Enhancement of Human Gingival Fibroblasts Bioactivity and Proliferation on Plasma Sprayed Yttria-Stabilised Zirconia/TiO2 Surface Coating of Titanium Alloys: An In-Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Powder Preparation
2.2. Plasma Spray Coating
2.3. Surface Characterisation
2.4. In Vitro Cytotoxicity Test
2.5. Cell Attachment and Cell Proliferation Analysis
2.6. Statistical Analysis
3. Results
3.1. Surface Composition and Microstructure
3.1.1. Surface Topography
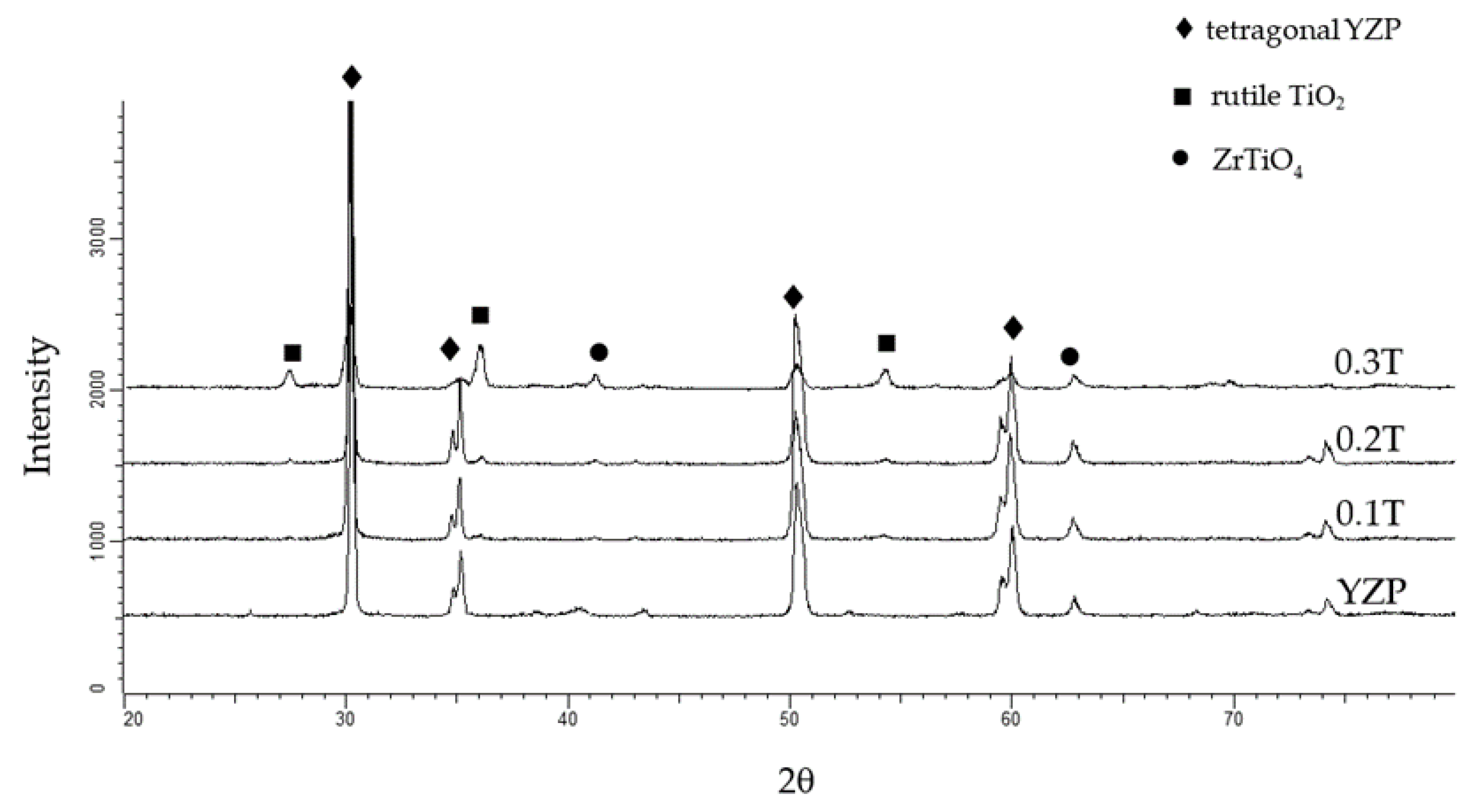
3.1.2. Phase Composition
3.1.3. Roughness and Wettability
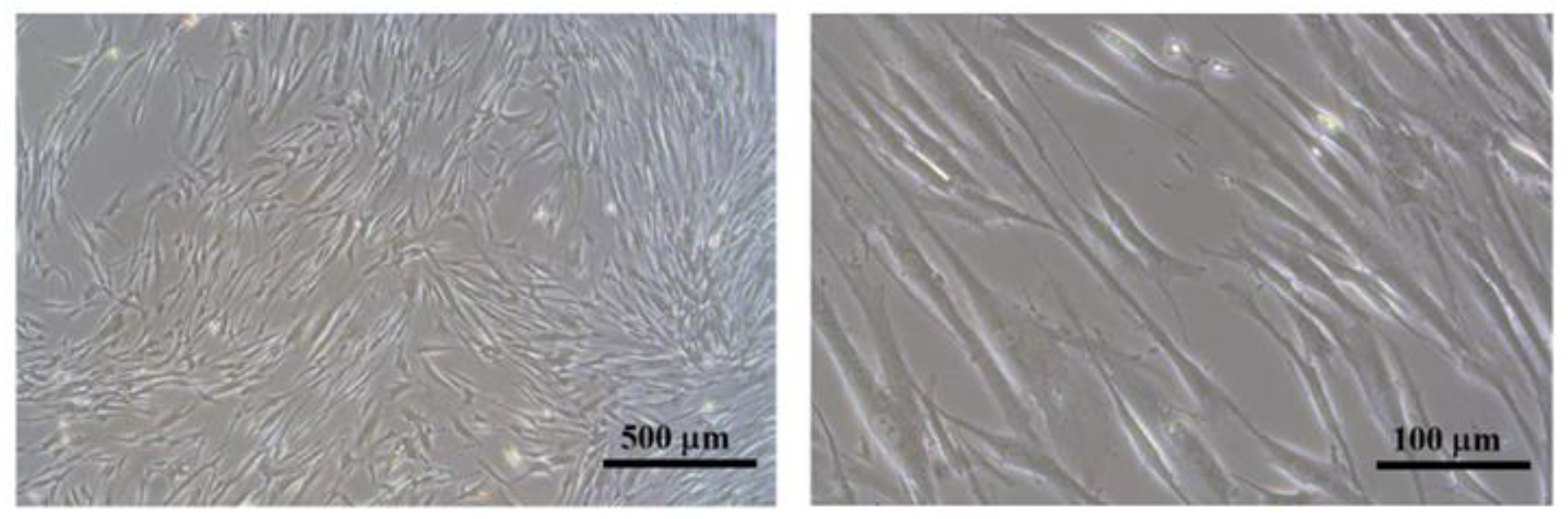
3.2. Cell Bioactivity
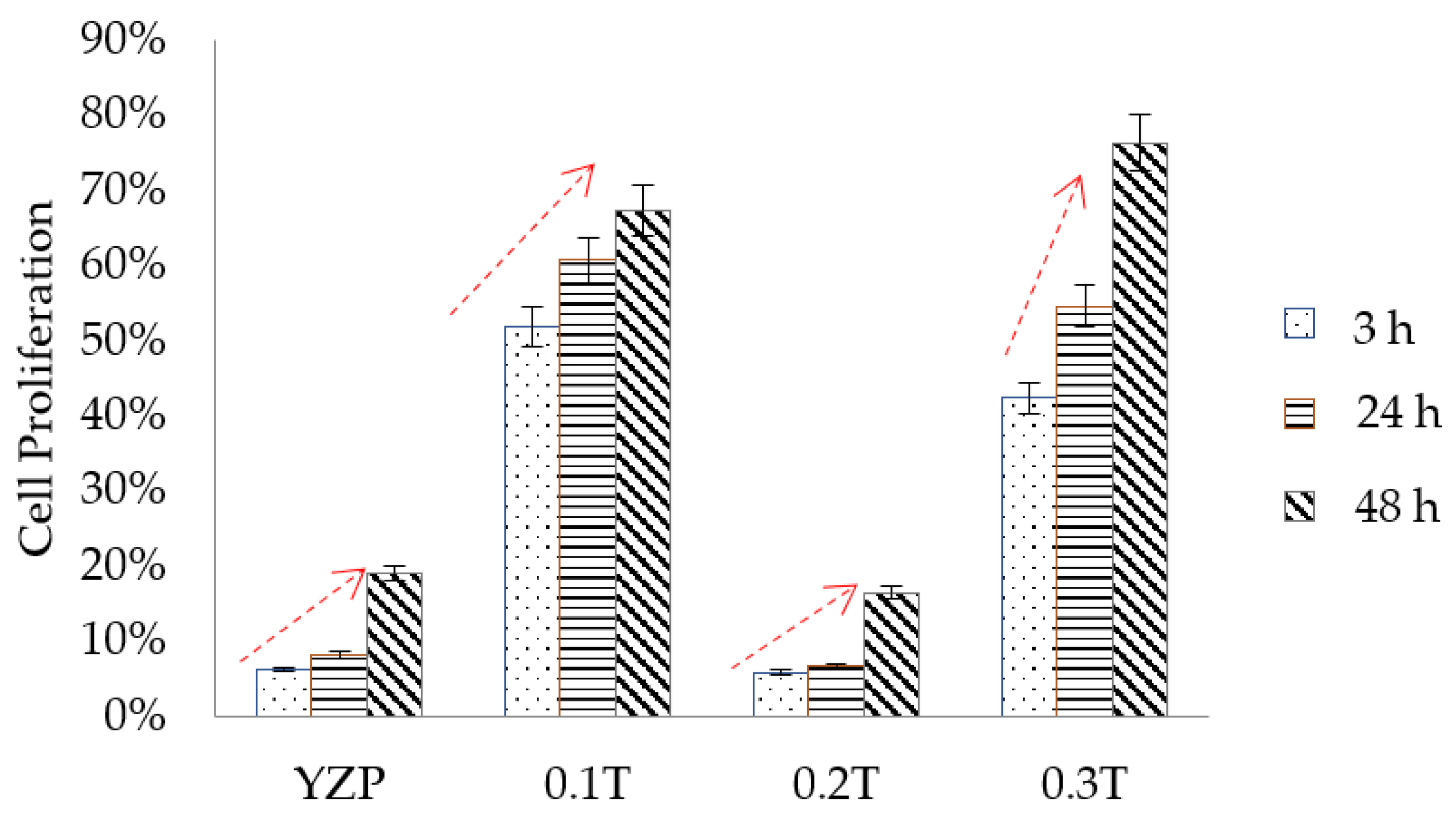
3.3. Cell Proliferation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fathi, A.M.; Ahmed, M.K.; Afifi, M.; Menazea, A.A.; Uskoković, V. Taking hydroxyapatite-coated titanium implants two steps forward: Surface modification using graphene mesolayers and a hydroxyapatite-reinforced polymeric scaffold. ACS Biomater. Sci. Eng. 2021, 7, 360–372. [Google Scholar] [CrossRef]
- Lee, T.M.; Yang, C.Y.; Chang, E.; Tsai, R.S. Comparison of plasma-sprayed hydroxyapatite coatings and zirconia-reinforced hydroxyapatite composite coatings: In vivo study. J. Biomed. Mater. Res. A 2004, 71, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Rapacz-Kmita, A.; Ślósarczyk, A.; Paszkiewicz, Z.; Paluszkiewicz, C. Phase stability of hydroxyapatite–zirconia (HAp–ZrO2) composites for bone replacement. J. Mol. Struct. 2004, 704, 333–340. [Google Scholar] [CrossRef]
- Nowicka, A.; El-Maghraby, H.F.; Švančárková, A.; Galusková, D.; Reveron, H.; Gremillard, L.; Chevalier, J.; Galusek, D. Corrosion and low temperature degradation of 3Y-TZP dental ceramics under acidic conditions. J. Eur. Ceram. Soc. 2020, 40, 6114–6122. [Google Scholar] [CrossRef]
- Afrashtehfar, K.I.; Del Fabbro, M. Clinical performance of zirconia implants: A meta-review. J. Prosthet. Dent. 2020, 123, 419–426. [Google Scholar] [CrossRef]
- Shahramian, K.; Gasik, M.; Kangasniemi, I.; Walboomers, X.F.; Willberg, J.; Abdulmajeed, A.; Närhi, T. Zirconia implants with improved attachment to the gingival tissue. J. Periodontol. 2020, 91, 1213–1224. [Google Scholar] [CrossRef]
- Schünemann, F.H.; Galárraga-Vinueza, M.E.; Magini, R.; Fredel, M.; Silva, F.; Souza, J.C.M.; Zhang, Y.; Henriques, B. Zirconia surface modifications for implant dentistry. Mater. Sci. Eng. C 2019, 98, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Pardun, K.; Treccani, L.; Volkmann, E.; Streckbein, P.; Heiss, C.; Destri, G.L.; Marletta, G.; Rezwan, K. Mixed zirconia calcium phosphate coatings for dental implants: Tailoring coating stability and bioactivity potential. Mater. Sci. Eng. C 2015, 48, 337–346. [Google Scholar] [CrossRef]
- Gouveia, P.F.; Mesquita-Guimarães, J.; Galárraga-Vinueza, M.E.; Souza, J.C.M.; Silva, F.S.; Fredel, M.C.; Boccaccini, A.R.; Detsch, R.; Henriques, B. In-vitro mechanical and biological evaluation of novel zirconia reinforced bioglass scaffolds for bone repair. J. Mech. Behav. Biomed. Mater. 2021, 114, 104164. [Google Scholar] [CrossRef]
- Novaes, A.B., Jr.; de Souza, S.L.; de Barros, R.R.; Pereira, K.K.; Iezzi, G.; Piattelli, A. Influence of implant surfaces on osseointegration. Braz. Dent. J. 2010, 21, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, R.; Shoja-Razavi, R.; Mozafarinia, R.; Jamali, H. Laser glazing of plasma-sprayed nanostructured yttria stabilized zirconia thermal barrier coatings. Ceram. Int. 2013, 39, 9483–9490. [Google Scholar] [CrossRef]
- Ctibor, P.; Boháč, P.; Stranyánek, M.; Čtvrtlík, R. Structure and mechanical properties of plasma sprayed coatings of titania and alumina. J. Eur. Ceram. Soc. 2006, 26, 3509–3514. [Google Scholar] [CrossRef]
- Hung, K.-Y.; Lo, S.-C.; Shih, C.-S.; Yang, Y.-C.; Feng, H.-P.; Lin, Y.-C. Titanium surface modified by hydroxyapatite coating for dental implants. Surf. Coat. Technol. 2013, 231, 337–345. [Google Scholar] [CrossRef]
- Khor, K.A.; Gu, Y.W.; Pan, D.; Cheang, P. Microstructure and mechanical properties of plasma sprayed HA/YSZ/Ti-6Al-4V composite coatings. Biomaterials 2004, 25, 4009–4017. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi-Fadaki, S.A.; Zangeneh-Madar, K.; Valefi, Z. The adhesion strength and indentation toughness of plasma-sprayed yttria stabilized zirconia coatings. Surf. Coat. Technol. 2010, 204, 2136–2141. [Google Scholar] [CrossRef]
- Sennerby, L.; Dasmah, A.; Larsson, B.; Iverhed, M. Bone tissue responses to surface-modified zirconia implants: A histomorphometric and removal torque study in the rabbit. Clin. Implant Dent. Relat. Res. 2005, 7, s13–s20. [Google Scholar] [CrossRef] [PubMed]
- Hadjicharalambous, C.; Buyakov, A.; Buyakova, S.; Kulkov, S.; Chatzinikolaidou, M. Porous alumina, zirconia and alumina/zirconia for bone repair: Fabrication, mechanical and in vitro biological response. Biomed. Mater. 2015, 10, 025012. [Google Scholar] [CrossRef]
- Ding, S.-J.; Lee, T.-L.; Chu, Y.-H. Environmental effect on bond strength of magnetron-sputtered hydroxyapatite/titanium coatings. J. Mater. Sci. Lett. 2003, 22, 479–482. [Google Scholar] [CrossRef]
- Romero, M.; Herrero-Climent, M.; Rios-Carrasco, B.; Brizuela, A.; Romero, M.M.; Gil, J. Investigation of the influence of roughness and dental implant design on primary stability via analysis of insertion torque and implant stability quotient: An in vitro study. J. Clin. Med. 2023, 12, 4190. [Google Scholar] [CrossRef]
- Lugscheider, E.; Knepper, M.; Heimberg, B.; Dekker, A.; Kirkpatrick, C.J. Cytotoxicity investigations of plasma sprayed calcium phosphate coatings. J. Mater. Sci. Mater. Med. 1994, 5, 371–375. [Google Scholar] [CrossRef]
- Shin, H.; Ko, H.; Kim, M. Cytotoxicity and biocompatibility of zirconia (Y-TZP) posts with various dental cements. Restor. Dent. Endod. 2016, 41, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Przekora, A.; Zarnowski, T.; Ginalska, G. A simple and effective protocol for fast isolation of human Tenon’s fibroblasts from a single trabeculectomy biopsy—A comparison of cell behaviour in different culture media. Cell. Mol. Biol. Lett. 2017, 22, 5. [Google Scholar] [CrossRef] [PubMed]
- ATSM F813-83; Standard practice for direct contact cell culture evaluation of materials for medical devices. American Society for Testing and Materials: West Conshohocken, PA, USA, 2020.
- Fu, L.; Khor, K.A.; Lim, J.P. Processing, microstructure and mechanical properties of yttria stabilized zirconia reinforced hydroxyapatite coatings. Mater. Sci. Eng. A 2001, 316, 46–51. [Google Scholar] [CrossRef]
- Chou, B.-Y.; Chang, E. Microstructural characterization of plasma-sprayed hydroxyapatite–10wt% ZrO2 composite coating on titanium. Biomaterials 1999, 20, 1823–1832. [Google Scholar] [CrossRef]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y.; Rajabi, A. Effects of TiO2 on microstructural, mechanical properties and in-vitro bioactivity of plasma sprayed yttria stabilised zirconia coatings for dental application. Ceram. Int. 2018, 44, 4271–4281. [Google Scholar] [CrossRef]
- ASTM E1920-03; Standard Guide for Metallographic Preparation of Thermal Sprayed Coatings. American Society for Testing and Materials: West Conshohocken, PA, USA, 2021.
- ATSM E2109; Standard Test Methods for Determining Area Percentage Porosity in Thermal Sprayed Coatings. American Society for Testing and Materials: West Conshohocken, PA, USA, 2021.
- Keira, S.M.; Ferreira, L.M.; Gragnani, A.; Duarte, I.d.S.; Santos, I.A.N. Experimental model for fibroblast culture. Acta Cirúrgica Bras. 2004, 19 (Suppl. 1), 11–16. [Google Scholar] [CrossRef]
- Si, J.; Zhang, J.; Liu, S.; Zhang, W.; Yu, D.; Wang, X.; Guo, L.; Shen, S.G. Characterization of a micro-roughened TiO2/ZrO2 coating: Mechanical properties and HBMSC responses in vitro. Acta Biochim. Biophys. Sin. 2014, 46, 572–581. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5; Biological Evaluation of Medical Devices–Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: London, UK, 2009.
- Mat-Baharin, N.H.; Razali, M.; Mohd-Said, S.; Syarif, J.; Muchtar, A. Influence of alloying elements on cellular response and in-vitro corrosion behavior of titanium-molybdenum-chromium alloys for implant materials. J. Prosthodont. Res. 2020, 64, 490–497. [Google Scholar] [CrossRef]
- Miao, X.; Sun, D.; Hoo, P.W.; Liu, J.; Hu, Y.; Chen, Y. Effect of titania addition on yttria-stabilised tetragonal zirconia ceramics sintered at high temperatures. Ceram. Int. 2004, 30, 1041–1047. [Google Scholar] [CrossRef]
- Saeidi, M.; Sarpoolaky, H.; Mirkazemi, S.M. Characterization and microstructure investigation of novel ternary ZrO2–Al2O3–TiO2 composites synthesized by citrate–nitrate process. J. Solgel Sci. Technol. 2015, 76, 436–445. [Google Scholar] [CrossRef]
- Lee, S.Y.; Fujioka, T.; Osuga, M.; Nishimura, T.; Suetsugu, S. Lamellipodia and filopodia. In Plasma Membrane Shaping; Suetsugu, S., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 245–263. [Google Scholar]
- Stammitti-Scarpone, A.; Acosta, E.J. Solid-liquid-liquid wettability and its prediction with surface free energy models. Adv. Colloid Interface Sci. 2019, 264, 28–46. [Google Scholar] [CrossRef] [PubMed]
- Rausch, M.A.; Shokoohi-Tabrizi, H.; Wehner, C.; Pippenger, B.E.; Wagner, R.S.; Ulm, C.; Moritz, A.; Chen, J.; Andrukhov, O. Impact of implant surface material and microscale roughness on the initial attachment and proliferation of primary human gingival fibroblasts. Biology 2021, 10, 356. [Google Scholar] [CrossRef]
- Fan, G.; Li, F. In situ crystallization to zinc aluminate films with controlled surface microstructure and anticorrosion performance. AIChE J. 2012, 58, 2639–2649. [Google Scholar] [CrossRef]
- Zhang, F.; Robinson, B.W.; de Villiers-Lovelock, H.; Wood, R.J.K.; Wang, S.C. Wettability of hierarchically-textured ceramic coatings produced by suspension HVOF spraying. J. Mater. Chem. A 2015, 3, 13864–13873. [Google Scholar] [CrossRef]
- Noro, A.; Kaneko, M.; Murata, I.; Yoshinari, M. Influence of surface topography and surface physicochemistry on wettability of zirconia (tetragonal zirconia polycrystal). J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, Y.; Ghazali, M.J.; Juoi, J.M.; Rahim, T.A.; Mustafa, Z. Plasma-sprayed TiO2 coatings: Hydrophobicity enhanced by ZnO additions. Int. J. Appl. Ceram. Technol. 2022, 19, 2213–2221. [Google Scholar] [CrossRef]
- Shanmuganantha, L.; Aslam Khan, M.U.; Sulong, A.B.; Ramli, M.I.; Baharudin, A.; Ariffin, H.M.; Abd Razak, S.I.; Ng, M.H. Characterization of titanium ceramic composite for bone implants applications. Ceram. Int. 2022, 48, 22808–22819. [Google Scholar] [CrossRef]
- Jamhari, F.I.; Foudzi, F.M.; Buhairi, M.A.; Sulong, A.B.; Mohd Radzuan, N.A.; Muhamad, N.; Mohamed, I.F.; Jamadon, N.H.; Tan, K.S. Influence of heat treatment parameters on microstructure and mechanical performance of titanium alloy in LPBF: A brief review. J. Mater. Res. Technol. 2023, 24, 4091–4110. [Google Scholar] [CrossRef]
- Paul, S.; Hanisch, O.; Nesic, D. Human gingival fibroblast proliferation on materials used for dental implant abutments: A systematic review. Int. J. Prosthodont. 2021, 34, 811–828. [Google Scholar] [CrossRef]
- Razali, M.; Ngeow, W.C.; Omar, R.A.; Chai, W.L. An integrated overview of ultraviolet technology for reversing titanium dental implant degradation: Mechanism of reaction and effectivity. Appl. Sci. 2020, 10, 1654. [Google Scholar] [CrossRef]
- Areid, N.; Peltola, A.; Kangasniemi, I.; Ballo, A.; Närhi, T.O. Effect of ultraviolet light treatment on surface hydrophilicity and human gingival fibroblast response on nanostructured titanium surfaces. Clin. Exp. Dent. Res. 2018, 4, 78–85. [Google Scholar] [CrossRef]
- Rohr, N.; Zeller, B.; Matthisson, L.; Fischer, J. Surface structuring of zirconia to increase fibroblast viability. Dent. Mater. 2020, 36, 779–786. [Google Scholar] [CrossRef]
- Narimatsu, I.; Atsuta, I.; Ayukawa, Y.; Oshiro, W.; Yasunami, N.; Furuhashi, A.; Koyano, K. Epithelial and connective tissue sealing around titanium implants with various typical surface finishes. ACS Biomater. Sci. Eng. 2019, 5, 4976–4984. [Google Scholar] [CrossRef] [PubMed]
- Jacquemet, G.; Hamidi, H.; Ivaska, J. Filopodia in cell adhesion, 3D migration and cancer cell invasion. Curr. Opin. Cell Biol. 2015, 36, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, M.; Yamakita, Y.; Kajiyama, H.; Senga, T.; Koya, Y.; Yamashita, M.; Nawa, A.; Kikkawa, F. Filopodia play an important role in the trans-mesothelial migration of ovarian cancer cells. Exp. Cell Res. 2020, 392, 112011. [Google Scholar] [CrossRef] [PubMed]
- Vrchovecká, K.; Kuta, J.; Uher, M.; Přibyl, J.; Pávková Goldbergová, M. Effect of titanium nanostructured surface on fibroblast behavior. J. Biomed. Mater. Res. A 2023, 111, 1333–1343. [Google Scholar] [CrossRef]
- Van den Borre, C.E.; Zigterman, B.G.R.; Mommaerts, M.Y.; Braem, A. How surface coatings on titanium implants affect keratinized tissue: A systematic review. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 1713–1723. [Google Scholar] [CrossRef]
- Bauer, S.; Park, J.; Faltenbacher, J.; Berger, S.; von der Mark, K.; Schmuki, P. Size selective behavior of mesenchymal stem cells on ZrO2 and TiO2 nanotube arrays. Integr. Biol. 2009, 1, 525–532. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, J.; Xu, Y.; Qian, S.; Wang, B.; Liu, F.; Liu, X. Biological behavior of osteoblast-like cells on titania and zirconia films deposited by cathodic arc deposition. Biointerphases 2012, 7, 60. [Google Scholar] [CrossRef]
- Garcia-Gareta, E.; Hua, J.; Knowles, J.C.; Blunn, G.W. Comparison of mesenchymal stem cell proliferation and differentiation between biomimetic and electrochemical coatings on different topographic surfaces. J. Mater. Sci. Mater. Med. 2013, 24, 199–210. [Google Scholar] [CrossRef]


| Parameter | Value |
|---|---|
| Velocity (r.p.m.) | 100 |
| Time for plasma spraying | 3 |
| Resting time in between spraying (min) | 10 |
| Amount of powder (g) | 100 |
| Powder to ethanol ratio | 1:1 |
| Designation | Powder Composition |
|---|---|
| YZP | 100% YZP |
| 0.1T | 90% YZP + 10%TiO2 |
| 0.2T | 80%YZP + 20%TiO2 |
| 0.3T | 70% YZP + 30%TiO2 |
| Properties | YZP | 0.01T | 0.2T | 0.3T |
|---|---|---|---|---|
| Roughness, Ra (μm) | 7.06 ± 0.24 | 6.96 ± 0.78 | 9.12 ± 0.33 | 5.86 ± 0.29 |
| Contact angle (°) | 129.7 | 113.3 | 112.5 | 81.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jemat, A.; Razali, M.; Otsuka, Y.; Ghazali, M.J. Enhancement of Human Gingival Fibroblasts Bioactivity and Proliferation on Plasma Sprayed Yttria-Stabilised Zirconia/TiO2 Surface Coating of Titanium Alloys: An In-Vitro Study. Coatings 2023, 13, 1746. https://doi.org/10.3390/coatings13101746
Jemat A, Razali M, Otsuka Y, Ghazali MJ. Enhancement of Human Gingival Fibroblasts Bioactivity and Proliferation on Plasma Sprayed Yttria-Stabilised Zirconia/TiO2 Surface Coating of Titanium Alloys: An In-Vitro Study. Coatings. 2023; 13(10):1746. https://doi.org/10.3390/coatings13101746
Chicago/Turabian StyleJemat, Afida, Masfueh Razali, Yuichi Otsuka, and Mariyam Jameelah Ghazali. 2023. "Enhancement of Human Gingival Fibroblasts Bioactivity and Proliferation on Plasma Sprayed Yttria-Stabilised Zirconia/TiO2 Surface Coating of Titanium Alloys: An In-Vitro Study" Coatings 13, no. 10: 1746. https://doi.org/10.3390/coatings13101746
APA StyleJemat, A., Razali, M., Otsuka, Y., & Ghazali, M. J. (2023). Enhancement of Human Gingival Fibroblasts Bioactivity and Proliferation on Plasma Sprayed Yttria-Stabilised Zirconia/TiO2 Surface Coating of Titanium Alloys: An In-Vitro Study. Coatings, 13(10), 1746. https://doi.org/10.3390/coatings13101746







