Design and Fabrication of Nanofiber-Coated Antenna with Electrospun Polyacrylonitrile (PAN) for Tissue Cancer Ablation
Abstract
:1. Introduction
2. Antenna Design
3. Experimental and Simulation
3.1. Modeling and Simulation
3.2. Antenna Fabrication
3.2.1. Electroplating Process
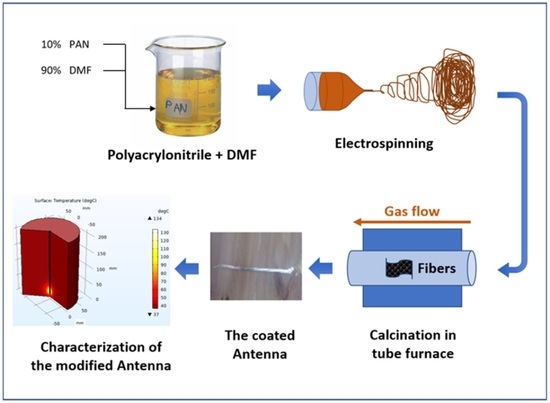
3.2.2. Electrospinning Process
3.2.3. Calcination Process
3.2.4. Inner Conductor Centering
4. Results and Discussion
4.1. Nanofiber Coating Characterization
4.2. Simulation Results
4.3. Testing the Proposed and Fabricated Antenna
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bertram, J.M.; Yang, D.; Converse, M.C.; Webster, J.G.; Mahvi, D.M. A Review of Coaxial-Based Interstitial Antennas for Hepatic Microwave Ablation. Crit. Rev. Biomed. Eng. 2006, 34, 187–213. [Google Scholar] [CrossRef] [PubMed]
- Livraghi, T.; Meloni, F.; Di Stasi, M.; Rolle, E.; Solbiati, L.; Tinelli, C.; Rossi, S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology 2008, 47, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Meloni, M.F.; Chiang, J.; Laeseke, P.F.; Dietrich, C.F.; Sannino, A.; Solbiati, M.; Nocerino, E.; Brace, C.L.; Lee, F.T. Microwave ablation in primary and secondary liver tumours: Technical and clinical approaches. Int. J. Hyperth. 2017, 33, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.J.; Dupuy, D.E.; Mayo-Smith, W.W. Microwave ablation: Principles and applications. Radiographics 2005, 25 (Suppl. S1), S69–S83. [Google Scholar] [CrossRef]
- Facciorusso, A.; El Aziz, M.A.A.; Tartaglia, N.; Ramai, D.; Mohan, B.P.; Cotsoglou, C.; Pusceddu, S.; Giacomelli, L.; Ambrosi, A.; Sacco, R. Microwave Ablation Versus Radiofrequency Ablation for Treatment of Hepatocellular Carcinoma: A Meta-Analysis of Randomized Controlled Trials. Cancers 2020, 12, 3796. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, A.O.; Nabeel, M.M.; Elbaz, T.M.; Shousha, H.I.; Hassan, E.M.; Mahmoud, S.H.; Rashed, N.A.; Ibrahim, M.M.; Abdelmaksoud, A.H. Microwave ablation versus transarterial chemoembolization in large hepatocellular carcinoma: Prospective analysis. Scand. J. Gastroenterol. 2015, 50, 479–484. [Google Scholar] [CrossRef]
- Poggi, G.; Tosoratti, N.; Montagna, B.; Picchi, C. Microwave ablation of hepatocellular carcinoma. World J. Hepatol. 2015, 7, 2578. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, H.; Nagasawa, T.; Fujiwara, Y.; Sato, H.; Abe, T.; Kooka, Y.; Endo, K.; Oikawa, T.; Sawara, K.; Takikawa, Y. Comparing the Safety and Efficacy of Microwave Ablation Using ThermosphereTM Technology versus Radiofrequency Ablation for Hepatocellular Carcinoma: A Propensity Score-Matched Analysis. Cancers 2021, 13, 1295. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Brace, C.L.; Lee, F.T.; Goldberg, S.N. Principles of and advances in percutaneous ablation. Radiology 2011, 258, 351–369. [Google Scholar] [CrossRef]
- NoroozOliaei, M.; Riazi Esfahani, H.; Abrishamian, M.S. Graphene coated dielectric resonator antenna for modeling the photoreceptors at visible spectrum. Heliyon 2022, 8, e09611. [Google Scholar] [CrossRef]
- Moghadasi, M.N.; Sadeghzadeh, R.A.; Toolabi, M.; Jahangiri, P.; Zarrabi, F.B. Fractal cross aperture nano-antenna with graphene coat for bio-sensing application. Microelectron. Eng. 2016, 162, 1–5. [Google Scholar] [CrossRef]
- Zarrabi, F.B.; Naser-Moghadasi, M.; Heydari, S.; Maleki, M.; Arezomand, A.S. Cross-slot nano-antenna with graphene coat for bio-sensing application. Opt. Commun. 2016, 371, 34–39. [Google Scholar] [CrossRef]
- Radjenović, B.; Sabo, M.; Šoltes, L.; Prnova, M.; Čičak, P.; Radmilović-Radjenović, M. On Efficacy of Microwave Ablation in the Thermal Treatment of an Early-Stage Hepatocellular Carcinoma. Cancers 2021, 13, 5784. [Google Scholar] [CrossRef] [PubMed]
- Farina, L.; Ruvio, G.; Shatwan, R.; Shalaby, A.; O’Halloran, M.; White, A.; Soo, A.; Breen, D.; Lowery, A.; Quinn, A.M. Histology-Validated Dielectric Characterisation of Lung Carcinoma Tissue for Microwave Thermal Ablation Applications. Cancers 2023, 15, 3738. [Google Scholar] [CrossRef] [PubMed]
- Paré, J.R.J.; Bélanger, J.M.R.; Cormier, G.; Foucher, D.; Thériault, A.; Savoie, J.C.; Rochas, J.F. Microwave-Assisted Chemical Ablation (MA-CA): A Novel Microwave-Assisted Tissue Ablation Procedure—Preliminary Assessment of Efficiency. Appl. Sci. 2023, 13, 7177. [Google Scholar] [CrossRef]
- Heat Transfer Modeling Software for Analyzing Thermal Effects. Available online: https://www.comsol.com/heat-transfer-module (accessed on 28 September 2023).
- COMSOL—Software for Multiphysics Simulation. Available online: https://www.comsol.com/ (accessed on 28 September 2023).
- Al-Gburi, A.; Zakaria, Z.; Wang, L. Microwave Imaging and Sensing Techniques for Breast Cancer Detection. Micromachines 2023, 14, 1462. [Google Scholar] [CrossRef]
- García Lampérez, A.; Hikmet, M.; Ucar, B.; Uras, E. A Compact Modified Two-Arm Rectangular Spiral Implantable Antenna Design for ISM Band Biosensing Applications. Sensors 2023, 23, 4883. [Google Scholar] [CrossRef]
- Yang, D.; Converse, M.C.; Mahvi, D.M.; Webster, J.G. Expanding the bioheat equation to include tissue internal water evaporation during heating. IEEE Trans. Biomed. Eng. 2007, 54, 1382–1388. [Google Scholar] [CrossRef]
- Prakash, P. Theoretical Modeling for Hepatic Microwave Ablation. Open Biomed. Eng. J. 2010, 4, 27. [Google Scholar] [CrossRef]
- Cavagnaro, M.; Pinto, R.; Lopresto, V. Numerical models to evaluate the temperature increase induced by ex vivo microwave thermal ablation. Phys. Med. Biol. 2015, 60, 3287–3311. [Google Scholar] [CrossRef] [PubMed]
- Stauffer, P.R.; Rossetto, F.; Prakash, M.; Neuman, D.G.; Lee, T. Phantom and animal tissues for modelling the electrical properties of human liver. Int. J. Hyperth. 2003, 19, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, J.H.; Yu, J.Y.; Zeng, H.M. Controlling numbers and sizes of beads in electrospun nanofibers. Polym. Int. 2008, 57, 632–636. [Google Scholar] [CrossRef]
- Kidoaki, S.; Kwon, I.K.; Matsuda, T. Mesoscopic spatial designs of nano- and microfiber meshes for tissue-engineering matrix and scaffold based on newly devised multilayering and mixing electrospinning techniques. Biomaterials 2005, 26, 37–46. [Google Scholar] [CrossRef]
- Stankus, J.J.; Guan, J.; Fujimoto, K.; Wagner, W.R. Microintegrating smooth muscle cells into a biodegradable, elastomeric fiber matrix. Biomaterials 2006, 27, 735–744. [Google Scholar] [CrossRef]
- Nilmoung, S.; Sinprachim, T.; Kotutha, I.; Kidkhunthod, P.; Yimnirun, R.; Rujirawat, S.; Maensiri, S. Electrospun carbon/CuFe2O4 composite nanofibers with improved electrochemical energy storage performance. J. Alloys Compd. 2016, 688, 1131–1140. [Google Scholar] [CrossRef]
- Thamer, B.M.; Al-aizari, F.A.; Abdo, H.S. Boosting anionic dyes removal performance of polyacrylonitrile nanofibers by incorporating nitrogen-rich conjugated polymer. Colloids Surf. A Physicochem. Eng. Asp. 2023, 677, 132361. [Google Scholar] [CrossRef]
- Aijaz, M.O.; Karim, M.R.; Alharbi, H.F.; Alharthi, N.H.; Al-Mubaddel, F.S.; Abdo, H.S. Magnetic/Polyetherimide-Acrylonitrile Composite Nanofibers for Nickel Ion Removal from Aqueous Solution. Membranes 2021, 11, 50. [Google Scholar] [CrossRef]
- Abdo, H.S.; Samad, U.A.; Abdo, M.S.; Alkhammash, H.I.; Aijaz, M.O. Electrochemical Behavior of Inductively Sintered Al/TiO2 Nanocomposites Reinforced by Electrospun Ceramic Nanofibers. Polymers 2021, 13, 4319. [Google Scholar] [CrossRef] [PubMed]
- Pech, O.; Maensiri, S. Effect of Calcining Temperature on Electrospun Carbon Nanofibers for Supercapacitor. J. Mater. Eng. Perform. 2020, 29, 2386–2394. [Google Scholar] [CrossRef]
- Izzo, F.; Granata, V.; Grassi, R.; Fusco, R.; Palaia, R.; Delrio, P.; Carrafiello, G.; Azoulay, D.; Petrillo, A.; Curley, S.A. Radiofrequency Ablation and Microwave Ablation in Liver Tumors: An Update. Oncologist 2019, 24, e990. [Google Scholar] [CrossRef]











| Symbol Name | Method of Entering | Value | Physical Property |
|---|---|---|---|
| Blood Density (Rho) | 1 × 103 [kg/m3] | 1000 kg/m3 | Blood Density |
| Specific heat (C) | 3639 [J/(kg·K)] | 3639 J/(kg·K) | Specific heat of blood |
| Omega blood | 3.6 × 10−3 [1/s] | 0.0036 1/s | Perfusion rate of blood |
| Blood Temperature | 37 [°C] | 310.15 K | Blood Temperature |
| Epsilon of liver | 43.03 | 43.03 | Relative permittivity of liver |
| Sigma liver | 1.69 [S/m] | 1.69 S/m | Electric conductivity of liver |
| Epsilon of dielectric | 3 | 3 | Relative permittivity of dielectric |
| Epsilon of catheter | 2.6 | 2.6 | Relative permittivity of catheter |
| Microwave frequency (F) | 2.45 [GHz] | 2.45 × 109 Hz | Microwave frequency |
| Input power (P) | 20 [W] | 20 W | Input microwave power |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdo, M.S.; Maher, A.; Fouly, A.; Almotairy, S.M.; Shar, M.A.; Abdo, H.S. Design and Fabrication of Nanofiber-Coated Antenna with Electrospun Polyacrylonitrile (PAN) for Tissue Cancer Ablation. Coatings 2023, 13, 1767. https://doi.org/10.3390/coatings13101767
Abdo MS, Maher A, Fouly A, Almotairy SM, Shar MA, Abdo HS. Design and Fabrication of Nanofiber-Coated Antenna with Electrospun Polyacrylonitrile (PAN) for Tissue Cancer Ablation. Coatings. 2023; 13(10):1767. https://doi.org/10.3390/coatings13101767
Chicago/Turabian StyleAbdo, Mohamed S., Ashraf Maher, Ahmed Fouly, Saud M. Almotairy, Muhammad A. Shar, and Hany S. Abdo. 2023. "Design and Fabrication of Nanofiber-Coated Antenna with Electrospun Polyacrylonitrile (PAN) for Tissue Cancer Ablation" Coatings 13, no. 10: 1767. https://doi.org/10.3390/coatings13101767
APA StyleAbdo, M. S., Maher, A., Fouly, A., Almotairy, S. M., Shar, M. A., & Abdo, H. S. (2023). Design and Fabrication of Nanofiber-Coated Antenna with Electrospun Polyacrylonitrile (PAN) for Tissue Cancer Ablation. Coatings, 13(10), 1767. https://doi.org/10.3390/coatings13101767