Microstructure, Mechanical Property, and Wear Behavior of NiAl-Based High-Entropy Alloy
Abstract
:1. Introduction
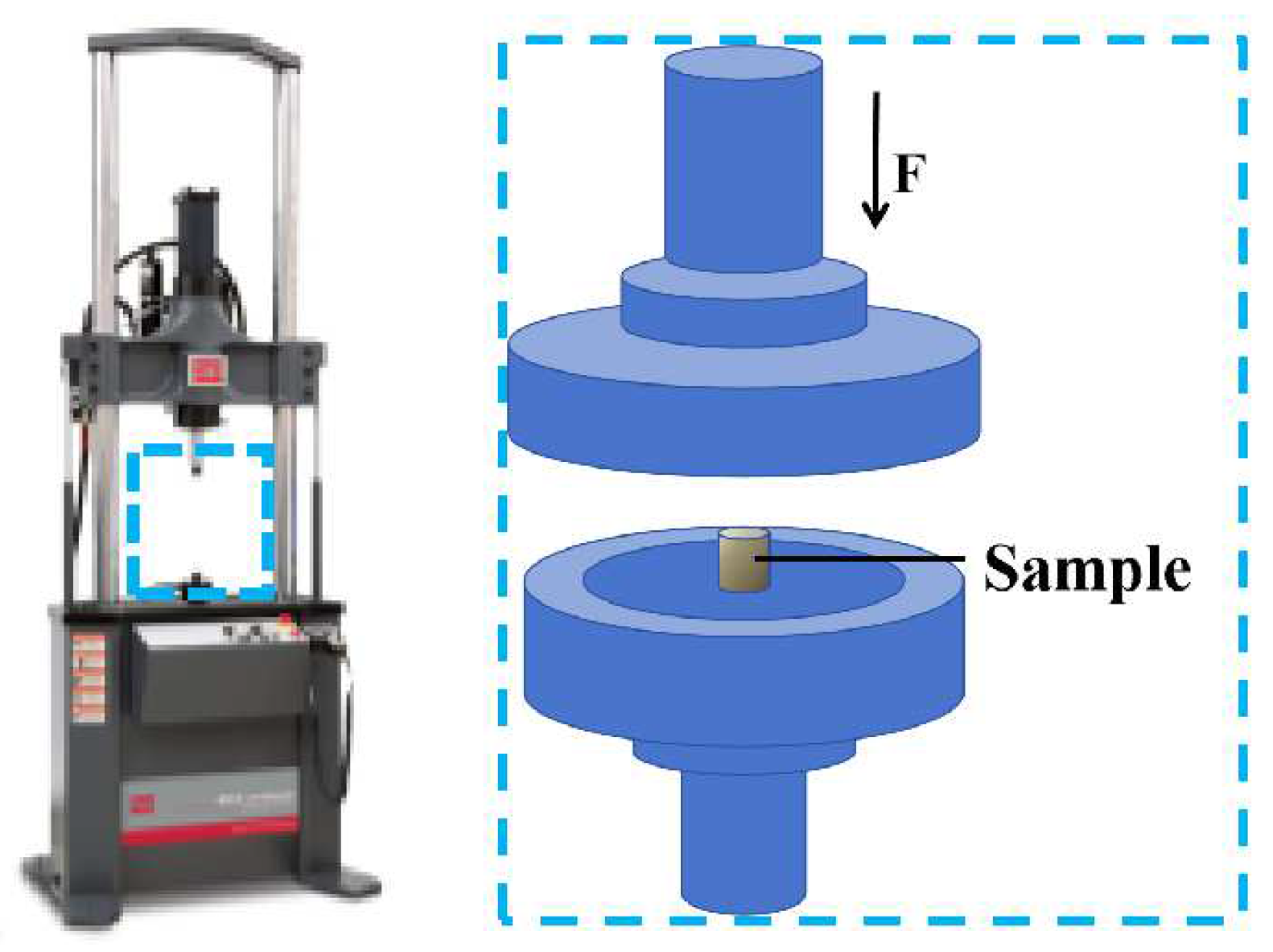
2. Experimental Procedures
3. Results and Discussion
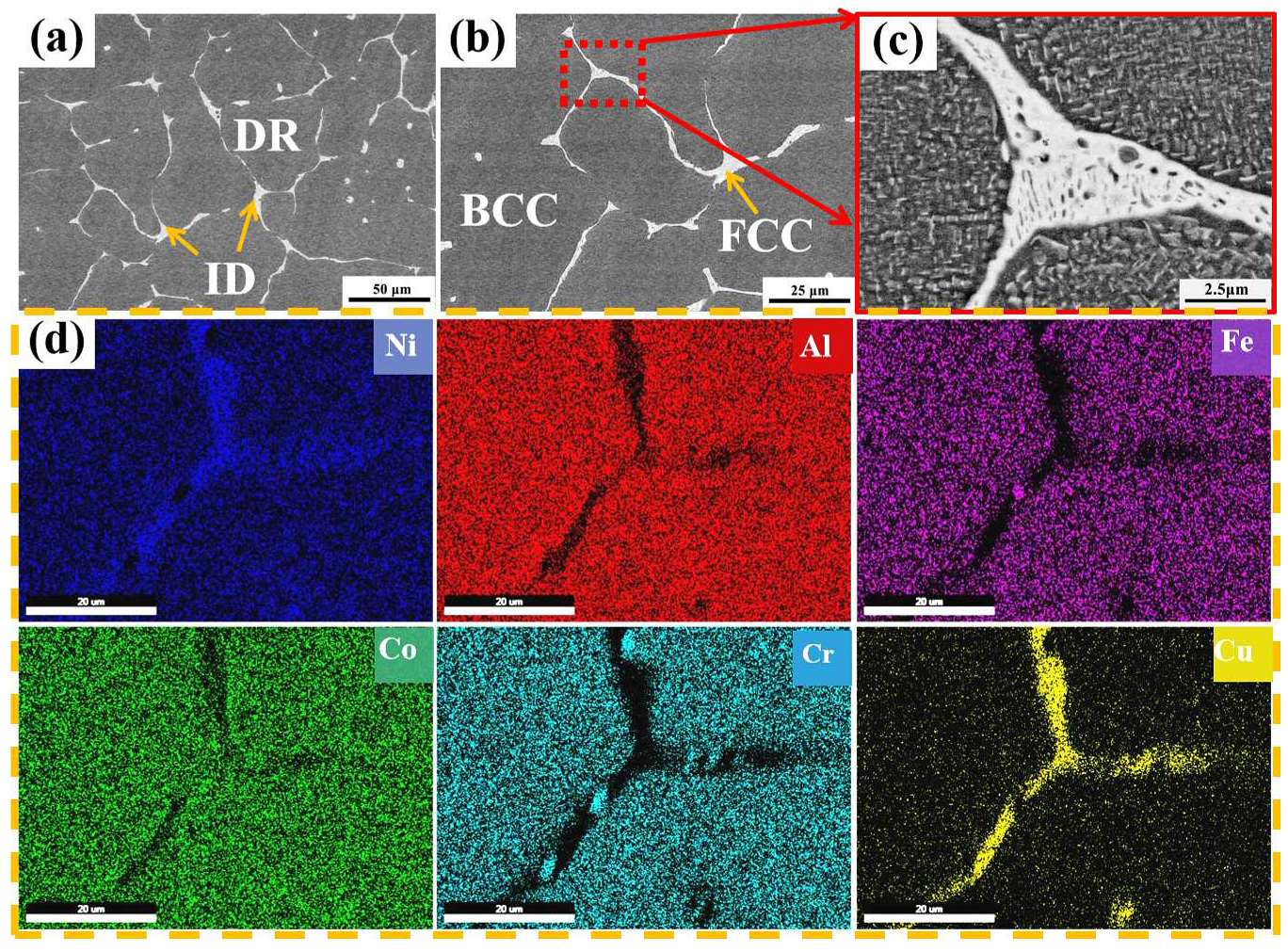
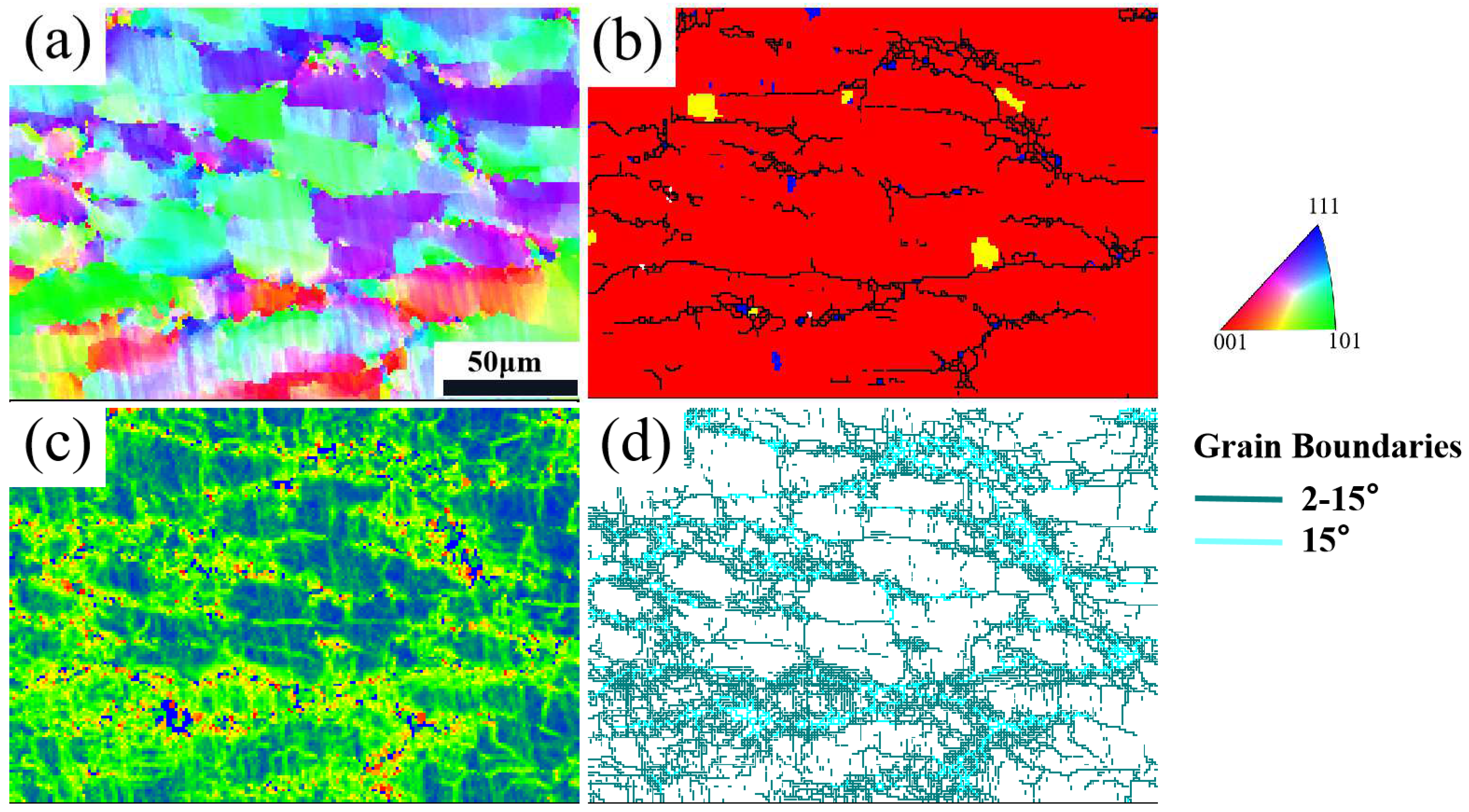
3.1. Microstructure and Phase Composition
3.2. Mechanical Properties
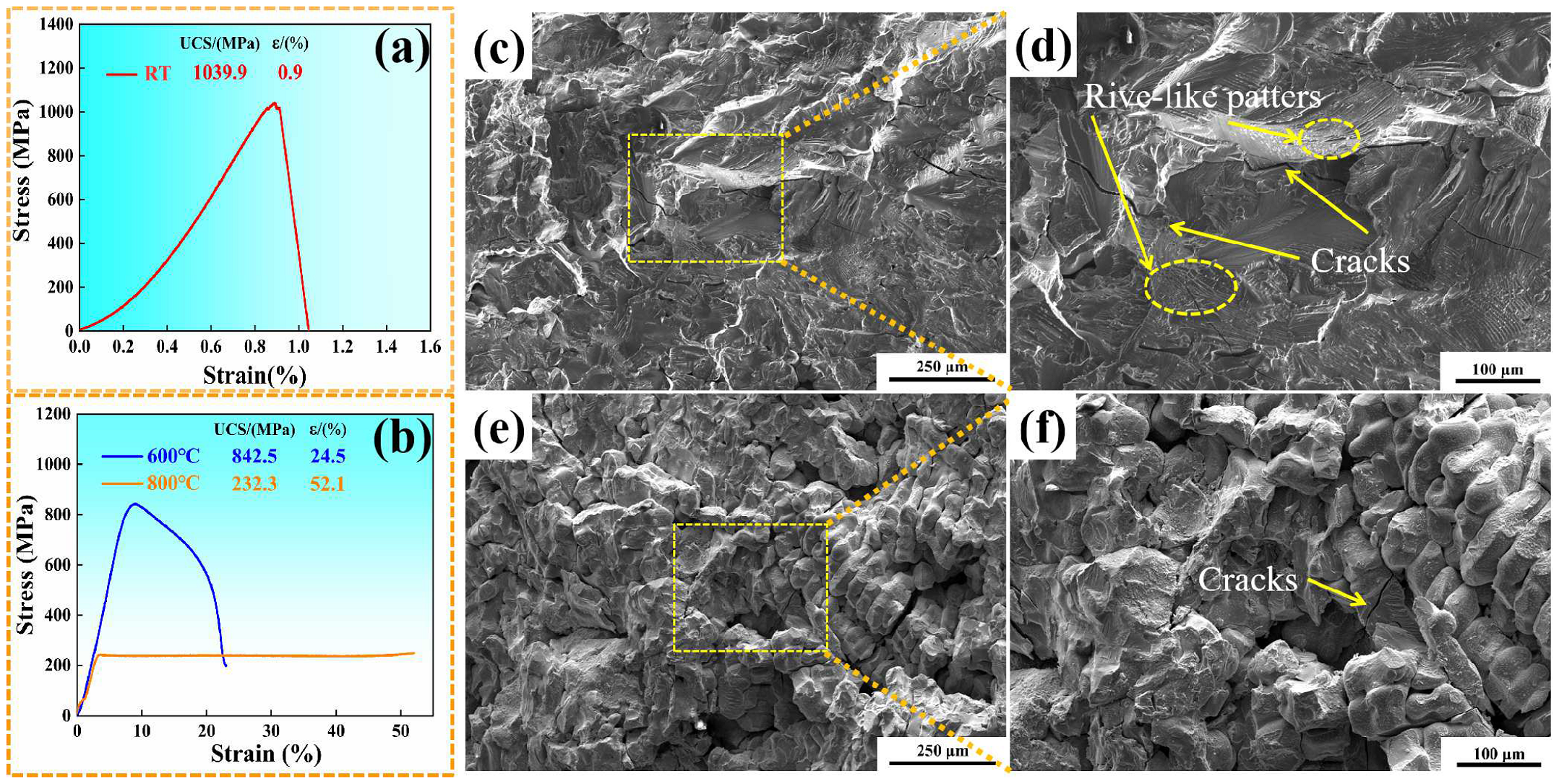
3.2.1. Compression Property
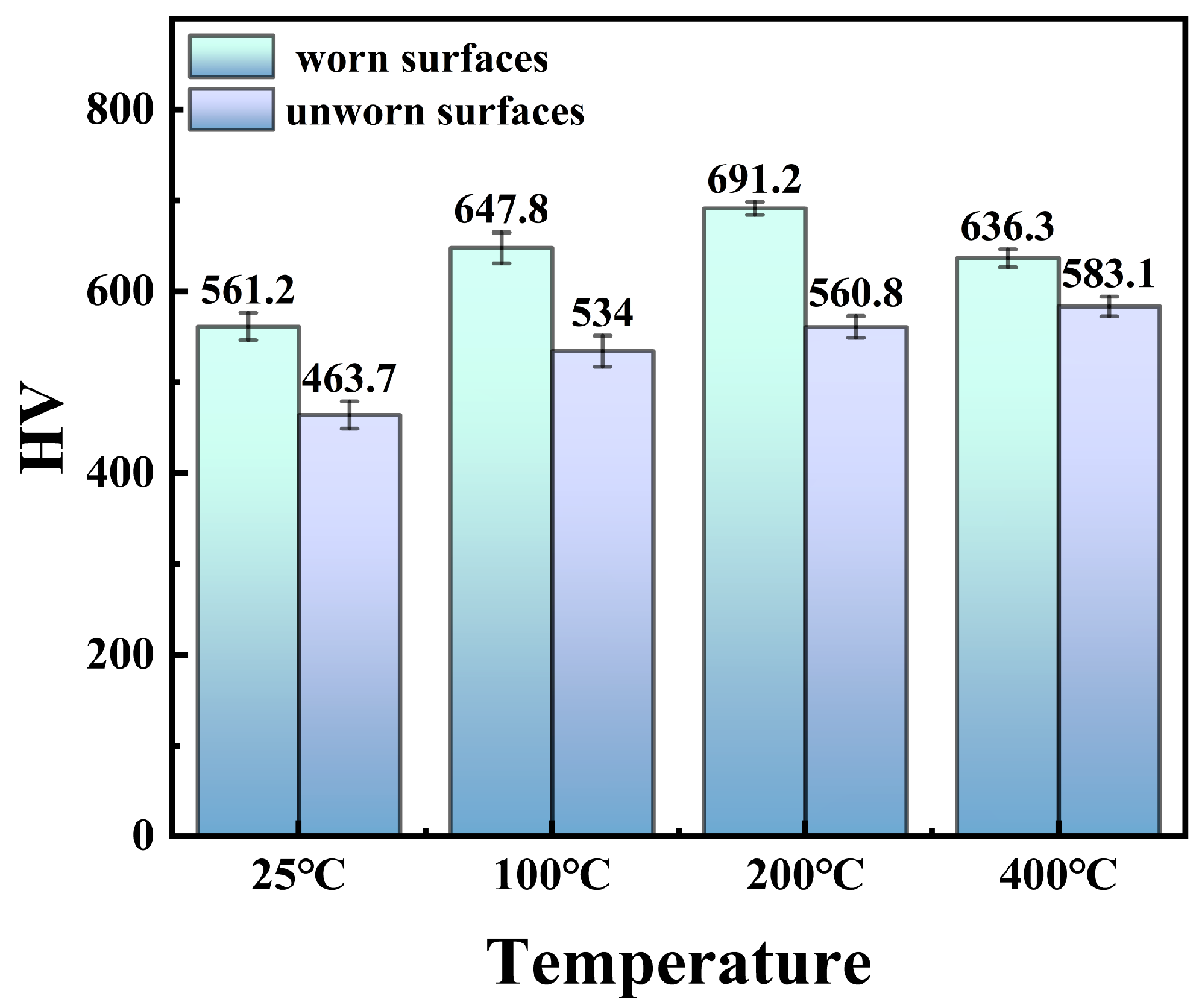
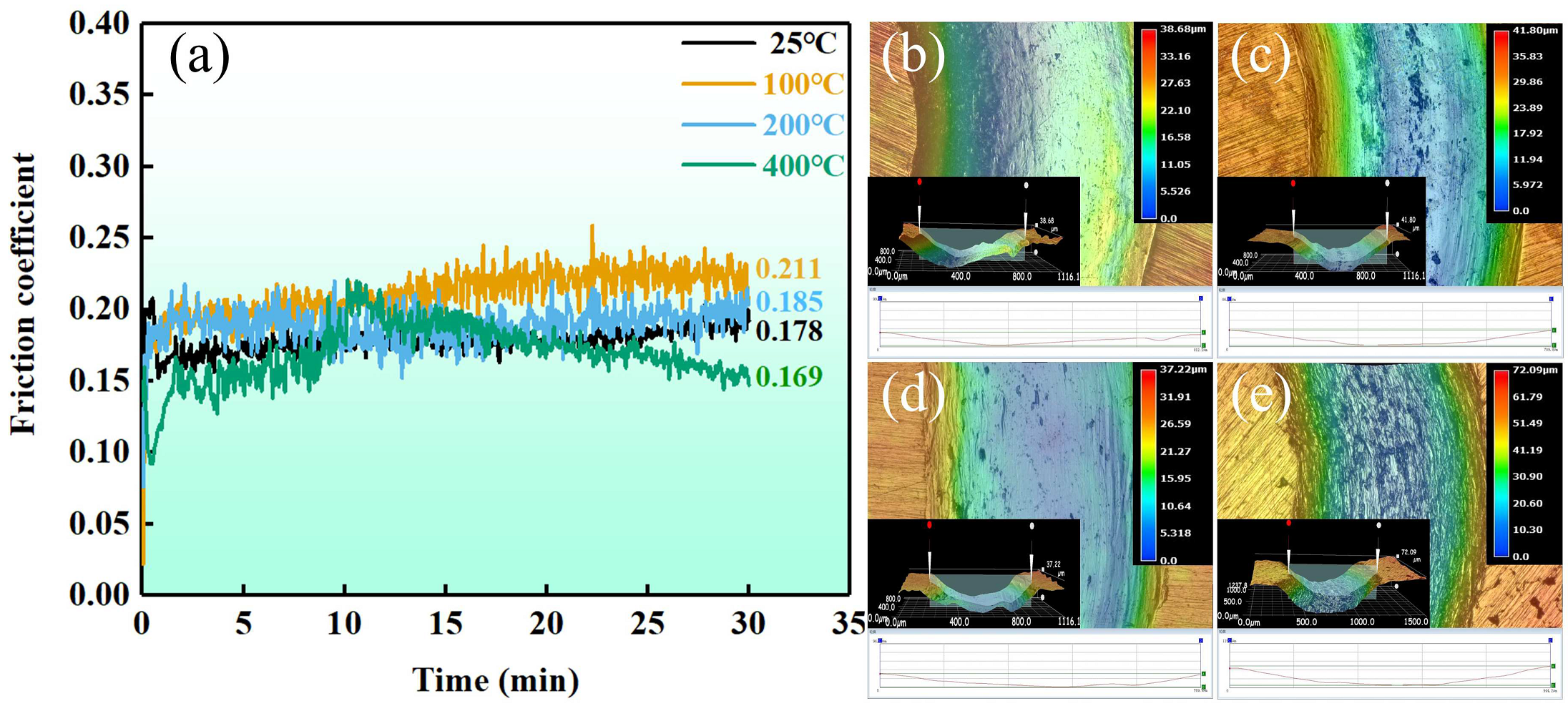
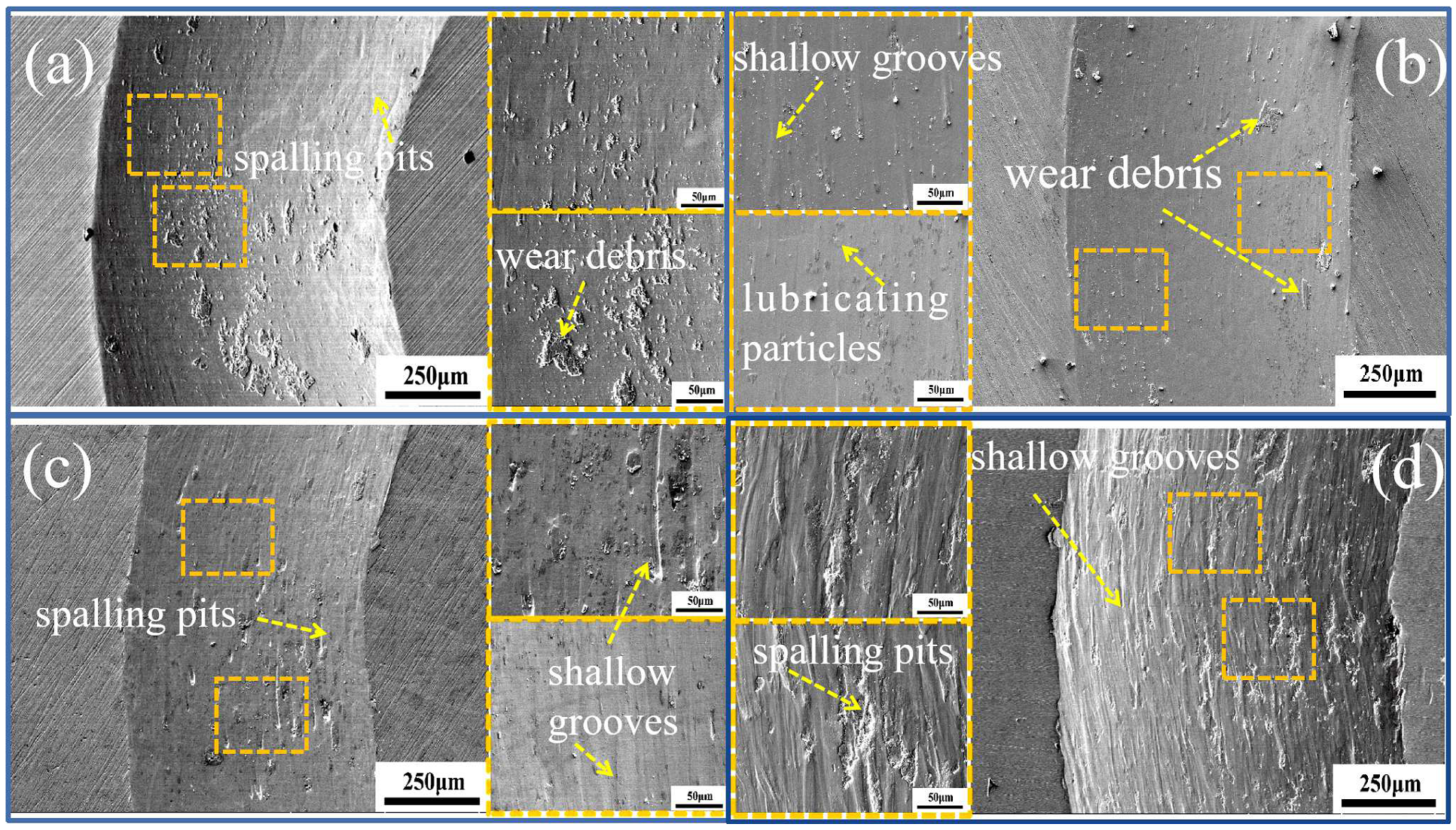
3.2.2. Wear Behavior
4. Conclusions
- (NiA1)78(CoCrFe)16.5Cu5.5 HEAs have a dendritic structure, with the gray BCC phase as the matrix and the white FCC phase at the interdendritic. Spinodal decomposition structure appears in the matrix, and (Cr, Fe) rich nano-precipitation in the FCC phase.
- The compression test shows that the (NiA1)78(CoCrFe)16.5Cu5.5 HEAs exhibits good comprehensive properties at 600 °C. Dynamic recovery and recrystallization occur at 800 °C.
- As the temperature of the wear test increases from RT to 400 °C, the surface grooves and plastic deformation of (NiA1)78(CoCrFe)16.5Cu5.5 HEAs increase, while the peeling and debris decrease. The wear mechanism is mainly abrasive wear.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375, 213–218. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Miracle, D.B.; Chuang, C.P.; Liaw, P.K. Refractory high-entropy alloys. Intermetallics 2010, 18, 1758–1765. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, M.; Yang, X. Advanced Technology in High-Entropy Alloys; Chemical Industry Publishing: Beijing, China, 2018. [Google Scholar]
- Chang, Y.J.; Yeh, A.C. The evolution of microstructures and high temperature properties of AlxCo1.5CrFeNi1.5Tiy high entropy alloys. J. Alloys Compd. 2015, 653, 379–385. [Google Scholar]
- Tong, C.J.; Chen, Y.L.; Yeh, J.W.; Lin, S.J.; Chen, S.K.; Shun, T.T.; Chang, S.Y. Microstructure characterization of AlxCoCrCuFeNi high-entropy alloy system with multiprincipal elements. Metall. Mater. Trans. A 2005, 36, 881–893. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, Y.; Guo, S.; Jiang, L.; Kang, H.; Wang, T. A Promising New Class of High-Temperature Alloys: Eutectic High-Entropy Alloys. Sci. Rep. 2014, 4, 6200. [Google Scholar] [CrossRef]
- Fan, Z.; Ru, L.B.; Jun, L.X.; Hui, W.; He, J.S.; Muhammed, N.; Li, W.X.; Yuan, W.; Ping, L.Z. Hydrogen embrittlement resistance of precipitation-hardened FeCoNiCr high entropy alloys. Intermetallics 2023, 153, 107800. [Google Scholar]
- He, J.Y.; Wang, H.; Wu, Y.; Liu, X.J.; Mao, H.H.; Nieh, T.G.; Lu, Z.P. Precipitation behavior and its effects on tensile properties of FeCoNiCr high-entropy alloys. Intermetallics 2016, 79, 41–52. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, H.; Wu, Y.; Liu, X.; Chen, H.; Yao, M. Ultrastrong steel via minimal lattice misfit and high-density nanoprecipitation. Nature 2017, 544, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.W.; Marceau, R.K.W.; Styles, M.J.; Barbier, D.; Hutchinson, C.R. G phase precipitation and strengthening in ultra-high strength ferritic steels: Towards lean maraging metallurgy. Acta Mater. 2017, 130, 28–46. [Google Scholar] [CrossRef]
- Jin, X.; Zhou, Y.; Zhang, L.; Du, X.; Li, B. A new pseudo binary strategy to design eutectic high entropy alloys using mixing enthalpy and valence electron concentration. Mater. Des. 2018, 143, 49–55. [Google Scholar] [CrossRef]
- Belov, N.A.; Alabin, A.N.; Eskin, D.G. Improving the properties of cold-rolled Al-6%Ni sheets by alloying and heat treatment. Scr. Mater. 2004, 50, 89–94. [Google Scholar] [CrossRef]
- Osório, W.R.; Peixoto, L.C.; Canté, M.V.; Garcia, A. Electrochemical corrosion characterization of Al-Ni alloys in a dilute sodium chloride solution. Electrochim. Acta 2010, 55, 4078–4085. [Google Scholar]
- Wang, Y.; Li, S.; Chen, F.; Yang, K.; Ge, G.; Tang, X. Unique dislocation loops distribution of AlCrFeNiTix eutectic high-entropy alloys under high-temperature ion irradiation. J. Alloys Compd. 2023, 958, 170373. [Google Scholar] [CrossRef]
- Liu, D.; Yu, P.; Li, G.; Liaw, P.K.; Liu, R. High-temperature high-entropy alloys AlxCo15Cr15Ni70-x based on the Al-Ni binary system. Mater. Sci. Eng. 2018, 724, 283–288. [Google Scholar]
- Wang, L.; Shen, J.; Zhang, Y.; Guo, L.; Xu, H.; Fu, H. Microstructure evolution and room temperature fracture toughness of as-cast and directionally solidified novel NiAl-Cr (Fe) alloy. Intermetallics 2017, 84, 11–19. [Google Scholar] [CrossRef]
- Misra, A.; Gibala, R. Room-temperature deformation behavior of directionally solidified multiphase Ni-Fe-Al alloys. Metall. Mater. Trans. A 1997, 28, 795–807. [Google Scholar]
- Jin, X.; Zhou, Y.; Zhang, L.; Du, X.; Li, B. A novel Fe20Co20Ni41Al19 eutectic high entropy alloy with excellent tensile properties. Mater. Lett. 2018, 216, 144–146. [Google Scholar] [CrossRef]
- Ishida, K.; Kainuma, R.; Ueno, N.; Nishizawa, T. Ductility enhancement in NiAl (B2)-base alloys by microstructural control. Metall. Trans. A 1991, 22, 441–446. [Google Scholar] [CrossRef]
- Yu, Z.; Yan, Y.; Qiang, J.; Gao, W.; Wang, X.; Liu, X.; Du, W. Microstructure evolution and compressive property variation of AlxCoCrFeNi high entropy alloys produced by directional solidification. Intermetallics 2023, 152, 107749. [Google Scholar] [CrossRef]
- Qiang, F.; Xin, S.; Guo, P.; Hou, H.; Wang, J.; Hou, W. Formation mechanism of interdiffusion layer and mechanical properties of Al0. 6CoCrFeNi high-entropy alloy/Ti composites. J. Alloys Compd. 2023, 943, 169151. [Google Scholar] [CrossRef]
- Guo, J.T.; Cui, C.Y.; Chen, Y.X.; Li, D.X.; Ye, H.Q. Microstructure, interface and mechanical property of the DS NiAl/Cr (Mo, Hf) composite. Intermetallics 2001, 9, 287–297. [Google Scholar] [CrossRef]
- Zaitsev, A.A.; Sentyurina, Z.A.; Levashov, E.A.; Pogozhev, Y.S.; Sanin, V.N.; Loginov, P.A.; Petrzhik, M.I. Structure and properties of NiAl-Cr (Co, Hf) alloys prepared by centrifugal SHS casting. Part 1–Room temperature investigations. Mater. Sci. Eng. A 2017, 690, 463–472. [Google Scholar] [CrossRef]
- Wang, L.; Yao, C.; Shen, J.; Zhang, Y.; Wang, T.; Xu, H.; Zhang, G. Microstructures and compressive properties of NiAl-Cr (Mo) and NiAl-Cr eutectic alloys with different Fe contents. Mater. Sci. Eng. A 2019, 744, 593–603. [Google Scholar] [CrossRef]
- Wang, L.; Su, Y.; Yao, C.; Huang, Y.; Shen, J.; Zhang, Y.; Liu, G.; Zhao, P.; Zhang, G. Microstructure and mechanical prop-erty of novel NiAl-based hypoeutectic/eutectic/hypereutectic high-entropy alloy. Intermetallics 2022, 143, 107476. [Google Scholar] [CrossRef]
- He, J.Y.; Liu, W.H.; Wang, H.; Wu, Y.; Liu, X.J.; Nieh, T.G.; Lu, Z.P. Effects of Al addition on structural evolution and tensile properties of the FeCoNiCrMn high-entropy alloy system. Acta Mater. 2014, 62, 105–113. [Google Scholar] [CrossRef]
- Zhang, X.; Li, B.; Zeng, L.; Yi, J.; Wang, B.; Hu, C.; Xia, M. Effect of Re addition on the microstructure and mechanical properties of AlCoCrFeNi2.1 eutectic high-entropy alloy. Intermetallics 2023, 154, 107808. [Google Scholar] [CrossRef]
- Huo, W.; Zhou, H.; Fang, F.; Zhou, X.; Xie, Z.; Jiang, J. Microstructure and properties of novel CoCrFeNiTax eutectic high-entropy alloys. J. Alloys Compd. 2018, 735, 897–904. [Google Scholar] [CrossRef]
- Chen, X.; Qi, J.Q.; Sui, Y.W.; He, Y.Z.; Wei, F.X.; Meng, Q.K.; Sun, Z. Effects of aluminum on microstructure and compressive properties of Al-Cr-Fe-Ni eutectic multi-component alloys. Mater. Sci. Eng. A 2017, 681, 25–31. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar]
- Hassan, M.A.; Yehia, H.M.; Mohamed, A.S.A.; El-Nikhaily, A.E.; Elkady, O.A. Effect of Copper Addition on the AlCoCrFeNi High Entropy Alloys Properties via the Electroless Plating and Powder Metallurgy Technique. Crystals 2021, 11, 540. [Google Scholar] [CrossRef]
- Yuan, Y.; Hui, Q.Z.; Bo, R.H.; Min, L.W. Research progress on friction and wear properties of high entropy alloys. Mater. Eng. 2022, 50, 1–17. [Google Scholar]
- Yan, G.X. Study on Solidification Structure and Properties of CoCrCuFeNi High Entropy Alloy. Master’s Thesis, Jiangsu University of Science and Technology, Zhenjiang, China, 2014. [Google Scholar]
- Ping, W.Y. Microstructure and Properties of AlCrFeCoNiCu System Multi principal Component Alloy and Its Composite Materials. Master’s thesis, Harbin Institute of Technology, Harbin, China, 2009. [Google Scholar]
- Yuan, L.; Min, C.; Xiang, L.Y.; Xiang, C. Microstructure and Mechanical Properties of AlxCoCrCuFeNi Multi principal Component High Entropy Alloy. Rare Met. Mater. Eng. 2009, 38, 1602–1607. [Google Scholar]
- Wei, S.; Na, L.X.; Tian, C.X.; Min, L.Z.; Hong, Z.Y.; Xiao, Y.Q.; Yv, W.C.; Chuang, D. Microstructure Evolution of Cux[Ni3Fe1] (x=4-12) Alloy. J. Vac. Sci. Technol. 2018, 38, 636–641. [Google Scholar]
- Na, M.J.; Dong, W.T.; Na, W. Microstructure Evolution of FeCrNiMn Duplex High Entropy Alloys During High-Temperature Compression. Rare Met. Mater. Eng. 2022, 51, 4496–4501. [Google Scholar]
- Mei, H.; Ding, J.C.; Zhao, Z.; Li, Q.; Song, J.; Li, Y.; Gong, W.; Ren, F.; Wang, Q. Effect of Cu content on high-temperature tribological properties and oxidation behavior of Al-Ti-V-Cu-N coatings deposited by HIPIMS. Surf. Coat. Technol. 2022, 434, 128–130. [Google Scholar] [CrossRef]



| Materials (at.%) | Yield Strength (MPa) | Compressive Strength (MPa) | Compressive Strain (%) |
|---|---|---|---|
| 52.4Ni-43.5Al-4.1Cr | - | 1026 ± 29 | 9.3 ± 0.5 |
| 51.1Ni-42.2Al-4.1Cr-2.6Fe | - | 1220 ± 37 | 11.8 ± 1.4 |
| 90Cr-4Ni-6Al | - | 736 ± 32 | 6.4 ± 0.7 |
| 82Cr-4Ni-6Al-8.0Fe | - | 964 ± 38 | 7.4 ± 0.3 |
| Ni-30Fe-20Al | 1065 | - | - |
| Ni-30Fe-23Al | 1120 | - | - |
| Ni-33Fe-21Al | 1150 | - | - |
| Fe20Co20Ni41Al19 | 577 | 1103 | 18.7 |
| Ni-26Al-50Co | - | - | 10.2 |
| Ni-25Al-18Fe | - | - | 6.1 |
| Ni-24Al-30Cu | - | - | 0 |
| Ni-20Al-20Cr | - | - | 2.5 |
| Al0.6CoCrFeNi | - | 1903.42 | 21.26 |
| Al1.2CoCrFeNi | - | 1462.36 | 18.05 |
| Ti + 10% Al0.6CoCrFeNi | - | 1642 | 21.7 |
| NiAl-28Cr-5.5Mo-0.5Hf(DS) | - | 255 | - |
| NiAl-12Cr-6Co | - | 1989 | 0.11 |
| NiAl-Cr(Mo)-5Fe | - | 2229 | 0.29 |
| Ni | Al | Co | Cr | Fe | Cu | |
|---|---|---|---|---|---|---|
| wt.% | 49.67 | 22.84 | 7.03 | 6.21 | 6.67 | 7.58 |
| at.% | 39 | 39 | 5.5 | 5.5 | 5.5 | 5.5 |
| Temperature | Wear Volume (mm3) | Specific Wear Rate (mm3/Nm) | Line Wear Rate (mm3/km) |
|---|---|---|---|
| RT | 1.70 × 10−1 | 7.53 × 10−5 | 0.38 |
| 100 °C | 1.59 × 10−1 | 7.05 × 10−5 | 0.36 |
| 200 °C | 1.74 × 10−1 | 7.69 × 10−5 | 0.39 |
| 400 °C | 4.00 × 10−1 | 1.77 × 10−4 | 0.90 |
| Temperature | Al | Ni | Co | Cr | Cu | Fe | O |
|---|---|---|---|---|---|---|---|
| 25 °C | 17.00 | 26.06 | 2.65 | 2.62 | 7.64 | 2.53 | 41.48 |
| 100 °C | 23.94 | 15.26 | 2.56 | 3.10 | 6.74 | 3.11 | 45.30 |
| 200 °C | 22.48 | 20.93 | 3.04 | 3.00 | 9.42 | 3.02 | 38.11 |
| 400 °C | 19.06 | 16.68 | 2.33 | 2.15 | 6.86 | 2.49 | 50.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Wang, X.; Huang, Y.; Xu, Z.; Deng, Y.; Jiang, X.; Yang, X. Microstructure, Mechanical Property, and Wear Behavior of NiAl-Based High-Entropy Alloy. Coatings 2023, 13, 1737. https://doi.org/10.3390/coatings13101737
Li Z, Wang X, Huang Y, Xu Z, Deng Y, Jiang X, Yang X. Microstructure, Mechanical Property, and Wear Behavior of NiAl-Based High-Entropy Alloy. Coatings. 2023; 13(10):1737. https://doi.org/10.3390/coatings13101737
Chicago/Turabian StyleLi, Ziyan, Xiaohong Wang, Yanyan Huang, Zhixin Xu, Yulei Deng, Xiaoying Jiang, and Xiaohong Yang. 2023. "Microstructure, Mechanical Property, and Wear Behavior of NiAl-Based High-Entropy Alloy" Coatings 13, no. 10: 1737. https://doi.org/10.3390/coatings13101737




