Sulfonated Pentablock Copolymer with Graphene Oxide for Co2+ Ions Removal: Efficiency, Interaction Mechanisms and Secondary Reaction Products
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
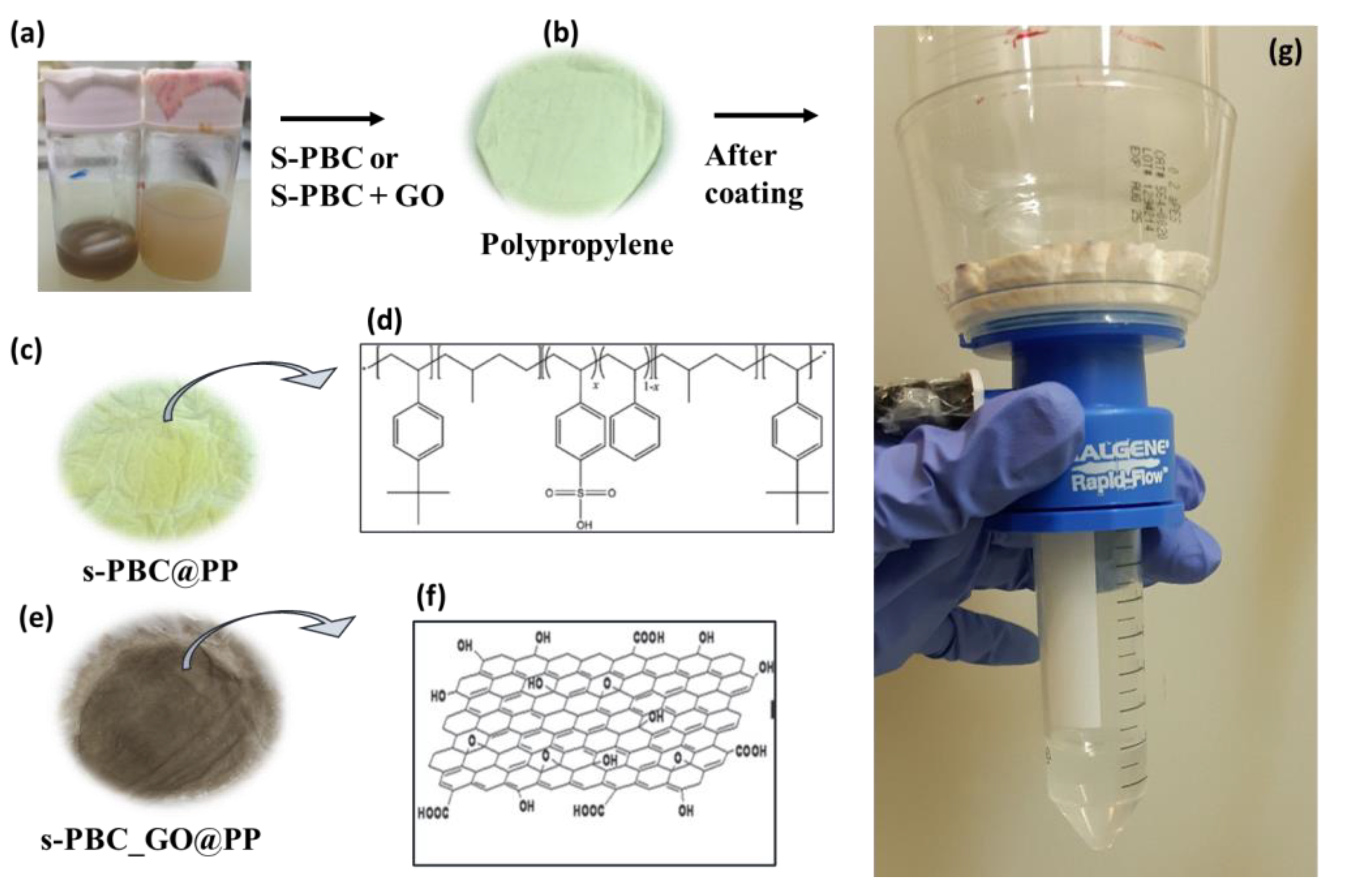
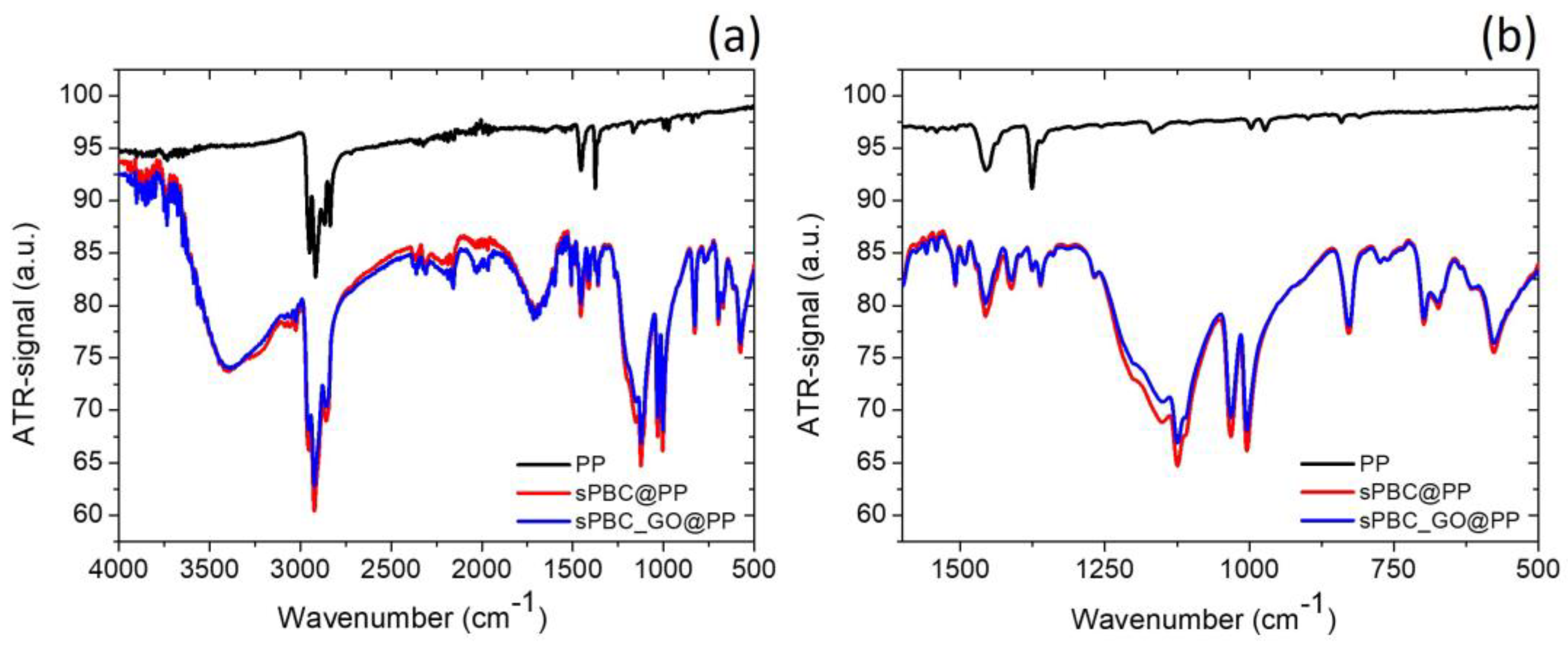
3.1. s-PBC/s-PBC_GO Coatings for Co2+ Ions Removal
2R-SO3+− Co(III) + 2R-SO3− → 2 R− SO3− Co(II) + 2SO3 .− + 2R.
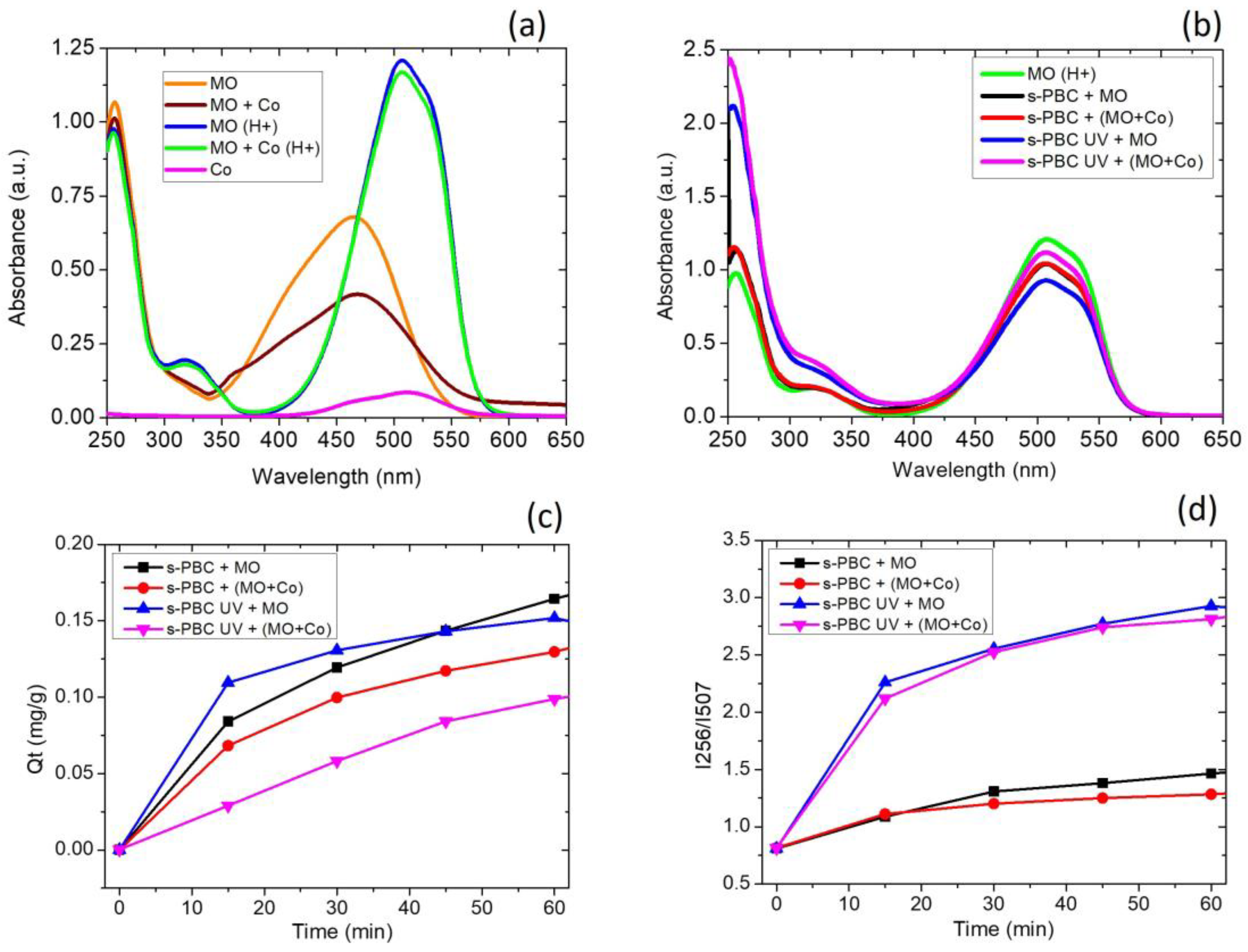
3.2. s-PBC Coatings for Multiple Contaminants Removal
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siddiqui, M.N.; Chanbasha, B.; Al-Arfaj, A.A.; Kon’kova, T.; Ali, I. Super-fast removal of cobalt metal ions in water using inexpensive mesoporous carbon obtained from industrial waste material. Environ. Technol. Innov. 2021, 21, 101257. [Google Scholar] [CrossRef]
- Hussain, J.; Husain, I.; Arif, M.; Gupta, N. Studies on heavy metal contamination in Godavari River basin. Appl. Water Sci. 2017, 7, 4539–4548. [Google Scholar] [CrossRef]
- Sayadi, M.H.; Rezaei, M.R.; Rezaei, A. Sediment Toxicity and Ecological Risk of Trace Metals from Streams Surrounding a Municipal Solid Waste Landfill. Bull. Environ. Contam. Toxicol. 2015, 94, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, O.M.L.; Basheer, A.A.; Khattab, R.A.; Ali, I. Health and environmental effects of persistent organic pollutants. J. Mol. Liq. 2018, 263, 442–453. [Google Scholar] [CrossRef]
- Sayadi, M.H.; Rezaei, M.R. Impact of land use on the distribution of toxic metals in surface soils in Birjand city. Iran. Proc. Int. Acad. Ecol. Environ. Sci. 2014, 4, 18–29. [Google Scholar]
- Bernabé, I.; Gómez, B.J.M.; Díez, E.; Sáez, P.; Rodríguez, A. Optimization and adsorption-based recovery of cobalt using activated disordered mesoporous carbons. Adv. Mate. Sci. Eng. 2019, 2019, 3430176. [Google Scholar] [CrossRef]
- Kara, M.; Yuzer, H.; Sabah, E.; Celik, M.S. Adsorption of cobalt from aqueous solutions onto sepiolite. Water Res. 2003, 37, 224–232. [Google Scholar] [CrossRef]
- Zhong, Q.; Zhao, Y.Q.; Shen, L.; Hao, B.; Xu, X.; Gao, B.Y.; Shang, Y.N.; Chu, K.Z.; Zhang, X.H.; Yue, Q.Y. Single and binary competitive adsorption of cobalt and nickel onto novel magnetic composites derived from green macroalgae. Environ. Eng. Sci. 2020, 37, 188–200. [Google Scholar] [CrossRef]
- Chenab, K.K.; Sohrabi, B.; Jafari, A.; Ramakrishna, S. Water treatment: Functional nanomaterials and applications from adsorption to photodegradation. Mater. Today Chem. 2020, 16, 100262. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, G.; Li, W.; Cui, Z.; Wu, J.; Akpinar, I.; Yu, L.; He, G.; Hu, J. Loofah activated carbon with hierarchical structures for high-efficiency adsorption of multi-level antibiotic pollutants. Appl. Surf. Sci. 2021, 550, 149313. [Google Scholar] [CrossRef]
- Armaković, S.J.; Savanović, M.M.; Armaković, S. Titanium Dioxide as the Most Used Photocatalyst for Water Purification: An Overview. Catalysts 2023, 13, 26. [Google Scholar] [CrossRef]
- Kumar, A.; Raizada, P.; Singh, P.; Saini, R.V.; Saini, A.K.; Hosseini-Bandegharaei, A. Perspective and status of polymeric graphitic carbon nitride-based Z-scheme photocatalytic systems for sustainable photocatalytic water purification. Chem. Eng. J. 2020, 391, 123496. [Google Scholar] [CrossRef]
- Scuderi, V.; Impellizzeri, G.; Zimbone, M.; Sanz, R.; Di Mauro, A.; Buccheri, M.A.; Miritello, M.; Terrasi, A.; Rappazzo, G.; Nicotra, G.; et al. Rapid synthesis of photoactive hydrogenated TiO2 nanoplumes. Appl. Catal. B Env. 2016, 183, 328–334. [Google Scholar] [CrossRef]
- Wang, T.; Wu, H.; Zhao, S.; Zhang, W.; Tahir, M.; Wang, Z.; Wang, J. Interfacial polymerized and pore-variable covalent organic framework composite membrane for dye separation. Chem. Eng. J. 2020, 384, 123347. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, C.; Liu, H.; Zhang, J.; Li, H.; Zhang, C. Contemporary antibiofouling modifications of reverse osmosis membranes: State-of-the-art insights on mechanisms and strategies. Chem. Eng. J. 2022, 429, 132400. [Google Scholar] [CrossRef]
- Zhao, D.L.; Zhao, Q.; Lin, H.; Chen, S.B.; Chung, T.-S. Pressure-assisted polydopamine modification of thin-film composite reverse osmosis membranes for enhanced desalination and antifouling performance. Desalination 2022, 530, 115671. [Google Scholar] [CrossRef]
- Saleh, T.A.; Mustaqeem, M.; Khaled, M. Water treatment technologies in removing heavy metal ions from wastewater: A review. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100617. [Google Scholar] [CrossRef]
- Dotto, G.L.; McKayb, G. Current scenario and challenges in adsorption for water treatment. J. Environ. Chem. Eng. 2020, 8, 103988. [Google Scholar] [CrossRef]
- Ali, I.; Gupta, V.K.; Aboul-Enein, H.Y. Metal ion speciation, and capillary electrophoresis: Application in the new millennium. Electrophoresis 2005, 26, 3988–4002. [Google Scholar] [CrossRef]
- Ali, I.; Basheer, A.A.; Mbianda, X.Y.; Burakov, A.; Galunin, E.; Burakova, I.; Mkrtchyan, E.; Tkachev, A.; Grachev, V. Graphene-based adsorbents for remediation of noxious pollutants from wastewater. Environ. Int. 2019, 127, 160–180. [Google Scholar] [CrossRef]
- Ali, I.; Alharbi, O.M.L.; Tkachev, A.; Galunin, E.; Burakov, A.; Grachev, V.A. Water treatment by new generation graphene materials: Hope for bright future. Environ. Sci. Pollut. Res. 2018, 25, 7315–7329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xing, G.; Chen, W.; Chen, L. Porous organic polymers: A promising platform for efficient photocatalysis. Mater. Chem. Front. 2020, 4, 332–353. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Z.; Liu, L.; Chen, Y. Construction of high performance thin-film nanocomposite nanofiltration membrane by incorporation of hydrophobic MOF-derived nanocages. Appl. Surf. Sci. 2021, 570, 151093. [Google Scholar] [CrossRef]
- Bai, Y.; Gao, P.; Fang, R.; Cai, J.; Zhang, L.D.; He, Q.Y.; Zhou, Z.H.; Sun, S.P.; Cao, X.L. Constructing positively charged acid-resistant nanofiltration membranes via surface postgrafting for efficient removal of metal ions from electroplating rinse wastewater. Sep. Purif. Technol. 2022, 297, 121500. [Google Scholar] [CrossRef]
- Giwa, A.; Ahmed, M.; Hasan, S.W. Polymers for Membrane Filtration in Water Purification. In Polymeric Materials for Clean Water; Springer: Berlin/Heidelberg, Germany, 2018; pp. 167–190. [Google Scholar]
- Dmitrieva, E.S.; Anokhina, T.S.; Novitsky, E.G.; Volkov, V.V.; Borisov, I.L.; Volkov, A.V. Polymeric Membranes for Oil-Water Separation: A Review. Polymers 2022, 14, 980. [Google Scholar] [CrossRef]
- Yu, T.; Zhou, J.; Liu, F.; Xu, B.M.; Pan, Y. Recent Progress of Adsorptive Ultrafiltration Membranes in Water Treatment—A Mini Review. Membranes 2022, 12, 519. [Google Scholar] [CrossRef]
- Filice, S.; D’Angelo, D.; Scarangella, A.; Iannazzo, D.; Compagnini, G.; Scalese, S. Highly effective and reusable sulfonated pentablock copolymer nanocomposites for water purification applications. RSC Adv. 2017, 7, 45521–45534. [Google Scholar] [CrossRef]
- Filice, S.; Mazurkiewicz-Pawlicka, M.; Malolepszy, A.; Stobinski, L.; Kwiatkowski, R.; Boczkowska, A.; Gradon, L.; Scalese, S. Sulfonated Pentablock Copolymer Membranes and Graphene Oxide Addition for Efficient Removal of Metal Ions from Water. Nanomaterials 2020, 10, 1157. [Google Scholar] [CrossRef]
- D’Angelo, D.; Filice, S.; Scarangella, A.; Iannazzo, D.; Compagnini, G.; Scalese, S. Bi2O3/Nexar® polymer nanocomposite membranes for visible photocatalytic applications. Catal. Today 2019, 321–322, 158–163. [Google Scholar] [CrossRef]
- Sciuto, E.L.; Filice, S.; Coniglio, M.A.; Faro, G.; Gradon, L.; Galati, C.; Spinella, N.; Libertino, S.; Scalese, S. Antimicrobial s-PBC Coatings for Innovative Multifunctional Water Filters. Molecules 2020, 25, 5196. [Google Scholar] [CrossRef]
- Filice, S.; Sciuto, E.L.; Scalese, S.; Faro, G.; Libertino, S.; Corso, D.; Timpanaro, R.M.; Laganà, P.; Coniglio, M.A. Innovative Antibiofilm Smart Surface against Legionella for Water Systems. Microorganisms 2022, 10, 870. [Google Scholar] [CrossRef] [PubMed]
- Filice, S.; Scuderi, V.; Libertino, S.; Zimbone, M.; Galati, C.; Spinella, N.; Gradon, L.; Falqui, L.; Scalese, S. Sulfonated Pentablock Copolymer Coating of Polypropylene Filters for Dye and Metal Ions Effective Removal by Integrated Adsorption and Filtration Process. Int. J. Mol. Sci. 2022, 23, 11777. [Google Scholar] [CrossRef] [PubMed]
- Kujawska, A.; Kiełkowska, U.; Atisha, A.; Yanful, E.; Kujawski, W. Comparative analysis of separation methods used for the elimination of pharmaceuticals and personal care products (PPCPs) from water—A critical review. Sep. Purif. Technol. 2022, 290, 120797. [Google Scholar] [CrossRef]
- Sikorska, E.; Gradoń, L. Biofouling reduction for improvement of depth Water filtration. Filter production and testing. Chem. Process Eng. 2016, 37, 319–330. [Google Scholar]
- Dai, Z.; Ansaloni, L.; Ryan, J.J.; Spontak, R.J.; Deng, L. Incorporation of an ionic liquid into a midblock-sulfonated multiblock polymer for CO2 capture. J. Membr. Sci. 2019, 588, 117193. [Google Scholar] [CrossRef]
- Available online: https://www.sigmaaldrich.com/IT/it/technical-documents/technical-article/analytical-chemistry/photometry-and-reflectometry/ir-spectrum-table (accessed on 1 August 2023).
- Chen, H.; Chang, K.; Men, X.; Sun, K.; Fang, X.; Ma, C.; Zhao, Y.; Yin, S.; Qin, W.; Wu, C. Covalent Patterning and Rapid Visualization of Latent Fingerprints with Photo-Cross-Linkable Semiconductor Polymer Dots. ACS Appl. Mater. Interfaces 2015, 7, 14477–14484. [Google Scholar] [CrossRef]
- Guo, H.; Peng, M.; Zhu, Z.; Sun, L. Preparation of reduced graphene oxide by infrared irradiation induced photothermal reduction. Nanoscale 2013, 5, 9040. [Google Scholar] [CrossRef]
- Dutta, D.; Dubey, R.; Dwivedi, S.K.; Puzari, A. Colorimetric Detection of Cobalt in Different Solvents by Using Their Solvatochromic Properties. Saudi J. Eng. Technol. 2020, 5, 144–149. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, D.; Li, J.; Wu, F.; Brigante, M.; Mailhot, G. Rapid oxidation of paracetamol by Cobalt(II) catalyzed sulfite at alkaline pH. Catal. Today 2018, 313, 155–160. [Google Scholar] [CrossRef]
- Yeh, T.-F.; Cihlář, J.; Chang, C.-Y.; Cheng, C.; Teng, H. Roles of graphene oxide in photocatalytic water splitting. Mater. Today 2013, 16, 78–84. [Google Scholar] [CrossRef]
- Liu, J.; Durstock, M.; Dai, L. Graphene oxide derivatives as hole- and electron-extraction layers for high-performance polymer solar cells. Energy Environ. Sci. 2014, 7, 1297–1306. [Google Scholar] [CrossRef]
- Velo-Gala, I.; Farre, M.J.; Radjenovic, J.; Gernjak, W. N-Nitrosodimethylamine (NDMA) degradation by the ultraviolet/peroxodisulfate process. Environ. Sci. Technol. Lett. 2019, 6, 106–111. [Google Scholar] [CrossRef]
- Hu, P.; Long, M. Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications. Appl. Catal. B Environ. 2016, 181, 103–117. [Google Scholar] [CrossRef]
- Wacławek, S.; Lutze, H.V.; Grübel, K.; Padil, V.V.T.; Cerník, M.; Dionysiou, D.D. Chemistry of persulfates in water and wastewater treatment: A review. Chem. Eng. J. 2017, 330, 44–62. [Google Scholar] [CrossRef]
- Luo, T.; Peng, Y.; Chen, L.; Li, J.; Wu, F.; Zhou, D. Metal-free electro-activated sulfite process for As(III) oxidation in water using graphite electrodes. Environ. Sci. Technol. 2020, 54, 10261–10269. [Google Scholar] [CrossRef]
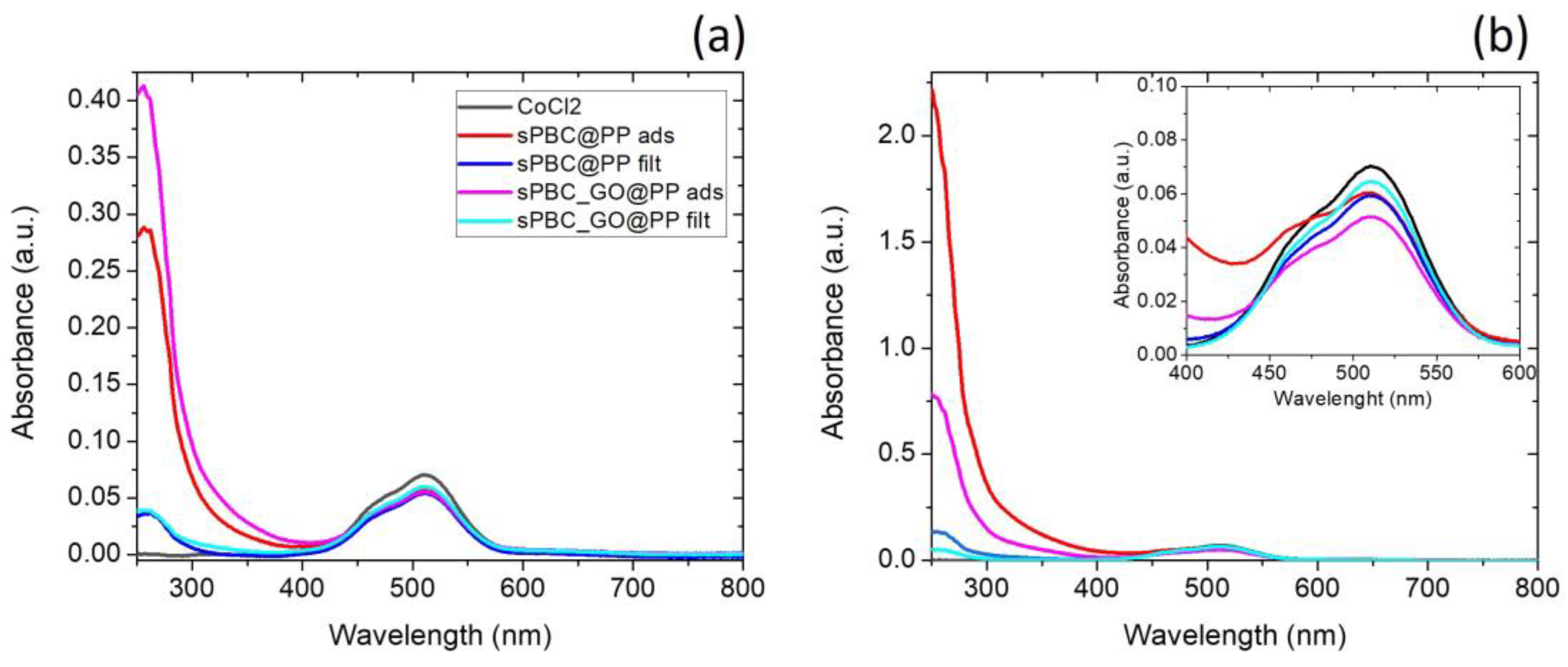


| Sample/Process | I250/I515 | Qt (mg/g) |
|---|---|---|
| s-PBC@PP/ads | 4.81 | 21 |
| s-PBC@PP/filt | 0.65 | 24 |
| s-PBC_GO@PP/ads | 7.44 | 37 |
| s-PBC_GO@PP/filt | 0.60 | 21 |
| s-PBC@PP UV/ads | 34.84 | 20 |
| s-PBC@PP UV/filt | 2.50 | 17 |
| s-PBC_GO@PP UV/ads | 12.63 | 20 |
| s-PBC_GO@PP UV/filt | 0.87 | 13 |
| Process | s-PBC@PP UV | s-PBC_GO@PP UV |
|---|---|---|
| 1st filtration | 2.26 | 0.80 |
| 2nd filtration | 3.59 | 1.19 |
| 3rd filtration | 4.29 | 1.55 |
| 4th filtration | 4.71 | 1.86 |
| 5th filtration | 5.02 | 2.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filice, S.; Scuderi, V.; Zimbone, M.; Libertino, S.; La Piana, L.; Farina, R.A.; Scalese, S. Sulfonated Pentablock Copolymer with Graphene Oxide for Co2+ Ions Removal: Efficiency, Interaction Mechanisms and Secondary Reaction Products. Coatings 2023, 13, 1715. https://doi.org/10.3390/coatings13101715
Filice S, Scuderi V, Zimbone M, Libertino S, La Piana L, Farina RA, Scalese S. Sulfonated Pentablock Copolymer with Graphene Oxide for Co2+ Ions Removal: Efficiency, Interaction Mechanisms and Secondary Reaction Products. Coatings. 2023; 13(10):1715. https://doi.org/10.3390/coatings13101715
Chicago/Turabian StyleFilice, Simona, Viviana Scuderi, Massimo Zimbone, Sebania Libertino, Luana La Piana, Roberta Agata Farina, and Silvia Scalese. 2023. "Sulfonated Pentablock Copolymer with Graphene Oxide for Co2+ Ions Removal: Efficiency, Interaction Mechanisms and Secondary Reaction Products" Coatings 13, no. 10: 1715. https://doi.org/10.3390/coatings13101715
APA StyleFilice, S., Scuderi, V., Zimbone, M., Libertino, S., La Piana, L., Farina, R. A., & Scalese, S. (2023). Sulfonated Pentablock Copolymer with Graphene Oxide for Co2+ Ions Removal: Efficiency, Interaction Mechanisms and Secondary Reaction Products. Coatings, 13(10), 1715. https://doi.org/10.3390/coatings13101715









