Microorganisms Involved in the Biodegradation and Microbiological Corrosion of Structural Materials
Abstract
:1. Introduction
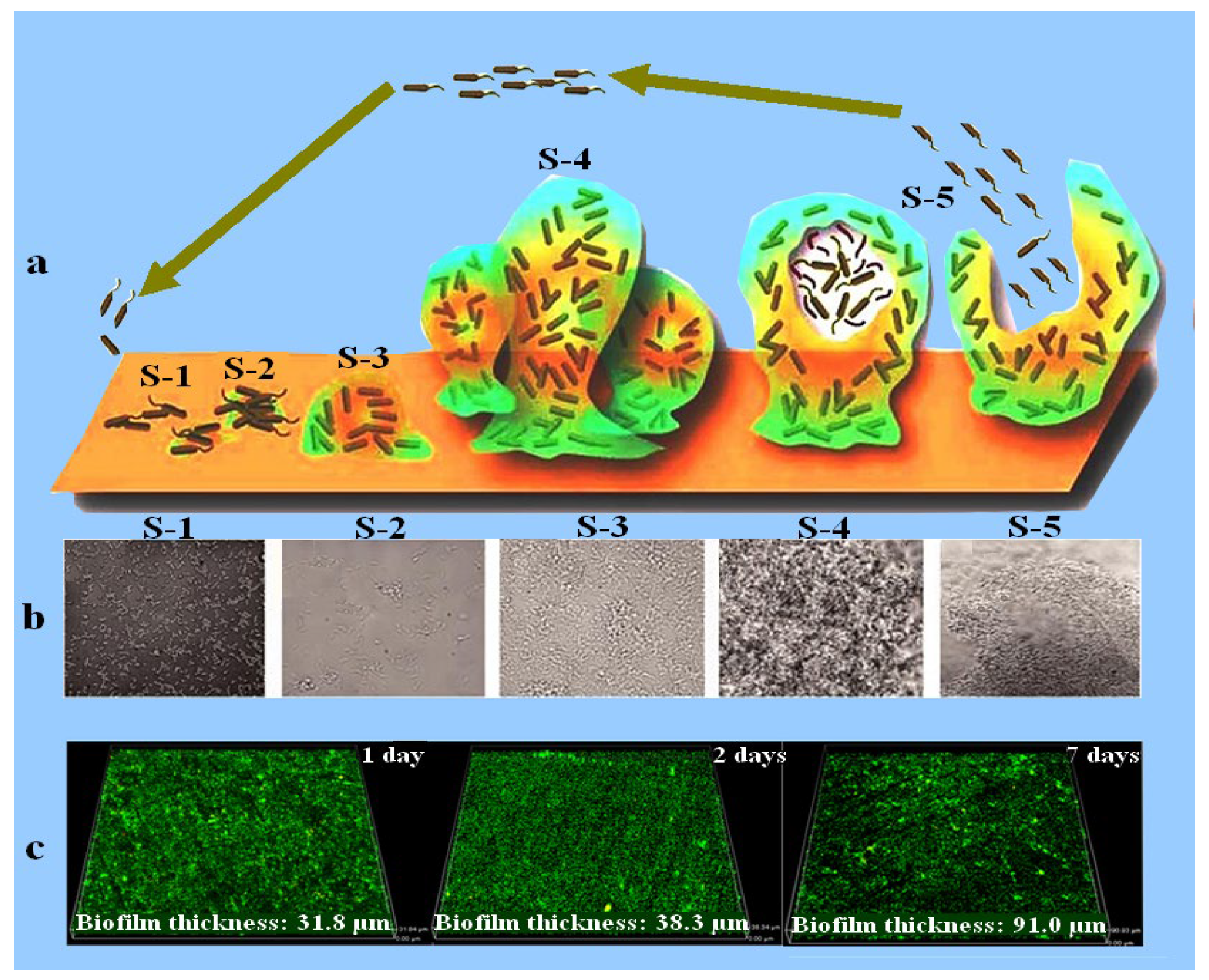
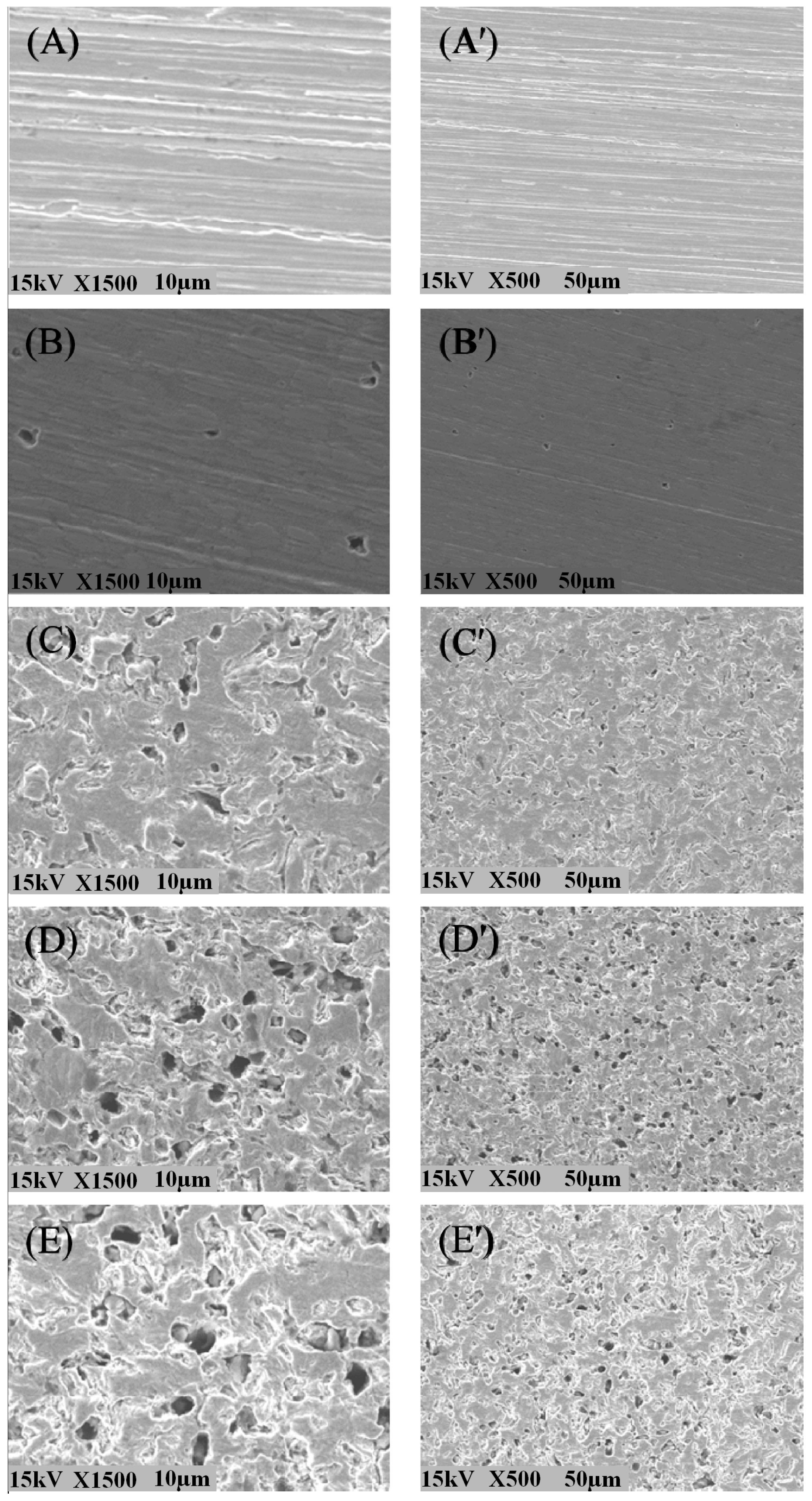
2. Bacteria
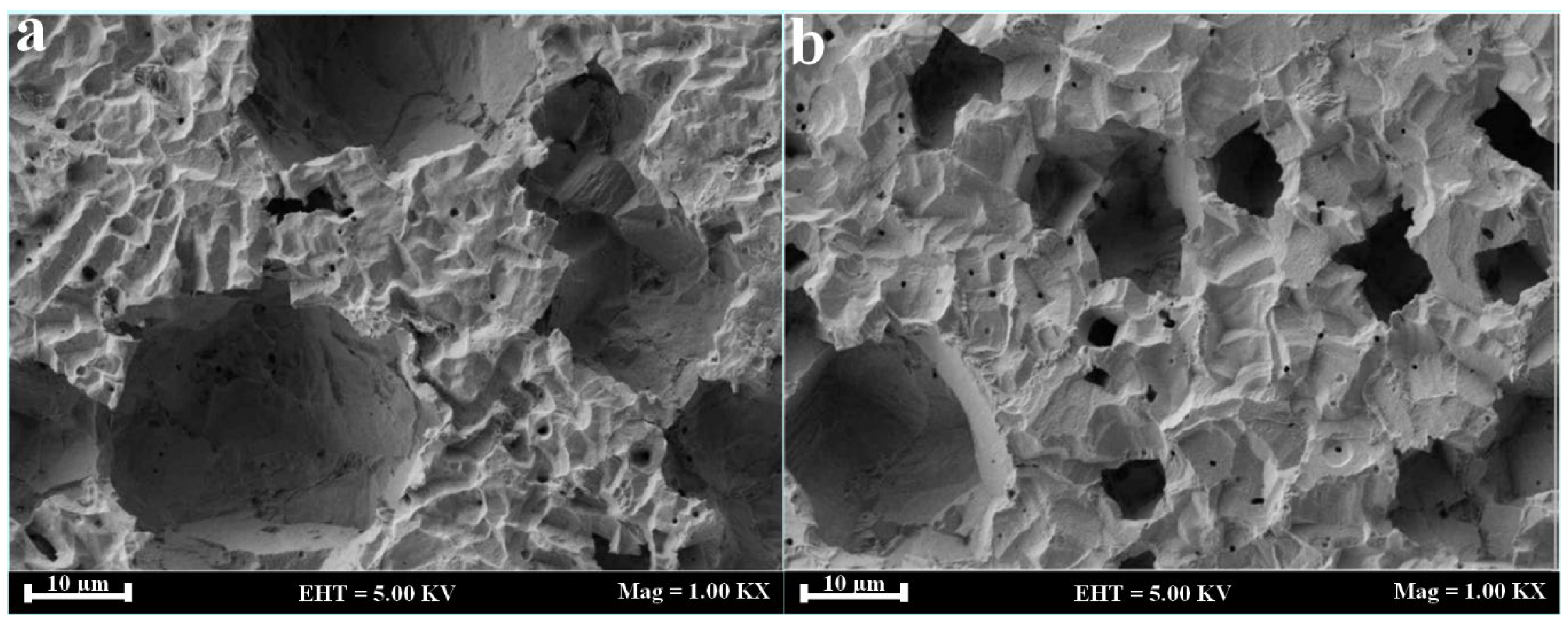
3. Fungi
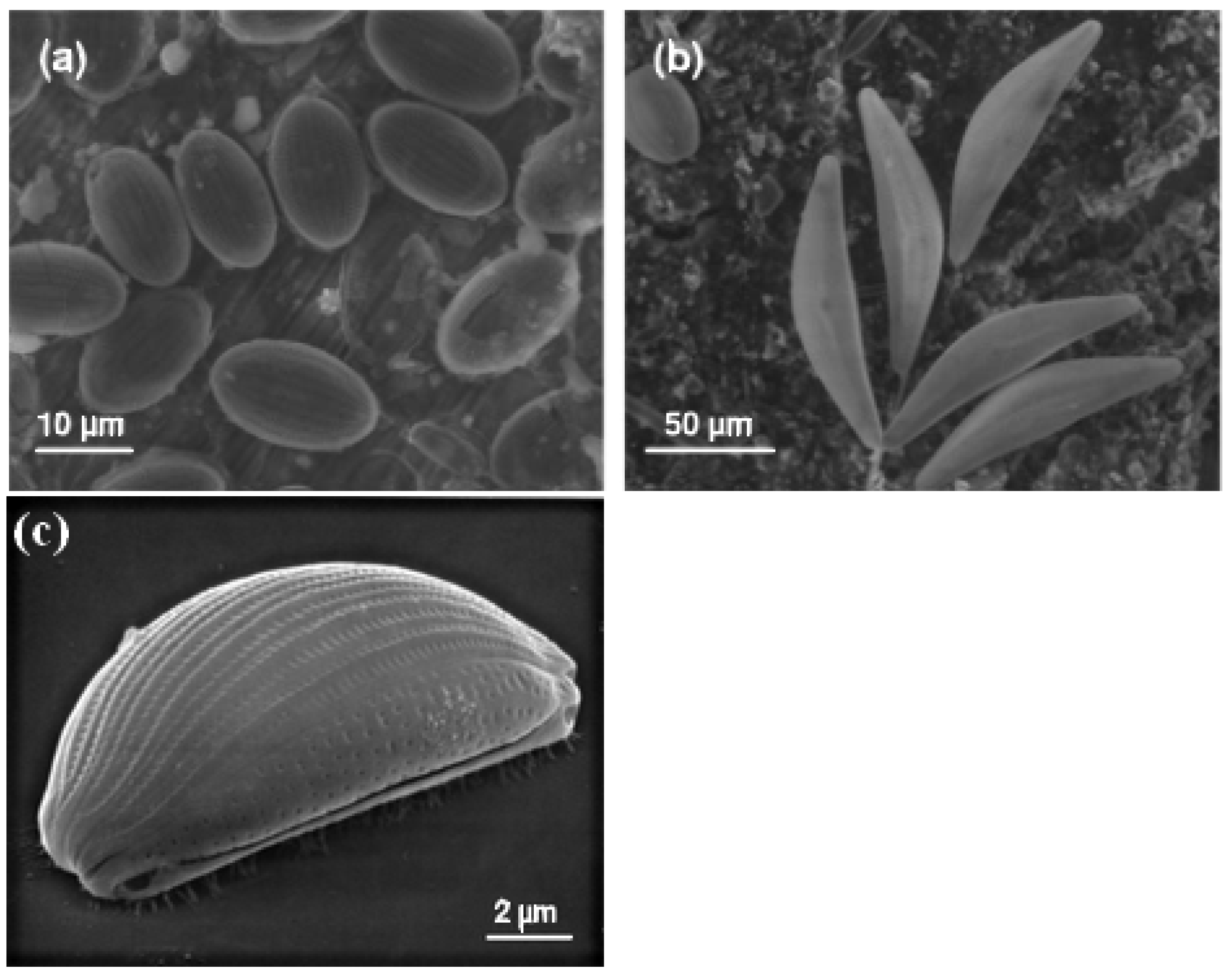
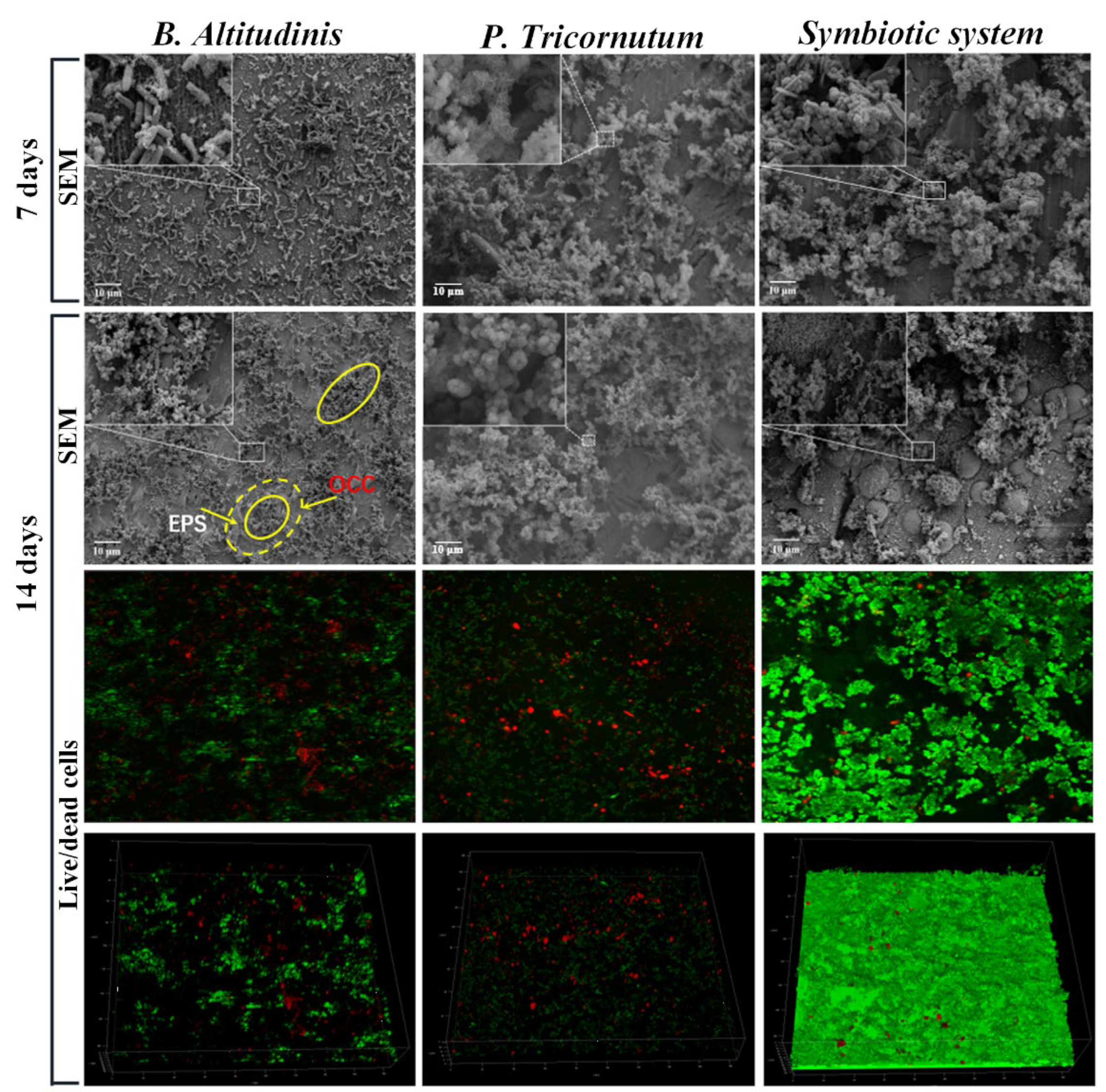
4. Algae
5. Archaea
6. Lichens
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- González, J.E.G.; Santana, F.J.H.; Mirza-Rosca, J.C. Effect of bacterial biofilm on 316 SS corrosion in natural seawater by EIS. Corros. Sci. 1998, 40, 2141–2154. [Google Scholar] [CrossRef]
- Gaines, H.A. Bacterial activity as a corrosion induced in the soil. J. Eng. Ind. Chem. 1910, 2, 128–130. [Google Scholar] [CrossRef]
- Telegdi, J.; Trif, L.; Roma, L. Smart anti-biofouling composite coatings for naval applications. In Smart Composite Coatings and Membranes; Elsevier: Amsterdam, The Netherlands, 2016; pp. 123–155. [Google Scholar] [CrossRef]
- Bessone, J.B.; Baldo, R.A.S.; de de Micheli, S.M. Sea water testing OF Al-Zn, Al-Zn-Sn, and Al-Zn-ln sacrificial anodes. Corrosion 1981, 37, 533–540. [Google Scholar] [CrossRef]
- Vu, B.; Chen, M.; Crawford, R.J.; Ivanova, E.P. Bacterial extracellular polysaccharides involved in biofilm formation. Molecules 2009, 14, 2535–2554. [Google Scholar] [CrossRef]
- Costerton, J.; Lewandownski, Z.; Caldwell, D.; Korber, D. Lappin-Scott H Microbial Biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef]
- Khan, M.S.; Liang, T.; Liu, Y.; Shi, Y.; Zhang, H.; Li, H.; Guo, S.; Pan, H.; Yang, K.; Zhao, Y. Microbiologically Influenced Corrosion Mechanism of Ferrous Alloys in Marine Environment. Metals 2022, 12, 1458. [Google Scholar] [CrossRef]
- Zobell, C.E. The Effect of Solid Surfaces upon Bacterial Activity. J. Bacteriol. 1943, 46, 39–56. [Google Scholar] [CrossRef]
- Beyenal, H.; Lewandowski, Z. Internal and external mass transfer in biofilms grown at various flow velocities. Biotechnol. Prog. 2002, 18, 55–61. [Google Scholar] [CrossRef]
- Allan, V.J.M.; Callow, M.E.; Macaskie, L.E.; Paterson-beedle, M.; Citrobacter, A. Effect of nutrient limitation on biofilm formation and phosphatase activity of a Citrobacter sp. Microbiology 2002, 148, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial Biofilm Eradication Agents: A Current Review. Front. Chem. 2019, 7, 824. [Google Scholar] [CrossRef]
- Saleem Khan, M.; Xu, D.; Liu, D.; Lekbach, Y.; Yang, K.; Yang, C. Corrosion Inhibition of X80 Steel in Simulated Marine Environment with Marinobacter aquaeolei. Acta Metall. Sin. 2019, 32, 1373–1384. [Google Scholar] [CrossRef]
- Yuan, S.J.; Pehkonen, S.O. AFM study of microbial colonization and its deleterious effect on 304 stainless steel by Pseudomonas NCIMB 2021 and Desulfovibrio desulfuricans in simulated seawater. Corros. Sci. 2009, 51, 1372–1385. [Google Scholar] [CrossRef]
- Miranda, E.; Bethencourt, M.; Botana, F.J.; Cano, M.J.; Sánchez-Amaya, J.M.; Corzo, A.; de Lomas, J.G.; Fardeau, M.L.; Ollivier, B. Biocorrosion of carbon steel alloys by an hydrogenotrophic sulfate-reducing bacterium Desulfovibrio capillatus isolated from a Mexican oil field separator. Corros. Sci. 2006, 48, 2417–2431. [Google Scholar] [CrossRef]
- McNamara, C.J.; Perry, T.D., IV; Bearce, K.A.; Hernandez-Duque, G.; Mitchell, R. Epilithic and endolithic bacterial communities in limestone from a Maya archaeological site. Microb. Ecol. 2006, 51, 51–64. [Google Scholar] [CrossRef] [PubMed]
- McNamara, C.J.; Perry, T.D., IV; Bearce, K.; Hernandez-Duque, G.; Mitchell, R. Measurement of limestone biodeterioration using the Ca2+ binding fluorochrome Rhod-5N. J. Microbiol. Methods 2005, 61, 245–250. [Google Scholar] [CrossRef]
- Rao, T.S. Microbial Fouling and Corrosion: Fundamentals and Mechanisms. In Operational and Environmental Consequences of Large Industrial Cooling Water Systems; Springer: Boston, MA, USA, 2012; ISBN 9781461416982. [Google Scholar]
- Rouvre, I.; Gauquelin, C.; Meynial-salles, I.; Basseguy, R. Impact of the chemicals, essential for the puri fi cation process of strict Fe-hydrogenase, on the corrosion of mild steel. Bioelectrochemistry 2016, 109, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Jia, R.; Li, Y.; Gu, T. Advances in the treatment of problematic industrial biofilms. World J. Microbiol. Biotechnol. 2017, 33, 97. [Google Scholar] [CrossRef]
- Kilbane, J.J. Determining the source of H2S on an offshore oil production platform. In Microbiologically Influenced Corrosion in the Upstream Oil and Gas Industry; CRC Press: Boca Raton, FL, USA, 2017; pp. 351–360. ISBN 9781498726603. [Google Scholar]
- Zhao, Y.; Zhou, E.; Liu, Y.; Liao, S.; Li, Z.; Xu, D.; Zhang, T.; Gu, T. Comparison of different electrochemical techniques for continuous monitoring of the microbiologically influenced corrosion of 2205 duplex stainless steel by marine Pseudomonas aeruginosa biofilm. Corros. Sci. 2017, 126, 142–151. [Google Scholar] [CrossRef]
- Sabel, C.F.; Victor, D.G. Governing global problems under uncertainty: Making bottom-up climate policy work. Clim. Chang. 2017, 144, 15–27. [Google Scholar] [CrossRef]
- Videla, H.A. Prevention and control of biocorrosion. Int. Biodeterior. Biodegrad. 2002, 49, 259–270. [Google Scholar] [CrossRef]
- Jacobson, G. Corrosion at Prudhoe Bay—A lesson on the line. Mater. Perform. 2007, 46, 26–34. [Google Scholar]
- Bhat, S.; Kumar, B.; Prasad, S.R.; Katarki, M.V. Failure of a new 8-in pipeline from group gathering station to central tank farm. Mater. Perform. 2011, 50, 50–54. [Google Scholar]
- Walsh, D.; Pope, D.; Danford, M.; Huff, T. The effect of microstructure on microbiologically influenced corrosion. J. Miner. Met. Mater. Soc. 1993, 45, 22–30. [Google Scholar] [CrossRef]
- Little, B.J.; Lee, J.S. Microbiologically Influenced Corrosion. In Oil and Gas Pipelines; John Wiley & Sons: Hoboken, NJ, USA, 2015; Volume 2, ISBN 9781119019213. [Google Scholar]
- Javaherdashti, R.; Alasvand, K. An Introduction to Microbial Corrosion. In Biological Treatment of Microbial Corrosion; Elsevier: Amsterdam, The Netherlands, 2019; pp. 25–70. ISBN 9780128161081. [Google Scholar]
- Fredrickson, J.K.; Zachara, J.M.; Balkwill, D.L.; Kennedy, D.; Li, S.M.W.; Kostandarithes, H.M.; Daly, M.J.; Romine, M.F.; Brockman, F.J. Geomicrobiology of high-level nuclear waste-contaminated vadose sediments at the Hanford Site, Washington State. Appl. Environ. Microbiol. 2004, 70, 4230–4241. [Google Scholar] [CrossRef]
- Metals Handbook, 9th ed.; ASM International: Metals park, OH, USA, 1987; Volume 13.
- Alabbas, F.M.; Williamson, C.; Bhola, S.M.; Spear, J.R.; Olson, D.L.; Mishra, B.; Kakpovbia, A.E. Microbial corrosion in linepipe steel under the influence of a sulfate-reducing consortium isolated from an oil field. J. Mater. Eng. Perform. 2013, 22, 3517–3529. [Google Scholar] [CrossRef]
- Videla, H.A.; Herrera, L.K. Microbiologically influenced corrosion: Looking to the future. Int. Microbiol. 2005, 8, 169–180. [Google Scholar]
- Shahryari, Z.; Gheisari, K.; Motamedi, H. Corrosion behavior of API X70 microalloyed pipeline steel in a simulated soil solution in the absence and presence of aerobic Pseudomonas species. Mater. Res. Express 2019, 6, 065409. [Google Scholar] [CrossRef]
- Stipaničev, M.; Turcu, F.; Esnault, L.; Schweitzer, E.W.; Kilian, R.; Basseguy, R. Corrosion behavior of carbon steel in presence of sulfate-reducing bacteria in seawater environment. Electrochim. Acta 2013, 113, 390–406. [Google Scholar] [CrossRef]
- El Hajj, H.; Abdelouas, A.; El Mendili, Y.; Karakurt, G.; Grambow, B.; Martin, C. Corrosion of carbon steel under sequential aerobic-anaerobic environmental conditions. Corros. Sci. 2013, 76, 432–440. [Google Scholar] [CrossRef]
- Lee, W.; Characklis, W.G. Corrosion of mild steel under anaerobic biofilm. Corrosion 1993, 49, 186–199. [Google Scholar] [CrossRef]
- Lee, W.; Lewandowski, Z.; Nielsen, P.H.; Allan Hamilton, W. Role of sulfate-reducing bacteria in corrosion of mild steel: A review. Biofouling 1995, 8, 165–194. [Google Scholar] [CrossRef]
- Chen, S.; Wang, P.; Zhang, D. Corrosion behavior of copper under biofilm of sulfate-reducing bacteria. Corros. Sci. 2014, 87, 407–415. [Google Scholar] [CrossRef]
- Marchal, R. Rôle des bactéries sulfurogènes dans la corrosion du fer. Oil Gas Sci. Technol. 1999, 54, 649–659. [Google Scholar] [CrossRef]
- Gu, T.; Zhao, K.; Nesic, S. A New Mechanistic Model for Mic Based on A Biocatalytic Cathodic Sulfate Reduction Theory. In Proceedings of the Corrosion 2009, Atlanta, GA, USA, 22–26 March 2009; pp. 1–12. [Google Scholar]
- Zhao, K. Investigation of Microbiologically Influenced Corrosion (Mic) and Biocide Treatment in Anaerobic Salt Water and Development of a Mechanistic Mic Model. Ph.D. Thesis, Ohio University, Athens, OH, USA, 2008. [Google Scholar]
- Chen, J.; Wu, J.; Wang, P.; Zhang, D.; Chen, S.; Tan, F. Correction to: Corrosion of 907 Steel Influenced by Sulfate-Reducing Bacteria. J. Mater. Eng. Perform. 2019, 28, 5913. [Google Scholar] [CrossRef]
- Fang, S.J.; Liu, Y.H.; Wang, Q.; Yu, S.R.; Song, Y.L. Influence of SRB on corrosion of AZ91 magnesium alloy in solution containing chlorine ions. Huanan Ligong Daxue Xuebao/J. South China Univ. Technol. (Nat. Sci.) 2008, 36, 92–96. [Google Scholar]
- Guan, F.; Zhai, X.; Duan, J.; Zhang, J.; Li, K.; Hou, B. Influence of sulfate-reducing bacteria on the corrosion behavior of 5052 aluminum alloy. Surf. Coatings Technol. 2017, 316, 171–179. [Google Scholar] [CrossRef]
- Zheng, X.; Zhuang, X.; Lei, Y.; Chu, Z.; Xu, J.; Gao, L.; Sun, X. Corrosion behavior of the Ti-6Al-4V alloy in sulfate-reducing bacteria solution. Coatings 2020, 10, 24. [Google Scholar] [CrossRef]
- Costello, J.A. Cathodic depolarization by sulphate reducing bacteria. S. Afr. J. Sci. 1974, 70, 202–204. [Google Scholar]
- Silva, D.; Basséguy, R.; Bergel, A. The role of hydrogenases in the anaerobic microbiologically influenced corrosion of steels. Bioelectrochemistry 2002, 56, 77–79. [Google Scholar] [CrossRef]
- Da Silva, S.; Basséguy, R.; Bergel, A. Electrochemical deprotonation of phosphate on stainless steel. Electrochim. Acta 2004, 49, 4553–4561. [Google Scholar] [CrossRef]
- Dinh, H.T.; Kuever, J.; Mußmann, M.; Hassel, A.W.; Stratmann, M.; Widdel, F. Iron corrosion by novel anaerobic microorganisms. Nature 2004, 427, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.J.; Pehkonen, S.O. Microbiologically influenced corrosion of 304 stainless steel by aerobic Pseudomonas NCIMB 2021 bacteria: AFM and XPS study. Colloids Surf. B Biointerfaces 2007, 59, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Lekbach, Y.; Liu, T.; Li, Y.; Moradi, M.; Dou, W.; Xu, D.; Smith, J.A.; Lovley, D.R. Microbial corrosion of metals: The corrosion microbiome. Adv. Microb. Physiol. 2021, 78, 317–390. [Google Scholar]
- Lv, M.; Du, M.; Li, X.; Yue, Y.; Chen, X. Mechanism of microbiologically influenced corrosion of X65 steel in seawater containing sulfate-reducing bacteria and iron-oxidizing bacteria. J. Mater. Res. Technol. 2019, 8, 4066–4078. [Google Scholar] [CrossRef]
- Ghali, E. Corrosion Resistance of Al and Mg Alloys; Wiley: Hoboken, NJ, USA, 2010; ISBN 9780471715764. [Google Scholar]
- Alekhova, T.A.; Aleksandrova, A.A.; Novozhilova, T.Y.; Lysak, L.V.; Zagustina, N.A.; Bezborodov, A.M. Monitoring of microbial degraders in manned space stations. Appl. Biochem. Microbiol. 2005, 41, 382–389. [Google Scholar] [CrossRef]
- Little, B.J.; Ray, R.I. The Role of Fungi in Microbiologically Influenced Corrosion. In Proceedings of the 15th International Corrosion Congress, Granada, Spain, 22–27 September 2002. [Google Scholar]
- Gadd, G.M. Metals, minerals and microbes: Geomicrobiology and bioremediation. Microbiology 2010, 156, 609–643. [Google Scholar] [CrossRef]
- Gadd, G.M. Geomycology: Biogeochemical transformations of rocks, minerals, metals and radionuclides by fungi, bioweathering and bioremediation. Mycol. Res. 2007, 111, 3–49. [Google Scholar] [CrossRef]
- Webb, J.S.; Nixon, M.; Eastwood, I.M.; Greenhalgh, M.; Robson, G.D.; Handley, P.S. Fungal colonization and biodeterioration of plasticized polyvinyl chloride. Appl. Environ. Microbiol. 2000, 66, 3194–3200. [Google Scholar] [CrossRef]
- There, I.; Polymers, E.; Pcl, F.; Corporation, U.C.; Brook, B. Scanning electron microscopic visualization of biodegradation of polycaprolactones by fungi. J. Polym. Sci. Polym. Lett. Ed. 1981, 19, 159–165. [Google Scholar]
- Date, R.; Use, A.; Leave, O.; No, J.O.; Little, B.; Ray, R. Fungal Influenced Corrosion of Metals in Humid Environments. NACE Int. Corros. Soc. 1999, 298, 1–6. [Google Scholar]
- Lugauskas, A.; Demčenko, I.; Selskiene, A.; Pakštas, V.; Jaskelevičius, B.; Narkevičius, A.; Bučinskiene, D. Resistance of chromated zinc coatings to the impact of microscopic fungi. Medziagotyra 2011, 17, 20–26. [Google Scholar] [CrossRef]
- Lee, J.S.; Ray, R.I.; Little, B.J. Microbiologically Influenced Corrosion in Military Environments. In Corrosion: Environments and Industries; ASM International: Materials Park, OH, USA, 2018. [Google Scholar]
- Little, B.; Staehle, R.; Davis, R. Fungal influenced corrosion of post-tensioned cables. Int. Biodeterior. Biodegrad. 2001, 47, 71–77. [Google Scholar] [CrossRef]
- McNamara, C.; Perry, T.; Leard, R.; Bearce, K.; Dante, J.; Mitchell, R. Corrosion of aluminum alloy 2024 by microorganisms isolated from aircraft fuel tanks. Biofouling 2005, 21, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Rauch, M.E.; Graef, H.W.; Rozenzhak, S.M.; Jones, S.E.; Bleckmann, C.A.; Kruger, R.L.; Naik, R.R.; Stone, M.O. Characterization of microbial contamination in United States Air Force aviation fuel tanks. J. Ind. Microbiol. Biotechnol. 2006, 33, 29–36. [Google Scholar] [CrossRef]
- Plassard, C.; Fransson, P. Regulation of low-molecular weight organic acid production in fungi. Fungal Biol. Rev. 2009, 23, 30–39. [Google Scholar] [CrossRef]
- Humphreys, P. The Potential Role of Fungi and Algae in the Atmospheric Corrosion of Waste Packages in Interim Storage and During the Operational Period of a GDF. Nuclear Decommissioning Authority: Oxfordshire, UK, 2012; pp. 1–40. [Google Scholar]
- Liu, H.; Xu, D.; Dao, A.Q.; Zhang, G.; Lv, Y.; Liu, H. Study of corrosion behavior and mechanism of carbon steel in the presence of Chlorella vulgaris. Corros. Sci. 2015, 101, 84–93. [Google Scholar] [CrossRef]
- Scotto, V.; Alabiso, G.; Marcenaro, G. An example of microbiologically influenced corrosion: The Behaviour of Stainless Steels in Natural Seawater. Bioelectrochemistry 1986, 212, 325–331. [Google Scholar]
- Mattila, K.; Carpen, L.; Hakkarainen, T.; Salkinoja-Salonen, M.S. Biofilm development during ennoblement of stainless steel in Baltic Sea water: A microscopic study. Int. Biodeterior. Biodegrad. 1997, 40, 1–10. [Google Scholar] [CrossRef]
- Videla, H.A. Biofilms and corrosion interactions on stainless steel in seawater. Int. Biodeterior. Biodegrad. 1994, 34, 245–257. [Google Scholar] [CrossRef]
- Mansfeld, F.; Liu, G.; Xiao, H.; Tsai, C.H.; Little, B.J. The corrosion behavior of copper alloys, stainless steels and titanium in seawater. Corros. Sci. 1994, 36, 2063–2095. [Google Scholar] [CrossRef]
- Motoda, S.; Suzuki, Y.; Shinohara, T.; Tsujikawa, S. The effect of marine fouling on the ennoblement of electrode potential for stainless steels. Corros. Sci. 1990, 31, 515–520. [Google Scholar] [CrossRef]
- Cooksey, B.; Cooksey, K.E. Calcium is Necessary for Motility in the Diatom Amphora coffeaeformis. Plant Physiol. 1980, 65, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Landoulsi, J.; Cooksey, K.E.; Dupres, V. Review—Interactions between diatoms and stainless steel: Focus on biofouling and biocorrosion. Biofouling 2011, 27, 1105–1124. [Google Scholar] [CrossRef]
- Edyvean, R.; Evans, L.V.; Hoagland, K.D. Algal Biofouling. J. Ecol. 1987, 28, 1206–1207. [Google Scholar] [CrossRef]
- Christie-Oleza, J.A.; Sousoni, D.; Lloyd, M.; Armengaud, J.; Scanlan, D.J. Nutrient recycling facilitates long-term stability of marine microbial phototroph-heterotroph interactions. Nat. Microbiol. 2017, 2, 17100. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Jiang, M.; Zhang, J.; Jiang, X.; Zheng, Z. The interactions of algae-bacteria symbiotic system and its effects on nutrients removal from synthetic wastewater. Bioresour. Technol. 2018, 247, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Song, G.L.; Zhang, J.; Gao, Y.; Wang, Z.M.; Zheng, D. Biocorrosion induced by red-tide alga-bacterium symbiosis and the biofouling induced by dissolved iron for carbon steel in marine environment. J. Mater. Sci. Technol. 2022, 128, 107–117. [Google Scholar] [CrossRef]
- Guzman, M.S.; Rengasamy, K.; Binkley, M.M.; Jones, C.; Ranaivoarisoa, T.O.; Singh, R.; Fike, D.A.; Meacham, J.M.; Bose, A. Phototrophic extracellular electron uptake is linked to carbon dioxide fixation in the bacterium Rhodopseudomonas palustris. Nat. Commun. 2019, 10, 1355. [Google Scholar] [CrossRef]
- Woese, C.R.; Fox, G.E. Phylogenetic structure of the prokaryotic domain: The primary kingdoms (archaebacteria/eubacteria/urkaryote/16S ribosomal RNA/molecular phylogeny). Proc. Natl. Acad. Sci. USA 1977, 74, 5088–5090. [Google Scholar] [CrossRef]
- Woese, C.R.; Kandler, O.; Wheelis, M.L. Towards a natural system of organisms: Proposal for the domains Archaea, Bacteria, and Eucarya. Proc. Natl. Acad. Sci. USA 1990, 87, 4576–4579. [Google Scholar] [CrossRef]
- Cavicchioli, R. Archaea—Timeline of the third domain. Nat. Rev. Microbiol. 2011, 9, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Yang, D.; Xu, D.; Gu, T. Carbon steel biocorrosion at 80 °C by a thermophilic sulfate reducing archaeon biofilm provides evidence for its utilization of elemental iron as electron donor through extracellular electron transfer. Corros. Sci. 2018, 145, 47–54. [Google Scholar] [CrossRef]
- Larsen, J.; Rasmussen, K.; Pedersen, H.; Sørensen, K.; Lundgaard, T. Consortia of MIC bacteria and archaea causing pitting corrosion in top side oil production facilities. In Proceedings of the Corrosion 2010, San Antonio, TX, USA, 14–18 March 2010. [Google Scholar]
- Stetter, K.O.; Huber, R.; Blöchl, E.; Kurr, M.; Eden, R.D.; Fielder, M.; Cash, H.; Vance, I. Hyperthermophilic archaea are thriving in deep North Sea and Alaskan oil reservoirs. Nature 1993, 365, 743–745. [Google Scholar] [CrossRef]
- Davidova, I.A.; Duncan, K.E.; Perez-Ibarra, B.M.; Suflita, J.M. Involvement of thermophilic archaea in the biocorrosion of oil pipelines. Environ. Microbiol. 2012, 14, 1762–1771. [Google Scholar] [CrossRef]
- Liang, R.; Grizzle, R.S.; Duncan, K.E.; McInerney, M.J.; Suflita, J.M. Roles of thermophilic thiosulfate-reducing bacteria and methanogenic archaea in the biocorrosion of oil pipelines. Front. Microbiol. 2014, 5, 89. [Google Scholar] [CrossRef] [PubMed]
- Usher, K.M.; Kaksonen, A.H.; MacLeod, I.D. Marine rust tubercles harbour iron corroding archaea and sulphate reducing bacteria. Corros. Sci. 2014, 83, 189–197. [Google Scholar] [CrossRef]
- Qian, H.; Ma, L.; Zhang, D.; Li, Z.; Huang, L.; Lou, Y.; Du, C. Microbiologically influenced corrosion of 304 stainless steel by halophilic archaea Natronorubrum tibetense. J. Mater. Sci. Technol. 2020, 46, 12–20. [Google Scholar] [CrossRef]
- Qian, H.; Zhang, D.; Lou, Y.; Li, Z.; Xu, D.; Du, C.; Li, X. Laboratory investigation of microbiologically influenced corrosion of Q235 carbon steel by halophilic archaea Natronorubrum tibetense. Corros. Sci. 2018, 145, 151–161. [Google Scholar] [CrossRef]
- Holmes, D.E.; Tang, H.; Woodard, T.; Liang, D.; Zhou, J.; Liu, X.; Lovley, D.R. Cytochrome-mediated direct electron uptake frommetallic iron by Methanosarcina acetivorans. mLife 2022, 1, 443–447. [Google Scholar] [CrossRef]
- Wang, Q.; Cen, Z.; Zhao, J. The survival mechanisms of thermophiles at high temperatures: An angle of omics. Physiology 2015, 30, 97–106. [Google Scholar] [CrossRef]
- Adamo, P.; Vingiani, S.; Violante, P. Lichen-rock interactions and bioformation of minerals. Dev. Soil Sci. 2002, 28, 377–391. [Google Scholar]
- Chen, J.; Blume, H.-P.; Beyer, L. Weathering of rocks induced by lichen colonization—A review. Catena 2000, 39, 121–146. [Google Scholar] [CrossRef]
- Radeka, M.; Ranogajec, J.; Kiurski, J.; Markov, S.; Marinković-Nedučin, R. Influence of lichen biocorrosion on the quality of ceramic roofing tiles. J. Eur. Ceram. Soc. 2007, 27, 1763–1766. [Google Scholar] [CrossRef]
- Seaward, M.R.D. Major impacts made by lichens in biodeterioration processes. Int. Biodeterior. Biodegrad. 1997, 40, 269–273. [Google Scholar] [CrossRef]
- Silva, B.; Prieto, B.; Rivas, T.; Sanchez-Biezma, M.J.; Paz, G.; Carballal, R. Rapid biological colonization of a granitic building by lichens. Int. Biodeterior. Biodegrad. 1997, 40, 263–267. [Google Scholar] [CrossRef]
- Salvadori, O.; Municchia, A.C. The Role of Fungi and Lichens in the Biodeterioration of Stone Monuments. Open Conf. Proc. J. 2016, 7, 39–54. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.S.; Yang, K.; Liu, Z.; Zhou, L.; Liu, W.; Lin, S.; Wang, X.; Shang, C. Microorganisms Involved in the Biodegradation and Microbiological Corrosion of Structural Materials. Coatings 2023, 13, 1683. https://doi.org/10.3390/coatings13101683
Khan MS, Yang K, Liu Z, Zhou L, Liu W, Lin S, Wang X, Shang C. Microorganisms Involved in the Biodegradation and Microbiological Corrosion of Structural Materials. Coatings. 2023; 13(10):1683. https://doi.org/10.3390/coatings13101683
Chicago/Turabian StyleKhan, M. Saleem, Ke Yang, Zifan Liu, Lujun Zhou, Wenle Liu, Siwei Lin, Xuelin Wang, and Chengjia Shang. 2023. "Microorganisms Involved in the Biodegradation and Microbiological Corrosion of Structural Materials" Coatings 13, no. 10: 1683. https://doi.org/10.3390/coatings13101683
APA StyleKhan, M. S., Yang, K., Liu, Z., Zhou, L., Liu, W., Lin, S., Wang, X., & Shang, C. (2023). Microorganisms Involved in the Biodegradation and Microbiological Corrosion of Structural Materials. Coatings, 13(10), 1683. https://doi.org/10.3390/coatings13101683









