reducing gamma-ray radiation [15,16].
This study investigated the shielding capabilities, mechanical properties, and morphology of various mixes of bentonite–barite–bismuth oxide specimens. The aim was to enhance the quality of radiation protection. Furthermore, the influence of the particle size and weight percentage of Bi2O3 particles on the gamma radiation shielding capability of bentonite–barite–bismuth oxide composites was examined. The radiation shielding parameters of different composites with varying weight fractions were evaluated using a NaI scintillation detector, covering photon energies ranging from 59.53 keV to 1408.01 keV. Additionally, a comparative analysis was conducted to assess the radiation shielding abilities of nano Bi2O3 and micro Bi2O3 composites.
2. Materials and Method
2.1. Materials
In this study, the primary material used was bentonite, which was obtained from Suez, Egypt. The bentonite was crushed and then ground into a fine powder. Barite and bismuth oxide were also included as ingredients. To determine the composition of the bentonite and barite chemically, energy dispersive X-ray (EDX) analysis was conducted. The results, including the average mass percentage of each metal, can be found in Figure 1 and Figure 2. Furthermore, two different sizes of Bi2O3 particles were utilized in this research. The first type was Bi2O3 microparticles (bulk), which are high-purity powders with a purity level of 99.9%. These microparticles were obtained from Loba Chemie in Mumbai, India. The second type was Bi2O3 nanoparticles, which were synthesized chemically by the Nanotech firm in Giza, Egypt.
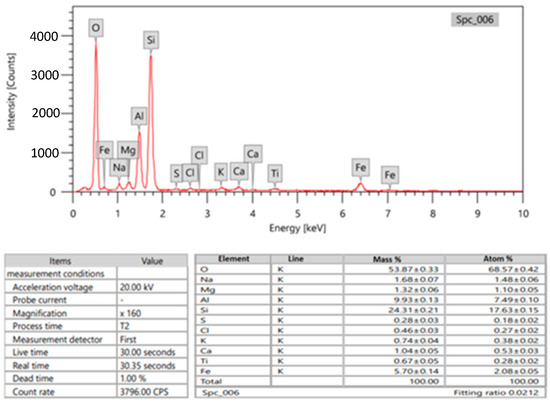
Figure 1.
Chemical composition of bentonite (Edx of bentonite as metal).
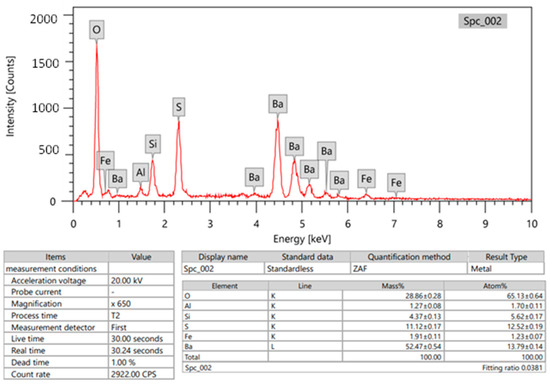
Figure 2.
Chemical composition of barite (Edx of barite composition as metal).
2.2. Samples Preparation
The specimens were created using a combination of finely ground bentonite powder, water, and additional substances, such as bismuth oxide (Bi2O3) and fine barite powder. To produce the samples, specific mass ratios of barite (20, 23.3, 27, and 30) were mixed with micro (bulk) and nano bismuth oxide (Bi2O3) in varying amounts of 0%, 6%, 13%, and 20%, respectively. The corresponding specimen codes and weight percentages (wt%) of bentonite, barite, and Bi2O3 are presented in Table 1. A coin-shaped mold with a diameter of 3 cm and a thickness of 0.5 cm was utilized to shape the specimens. The mixture was poured into the mold and left to dry and solidify in the open air. Each specimen’s average density (g/cm3) was then determined using the Archimedes method with water serving as the immersion medium.

Table 1.
Specimen codes and weight fraction in percentage (wt%) of bentonite, barite, and Bi2O3.
2.3. Morphology Test
In order to distinguish between micro- and nanosized Bi2O3 particles, we utilized the JEM-2100F transmission electron microscope, JEOL, Japan with a 200 kV acceleration voltage to conduct the TEM investigation. Figure 3a,b reveals that the average size of microparticles is 3 µm, while nanoparticles measure 12 nm. The cross-section shape and distribution of the Bi2O3 inside the samples could be studied using scanning electron microscopy (SEM; JEOL-JFC-1100E). Images of the scanned samples for bentonite–barite of 0, 6, 13, and 20 wt% bulk and nano of Bi2O3 are shown in Figure 4. The morphology of the composites under testing was revealed with the scanning electron microscope in Figure 4a where bentonite with fluffy platelets and a porous structure was combined with barite as the plate lumps formed. Figure 4b–d shows composites of bentonite and barite intercalated with spherical-shaped bulk Bi2O3 of 6, 13, and 20 wt%, while Figure 4e–g showed composites of bentonite and barite intercalated with spherical-shaped nano Bi2O3 of 6, 13, and 20 wt%. This demonstrates that the high nanofiller Bi2O3 composites reduced the bentonite pores, increased the composite’s density, and improved the attributes of these nanocomposites as gamma-ray shielding materials.
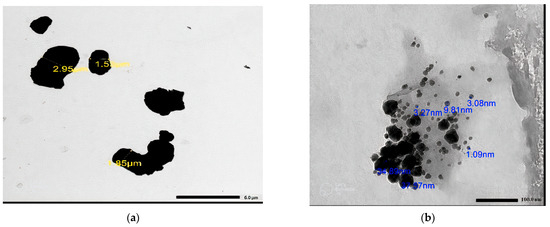
Figure 3.
The TEM image of (a) micro Bi2O3 particles, (b) nano Bi2O3 particles.
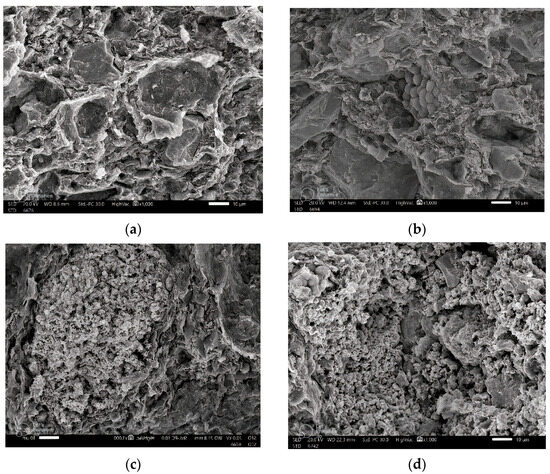
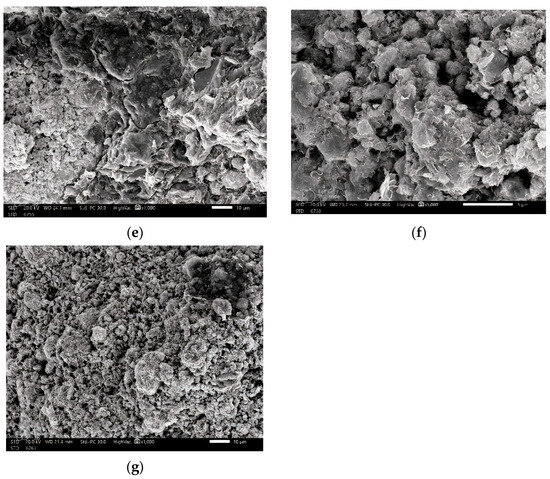
Figure 4.
SEM images of (a) BB-0 (0 wt% Bi2O3), (b) BB-1 (6 wt% micro Bi2O3), (c) BB-2 (13 wt% micro Bi2O3), (d) BB-5 (20 wt% micro Bi2O3), (e) BB-1(6 wt% nano Bi2O3), (f) BB-2 (13 wt% nano Bi2O3), and (g) BB-5 (20 wt% nano Bi2O3).
2.4. Mechanical Properties
The material’s mechanical behavior reflects the link between a material’s response and deformation caused by a force or load applied. Strength, ductility, toughness, hardness, and stiffness are important mechanical design characteristics. The type of applied load, its duration, and environmental conditions are all factors that should be taken into account when determining how the mechanical properties of materials will behave. The load may be constant in magnitude and classified as tensile, compressive, or shear throughout time or continuously fluctuate. With the help of the universal testing machine (see Figure 5), the most typical mechanical stress-strain tests were carried out in tension for all test specimens at room temperature. A steadily increasing tensile force applied uniaxially along one of the specimen’s lengths causes deformation, typically leading to fracture. The test subjects’ tensile specimens were made into cylindrical specimens with a 23 mm diameter and an area of 415 mm2.
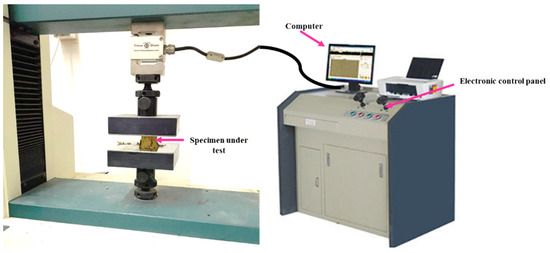
Figure 5.
The experimental setup configuration of the specimen in the universal testing machine to perform the stress-strain tests.
Comparing samples with similar Bi2O3 concentrations and adding or fixing barite (BB-1 and BB-3 or BB-2 and BB-4) for micro samples, it is obvious that the break distance decreases while the ultimate tension grows. When contrasting BB-5 (highest concentration of Bi2O3 20 wt%) with BB-4 (lowest concentration of Bi2O3 (13 wt%) and descending barite concentration, data in Table 2 demonstrate that the break distance increased, and the ultimate tension decreased. When BB-1 and BB-3, which have the same concentration of Bi2O3 (6 wt%), or BB-2 and BB-4, which have the same concentration of Bi2O3 (13 wt%), are compared, it can be seen that the extra barite increases final stress while decreasing break distance. When BB-5 and BB-4 are compared, bentonite somewhat increases while barite slightly reduces, and by adding more Bi2O3 (20 wt%), the final stress is reduced, and the break distance is increased (see Table 2).

Table 2.
The table shows the ultimate force and ultimate stress with the break distance for each sample.
2.5. Radiation Measurements
An experiment was conducted to determine the shielding characteristics and quantity of γ-rays that penetrated the sample. The experiment involved a focused beam of gamma rays produced by a gamma source, which passed through the sample and reached the detector. In this experiment, a scintillation detector of the NaI (Tl) type was used. The setup arrangement of the source–detector system is illustrated in Figure 6. Four radioactive sources (Am-241, Ba-133, Cs-137, and Co-60) were utilized for this study. These sources emit a wide range of radiation energy from 0.0595 MeV to 1.332 MeV. The photons that exited the sample interacted with the detector crystal, converting them into signals. The Genie 2000 software, 9233652E V3.0 was employed to display these signals as peaks in a spectrum [17,18], Figure 7.
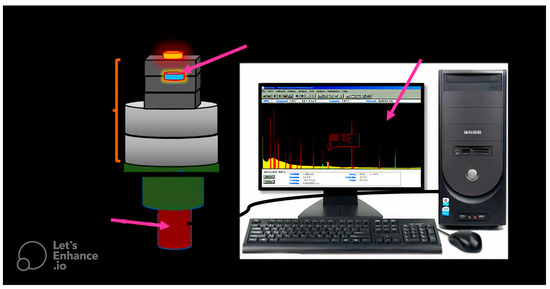
Figure 6.
The experimental setup configuration of the specimen with respect to the radioactive source and the detector.

Figure 7.
The spectrum of Cobalt-60.
2.6. Gamma-Ray Attenuation Characteristics
The LAC describes how easily a beam of gamma ray can enter the body of material. The values of the linear attenuation coefficients were computed using Beer–Lambert’s law [19]:
where µ (cm−1) is the linear attenuation coefficient (LAC), x is the material’s thickness, I is the intensity of an attenuated photon, and Io is the intensity of an input photon.
I = Io e−µx
The average distance between two consecutive photon collisions is known as the mean free path, and it is determined using Equation (2) [20,21,22,23]:
MFP = 1/µ
The half value layer (HVL) was calculated to determine the specimen’s gamma shielding penetration strength. HVL is described as the thickness that reduces the incident photon’s intensity to 50% of its original intensity. Below, Formula (3) was used to calculate the HVL for the current specimens. [24,25]:
HVL = ln2/µ
The following formula was used to calculate the tenth value layer (TVL), which is the thickness of material needed to attenuate incident radiation to 90% of its original intensity [26,27,28]:
TVL = ln10/µ
The most crucial shielding metric, the mass attenuation coefficient (μm), is utilized to compare the shielding effects of various materials. The mass attenuation coefficient (µ/), is the division of the LAC by the composite density of unit (cm2 g−1) as shown in the following equation:
where Wi is a weight fraction of i-th constituent element in the shielding material sample, and (µ/)I is the total mass attenuation coefficient of that element at certain photon energy.
The effective atomic number Zeff is a crucial radiation interaction metric used to describe how radiation attenuation in composite materials varies depending on the specimen’s composition and photon energy. Formula (6) given below can be used to calculate the Zeff values [29]:
where , Ai, and Zi are weight fraction, atomic weight, and the atomic number of each constituent element in each specimen, respectively.
The correction factor is the buildup factor that takes into consideration the increased radiation transmission through shielding. The buildup factor is affected by the energy of the primary radiation, the composition, and the thickness of the shielding substance.
For each sample, the equivalent atomic number (Zeq), which represents the attributes of the composite material in terms of an equivalent element, must be computed in order to derive the energy absorption accumulation factor. The following formula can be used to calculate the values of Zeq, which are affected by the photon energy values [30,31,32]:
where Z1 and Z2 are the atomic numbers of elements corresponding to the ratios R1 and R2, respectively, and R is the ratio of Compton partial mass attenuation coefficients (µCompton) to the total mass attenuation coefficients (µtotal) for the selected composite at specific values of energies.
Then, the five G-P fitting parameters (b, c, a, Xk, and d) for the selected composite must be determined using the same logarithmic interpolation as well as Zeq (8) [33]:
where h represents the G-P fitting coefficient function, which corresponds to the obtained Zeq, and h1 and h2 represent the values of G-P fitting parameters that correspond to the atomic numbers Z1 and Z2, respectively, within the range of energy between 0.015 and 15 MeV for penetration depth MFP up to 40.
Finally, the calculated G-P parameters were used to compute the EABF from the following G-P fitting formula [34]:
where x is the depth of penetration in mfp, K is the photon dose multiplicative factor and represents the spectrum shape change, E is the incident photon energy, and (b, c, a, Xk, d) are the calculated G-P fitting parameters in the previous step.
B (E, x) = 1+ (b – 1) x for K = 1
3. Results and Discussion
Without a doubt, the linear attenuation coefficient (μ) is the most basic parameter employed in experiments to gauge a substance’s capacity to absorb input gamma radiation. It shows the likelihood of a photon interacting with any material for a given travel path length. The LAC (μ) of the absorbing substance is proportional to how the photons interact predominantly with atomic electrons. The linear attenuation coefficient of the specimen increases with the increase in the wt% of the reinforcement materials (Bi2O3—Barite) due to the rise in density ( of the samples while decreasing with the rise in photon energy. Therefore, at a photon energy of around 0.05953 MeV, the LAC shows larger values in Figure 8. The graph clearly shows that for the same concentrations and photon energy, the LAC values of the nano Bi2O3 particles are greater than those of the micro Bi2O3 particles. More barite concentrations also result in higher sample densities, which raise the linear attenuation coefficient (LAC) values for constant bismuth oxide (Bi2O3) concentrations at the same photon energy.
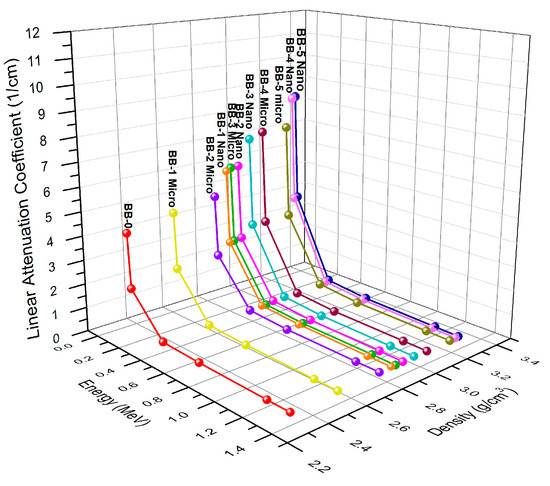
Figure 8.
The variation of LAC as a function of energy and density of micro- and nano Bi2O3-sized particles.
When barite and bismuth oxide are added to the bentonite matrix, they do not suffer any chemical reactions and instead choose to occupy the matrix’s interstitial spaces. Table 3 displays the improvement in the μm values due to the energy of the photons and the chemical makeup of the system composed of bentonite, Bi2O3, and barite. Table 3 shows that the μm values decrease as the photon energy and the amount of bismuth oxide–barite in the samples rise. At energies below 356.01 keV, the μm values drop off due to the photoelectric absorption process. This behavior is linked to the energy of the associated photon [35]. Due to the Compton scattering effect phenomenon, the values of μm also drop, although with minor deviations, as the energy level increases for the energy range of 661.66 to 1.332.5 keV [36].

Table 3.
The mass attenuation coefficient values at different photon energies for bentonite–bismuth oxide and barite in different concentrations.
The MFP exhibits high values at higher photon energies and decreases at lower photon energies, which is a straightforward observation. Moreover, the inclusion of bismuth/barite in the samples affects the MFP trend, resulting in decreased MFP values as the bismuth/barite content increases. These results imply that as the bismuth/barite level rises, the MFP values decline. This relationship is illustrated in Figure 9 where the BB-0 sample with no bismuth oxide content displays the highest MFP values, while the BB-5 sample with the highest concentration of bismuth oxide exhibits the lowest MFP values. Additionally, the MFP values of the nanoparticles are lower than the microparticles.
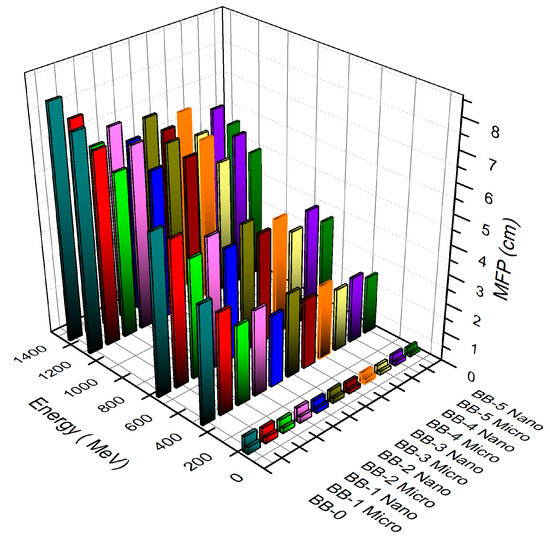
Figure 9.
The relation of the mean free path with the photon energy and concentrations of micro- and nano Bi2O3-sized particles.
Another parameter for assessing the shielding capacity of gamma photons is the half value layer (HVL), which provides specific information about the samples. A lower HVL indicates more effective radiation shielding. Figure 10 illustrates the HVL trend for the sample composition at photon energies of 59.53, 661.66, and 1332.5 keV. It is evident that the values of HVL for BB-0 containing 0 wt% of bulk and NPs Bi2O3 are higher than those of the BB-1, BB-2, BB-3, BB-4, and BB-5 composites at the same values of photon energy. As the concentration of bismuth oxide increases, the HVL decreases. Furthermore, nanosized bismuth oxide particles in the bentonite–barite composites significantly reduce the HVL compared to microsized particles of the same concentration and photon energy. This indicates that nanocomposites outperform micro composites of the same concentration in shielding against gamma radiation at the same values of photon energy. Additionally, it was observed that higher barite concentrations lead to lower HVL levels at the same photon energies when comparing samples.
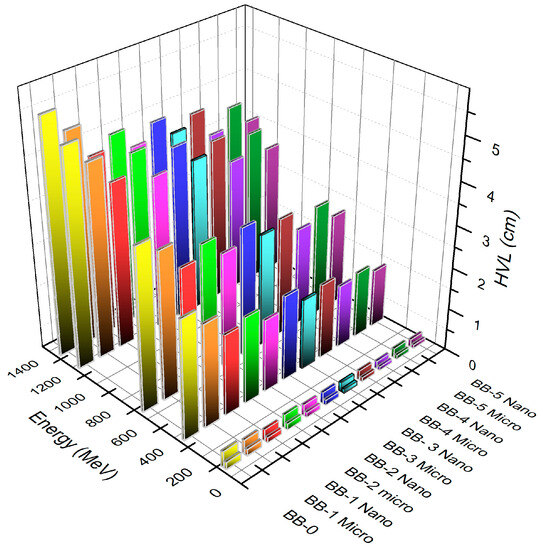
Figure 10.
The relation of the HVL versus the photon energy and concentration of micro- and nano Bi2O3-sized particles.
Similarly, the values of the tenth value layer (TVL), which show a greater reduction in the incident photon intensity to a tenth of its initial intensity value when the shielding material thickness grows to particular levels, have a direct link with photon energy. Additionally, it is noted that the composite without Bi2O3 has a larger TVL. When Bi2O3 is added to the composites in varying amounts (6, 13, or 20 wt%), the tenth value layer decreases with the increase in photon energy.
Additionally, the impact of the particle sizes on the tenth value layer values is demonstrated in samples of nano Bi2O3–bentonite–barite with substantially lower TVL values than samples of micro Bi2O3–bentonite–barite at the same photon energy. Accordingly, increasing the amount of barite in samples while maintaining the same photon energy levels results in a drop in TVL, as seen in Figure 11.
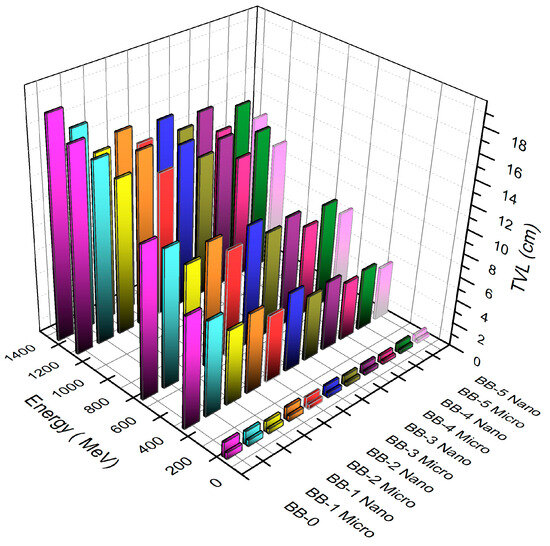
Figure 11.
The TVL function of the photon energy and relation with concentration of micro- and nano Bi2O3-sized particles.
Figure 12 shows the relationship between the effective atomic number, the photon energy, and the density of each sample. It was noticed that the photon energy and sample composition generally affect the effective atomic number (Zeff) values. By increasing the energy of the photon, the values of Zeff decrease. The examined composites have Zeff values ranging from 12.65 to 64.32. When comparing the Zeff values for samples BB-1 and BB-3 with the same Bi2O3 concentration (6 wt%), it is obvious that Zeff is higher for BB-3 than BB-1. Similar to this, comparing BB-2 and BB-4 with the same amount of Bi2O3 (13 wt%), it is noticed that BB-4’s Zeff values are greater than that of BB-2’s. This is due to the samples BB-3 and BB-4 containing more barite than the samples BB-1 and BB-2, respectively. Additionally, it was discovered that the bentonite–barite sample BB-5, which contains the highest amount of Bi2O3 (20 wt%), has the highest Zeff, whereas the BB-0 (0 wt% Bi2O3) bentonite–barite sample has the lowest Zeff. Consequently, the shielding material efficiency against the incident gamma ray increases with the increase in the gamma rays’ interaction with the samples that have a more effective atomic number. It is evident that the higher the Zeff, the better the shielding material performance.
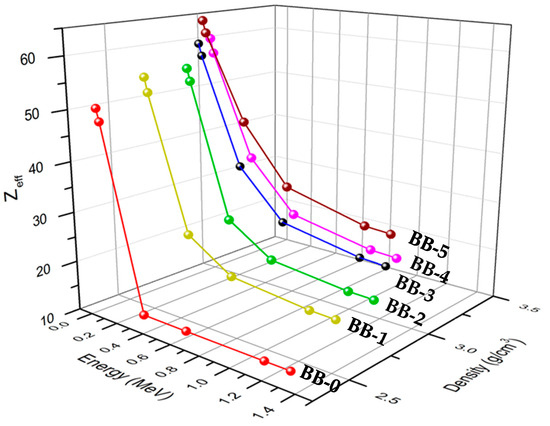
Figure 12.
The effective atomic number vs. energy and its relation to the composite’s density.
The primary radiation’s energy, the shielding material’s makeup, and the shielding material’s thickness all affect buildup factors. Figure 13 shows the relation between the EABF and the photon energy at 1, 15, 25, and 40 mfp. It is observed that the EABF in the lower and higher energies has a lower level according to the photoelectric absorption that predominates in regions of lower photon energy and the pair production that predominates in regions of higher photon energy. At moderate photon energies where the buildup factor rises, Compton scattering predominates. The multiple Compton scattering increases the buildup factors’ values to their maximum. A result of the K-edge of barium (37.44 keV) and bismuth (90.5 keV), there is a dramatic change in the buildup factor’s behavior at lower photon energies. The abrupt rise in the energy is due to the fact that the incident photon energy must exceed the electron’s binding energy in the K shell of the atom with which it interacts. At energy levels exceeding 10 MeV, the values exhibited a consistent trend of gradual increase with the incident energy. Moreover, the material’s penetration depth increases, resulting in an augmented thickness of the interacting material. Consequently, this leads to an increase in scattering events within the interacting medium, especially for the material that has the highest equivalent atomic number. As a result, significant values of EABF are observed.
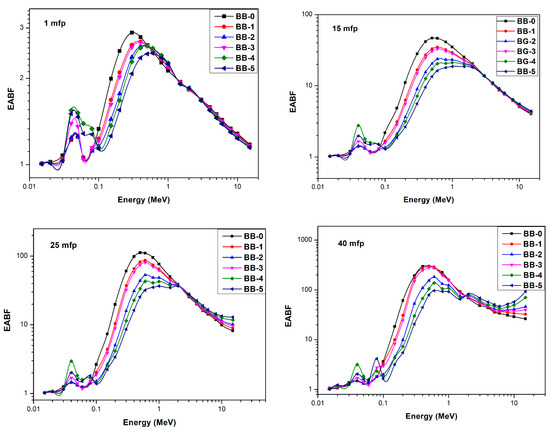
Figure 13.
The energy absorption buildup factor vs. energy at 1, 15, 25, and 40 mfps.
In a comparison between this study, which used the bentonite–barite matrix and Bi2O3 bulk and Np’s (filler), and the study of Abbas et al. [34], which used bulk and nanoscale Bi2O3 as filler in the bentonite matrix, at the same Bi2O3 microparticle concentrations and photon energies (59.53, 80.99, 356.01 keV) and the bentonite–barite composites of the current work for (6 wt% and 20 wt%), the Bi2O3 microparticles have a higher linear attenuation coefficient than that of the bentonite–gypsum composite of Abbas et al. [34], as seen in Table 4. This means that the presence of barite in the bentonite composites increases the linear attenuation coefficient, which confirms the higher shielding performance of the bentonite in the nano Bi2O3 composites.

Table 4.
The comparison in the linear attenuation coefficient at photon energies 59.53, 80.99, and 356.01 keV and at the same concentrations of bismuth oxide (6 wt% and 20 wt%) between bentonite–gypsum composites and bentonite–barite composites.
4. Conclusions
Due to their good radiation-shielding qualities, bentonite and barite were chosen as the primary matrix in this investigation. Bismuth oxide, both in bulk and in nanoscale form, was employed as a filler with varying weight percentages. The comparison that was carried out between the determined experimental and theoretical (XCOM) mass attenuation coefficients produced positive findings. The composites with higher concentrations of bismuth oxide were found to have higher mass attenuation coefficient values. Composites with a higher barite content had greater mass attenuation coefficient values as well due to their high density value. Moreover, the mass attenuation coefficients of the nanosized particles were substantially higher when compared to bulk samples of the same Bi2O3 weight percentages and at the same values of photon energies, which ranged from 0.0595 MeV to 1.3325 MeV. When compared to bulk samples of the same Bi2O3 oxide weight percentages and at a range of γ-ray photon energies from 0.0595 MeV to 1.3325 MeV, the nanosized particles exhibited significantly better mass attenuation coefficients. The determination of the HVL, TVL, MFP, and the effective atomic number for all samples at variant gamma energies confirmed the obtained results that were calculated using the mass attenuation coefficient. Moreover, the estimation of the values for the EABF for all composites grew at low photon energies and showed the maximum at middle photon energies. Furthermore, composites with higher bismuth oxide content had lower absorption build-up factors. Additionally, at deeper penetration depths, the energy absorption build-up factor values were higher. Finally, barite-containing bentonite composites improved attenuation efficiency, especially when nanofiller Bi2O3 was present in high concentrations.
Author Contributions
Methodology, A.M.E.-K.; Software, M.I.A. and M.F.D.; Formal analysis, M.M.G., H.M.A. and R.B.; Data curation, S.N.E.-S.; Writing—original draft, S.N.E.-S.; Visualization, M.F.D., H.M.A. and R.B.; Supervision, M.I.A., M.M.G., M.E. and A.M.E.-K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mahmoud, K.; Sayyed, M.; Tashlykov, O. Comparative studies between the shielding parameters of concretes with different additive aggregates using MCNP-5 simulation code. Radiat. Phys. Chem. 2019, 165, 108426. [Google Scholar] [CrossRef]
- Kamislioglu, M. Research on the effects of bismuth borate glass system on nuclear radiation shielding parameters. Results Phys. 2021, 22, 103844. [Google Scholar] [CrossRef]
- Sazali, M.A.; Rashid, N.K.; Hamzah, K. A review on multilayer radiation shielding. IOP Conf. Ser. Mater. Sci. Eng 2019, 555, 012008. [Google Scholar] [CrossRef]
- Sikora, P.; El-Khayatt, A.M.; Saudi, H.M.; Chung, S.Y.; Stephan, D.; Abd Elrahman, M. Evaluation of the effects of bismuth oxide (Bi2O3) micro and nanoparticles on the mechanical, microstructural and c-ray/neutron shielding properties of Portland cement pastes. Constr. Build. Mater 2021, 284, 122758. [Google Scholar] [CrossRef]
- Ganguly, S.; Bhawal, P.; Ravindren, R.; Das, N.C. Polymer nanocomposites for electromagnetic interference shielding: A review. J. Nanosci. Nanotechnol. 2018, 18, 7641–7669. [Google Scholar] [CrossRef]
- Hayouni, Y.; Gallala, W.; Gaied, M.E.; Plank, J.; Bourham, M.; Alsmadi, Z.Y. Enhanced Shielding and Mechanical Properties of White Cement Mortars Via Celestobarite Fine Aggregate. Int. J. Eng. Technol. 2021, 10, 1–16. [Google Scholar]
- ALMisned, G.; Zakaly, H.M.H.; Issa, S.A.; Eng, A.; Kilic, G.; Bawazeer, O.; Almatar, A.; Shamsi, D.; Rabaa, E.; Sideig, Z. Gamma-Ray Protection Properties of Bismuth-Silicate Glasses against Some Diagnostic Nuclear Medicine Radioisotopes: A Comprehensive Study. Materials 2021, 14, 6668. [Google Scholar] [CrossRef]
- Zaid, M.H.M.; Matori, K.A.; Sidek, H.A.A.; Ibrahim, I.R. Bismuth modified gamma radiation shielding properties of titanium vanadium sodium tellurite glasses as a potent transparent radiation-resistant glass applications. Nucl. Eng. Technol. 2021, 53, 1323–1330. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, P.; Saha, A.; Noked, M.; Gedanken, A.; Margel, S. Mussel-inspired polynorepinephrine/MXene-based magnetic nanohybrid for electromagnetic interference shielding in X-band and strain-sensing performance. Langmuir 2022, 38, 3936–3950. [Google Scholar] [CrossRef]
- Gallala, W.; Hayoun, Y.; Gaied, M.E.; Fusco, M.; Alsaied, J.; Bailey, K.; Bourham, M. Mechanical and radiation shielding properties of mortars with additive fne aggregate mine waste. Ann. Nucl. Energy 2017, 101, 600–606. [Google Scholar] [CrossRef]
- Ouda, A.S. Development of high-performance heavy-density concrete using different aggregates for gamma-ray shielding. Prog. Nucl. Energy 2015, 79, 48–55. [Google Scholar] [CrossRef]
- McConn, R.J.; Gesh, C.J.; Pagh, R.T.; Rucker, R.A.; Williams, R.G. Compendium of Material Composition Data for Radiation Transport Modeling; Homeland Security: Washington, DC, USA, 2011. [Google Scholar]
- Sallem, F.H.; Sayyed, M.I.; Aloraini, D.A.; Almuqrin, A.H.; Mahmoud, K.A. Characterization and Gamma-ray Shielding Performance of Calcinated and Ball-Milled Calcinated Bentonite Clay Nanoparticles. Crystals 2022, 12, 1178. [Google Scholar] [CrossRef]
- Al-Ghamdi, H.; Elsafi, M.; Almuqrin, A.H.; Yasmin, S.; Sayyed, M.I. Investigation of the Gamma-ray Shielding Performance of CuO-CdO-Bi2O3 Bentonite Ceramics. Materials 2022, 15, 5310. [Google Scholar] [CrossRef]
- Cheng, J.; Li, C.; Xiong, Y.; Zhang, H.; Raza, H.; Ullah, S.; Wu, J.; Zheng, G.; Cao, Q.; Zhang, D.; et al. Recent advances in design strategies and multifunctionality of flexible electromagnetic interference shielding materials. Nano-Micro Lett. 2022, 14, 80. [Google Scholar] [CrossRef]
- Wang, X.Y.; Liao, S.Y.; Wan, Y.J.; Zhu, P.L.; Hu, Y.G.; Zhao, T.; Sun, R.; Wong, C.P. Electromagnetic interference shielding materials: Recent progress, structure design, and future perspective. J. Mater. Chem. C 2022, 10, 44–72. [Google Scholar] [CrossRef]
- Gouda, M.M. Calibration of NaI (Tl) Cylindrical Detector Using Axially Shifted Radioactive Cylindrical Sources. Nucl. Technol. Radiat. Prot. 2019, 34, 353–360. [Google Scholar] [CrossRef]
- Elsafi, M.; Alzahrani, J.S.; Abbas, M.I.; Gouda, M.M.; Thabet, A.A.; Badawi, M.S.; El-Khatib, A.M. Geant4 Tracks of NaI Cubic Detector Peak Efficiency, Including Coincidence Summing Correction for Rectangular Sources. Nucl. Sci. Eng. 2021, 195, 1008–1016. [Google Scholar] [CrossRef]
- Han, I.; Demir, L. Studies on effective atomic numbers, electron densities from mass attenuation coefficients in TixCo1−x and CoxCu1−x alloys. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2009, 267, 3505–3510. [Google Scholar] [CrossRef]
- Kurudirek, M. Heavy metal borate glasses: Potential use for radiation shielding. J. Alloys Compd. 2017, 727, 1227–1236. [Google Scholar] [CrossRef]
- Abouhaswa, A.S.; Mhareb, M.H.A.; Alalawi, A.; Al-Buriahi, M.S. Physical, structural, optical, and radiation shielding properties of B2O3-20Bi2O3-20Na2O2-Sb2O3 glasses: Role of Sb2O3. J. Non-Cryst. Solids 2020, 543, 120130. [Google Scholar] [CrossRef]
- Issa, S.A.M. Effective atomic number and mass attenuation coefficient of PbO–BaO–B2O3 glass system. Radiat. Phys. Chem. 2016, 120, 33–37. [Google Scholar] [CrossRef]
- Kaur, P.; Singh, K.; Kurudirek, M.; Thakur, S. Study of environment friendly bismuth incorporated lithium borate glass system for structural, gamma-ray and fast neutron shielding properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 223, 117309. [Google Scholar] [CrossRef]
- Al-Buriahi, M.S.; Arslan, H.; Tonguc, B.T. Investigation of photon energy absorption properties for some biomolecules. Nucl. Sci. Tech. 2019, 30, 103. [Google Scholar] [CrossRef]
- Hassan, H.E.; Badran, H.M.; Aydarous, A.; Sharshar, T. Studying the effect of nano lead compounds additives on the concrete shielding properties for γ-rays. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2015, 360, 81–89. [Google Scholar] [CrossRef]
- Nagaraja, N.; Manjunatha, H.C.; Seenappa, L.; Sridhar, K.N.; Ramalingam, H.B. Radiation shielding properties of silicon polymers. Radiat. Phys. Chem. 2020, 171, 108723. [Google Scholar] [CrossRef]
- Martin, A.; Harbison, S.; Beach, K.; Cole, P. An Intro-Duction to Radiation Protection, 6th ed.; CRC Press: Boca Raton, FL, USA, 2012; p. 256. [Google Scholar]
- Kaçal, M.R.; Akman, F.; Sayyed, M.I. Evaluation of gamma-ray and neutron attenuation properties of some polymers. Nucl. Eng. Technol. 2019, 51, 818–824. [Google Scholar] [CrossRef]
- El-Bashir, B.O.; Sayyed, M.I.; Zaid, M.H.M.; Matori, K.A. Comprehensive study on physical, elastic and shielding properties of ternary BaO-Bi2O3-P2O5 glasses as a potent radiation shielding material. J. Non-Cryst. Solids 2017, 468, 92–99. [Google Scholar] [CrossRef]
- Feng, L.; Li, S.; Feng, S. Preparation and characterization of silicone rubber with high modulus via tension spring-type crosslinking. RSC Adv. 2017, 7, 13130. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Lakshminarayana, G.; Kityk, I.V.; Mahdi, M.A. Evaluation of shielding parameters for heavy metal fluoride based tellurite-rich glasses for gamma ray shielding applications. Radiat. Phys. Chem. 2017, 139, 33–39. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Elmahroug, Y.; El-bashir, B.O.; Issa, S.A.M. Gamma-ray shielding properties of zinc oxide soda lime silica glasses. J. Mater. Sci. Mater. Electron. 2017, 28, 4064–4074. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Askari, M.; Ezzati, S.N. X-ray attenuating nanocomposite based on polyaniline using Pb nanoparticles. Synth. Met. 2014, 196, 68–75. [Google Scholar] [CrossRef]
- Abbas, M.I.; El-Khatib, A.M.; Elsafi, M.; El-Shimy, S.N.; Dib, M.F.; Abdellatif, H.M.; Baharoon, R.; Gouda, M.M. Investigation of Gamma-Ray Shielding Properties of Bismuth Oxide Nanoparticles with a Bentonite–Gypsum Matrix. Materials 2023, 16, 2056. [Google Scholar] [CrossRef] [PubMed]
- Şakar, E. Determination of photon-shielding features and build-up factors of nickelesilver alloys. Radiat. Phys. Chem. 2020, 172, 108778. [Google Scholar] [CrossRef]
- Manohara, S.R.; Hanagodimath, S.M.; Gerward, L. Photon interaction and energy absorption in glass: A transparent gamma ray shield. J. Nucl. Mater. 2009, 393, 465–472. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).