Strong Interface Interaction of ZnO Nanosheets and MnSx Nanoparticles Triggered by Light over Wide Ranges of Wavelength to Enhance Their Removal of VOCs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of ZnO Nanosheets
2.3. Preparation of ZnO/MnSx Nanocomposites
2.4. Morphology Observation with SEM
2.5. Energy Dispersive Spectroscopy (EDS) Examination
2.6. Morphology Observation with TEM
2.7. XRD Characterization
2.8. Measurement of UV-Vis-NIR Spectrum
2.9. Photocurrent Response of Nanocomposite
2.10. Construction of Prototype Sensors and Arrays
2.11. Characterization of Surface and Interface Features of Nanocomposite with the Help of QCM Arrays
2.12. Comparison of the Nanocomposites to the Removal of Organic Pollutants
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, U.; Qureshi, A.A.; Javed, S.; Rehman, G.; Akram, M.A. Graphene oxide incorporation in Ag-doped ZnO nanocomposite as efficient electron extraction material for planar perovskite solar cells. Results Opt. 2023, 12, 100486. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Q.; Xu, L.; Zhang, Z.; Kang, Z.; Liao, Q.; Zhang, Y. Interface Engineering in 1D ZnO-Based Heterostructures for Photoelectrical Devices. Adv. Funct. Mater. 2021, 32, 112106887. [Google Scholar] [CrossRef]
- Tian, F.; Han, W.; Hu, J.; Wang, H.; Li, H.; Geng, F.; Wei, T.; Li, D. Oxygen vacancy-enriched bilayer flower-like structure of ZnO&NiO@C-ZnO nanorod arrays on carbon cloth with improved electrochemical performance. J. Energy Storage Part B 2023, 72, 108316. [Google Scholar]
- Kumar, R.D.; Kumar, A.J.; Balachandran, S.; Kusmartsev, F.V.; Trabelsi, A.B.G.; Alkallas, F.H.; Nagarani, S.; Sethuraman, V.; Lee, B. High-performance chrysanthemum flower-like structure of Ni-doped ZnO nanoflowers for pseudo-supercapacitors. J. Energy Storage Part B 2023, 72, 108441. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, F.; Mou, Z.; Liu, X.; Sun, J.; Lei, W. A facile one-step synthesis of ZnO quantum dots modified poly (triazine imide) nanosheets for enhanced hydrogen evolution under visible light. Chem. Commun. 2016, 52, 13020–13023. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Liu, P.; Liu, R.; Liu, M.; Tao, K.; Zhu, S.; Wu, M.; Yi, F.; Han, L. MOF-derived hierarchical double-shelled NiO/ZnO hollow spheres for high-performance supercapacitors. Dalton Trans. 2016, 45, 13311–13316. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.; Yu, H.; Zhang, K.; Zhou, Y.; Meng, D.; Sun, Y. Integration of ZnO and Au/ZnO Nanostructures into Gas Sensor Devices for Sensitive Ethanolamine Detection. ACS Appl. Nano Mater. 2023, 6, 5994–6001. [Google Scholar] [CrossRef]
- Hyun, S.K.; Nam, B.; Ko, T.K.; Lee, C.; Choi, S.B.; Lee, W.I. Optimal Composition of ZnO/WO3 Composite Nanoparticle Gas Sensors. Phys. Status Solidi A 2020, 217, 1900874. [Google Scholar] [CrossRef]
- Ramakrishnan, V.; Nair, K.G.; Dhakshinamoorthy, J.; Ravi, K.R.; Pullithadathil, B.I. Porous, n–p type ultra-long, ZnO@Bi2O3 heterojunction nanorods—Based NO2 gas sensor: New insights towards charge transport characteristics. Phys. Chem. Chem. Phys. 2020, 22, 7524–7536. [Google Scholar] [CrossRef]
- Cao, P.; Cai, Y.; Pawar, D.; Han, S.; Xu, W.; Fang, M.; Liu, X.; Zeng, Y.; Liu, W.; Lu, Y.; et al. Au@ZnO/rGO nanocomposite-based ultra-low detection limit highly sensitive and selective NO2 gas sensor. J. Mater. Chem. C 2022, 10, 4295–4305. [Google Scholar] [CrossRef]
- Vishnuraj, R.; Aleem, M.; Nair, K.G.; Pullithadathil, B. 1D aligned, n–p and n–n type ZnO heterojunction nanofibers for NO2 sensors: Exploration of conduction mechanism using in situ impedance spectroscopy. Mater. Adv. 2023, 4, 3010–3025. [Google Scholar] [CrossRef]
- Beitollahi, H.; Tajik, S.; Nejad, F.G.; Safaei, M. Recent advances in ZnO nanostructure-based electrochemical sensors and biosensors. J. Mater. Chem. B 2020, 8, 5826–5844. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.S.; Singh, S.; Batool, M.; Fahmy, H.M.; Seku, K.; Shalan, A.E.; Lanceros-Mendez, S.; Zafar, M.N. A review on 2D-ZnO nanostructure-based biosensors: From materials to devices. Mater. Adv. 2023, 4, 320–354. [Google Scholar] [CrossRef]
- Tang, K.; Jiang, M.; Yang, B.; Xu, T.; Liu, Z.; Wan, P.; Kan, C.; Shi, D. Enhancing UV photodetection performance of an individual ZnO microwire p–n homojunction via interfacial engineering. Nanoscale 2023, 15, 2292–2304. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.; Kumar, S.; Misra, A. Zinc oxide heterostructures: Advances in devices from self-powered photodetectors to self-charging supercapacitors. Mater. Adv. 2021, 2, 6768–6799. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Li, L.; Gu, Y.; Kim, B.; Huang, J. Self-Powered Broadband Photodetectors Material Based on Co3O4–ZnO Heterojunction with Bottlebrush Nanostructure. ACS Appl. Electron. Mater. 2023, 5, 3224–3231. [Google Scholar] [CrossRef]
- Kim, H.; Baek, S.; Lee, S. Surface Plasmon-Enhanced High-Performance ZnO/Ni/ZnO Ultraviolet Photodetectors. Phys. Status Solidi Rapid Res. Lett. 2020, 14, 1900685. [Google Scholar] [CrossRef]
- Ning, Y.; Zhang, Z.; Teng, F.; Fang, X. Novel Transparent and Self-Powered UV Photodetector Based on Crossed ZnO Nanofiber Array Homojunction. Small 2018, 14, 1703754. [Google Scholar] [CrossRef]
- Zhou, X.; Yan, C.; Deng, R.; Hu, Y.; Deng, Z.; Cui, Q.; Lou, Z.; Hou, Y.; Teng, F. High-Performance Polymer Photodetectors using ZnO Nanocrystal Trap States. Phys. Status Solidi Rapid Res. Lett. 2021, 15, 2100003. [Google Scholar] [CrossRef]
- Yashaswini, M.; Naik, K.G. Fabrication of ZnO nanorods and ZnO/CdS core-shell nanorods for the ultraviolet photo-sensing application. Inorg. Chem. Commun. 2023, 154, 110975. [Google Scholar] [CrossRef]
- Zhou, J.; Qiao, Q.; Tan, Y.; Wu, C.; Hu, J.; Qiu, X.; Wu, S.; Zheng, J.; Wang, R.; Zhang, C.; et al. The improvement of polymer photodetector based on 1D-ZnO nanorod arrays/0D-ZnO quantum dots composite film. Opt. Mater. 2023, 142, 114086. [Google Scholar] [CrossRef]
- Peng, Y.; Jiang, D.; Zhao, M.; Duan, Y.; Wei, H.; Li, H.; Liang, Q.; Wang, S. High-performance UV–visible photodetectors based on ZnO/perovskite heterostructures. J. Alloys Compd. 2023, 965, 171372. [Google Scholar] [CrossRef]
- Boruah, B.D. Zinc oxide ultraviolet photodetectors: Rapid progress from conventional to self-powered photodetectors. Nanoscale Adv. 2019, 1, 2059–2085. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Fu, X.; Guo, Y.; Zhang, J.; Tian, W. A study on Ag or Ce doped and co-doped ZnO for the photocatalytic degradation of RhB dye. Vacuum 2023, 215, 112337. [Google Scholar] [CrossRef]
- Vo, N.T.T.; You, S.; Pham, M.; Pham, V.V. A green synthesis approach of p-n CuO/ZnO junctions for multifunctional photocatalysis towards the degradation of methyl orange, phenol, and nitric oxide. Environ. Technol. Innov. 2023, 32, 103285. [Google Scholar] [CrossRef]
- Shi, Q.; Luo, Z.; Jiang, L.; Li, X.; Bai, C.; Yu, Q. Fabrication and photocatalytic properties of Co-doped ZnO nanomaterials. Mater. Lett. 2023, 350, 134952. [Google Scholar] [CrossRef]
- Al-Zaqri, N. Highly operative near full spectrum Cu doped NiO/ZnO photocatalyst: Photodegradation of phenol and organic dyes. Opt. Mater. 2023, 143, 114139. [Google Scholar] [CrossRef]
- Alawamleh, H.K.; Amin, A.H.; Ali, A.M.; Alreda, B.A.; Lagum, A.A.; Pecho, R.D.C.; Taqi, N.; Salman, H.M.; Nassar, M.F. Solar light driven enhanced photocatalytic treatment of azo dye contaminated water based on Co-doped ZnO/g-C3N4 nanocomposite. Chemosphere 2023, 335, 139104. [Google Scholar] [CrossRef]
- Bi, K.; Qin, X.; Cheng, S.; Liu, S. ZnO/g-C3N4 S scheme photocatalytic material with visible light response and enhanced photocatalytic performance. Diam. Relat. Mater. 2023, 137, 110143. [Google Scholar] [CrossRef]
- Yan, M.; An, B.; Li, X.; Zai, Z.; Wu, S.; Ma, J.; Zhang, L. Effect of different electronegative oxygen atoms of cellulose nanofibrils on the formation and photocatalytic property of ZnO/cellulose composite. Appl. Surf. Sci. 2023, 637, 157974. [Google Scholar] [CrossRef]
- Xie, Z.; Xie, L.; Qi, F.; Liu, H.; Meng, L.; Wang, J.; Xie, Y.; Chen, J.; Lu, C. Efficient photocatalytic hydrogen production by space separation of photo-generated charges from S-scheme ZnIn2S4/ZnO heterojunction. J. Colloid Interface Sci. Part A 2023, 650, 784–797. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wang, Y. Optimizing crystal characterization of WO3–ZnO composites for boosting photoactive performance via manipulating crystal formation conditions. CrystEngComm 2021, 23, 3498–3509. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, K.; Wang, S.; Jiang, D.; Ma, C.; Zhu, L.; Xu, X. An in situ-fabricated p-Co3O4@n-ZnO surface heterojunction photocatalyst for solar-to-fuel conversion of CO2. Mater. Chem. Front. 2023, 7, 523–534. [Google Scholar] [CrossRef]
- Feng, C.; Chen, Z.; Jing, J.; Hou, J. The photocatalytic phenol degradation mechanism of Ag-modified ZnO nanorods. J. Mater. Chem. C 2020, 8, 3000–3009. [Google Scholar] [CrossRef]
- Jayswal, S.; Moirangthem, R.S. Construction of a solar spectrum active SnS/ZnO p–n heterojunction as a highly efficient photocatalyst: The effect of the sensitization process on its performance. New J. Chem. 2018, 42, 13689–13701. [Google Scholar] [CrossRef]
- Sher, M.; Javed, M.; Shahid, S.; Iqbal, S.; Qamar, M.A.; Bahadur, A.; Qayyum, M.A. The controlled synthesis of g-C3N4/Cd-doped ZnO nanocomposites as potential photocatalysts for the disinfection and degradation of organic pollutants under visible light irradiation. RSC Adv. 2021, 11, 2025–2039. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Lin, J.; Li, R.; Su, Y.; Zhao, X.; Liu, Y.; Chen, W.; Lian, X.; Chen, X.; Pan, X. Hierarchical ZnO Nanosheet-Reduced Graphene Oxide Composites for Photocatalytic Ethylene Oxidation. ACS Appl. Nano Mater. 2022, 5, 1828–1835. [Google Scholar] [CrossRef]
- Lonkar, S.L.P.; Pillai, V.; Abdala, A.; Mittal, V. In situ formed graphene/ZnO nanostructured composites for low-temperature hydrogen sulfide removal from natural gas. RSC Adv. 2016, 6, 81142–81150. [Google Scholar] [CrossRef]
- Kegel, J.; Zubialevich, V.Z.; Schmidt, M.; Povey, I.M.; Pemble, M.E. Effect of Surface and Defect Chemistry on the Photocatalytic Properties of Intentionally Defect-Rich ZnO Nanorod Arrays. ACS Appl. Mater. Interfaces 2018, 10, 17994–18004. [Google Scholar] [CrossRef]
- Cox, J.W.; Foster, G.M.; Jarjour, A.; Wenckstern, H.; Grundmann, M.; Brillson, L.J. Defect Manipulation To Control ZnO Micro-/Nanowire-Metal Contacts. Nano Lett. 2018, 18, 6974–6980. [Google Scholar] [CrossRef]
- Lai, J.; Farooq, M.U.; Sun, Y.; Tan, P.; Zhang, J. Multiphonon Process in Mn-Doped ZnO Nanowires. Nano Lett. 2022, 22, 5385–5391. [Google Scholar] [CrossRef] [PubMed]
- Labégorre, J.; Lebedev, O.I.; Bourgès, C.; Rečnik, A.; Košir, M.; Bernik, S.; Maignan, A.; Mercier, T.L.; Pautrot-d’Alençon, L.; Guilmeau, E. Phonon Scattering and Electron Doping by 2D Structural Defects in In/ZnO. ACS Appl. Mater. Interfaces 2018, 10, 6415–6423. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Chen, Y.; Lin, T.; Mou, C. Defective Mesocrystal ZnO-Supported Gold Catalysts: Facilitating CO Oxidation via Vacancy Defects in ZnO. ACS Catal. 2018, 8, 6862–6869. [Google Scholar] [CrossRef]
- Huang, P.; Qin, F.; Lee, J. Role of the Interface between Ag and ZnO in the Electric Conductivity of Ag Nanoparticle-Embedded ZnO. ACS Appl. Mater. Interfaces 2020, 12, 4715–4721. [Google Scholar] [CrossRef] [PubMed]
- Wolf, E.H.; Millet, M.; Seitz, F.; Redeker, F.A.; Riedel, W.; Scholz, G.; Hetaba, W.; Teschner, D.; Wrabetz, S.; Girgsdies, F.; et al. F-doping of nanostructured ZnO: A way to modify structural, electronic, and surface properties. Phys. Chem. Chem. Phys. 2020, 22, 11273–11285. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Gao, Y.; Xie, C.; Tong, X.; Li, Z.; Luo, L. Recent advances in the fabrication of graphene–ZnO heterojunctions for optoelectronic device applications. J. Mater. Chem. C 2018, 6, 3815–3833. [Google Scholar] [CrossRef]
- Pham, T.T.H.; Vu, X.H.; Dien, N.D.; Trang, T.T.; Chi, T.T.K.; Phuong, P.H.; Nghia, N.T. Ag nanoparticles on ZnO nanoplates as a hybrid SERS-active substrate for trace detection of methylene blue. RSC Adv. 2022, 12, 7850–7863. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.; Shih, C.; Sauter, E.; Das, S.K.; Liang, Y.; Lien, H.; Chang, S.; Zharnikov, M.; Tai, Y. ZnO as an effective hole transport layer for water resistant organic solar cells. J. Mater. Chem. A 2018, 6, 6542–6550. [Google Scholar] [CrossRef]
- Wu, Z.; Yu, H.; Shi, S.; Li, Y. Bismuth oxysulfide modified ZnO nanorod arrays as an efficient electron transport layer for inverted polymer solar cells. J. Mater. Chem. A 2019, 7, 14776–14789. [Google Scholar] [CrossRef]
- Du, L.; Qian, K.; Zhu, X.; Yan, X.; Kobayashi, H.; Liu, Z.; Lou, Y.; Li, R. Interface engineering of palladium and zinc oxide nanorods with strong metal–support interactions for enhanced hydrogen production from base-free formaldehyde solution. J. Mater. Chem. A 2019, 7, 8855–8864. [Google Scholar] [CrossRef]
- Weng, Z.; Xu, Y.; Gao, J.; Wang, X. Research progress of stimuli-responsive ZnO-based nanomaterials in biomedical applications. Biomater. Sci. 2023, 11, 76–95. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Banerjee, P.; Kar, A.K. Insights into the impact of photophysical processes and defect state evolution on the emission properties of surface-modified ZnO nanoplates for application in photocatalysis and hybrid LEDs. Phys. Chem. Chem. Phys. 2022, 24, 2424–2440. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lin, H.; Jing, M.; Zhang, L.; Yuan, W.; Li, C. ZnO nanowire arrays with in situ sequentially self-assembled vertically oriented CdS nanosheets as superior photoanodes for photoelectrochemical water splitting. Sustain. Energy Fuels 2022, 6, 3240–3248. [Google Scholar] [CrossRef]
- Indubala, E.; Dhanasekar, M.; Sudha, V.; Malar, E.J.P.; Divya, P.; Sherine, J.; Rajagopal, R.; Bhat, S.V.; Harinipriya, S. L-Alanine capping of ZnO nanorods: Increased carrier concentration in ZnO/CuI heterojunction diode. RSC Adv. 2018, 8, 5350–5361. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.R.; Akram, R.; Aamir, M.; Malik, M.A.; Tahir, A.A.; Choudhary, M.A.; Akhtar, J. Investigations of photoelectrochemical performance of polycrystalline Bi-doped ZnO thin films. J. Phys. Chem. Solids 2023, 181, 111529. [Google Scholar] [CrossRef]
- Kumar, E.T.D.; Easwaramoorthi, S.; Rao, J.R. Gold-reduced graphene oxide intimated BiVO4–ZnO mixed oxide composite with leveraged charge carrier transport under solar radiation. Opt. Mater. 2023, 142, 114054. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, C.; Zhao, W.; Li, Z.; Kou, L.; Zhang, Z.; Stiens, J. Effect of graphene film thickness on photoluminescence properties of ZnO/graphene composite films. Ceram. Int. 2023, 49, 30864–30874. [Google Scholar] [CrossRef]
- Wang, C. Fabrication and optical-electrical characteristics of ZnS/ZnO films by pulsed laser deposition. Thin Solid Film. 2023, 780, 139971. [Google Scholar] [CrossRef]
- Khoshsang, H.; Abbasi, K.; Ghaffarinejad, A. Biosynthesis of ZnO and CuO nanoparticles using sunflower petal extract. Inorg. Chem. Commun. 2023, 155, 111083. [Google Scholar] [CrossRef]
- Raha, S.; Ahmaruzzaman, M. ZnO nanostructured materials and their potential applications: Progress, challenges, and perspectives. Nanoscale Adv. 2022, 4, 1868–1925. [Google Scholar] [CrossRef]
- Mota, I.C.; Marques, M.F.V. Synthesis of Polyvinylcarbazole/Reduced Graphite Oxide-ZnO Nanocomposites. Macromol. Symp. 2019, 383, 1700081. [Google Scholar] [CrossRef]
- Camarda, P.; Messina, F.; Vaccaro, L.; Agnello, S.; Buscarino, G.; Schneider, R.; Popescu, R.; Gerthsen, D.; Lorenzi, R.; Gelardi, F.M.; et al. Luminescence mechanisms of defective ZnO nanoparticles. Phys. Chem. Chem. Phys. 2016, 18, 16237–16244. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Takahashi, M.; Malfattiac, L.; Innocenzi, P. Carbon dots in ZnO macroporous films with controlled photoluminescence through defects engineering. RSC Adv. 2016, 6, 55393–55400. [Google Scholar] [CrossRef]
- Sawant, S.Y.; Cho, M.H. Facile and single-step route towards ZnO@C core-shell nanoparticles as an oxygen vacancy induced visible light active photocatalyst using the thermal decomposition of Zn(an)2(NO3)2. RSC Adv. 2016, 6, 70644–70652. [Google Scholar] [CrossRef]
- Bitenc, M.; Horvat, B.; Likozar, B.; Dra, G.; Orel, Z.C. The impact of ZnO load, stability and morphology on the kinetics of the photocatalytic degradation of caffeine and resazurin. Appl. Catal. B Environ. 2013, 136–137, 202–209. [Google Scholar] [CrossRef]
- Farooq, M.H.; Aslam, I.; Anam, H.S.; Tanveer, M.; Ali, Z.; Ghani, U.; Boddula, R. Improved photocatalytic performance of reduced zinc oxide (ZnO) novel morphology of astray like microstructure under solar light irradiation. Mater. Sci. Energy Technol. 2019, 2, 181–186. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, C.; Hu, C.; Kuo, J.; Boddula, R. Fabrication and characterization of ZnO nanowires array electrodes with high photocurrent densities: Effects of the seed layer calcination time. Mater. Chem. Phys. 2017, 189, 56–63. [Google Scholar] [CrossRef]
- Banik, A.; Ansari, M.S.; Sahu, T.K.; Qureshi, M. Understanding the role of silica nanospheres with their light scattering and energy barrier properties in enhancing the photovoltaic performance of ZnO based solar cells. Phys. Chem. Chem. Phys. 2016, 18, 27818–27828. [Google Scholar] [CrossRef]
- Gupta, R.; Eswar, N.K.; Modaka, J.M.; Madras, G. Visible light driven efficient N and Cu co-doped ZnO for photoinactivation of Escherichia coli. RSC Adv. 2016, 6, 85675–85687. [Google Scholar] [CrossRef]
- Luo, J.; Wang, Y.; Sun, J.; Yang, Z.; Zhang, Q. MnS passivation layer for highly efficient ZnO–based quantum dot-sensitized solar cells. Environ. Res. 2023, 231, 2116218. [Google Scholar] [CrossRef]
- An, S.; Gao, Q.; Zhang, X.; Li, X.; Duan, L.; Lü, W. Introducing of MnS passivation layer on TiO2 mesoporous film for improving performance of quantum dot sensitized solar cells. Appl. Catal. B Environ. 2021, 283, 119638. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Zhu, J.; Meng, T.; Ma, L.; Zhang, H.; Xu, M.; Jiang, J.; Li, C. One-Dimensional Integrated MnS@Carbon Nanoreactors Hybrid: An Alternative Anode for Full-Cell Li-Ion and Na-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 27911–27919. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Zhang, X.; Wang, J. Mesoporous γ-MnS nanospheres as anode materials for Li-ion batteries. Mater. Lett. 2017, 188, 13–16. [Google Scholar] [CrossRef]
- Ha, D.; Ly, T.; Caron, J.M.; Zhang, H.; Fritz, K.E.; Robinson, R.D. A General Method for High-Performance Li-Ion Battery Electrodes from Colloidal Nanoparticles without the Introduction of Binders or Conductive-Carbon Additives: The Cases of MnS, Cu2−xS, and Ge. ACS Appl. Mater. Interfaces 2015, 7, 25053–25060. [Google Scholar] [CrossRef] [PubMed]
- Naveenkumar, P.; Maniyazagan, M.; Yesuraj, J.; Yang, H.; Kang, N.; Kim, K.; Kalaignan, G.P.; Kang, W.S.; Kim, S. Electrodeposited MnS@Ni(OH)2 core-shell hybrids as an efficient electrode materials for symmetric supercapacitor applications. Optik 2021, 243, 167457. [Google Scholar] [CrossRef]
- Zhang, C.; Lin, Z.; Huang, C.; Zheng, B.; Li, Y.; Wang, J.; Deng, M.; Tang, S.; Du, Y. Surface Sulfur Vacancies Induced the Direct Growth of Mesoporous MnS Nanosheets on Three-Dimensional Reduced Graphene Oxide with Ultrahigh Capacity as Electrode Materials for Supercapacitors. ACS Appl. Energy Mater. 2019, 2, 6599–6607. [Google Scholar] [CrossRef]
- Quan, H.; Cheng, B.; Chen, D.; Su, X.; Xiao, Y.; Lei, S. One-pot synthesis of a-MnS/nitrogen-doped reduced graphene oxide hybrid for high-performance asymmetric supercapacitors. Electrochim. Acta 2016, 210, 557–566. [Google Scholar] [CrossRef]
- Pujari, R.B.; Lokhande, A.C.; Yadav, A.A.; Kim, J.H.; Lokhande, C.D. Synthesis of MnS microfibers for high-performance flexible supercapacitors. Mater. Des. 2016, 108, 510–517. [Google Scholar] [CrossRef]
- Kadhm, A.J.; Ismail, R.A.; Atwan, A.F. Preparation of nanostructured cerium-doped MnS/Si heterojunction photodetector by chemical spray pyrolysis: Influence of doping concentration. J. Alloys Compd. 2021, 889, 161662. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Q.; Yi, X.; Zhong, X.; Song, G.; Chai, Z.; Liu, Z.; Yang, K. Au@MnS@ZnS Core/Shell/Shell Nanoparticles for Magnetic Resonance Imaging and Enhanced Cancer Radiation Therapy. ACS Appl. Mater. Interfaces 2016, 8, 9557–9564. [Google Scholar] [CrossRef]
- Meng, J.; Zhao, Y.; Li, Z.; Wang, L.; Tian, Y. Phase transfer preparation of ultrasmall MnS nanocrystals with a high-performance MRI contrast agent. RSC Adv. 2016, 6, 6878–6887. [Google Scholar] [CrossRef]
- Zhao, Y.; Meng, J.; Sheng, X.; Tian, Y. Synthesis of Ultrathin MnS Shell on ZnS: Mn Nanorods by One-Step Coating and Doping for MRI and Fluorescent Imaging. Adv. Opt. Mater. 2016, 4, 1115–1123. [Google Scholar] [CrossRef]
- Saleem, A.; Alharbi, F.F.; Ashiq, M.N.; Manzoor, S.; Shah, S.I.A.; Khan, K.Z.; Aman, S.; Ahmad, N.; Alzahrani, H.A.; Messali, M. Facile Hydrothermal Synthesis of Visible-Light-Driven MnS/rGO Nanocomposite for Photocatalytic Degradation of Methylene Blue Dye. Phys. Status Solidi A 2023, 220, 2200734. [Google Scholar] [CrossRef]
- Wang, Y.; He, Y.; Chi, Y.; Yin, P.; Wei, L.; Liu, W.; Wang, X.; Zhang, H.; Song, H. Construction of S-scheme p-n heterojunction between protonated g-C3N4 and α-MnS nanosphere for photocatalytic H2O2 production and in situ degradation of oxytetracycline. Inorg. Chem. Commun. 2023, 155, 111003. [Google Scholar] [CrossRef]
- Farid, H.M.T.; Altuijri, R.; Maati, L.A.E.; Jawhari, A.H. One pot development of MnS/g-C3N4 nanocomposite via hydrothermal synthesis for photodegradation of methylene blue. Chem. Phys. Lett. 2021, 779, 138877. [Google Scholar]
- Yin, J.; Wu, Z.; Fang, M.; Xu, Y.; Zhu, W.; Li, C. In situ synthesis of C3N4/Bi2S3 composites with enhanced photocatalytic degradation performance under visible light irradiation. J. Chin. Chem. Soc. 2018, 65, 1044–1052. [Google Scholar] [CrossRef]
- Heiba, Z.K.; Mohamed, M.B.; Badawi, A.; Farag, N.M. Effect of sulfur deficiency on the structural, optical, and electronic properties of MnS nanostructures. Electrochim. Acta 2021, 412, 140138. [Google Scholar] [CrossRef]
- Caillet, D.A.; Harrison, D.P. Structural property variations in the MnO/MnS system. J. Ind. Eng. Chem. 2023, 120, 1–26. [Google Scholar] [CrossRef]
- Ma, X.; Li, C.; Zhang, X.; Gao, M.; Li, G. Broadband Spectrum Light-Driven PANI/Au/Beta-Cyclodextrin Nanocomposite and Its Light-Triggered Interfacial Carrier Transfer. Coatings 2022, 12, 1401. [Google Scholar] [CrossRef]
- Ma, X.; Li, C.; Gao, M.; Zhang, X.; Wang, Y.; Li, G. Interface Optimization of Metal Quantum Dots/Polymer Nanocomposites and Their Properties: Studies of Multi-Functional Organic/Inorganic Hybrid. Materials 2023, 16, 150. [Google Scholar] [CrossRef]
- Ma, X.; Gao, M.; Zhang, X.; Wang, Y.; Li, G. Polymer-Derived Carbon Nanofiber and Its Photocurrent-Switching Responses of Carbon Nanofiber/Cu Nanocomposite in Wide Ranges of Excited Light Wavelength. Polymers 2023, 15, 3528. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, B.; Cong, Q.; He, X.; Gao, M.; Li, G. Organic/inorganic nanocomposites of ZnO/CuO/chitosan with improved properties. Mater. Chem. Phys. 2016, 178, 88–97. [Google Scholar] [CrossRef]
- Cong, Q.; He, X.; Gao, M.; Ma, X.; Li, G. ZnO/CuS heterostructured nanocomposite and its organic functionalization. Mater. Res. Innov. 2014, 18, 740–746. [Google Scholar] [CrossRef]
- Cong, Q.; Geng, H.; He, X.; Gao, M.; Ma, X.; Li, G. Surface modification of ZnO nanosheets with Au/polyaniline and their properties. Mater. Res. Innov. 2014, 18, 30–36. [Google Scholar]
- Ma, X.; Wang, M.; Li, G.; Chen, H.; Bai, R. Preparation of Polyaniline-TiO2 Composite Film with in-situ Polymerization Approach and Its Gas-sensitivity at Room Temperature. Mater. Chem. Phys. 2006, 98, 241–247. [Google Scholar] [CrossRef]
- Ma, X.; Zhu, T.; Xu, H.; Li, G.; Zheng, J.; Liu, A.; Zhang, J.; Du, H. Rapid response behavior of nanofiber-structured TiO2 sensor to selected stimulant chemical warfare agents operating at room temperature. Anal. Bioanal. Chem. 2008, 390, 1133–1137. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Y.; Gao, M.; Xu, H.; Li, G. A novel strategy to prepare ZnO/PbS heterostructured functional nanocomposite utilizing the surface adsorption property of ZnO nanosheets. Catal. Today 2010, 158, 459–463. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, B.; Cong, Q.; He, X.; Gao, M.; Li, G. Highly-Enhanced Performance of TiO2 Nanotube Attached CdS Quantum Dots. Curr. Nanosci. 2016, 12, 500–507. [Google Scholar] [CrossRef]
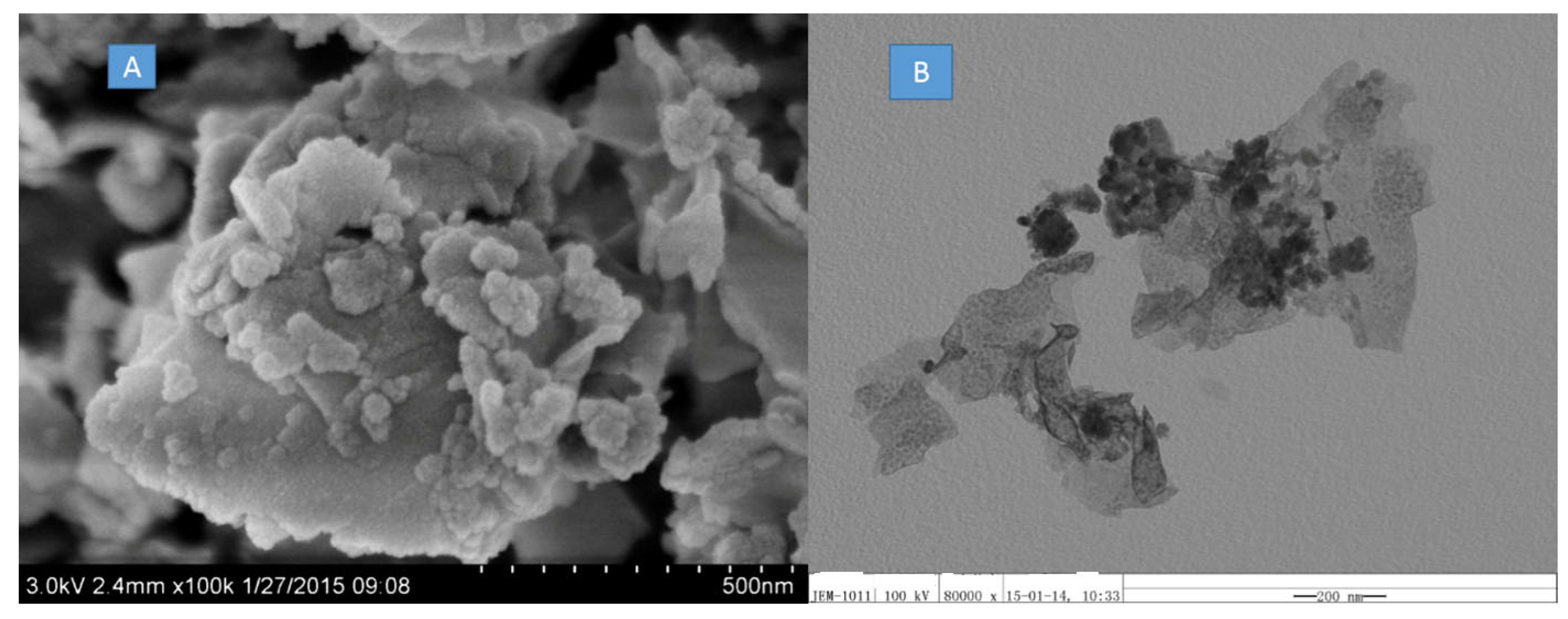

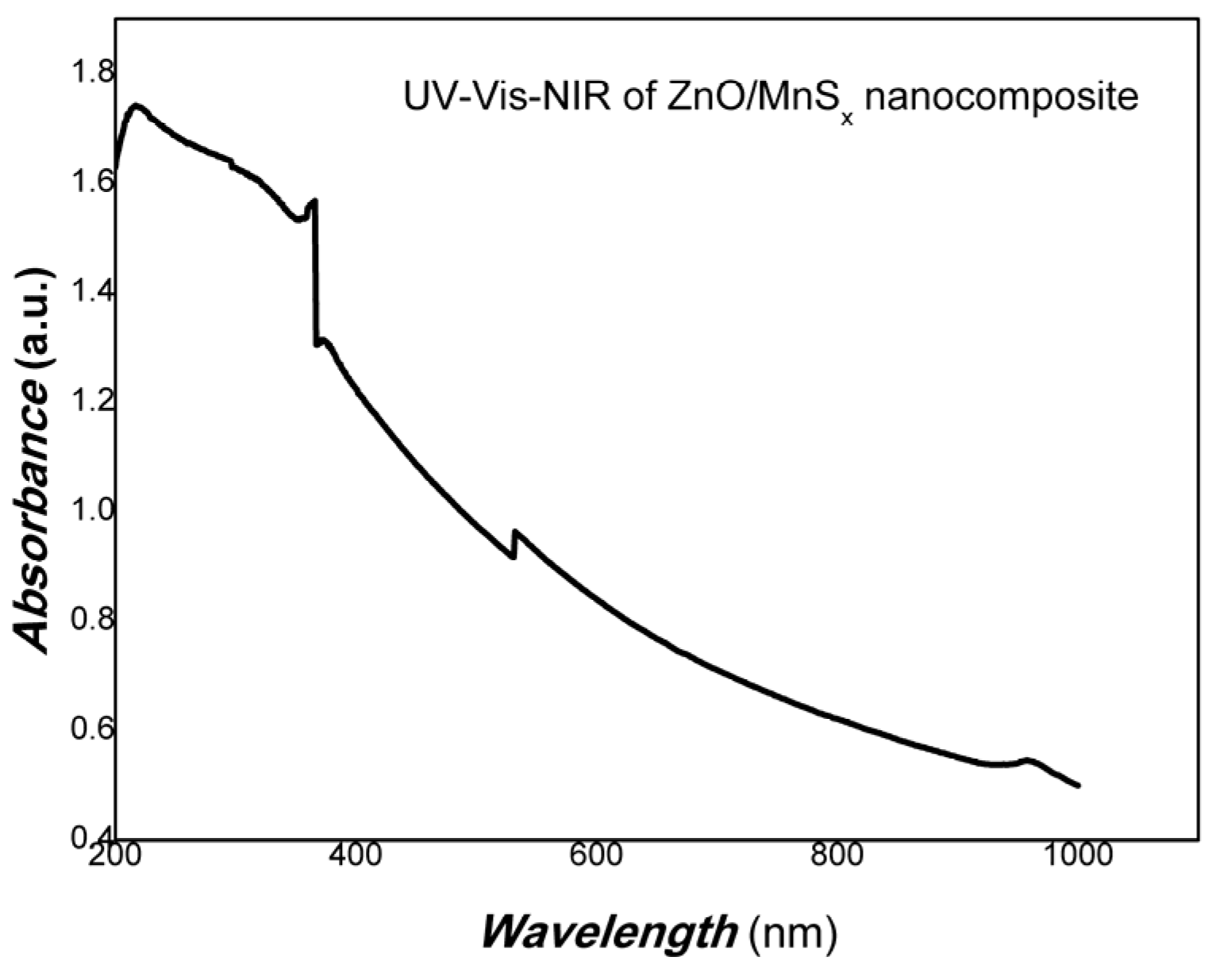

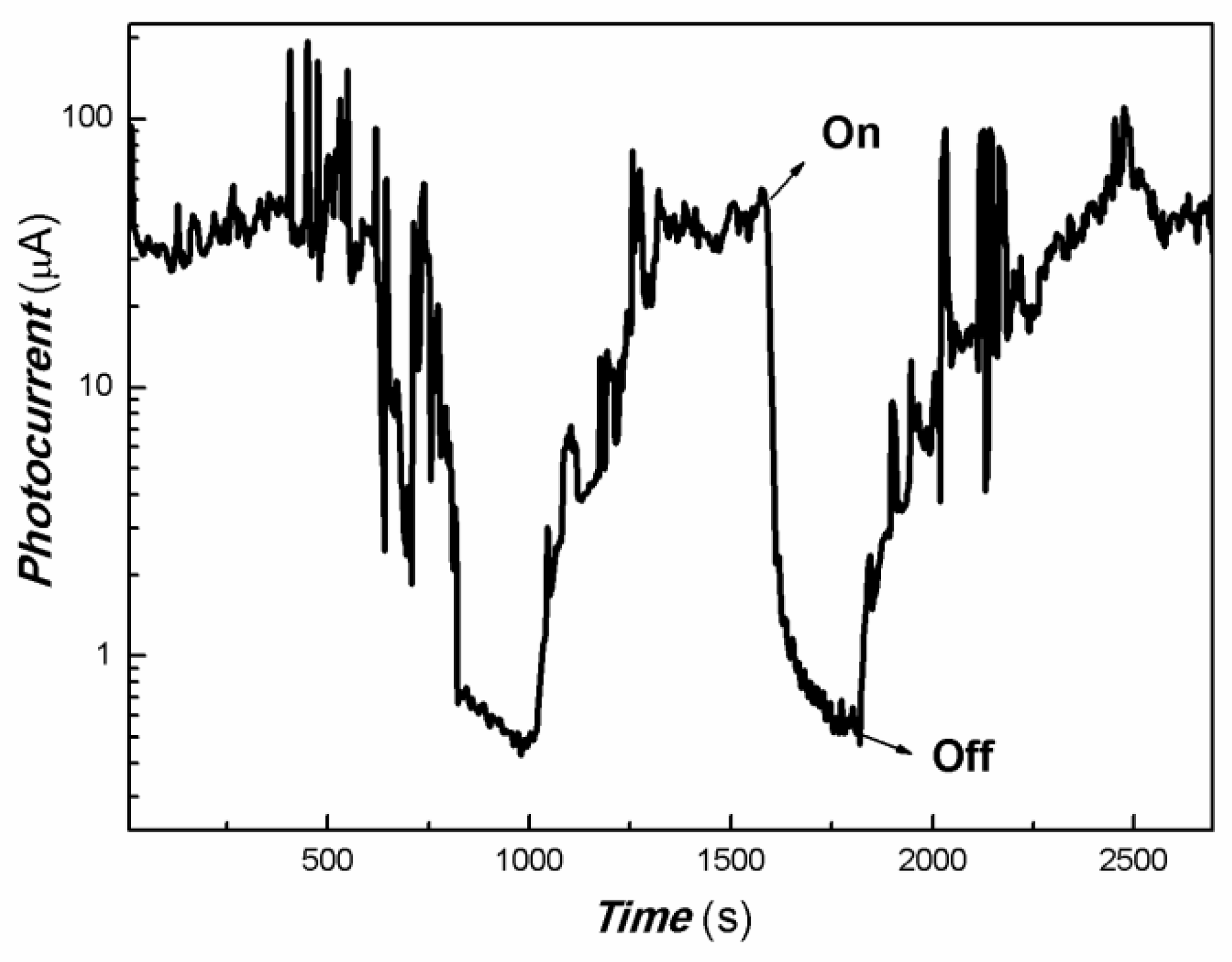


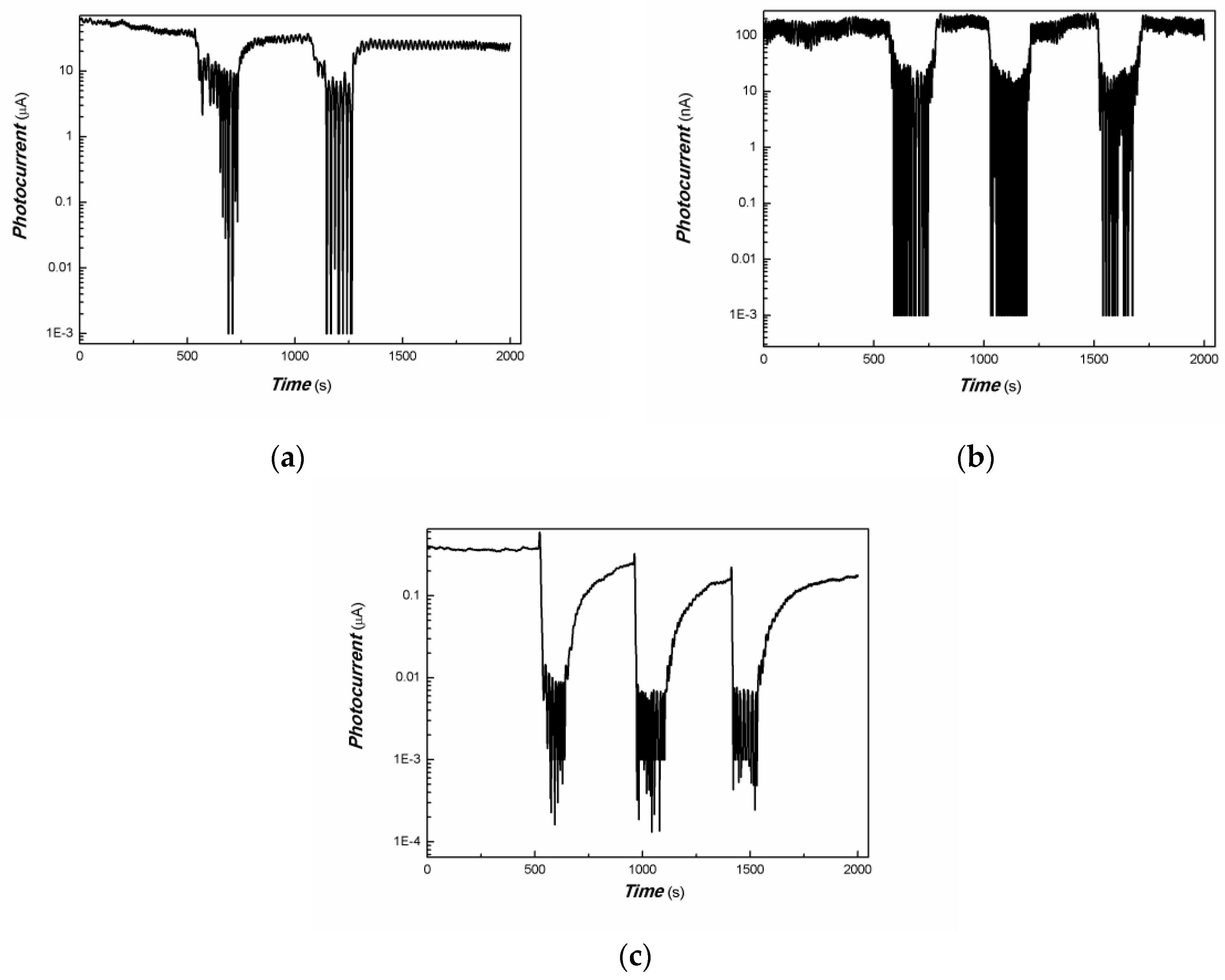

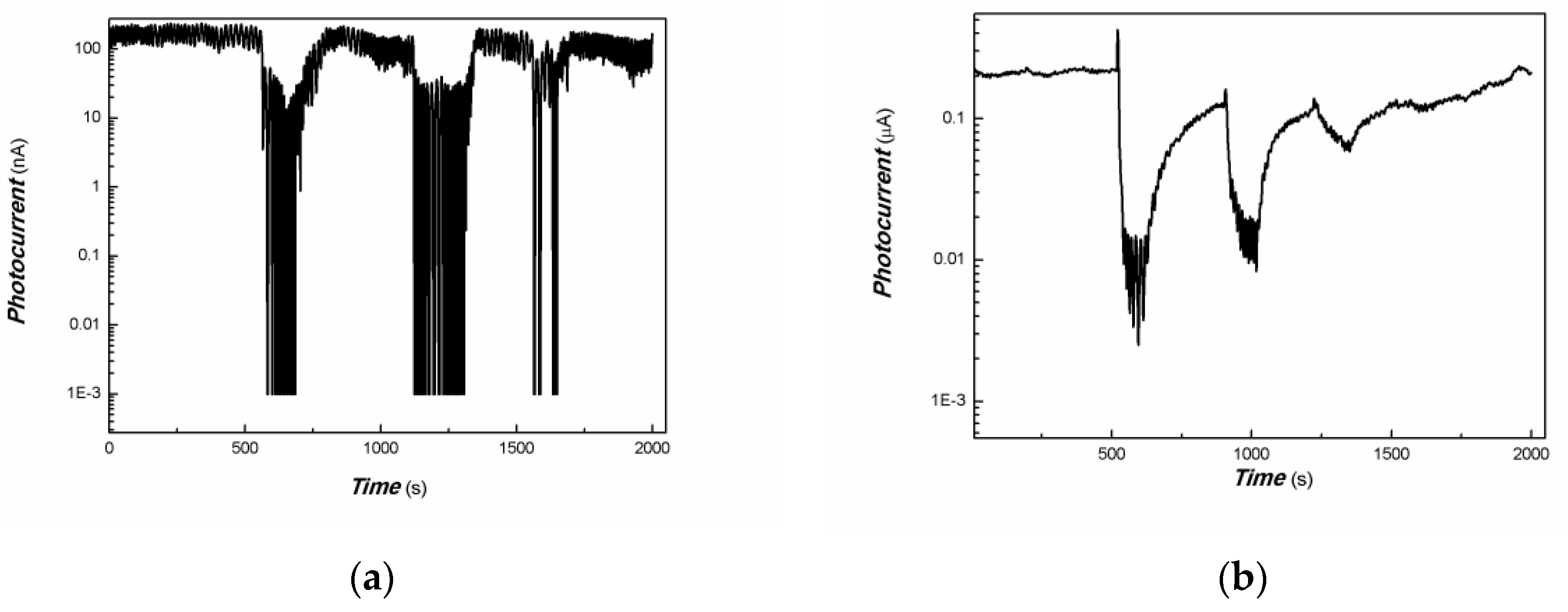


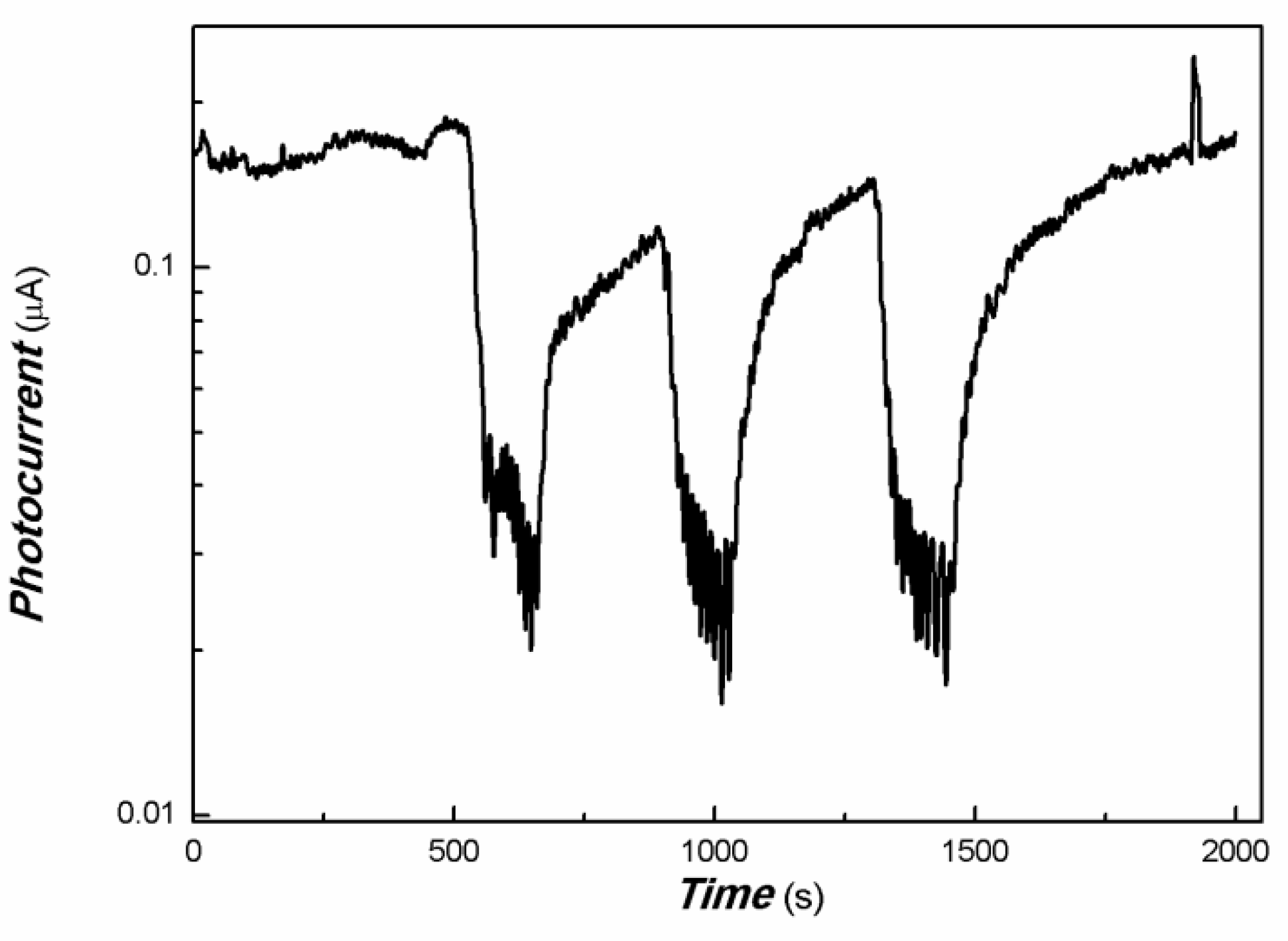

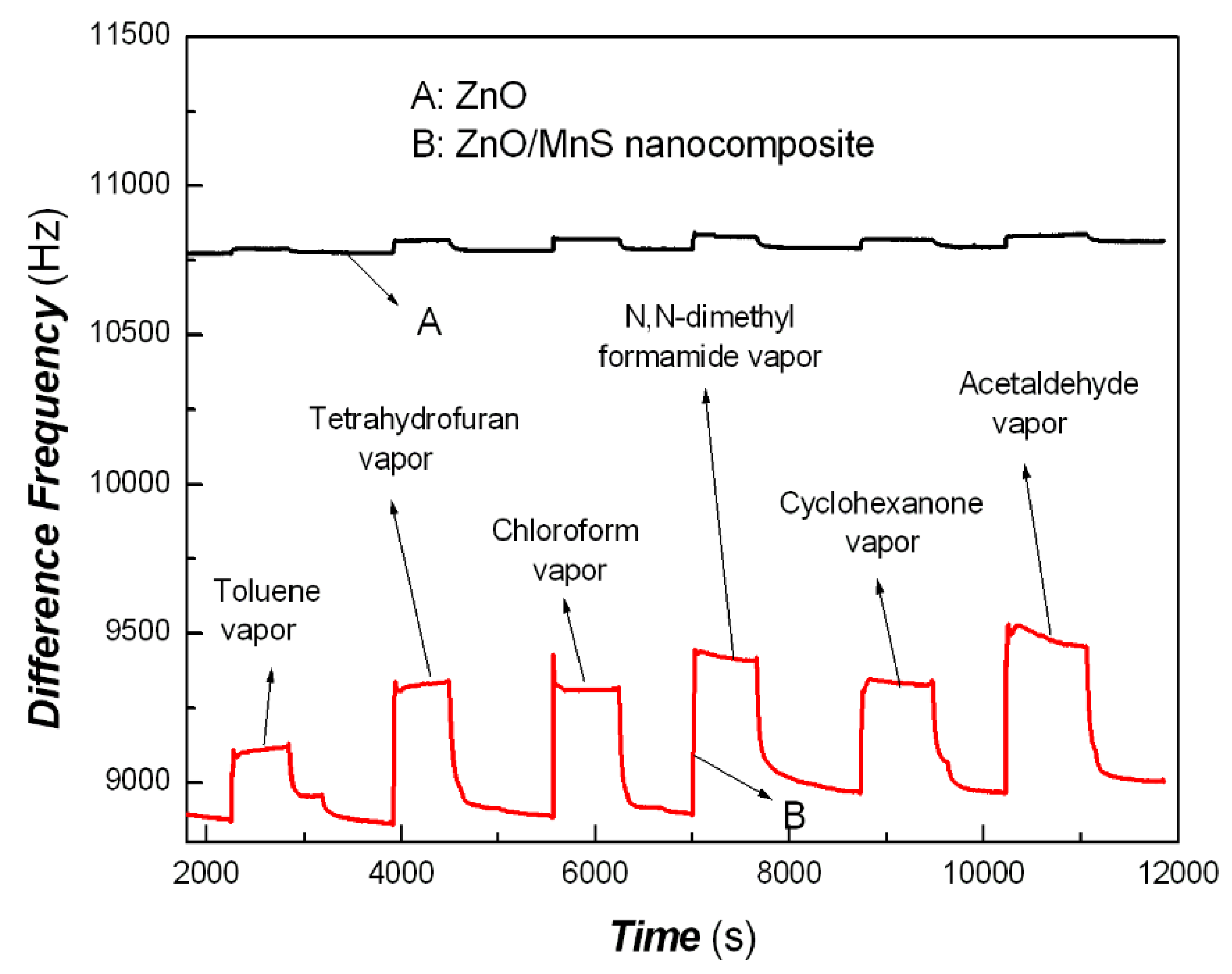
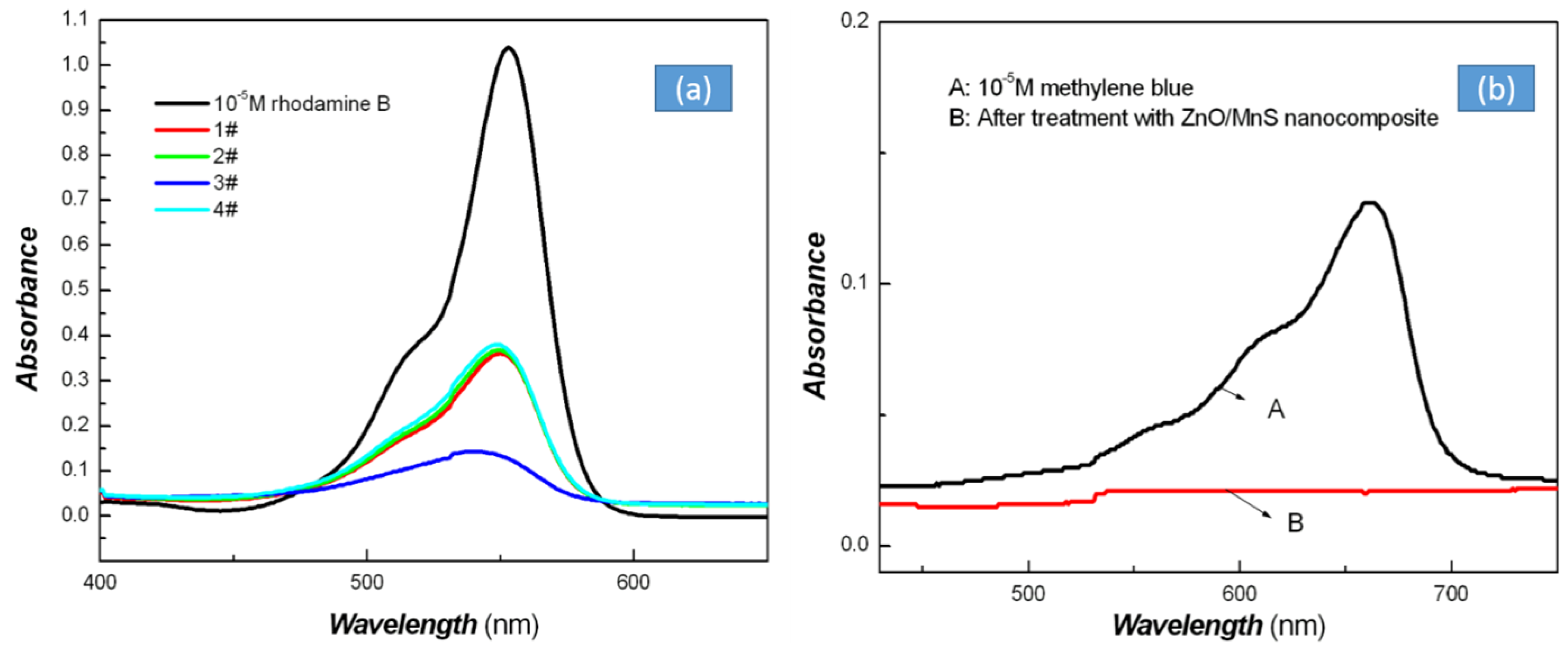

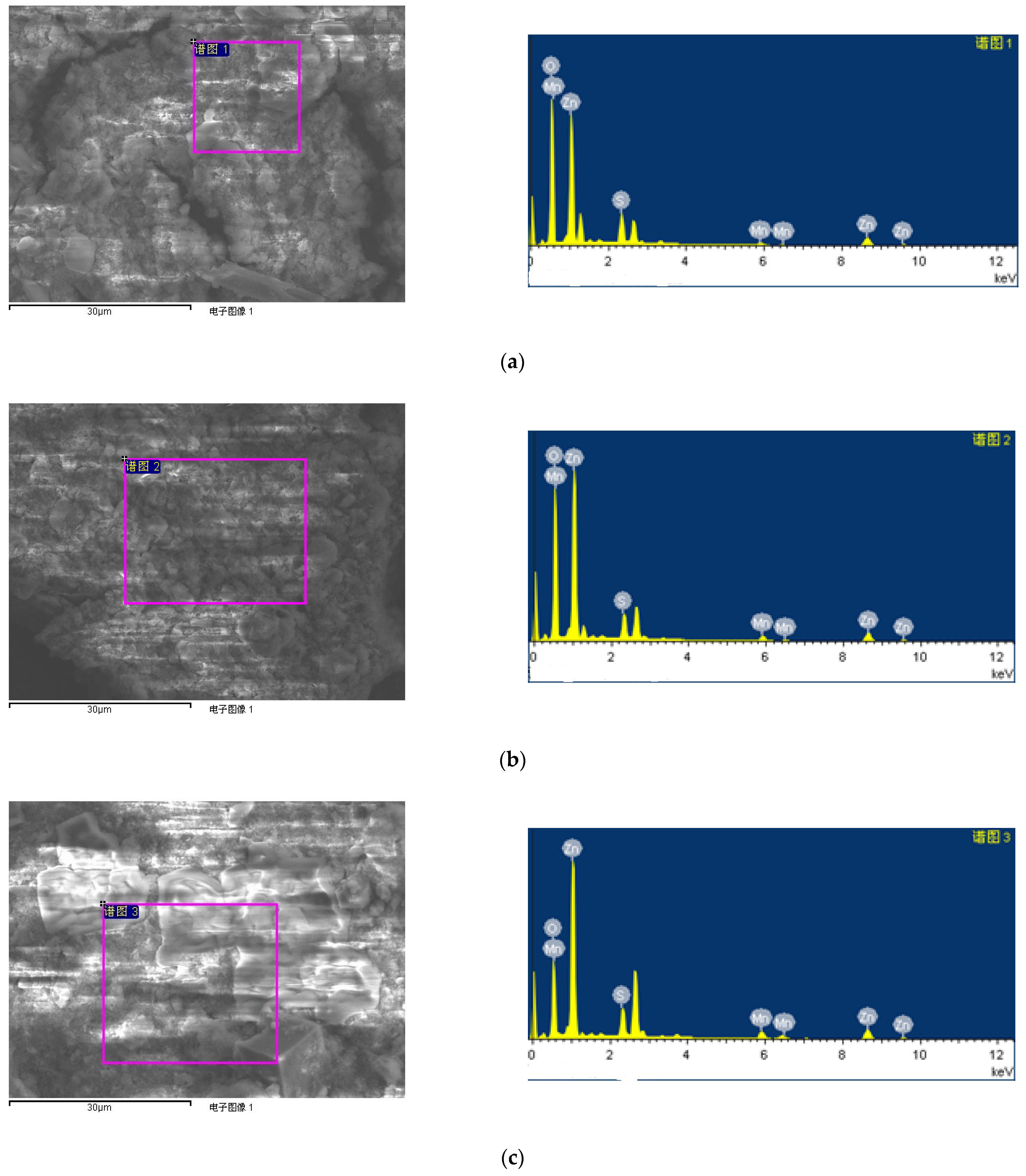
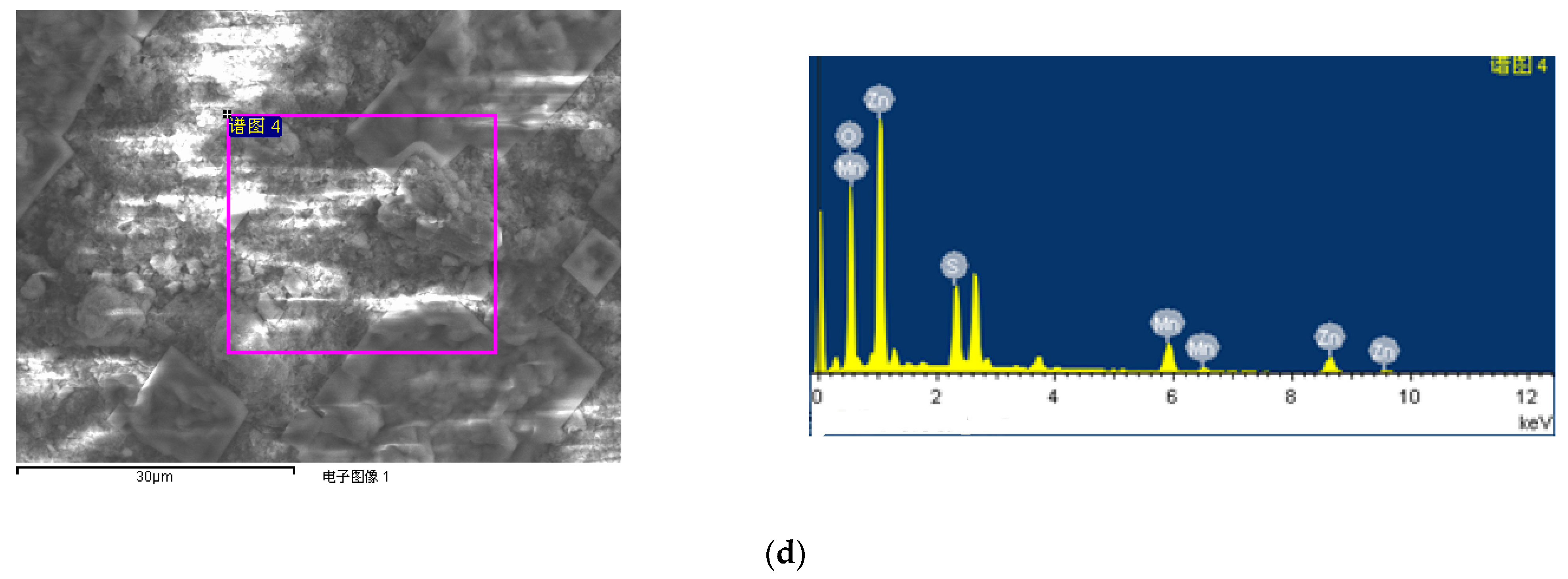
| Sample No. | Zn Element | O Element | The Molar Ratio of O/Zn | Mn Element | S Element | The Molar Ratio of S/Mn | Total | |
|---|---|---|---|---|---|---|---|---|
| Atomic Percent (%) | 1# ZnO/MnSx nanocomposite | 18.94 | 74.55 | 3.94 | 0.99 | 5.52 | 5.58 | 100 |
| 2# ZnO/MnSx nanocomposite | 22.06 | 71.57 | 3.24 | 1.96 | 4.41 | 2.25 | 100 | |
| 3# ZnO/MnSx nanocomposite | 30.39 | 58.17 | 1.91 | 4.18 | 7.27 | 1.74 | 100 | |
| 4# ZnO/MnSx nanocomposite | 22.37 | 61.33 | 2.74 | 7.07 | 9.21 | 1.30 | 100 | |
| Weight percent (%) | 1# ZnO/MnSx nanocomposite | 46.51 | 44.80 | - | 2.04 | 6.64 | - | 100 |
| 2# ZnO/MnSx nanocomposite | 50.85 | 40.37 | - | 3.80 | 4.99 | - | 100 | |
| 3# ZnO/MnSx nanocomposite | 58.78 | 27.53 | - | 6.79 | 6.89 | - | 100 | |
| 4# ZnO/MnSx nanocomposite | 46.74 | 31.37 | - | 12.46 | 9.44 | - | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Zhang, X.; Gao, M.; Wang, Y.; Li, G. Strong Interface Interaction of ZnO Nanosheets and MnSx Nanoparticles Triggered by Light over Wide Ranges of Wavelength to Enhance Their Removal of VOCs. Coatings 2023, 13, 1727. https://doi.org/10.3390/coatings13101727
Ma X, Zhang X, Gao M, Wang Y, Li G. Strong Interface Interaction of ZnO Nanosheets and MnSx Nanoparticles Triggered by Light over Wide Ranges of Wavelength to Enhance Their Removal of VOCs. Coatings. 2023; 13(10):1727. https://doi.org/10.3390/coatings13101727
Chicago/Turabian StyleMa, Xingfa, Xintao Zhang, Mingjun Gao, You Wang, and Guang Li. 2023. "Strong Interface Interaction of ZnO Nanosheets and MnSx Nanoparticles Triggered by Light over Wide Ranges of Wavelength to Enhance Their Removal of VOCs" Coatings 13, no. 10: 1727. https://doi.org/10.3390/coatings13101727







