Recent Advances in Surface Functionalization of Magnetic Nanoparticles
Abstract
:1. Introduction
2. Synthesis of Magnetic Nanoparticles
3. Characterization Techniques
4. Surface Functionalization Strategies
5. Surface Functionalization for Drug Delivery
6. Challenges and Future Perspectives
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, K.Y.; Zhang, L.L.; Bao, G. Magnetic iron oxide nanoparticles for biomedical applications. Curr. Opin. Biomed. Eng. 2021, 20, 100330. [Google Scholar] [CrossRef] [PubMed]
- Shankar, M.; Kesavan, S.S.; Biswas, K. Exploring the Potentials of Magnetic Nanoscale Material for Different Biomedical Applications: A Review. Bionanoscience 2023. [Google Scholar] [CrossRef]
- Matveeva, V.G.; Bronstein, L.M. Magnetic Nanoparticle-Containing Supports as Carriers of Immobilized Enzymes: Key Factors Influencing the Biocatalyst Performance. Nanomaterials 2021, 11, 2257. [Google Scholar] [CrossRef] [PubMed]
- Palade, P.; Comanescu, C.; Radu, C. Synthesis of Nickel and Cobalt Ferrite-Doped Graphene as Efficient Catalysts for Improving the Hydrogen Storage Kinetics of Lithium Borohydride. Materials 2023, 16, 427. [Google Scholar] [CrossRef]
- Mehak; Thummer, R.P.; Pandey, L.M. Surface modified iron-oxide based engineered nanomaterials for hyperthermia therapy of cancer cells. Biotechnol. Genet. 2023. [Google Scholar] [CrossRef]
- Pryazhnikov, D.V.; Kubrakova, I.V. Surface-Modified Magnetic Nanoscale Materials: Preparation and Study of Their Structure, Composition, and Properties. J. Anal. Chem. 2021, 76, 685–706. [Google Scholar] [CrossRef]
- Ansari, M.J.; Kadhim, M.M.; Hussein, B.A.; Lafta, H.A.; Kianfar, E. Synthesis and Stability of Magnetic Nanoparticles. Bionanoscience 2022, 12, 627–638. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C.L. Recent Advances in Functionalized Nanoparticles in Cancer Theranostics. Nanomaterials 2022, 12, 2826. [Google Scholar] [CrossRef]
- Comanescu, C. Magnetic Nanoparticles: Current Advances in Nanomedicine, Drug Delivery and MRI. Chemistry 2022, 4, 872–930. [Google Scholar] [CrossRef]
- Senthilkumar, N.; Sharma, P.K.; Sood, N.; Bhalla, N. Designing magnetic nanoparticles for in vivo applications and understanding their fate inside human body. Coordin. Chem. Rev. 2021, 445, 214082. [Google Scholar] [CrossRef]
- Liu, Y.K.; Su, G.M.Y.; Zhang, R.Y.; Dai, R.J.; Li, Z. Nanomaterials-Functionalized Hydrogels for the Treatment of Cutaneous Wounds. Int. J. Mol. Sci. 2023, 24, 336. [Google Scholar] [CrossRef] [PubMed]
- Gambhir, R.P.; Rohiwal, S.S.; Tiwari, A.P. Multifunctional surface functionalized magnetic iron oxide nanoparticles for biomedical applications: A review. Appl. Surf. Sci. Adv. 2022, 11, 100303. [Google Scholar] [CrossRef]
- Gwon, H.; Park, S.; Lu, Q.; Choi, H.J.; Lee, S. Size effect of iron oxide nanorods with controlled aspect ratio on magneto-responsive behavior. J. Ind. Eng. Chem. 2023, 124, 279–286. [Google Scholar] [CrossRef]
- Jimenez-Carretero, M.; Rodriguez-Lopez, J.; Ropero-Moreno, C.; Granada, J.; Delgado-Martin, J.; Martinez-Bueno, M.; Fernandez-Vivas, A.; Jimenez-Lopez, C. Biomimetic magnetic nanoparticles for bacterial magnetic concentration in liquids and qPCR-detection. Food Control 2023, 147, 109623. [Google Scholar] [CrossRef]
- Franzel, L.; Bertino, M.F.; Huba, Z.J.; Carpenter, E.E. Synthesis of magnetic nanoparticles by pulsed laser ablation. Appl. Surf. Sci. 2012, 261, 332–336. [Google Scholar] [CrossRef]
- Liu, J.; Su, D.; Wu, K.; Wang, J.P. High-moment magnetic nanoparticles. J. Nanopart. Res. 2020, 22, 66. [Google Scholar] [CrossRef]
- Carvallo, C.; Fondet, A.; Le Fèvre, R.; Taverna, D.; Guyodo, Y.; Chebbi, I.; Dupuis, V.; Lagroix, F.; Khelfallah, M.; Guigner, J.-M.; et al. Magnetic and structural properties of biogenic magnetic nanoparticles along their production process for use in magnetic hyperthermia. J. Magn. Magn. Mater. 2023, 575, 170726. [Google Scholar] [CrossRef]
- Iacob, N.; Kuncser, A.; Comanescu, C.; Palade, P.; Kuncser, V. Optimization of magnetic fluid hyperthermia with respect to nanoparticle shape-related parameters: Case of magnetite ellipsoidal nanoparticles. J. Nanopart. Res. 2020, 22, 138. [Google Scholar] [CrossRef]
- Pedroso-Santana, S.; Fleitas-Salazar, N. The Use of Capping Agents in the Stabilization and Functionalization of Metallic Nanoparticles for Biomedical Applications. Part. Part. Syst. Charact. 2023, 40, 2200146. [Google Scholar] [CrossRef]
- Lu, A.H.; Salabas, E.E.; Schüth, F. Magnetic Nanoparticles: Synthesis, Protection, Functionalization, and Application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef]
- Majidi, S.; Zeinali, F.; Samad, S.; Farkhani, M.; Soleymani, M.; Akbarzadeh, A. Current methods for synthesis of magnetic nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Gareev, K.; Tagaeva, R.; Bobkov, D.; Yudintceva, N.; Goncharova, D.; Combs, S.E.; Ten, A.; Samochernych, K.; Shevtsov, M. Passing of Nanocarriers across the Histohematic Barriers: Current Approaches for Tumor Theranostics. Nanomaterials 2023, 13, 1140. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.S.; Chen, Y.; Wu, M.Y. Biomimetic nanomedicine toward personalized disease theranostics. Nano Res. 2021, 14, 2491–2511. [Google Scholar] [CrossRef]
- Palade, P.; Comanescu, C.; Kuncser, A.; Berger, D.; Matei, C.; Iacob, N.; Kuncser, V. Mesoporous Cobalt Ferrite Nanosystems Obtained by Surfactant-Assisted Hydrothermal Method: Tuning Morpho-structural and Magnetic Properties via pH-Variation. Nanomaterials 2020, 10, 476. [Google Scholar] [CrossRef] [PubMed]
- Aslam, H.; Shukrullah, S.; Naz, M.Y.; Fatima, H.; Hussain, H.; Ullah, S.; Assiri, M.A. Current and future perspectives of multifunctional magnetic nanoparticles based controlled drug delivery systems. J. Drug Deliv. Sci. Technol. 2022, 67, 102946. [Google Scholar] [CrossRef]
- Hur, J.U.; Shin, J.R.; Han, J.S.; Kim, Y.H.; An, G.S. Self-assembled core-shell Fe3O4-Pt nanoparticles via silylation/polymerization-based amino-functionalization. Colloid Interfac. Sci. 2022, 50, 100655. [Google Scholar] [CrossRef]
- Low, L.E.; Lim, H.P.; Ong, Y.S.; Siva, S.P.; Sia, C.S.; Goh, B.H.; Chan, E.S.; Tey, B.T. Stimuli-controllable iron oxide nanoparticle assemblies: Design, manipulation and bio-applications. J. Control Release 2022, 345, 231–274. [Google Scholar] [CrossRef]
- Strassburg, S.; Mayer, K.; Scheibel, T. Functionalization of biopolymer fibers with magnetic nanoparticles. Phys. Sci. Rev. 2022, 7, 1091–1117. [Google Scholar] [CrossRef]
- Darroudi, M.; Gholami, M.; Rezayi, M.; Khazaei, M. An overview and bibliometric analysis on the colorectal cancer therapy by magnetic functionalized nanoparticles for the responsive and targeted drug delivery. J. Nanobiotechnol. 2021, 19, 399. [Google Scholar] [CrossRef]
- Mahajan, R.; Suriyanarayanan, S.; Nicholls, I.A. Improved Solvothermal Synthesis of gamma-Fe2O3 Magnetic Nanoparticles for SiO2 Coating. Nanomaterials 2021, 11, 1889. [Google Scholar] [CrossRef]
- Elahi, N.; Rizwan, M. Progress and prospects of magnetic iron oxide nanoparticles in biomedical applications: A review. Artif. Organs 2021, 45, 1272–1299. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Faria, I.; Yousefiasl, S.; Macario-Soares, A.; Pereira-Silva, M.; Peixoto, D.; Zafar, H.; Raza, F.; Faneca, H.; Veiga, F.; Hamblin, M.R.; et al. Stem cell membrane-coated abiotic nanomaterials for biomedical applications. J. Control Release 2022, 351, 174–197. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Jana, N.R. Biomedical Applications of Functional Polyaspartamide-Based Materials. ACS Appl. Polym. Mater. 2021, 3, 4791–4811. [Google Scholar] [CrossRef]
- Klekotka, U.; Zambrzycka-Szelewa, E.; Satula, D.; Kalska-Szostko, B. Stability Studies of Magnetite Nanoparticles in Environmental Solutions. Materials 2021, 14, 5069. [Google Scholar] [CrossRef] [PubMed]
- Zakhireh, S.; Barar, J.; Adibkia, K.; Beygi-Khosrowshahi, Y.; Fathi, M.; Omidain, H.; Omidi, Y. Bioactive Chitosan-Based Organometallic Scaffolds for Tissue Engineering and Regeneration. Topics Curr. Chem. 2022, 380, 13. [Google Scholar] [CrossRef] [PubMed]
- Kwizera, E.A.; Stewart, S.; Mahmud, M.M.; He, X.M. Magnetic Nanoparticle-Mediated Heating for Biomedical Applications. J. Heat Trans.-T Asme 2022, 144, 030801. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Rotherham, M.; Farrow, N.; Roach, P.; Dobson, J.; El Haj, A.J. Immobilization of Wnt Fragment Peptides on Magnetic Nanoparticles or Synthetic Surfaces Regulate Wnt Signaling Kinetics. Int. J. Mol. Sci. 2022, 23, 10164. [Google Scholar] [CrossRef]
- Mohapatra, A.; Uthaman, S.; Park, I.K. External and Internal Stimuli-Responsive Metallic Nanotherapeutics for Enhanced Anticancer Therapy. Front. Mol. Biosci. 2021, 7, 597634. [Google Scholar] [CrossRef]
- Kothandaraman, H.; Kaliyamoorthy, A.; Rajaram, A.; Kalaiselvan, C.R.; Sahu, N.K.; Govindasamy, P.; Rajaram, M. Functionalization and Haemolytic analysis of pure superparamagnetic magnetite nanoparticle for hyperthermia application. J. Biol. Phys. 2022, 48, 383–397. [Google Scholar] [CrossRef]
- Duong, H.D.T.; Yoon, S.H.; Nguyen, D.T.; Kim, K.S. Magnetic heating of water dispersible and size-controlled superparamagnetic cobalt iron oxide nanoparticles. Powder Technol. 2023, 427, 118720. [Google Scholar] [CrossRef]
- Ghosal, K.; Chatterjee, S.; Thomas, S.; Roy, P. A Detailed Review on Synthesis, Functionalization, Application, Challenges, and Current Status of Magnetic Nanoparticles in the Field of Drug Delivery and Gene Delivery System. AAPS PharmSciTech 2022, 24, 25. [Google Scholar] [CrossRef]
- Arias-Ramos, N.; Ibarra, L.E.; Serrano-Torres, M.; Yague, B.; Caverzan, M.D.; Chesta, C.A.; Palacios, R.E.; Lopez-Larrubia, P. Iron Oxide Incorporated Conjugated Polymer Nanoparticles for Simultaneous Use in Magnetic Resonance and Fluorescent Imaging of Brain Tumors. Pharmaceutics 2021, 13, 1258. [Google Scholar] [CrossRef]
- Avarand, S.; Morsali, A.; Heravi, M.M.; Beyramabadi, S.A. A quantum chemical study on the magnetic nanocarrier-tirapazamine drug delivery system. Nanosyst.-Phys. Chem. M 2021, 12, 167–174. [Google Scholar] [CrossRef]
- Beagan, A.M.; Alghamdi, A.A.; Lahmadi, S.S.; Halwani, M.A.; Almeataq, M.S.; Alhazaa, A.N.; Alotaibi, K.M.; Alswieleh, A.M. Folic Acid-Terminated Poly(2-Diethyl Amino Ethyl Methacrylate) Brush-Gated Magnetic Mesoporous Nanoparticles as a Smart Drug Delivery System. Polymers 2021, 13, 59. [Google Scholar] [CrossRef]
- Valls-Chivas, A.; Gomez, J.; Garcia-Peiro, J.I.; Hornos, F.; Hueso, J.L. Enzyme-Iron Oxide Nanoassemblies: A Review of Immobilization and Biocatalytic Applications. Catalysts 2023, 13, 980. [Google Scholar] [CrossRef]
- Belleti, E.; Bevilaqua, V.R.; Brito, A.M.M.; Modesto, D.A.; Lanfredi, A.J.C.; Viviani, V.R.; Nantes-Cardoso, I.L. Synthesis of bioluminescent gold nanoparticle-luciferase hybrid systems for technological applications. Photoch. Photobio Sci. 2021, 20, 1439–1453. [Google Scholar] [CrossRef] [PubMed]
- Boosz, P.; Pfister, F.; Stein, R.; Friedrich, B.; Fester, L.; Band, J.; Muhlberger, M.; Schreiber, E.; Lyer, S.; Dudziak, D.; et al. Citrate-Coated Superparamagnetic Iron Oxide Nanoparticles Enable a Stable Non-Spilling Loading of T Cells and Their Magnetic Accumulation. Cancers 2021, 13, 4143. [Google Scholar] [CrossRef]
- Cheah, P.; Qu, J.; Li, Y.; Cao, D.M.; Zhu, X.C.; Zhao, Y.F. The key role of reaction temperature on a polyol synthesis of water-dispersible iron oxide nanoparticles. J. Magn. Magn. Mater. 2021, 540, 168481. [Google Scholar] [CrossRef]
- Correa, T.; Bazylinski, D.A.; Garcia, F.; Abreu, F. A rapid and simple preparation of amphotericin B-loaded bacterial magnetite nanoparticles. RSC Adv. 2021, 11, 28000–28007. [Google Scholar] [CrossRef] [PubMed]
- Dhavale, R.P.; Dhavale, R.P.; Sahoo, S.C.; Kollu, P.; Jadhav, S.U.; Patil, P.S.; Dongale, T.D.; Chougale, A.D.; Patil, P.B. Chitosan coated magnetic nanoparticles as carriers of anticancer drug Telmisartan: pH-responsive controlled drug release and cytotoxicity studies. J. Phys. Chem. Solids 2021, 148, 109749. [Google Scholar] [CrossRef]
- Domagalski, J.T.; Xifre-Perez, E.; Tabrizi, M.A.; Ferre-Borrull, J.; Marsal, L.F. Magnetic nanoparticle decorated anodic alumina nanotubes for fluorescent detection of cathepsin B. J. Colloid Interf. Sci. 2021, 584, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Doswald, S.; Stark, W.J. Preparation of Functionalized Carbon-Coated Cobalt Nanoparticles with Sulfonated Arene Derivatives, a Study on Surface Functionalization and Stability. Chem.-Eur. J. 2021, 27, 4108–4114. [Google Scholar] [CrossRef] [PubMed]
- Ehsanimehr, S.; Moghadam, P.N.; Dehaen, W.; Shafiei-Irannejad, V. PEI grafted Fe3O4@SiO2@SBA-15 labeled FA as a pH-sensitive mesoporous magnetic and biocompatible nanocarrier for targeted delivery of doxorubicin to MCF-7 cell line. Colloid Surface A 2021, 615, 126302. [Google Scholar] [CrossRef]
- Fan, X.M.; Shen, J.J.; Xu, Y.Y.; Gao, J.; Zhang, Y.W. Metabolic integration of azide functionalized glycan on Escherichia coli cell surface for specific covalent immobilization onto magnetic nanoparticles with click chemistry. Bioresour. Technol. 2021, 324, 124689. [Google Scholar] [CrossRef]
- Fernandez-Ponce, C.; Manuel, J.M.; Fernandez-Cisnal, R.; Felix, E.; Beato-Lopez, J.; Munoz-Miranda, J.P.; Beltran, A.M.; Santos, A.J.; Morales, F.M.; Yeste, M.P.; et al. Superficial Characteristics and Functionalization Effectiveness of Non-Toxic Glutathione-Capped Magnetic, Fluorescent, Metallic and Hybrid Nanoparticles for Biomedical Applications. Metals 2021, 11, 383. [Google Scholar] [CrossRef]
- Habra, K.; McArdle, S.E.B.; Morris, R.H.; Cave, G.W.V. Synthesis and Functionalisation of Superparamagnetic Nano-Rods towards the Treatment of Glioblastoma Brain Tumours. Nanomaterials 2021, 11, 2157. [Google Scholar] [CrossRef]
- Jose, R.; Rinita, J.; Jothi, N.S.N. The synthesis and characterisation of curcumin loaded Ag ((1-X)) Ni (X) Fe-2 O-4 for drug delivery. Mater. Technol. 2021, 36, 339–346. [Google Scholar] [CrossRef]
- Kannan, K.; Mukherjee, J.; Mishra, P.; Gupta, M.N. Nickel Ferrite Nanoparticles as an Adsorbent for Immobilized Metal Affinity Chromatography of Proteins. J. Chromatogr. Sci. 2021, 59, 262–268. [Google Scholar] [CrossRef]
- Leitner, N.S.; Schroffenegger, M.; Reimhult, E. Polymer Brush-Grafted Nanoparticles Preferentially Interact with Opsonins and Albumin. ACS Appl. Bio Mater. 2021, 4, 795–806. [Google Scholar] [CrossRef]
- Martin, D.S.; Oropesa-Nunez, R.; de la Torre, T.Z.G. Evaluating the Performance of a Magnetic Nanoparticle-Based Detection Method Using Circle-to-Circle Amplification. Biosensors 2021, 11, 173. [Google Scholar] [CrossRef]
- Moskvin, M.; Huntosova, V.; Herynek, V.; Matous, P.; Michalcova, A.; Lobaz, V.; Zasonska, B.; Slouf, M.; Seliga, R.; Horak, D. In vitro cellular activity of maghemite/cerium oxide magnetic nanoparticles with antioxidant properties. Colloid Surface B 2021, 204, 111824. [Google Scholar] [CrossRef] [PubMed]
- Nayeem, J.; Al-Bari, M.A.A.; Mahiuddin, M.; Rahman, M.A.; Mefford, O.T.; Ahmad, H.; Rahman, M.M. Silica coating of iron oxide magnetic nanoparticles by reverse microemulsion method and their functionalization with cationic polymer P (NIPAm-co-AMPTMA) for antibacterial vancomycin immobilization. Colloid Surface A 2021, 611, 125857. [Google Scholar] [CrossRef]
- Reyes-Ortega, F.; Delgado, A.V.; Iglesias, G.R. Modulation of the Magnetic Hyperthermia Response Using Different Superparamagnetic Iron Oxide Nanoparticle Morphologies. Nanomaterials 2021, 11, 627. [Google Scholar] [CrossRef]
- Rezaei, A.; Morsali, A.; Bozorgmehr, M.R.; Nasrabadi, M. Quantum chemical analysis of 5-aminolevulinic acid anticancer drug delivery systems: Carbon nanotube, -COOH functionalized carbon nanotube and iron oxide nanoparticle. J. Mol. Liq. 2021, 340, 117182. [Google Scholar] [CrossRef]
- Veloso, S.R.S.; Silva, J.F.G.; Hilliou, L.; Moura, C.; Coutinho, P.J.G.; Martins, J.A.; Testa-Anta, M.; Salgueirino, V.; Correa-Duarte, M.A.; Ferreira, P.M.T.; et al. Impact of Citrate and Lipid-Functionalized Magnetic Nanoparticles in Dehydropeptide Supramolecular Magnetogels: Properties, Design and Drug Release. Nanomaterials 2021, 11, 16. [Google Scholar] [CrossRef]
- Wu, K.; Liu, J.M.; Saha, R.; Ma, B.; Su, D.Q.; Chugh, V.K.; Wang, J.P. Stable and Monodisperse Iron Nitride Nanoparticle Suspension for Magnetic Diagnosis and Treatment: Development of Synthesis and Surface Functionalization Strategies. ACS Appl. Nano Mater. 2021, 4, 4409–4418. [Google Scholar] [CrossRef]
- Zare, M.; Sarkati, M.N. Chitosan-functionalized Fe3O4 nanoparticles as an excellent biocompatible nanocarrier for silymarin delivery. Polym. Adv. Technol. 2021, 32, 4094–4100. [Google Scholar] [CrossRef]
- Zarinwall, A.; Asadian-Birjand, M.; Seleci, D.A.; Maurer, V.; Trautner, A.; Garnweitner, G.; Fuchs, H. Magnetic Nanoparticle-Based Dianthin Targeting for Controlled Drug Release Using the Endosomal Escape Enhancer SO1861. Nanomaterials 2021, 11, 1057. [Google Scholar] [CrossRef]
- Zhalechin, M.; Dehaghi, S.M.; Najafi, M.; Moghimi, A. Magnetic polymeric core-shell as a carrier for gradual release in-vitro test drug delivery. Heliyon 2021, 7, e06652. [Google Scholar] [CrossRef]
- Zuk, M.; Gaweda, W.; Majkowska-Pilip, A.; Osial, M.; Wolski, M.; Bilewicz, A.; Krysinski, P. Hybrid Radiobioconjugated Superparamagnetic Iron Oxide-Based Nanoparticles for Multimodal Cancer Therapy. Pharmaceutics 2021, 13, 1843. [Google Scholar] [CrossRef]
- Ali, T.H.; Mandal, A.M.; Heidelberg, T.; Hussen, R.S.D. Sugar based cationic magnetic core-shell silica nanoparticles for nucleic acid extraction. RSC Adv. 2022, 12, 13566–13579. [Google Scholar] [CrossRef]
- Behr, J.; Carnell, L.R.; Stein, R.; Pfister, F.; Friedrich, B.; Huber, C.; Lyer, S.; Band, J.; Schreiber, E.; Alexiou, C.; et al. In Vitro Setup for Determination of Nanoparticle-Mediated Magnetic Cell and Drug Accumulation in Tumor Spheroids under Flow Conditions. Cancers 2022, 14, 5978. [Google Scholar] [CrossRef]
- Bin Jang, S.; Jin, S.M.; Kim, H.S.; Jeong, Y.Y.; Lee, S.J.; Hahn, S.; Lee, H.; Lee, H.S.; Kim, J.H.; Lee, D.Y. DAMP-modulating nanoparticle for successful pancreatic islet and stem cell transplantation. Biomaterials 2022, 287, 121679. [Google Scholar] [CrossRef]
- Gupta, R.; Gupta, P.; Footer, C.; Stenning, G.B.G.; Darr, J.A.; Pancholi, K. Tuneable magnetic nanocomposites for remote self-healing. Sci. Rep. 2022, 12, 10180. [Google Scholar] [CrossRef]
- Ikram, H.; Al Rashid, A.; Koc, M. Synthesis and characterization of hematite (alpha-Fe2O3) reinforced polylactic acid (PLA) nanocomposites for biomedical applications. Compos. Part C-Open 2022, 9, 100331. [Google Scholar] [CrossRef]
- Liu, C.H.; Lin, C.H.; Chen, Y.J.; Wu, W.C.; Wang, C.C. Multifunctional magnetic nanocarriers for delivery of siRNA and shRNA plasmid to mammalian cells: Characterization, adsorption and release behaviors. Colloid Surface B 2022, 219, 112861. [Google Scholar] [CrossRef]
- Miola, M.; Verne, E. In situ reduction of Ag on magnetic nanoparticles with gallic acid: Effect of the synthesis parameters on morphology. Nanomedicine 2022, 17, 499–511. [Google Scholar] [CrossRef]
- Mun, H.; Chaban, Y.; Tabish, T.A.; Thorat, N.; Cowieson, N.; Owen, C.D.; Townley, H.E. CD44 and CD221 directed magnetic cubosomes for the targeted delivery of helenalin to rhabdomyosarcoma cells. Nano Res. 2023, 16, 2915–2926. [Google Scholar] [CrossRef]
- Mushtaq, S.; Shahzad, K.; Rizwan, M.; Ul-Hamid, A.; Abbasi, B.H.; Khalid, W.; Atif, M.; Ahmad, N.; Ali, Z.; Abbasi, R. Magnetoelectric core-shell CoFe2O4@BaTiO3 nanorods: Their role in drug delivery and effect on multidrug resistance pump activity in vitro. RSC Adv. 2022, 12, 24958–24979. [Google Scholar] [CrossRef]
- Nayak, J.; Prajapati, K.S.; Kumar, S.; Vashistha, V.K.; Sahoo, S.K.; Kumar, R. Thiolated β-cyclodextrin modified iron oxide nanoparticles for effective targeted cancer therapy. Mater. Today Commun. 2022, 33, 104644. [Google Scholar] [CrossRef]
- Rocha, J.M.V.; de Souza, V.B.; Panunto, P.C.; Nicolosi, J.S.; da Silva, E.D.; Cadore, S.; Londono, O.M.; Muraca, D.; Tancredi, P.; de Brot, M.; et al. In vitro and in vivo acute toxicity of a novel citrate-coated magnetite nanoparticle. PLoS ONE 2022, 17, e0277396. [Google Scholar] [CrossRef]
- Swain, S.K.; Phaomei, G.; Tripathy, S.K.; Yaiphaba, N.; Devi, R.B.; Nayak, S.; Parida, B.B. Effect of beta-cyclodextrin decoration on structural, optical and magnetic properties of luminescent magnetic nanoparticles and its application as a drug carrier. J. Mol. Struct. 2022, 1247, 131330. [Google Scholar] [CrossRef]
- Szymczyk, A.; Drozd, M.; Kaminska, A.; Matczuk, M.; Trzaskowski, M.; Mazurkiewicz-Pawlicka, M.; Ziolkowski, R.; Malinowska, E. Comparative Evaluation of Different Surface Coatings of Fe3O4-Based Magnetic Nano Sorbent for Applications in the Nucleic Acids Extraction. Int. J. Mol. Sci. 2022, 23, 8860. [Google Scholar] [CrossRef]
- Tang, X.Z.; Manamanchaiyaporn, L.; Zhou, Q.; Huang, C.Y.; Li, L.H.; Li, Z.Q.; Wang, L.C.; Wang, J.N.; Ren, L.; Xu, T.T.; et al. Synergistic Integration and Pharmacomechanical Function of Enzyme-Magnetite Nanoparticle Swarms for Low-Dose Fast Thrombolysis. Small 2022, 18, 2202848. [Google Scholar] [CrossRef]
- Toyos-Rodriguez, C.; Llamedo-Gonzalez, A.; Pando, D.; Garcia, S.; Garcia, J.A.; Garcia-Alonso, F.J.; De la Escosura-Muniz, A. Novel magnetic beads with improved performance for Alzheimer’s disease biomarker detection. Microchem. J. 2022, 175, 107211. [Google Scholar] [CrossRef]
- Treder, N.; Roszkowaka, A.; Oledzka, I.; Baczek, T.; Plenis, A. Effects of Fe3O4 Magnetic Nanoparticle Functionalization with Ionic Liquids and a Double-Chained Surfactant on the Pretreatment of Plasma Samples during Drug Extraction. Anal. Chem. 2022, 94, 16587–16595. [Google Scholar] [CrossRef]
- Urquizo, I.A.F.; Garcia, T.C.H.; Loredo, S.L.; Galindo, J.T.E.; Casillas, P.E.G.; Barron, J.C.S.; Gonzalez, C.C. Effect of Aminosilane Nanoparticle Coating on Structural and Magnetic Properties and Cell Viability in Human Cancer Cell Lines. Part. Part. Syst. Charact. 2022, 39, 2200106. [Google Scholar] [CrossRef]
- Valdivia, V.; Gimeno-Ferrero, R.; Leal, M.P.; Paggiaro, C.; Fernandez-Romero, A.M.; Gonzalez-Rodriguez, M.L.; Fernandez, I. Biologically Relevant Micellar Nanocarrier Systems for Drug Encapsulation and Functionalization of Metallic Nanoparticles. Nanomaterials 2022, 12, 1753. [Google Scholar] [CrossRef]
- Zohreh, N.; Karimi, N.; Hosseini, S.H.; Istrate, C.; Busuioc, C. Fabrication of a magnetic nanocarrier for doxorubicin delivery based on hyperbranched polyglycerol and carboxymethyl cellulose: An investigation on the effect of borax cross-linker on pH-sensitivity. Int. J. Biol. Macromol. 2022, 203, 80–92. [Google Scholar] [CrossRef]
- Beltrame, J.M.; Ribeiro, B.B.P.; Guindani, C.; Candiotto, G.; Felipe, K.B.; Lucas, R.; Zottis, A.D.; Isoppo, E.; Sayer, C.; de Araujo, P.H.H. Coating of SPIONs with a Cysteine-Decorated Copolyester: A Possible Novel Nanoplatform for Enzymatic Release. Pharmaceutics 2023, 15, 1000. [Google Scholar] [CrossRef]
- Chauhan, M.; Basu, S.M.; Qasim, M.; Giri, J. Polypropylene sulphide coating on magnetic nanoparticles as a novel platform for excellent biocompatible, stimuli-responsive smart magnetic nanocarriers for cancer therapeutics. Nanoscale 2023, 15, 7384–7402. [Google Scholar] [CrossRef] [PubMed]
- Jalali, S.; Moghadam, P.N.; Shafiei-Irannejad, V. Synthesis of Magnetic Nanocarrier Conjugated by Folate Based on Tragacanth and In Vitro Investigation of their Efficiency on Breast Cancer Cells. Starch-Starke 2023, 75, 2200092. [Google Scholar] [CrossRef]
- Liu, L.; Yang, Z.J.; Liu, C.B.; Wang, M.Y.; Chen, X. Preparation of PEI-modified nanoparticles by dopamine self-polymerization for efficient DNA delivery. Biotechnol. Appl. Biochem. 2023, 70, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.; Tran, Q.H.; Terki, F.; Charnay, C.; Dumail, X.; Reibel, C.; Cazals, G.; Valette, G.; Jay-Allemand, C.; Bidel, L.P.R. Aggregation of magnetic nanoparticles functionalized with trans-resveratrol in aqueous solution. Discov. Nano 2023, 18, 64. [Google Scholar] [CrossRef] [PubMed]
- Kovrigina, E.; Poletaeva, Y.; Zheng, Y.; Chubarov, A.; Dmitrienko, E. Nylon-6-Coated Doxorubicin-Loaded Magnetic Nanoparticles and Nanocapsules for Cancer Treatment. Magnetochemistry 2023, 9, 106. [Google Scholar] [CrossRef]
- Baldea, I.; Petran, A.; Florea, A.; Sevastre-Berghian, A.; Nenu, I.; Filip, G.A.; Cenariu, M.; Radu, M.T.; Iacovita, C. Magnetic Nanoclusters Stabilized with Poly[3,4-Dihydroxybenzhydrazide] as Efficient Therapeutic Agents for Cancer Cells Destruction. Nanomaterials 2023, 13, 933. [Google Scholar] [CrossRef]
- Urquizo, I.A.F.; Tozcano, D.I.M.; Gómez, L.E.V.; Pérez, J.A.R.; González, C.C. Enhancing the Cytocompatibility of Cobalt-Iron Ferrite Nanoparticles Through Chemical Substitution and Surface Modification. Adv. Mater. Interfaces 2023, 10, 2300206. [Google Scholar] [CrossRef]
- Toderascu, L.I.; Sima, L.E.; Orobeti, S.; Florian, P.E.; Icriverzi, M.; Maraloiu, V.-A.; Comanescu, C.; Iacob, N.; Kuncser, V.; Antohe, I.; et al. Synthesis and Anti-Melanoma Activity of L-Cysteine-Coated Iron Oxide Nanoparticles Loaded with Doxorubicin. Nanomaterials 2023, 13, 621. [Google Scholar] [CrossRef]
- Xu, X.; Xiang, H.J.; Wang, Z.J.; Wu, C.J.; Lu, C.C. Doping engineering and functionalization of iron oxide nanoclusters for biomedical applications. J. Alloys Compd. 2022, 923, 166459. [Google Scholar] [CrossRef]
- Upadhyay, K.; Tamrakar, R.K.; Thomas, S.; Kumar, M. Surface functionalized nanoparticle A boon to biomedical science. Chem.-Biol. Interact. 2023, 380, 110537. [Google Scholar] [CrossRef]
- Popova, V.; Dmitrienko, E.; Chubarov, A. Magnetic Nanocomposites and Imprinted Polymers for Biomedical Applications of Nucleic Acids. Magnetochemistry 2023, 9, 12. [Google Scholar] [CrossRef]
- Mulens-Arias, V.; Rojas, J.M.; Barber, D.F. The Use of Iron Oxide Nanoparticles to Reprogram Macrophage Responses and the Immunological Tumor Microenvironment. Front. Immunol. 2021, 12, 693709. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Iqbal, H.M.N.; Adil, S.F.; Shaik, M.R.; Abdelgawad, A.; Hatshan, M.R.; Khan, M. Surface-coated magnetic nanostructured materials for robust bio-catalysis and biomedical applications-A review. J. Adv. Res. 2022, 38, 157–177. [Google Scholar] [CrossRef] [PubMed]
- Bohara, R.A.; Thorat, N.D.; Pawar, S.H. Role of functionalization: Strategies to explore potential nano-bio applications of magnetic nanoparticles. RSC Adv. 2016, 6, 43989–44012. [Google Scholar] [CrossRef]
- Mittal, N.; Kundu, A.; Pathania, A.R. A review of the chemical synthesis of magnetic nano-particles and biomedical applications. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
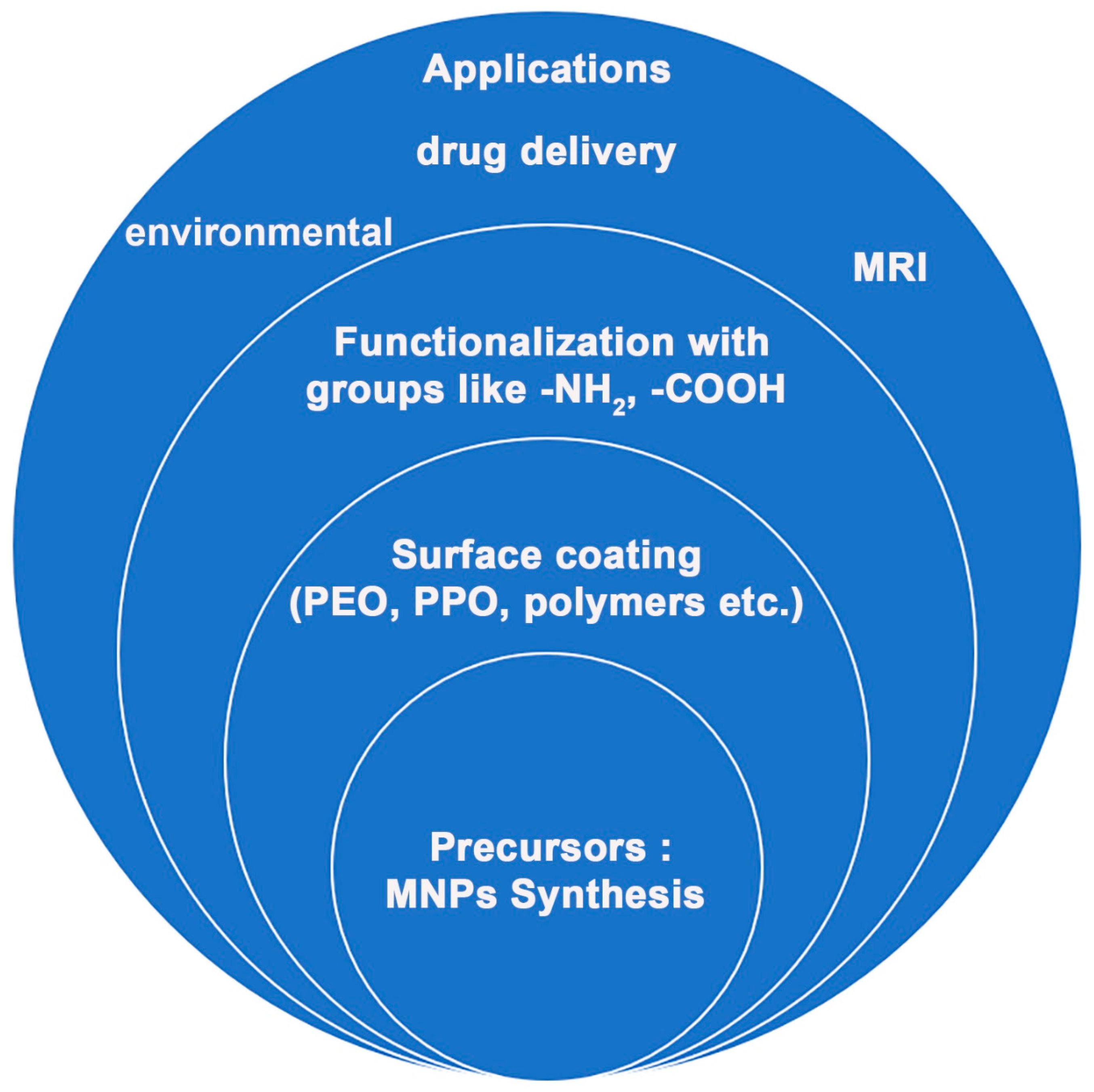

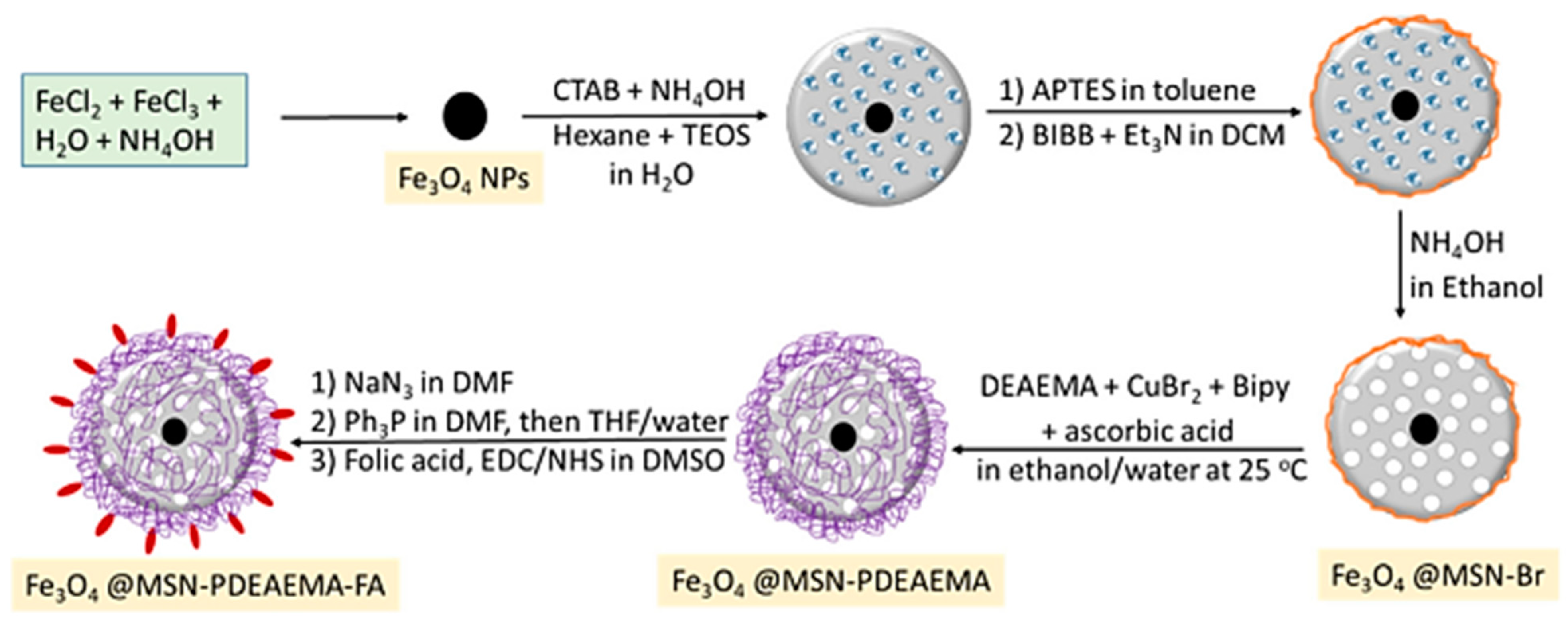
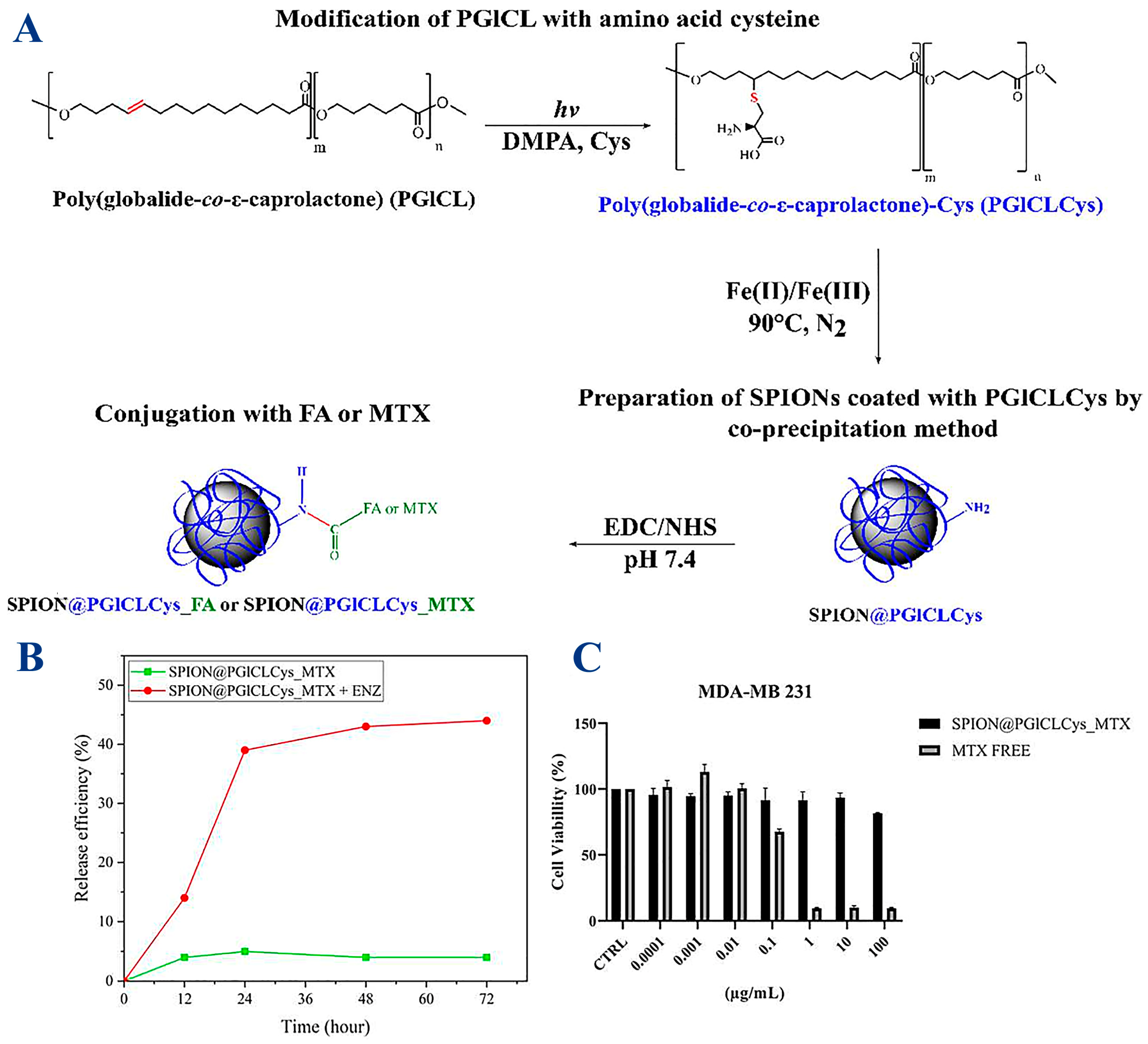
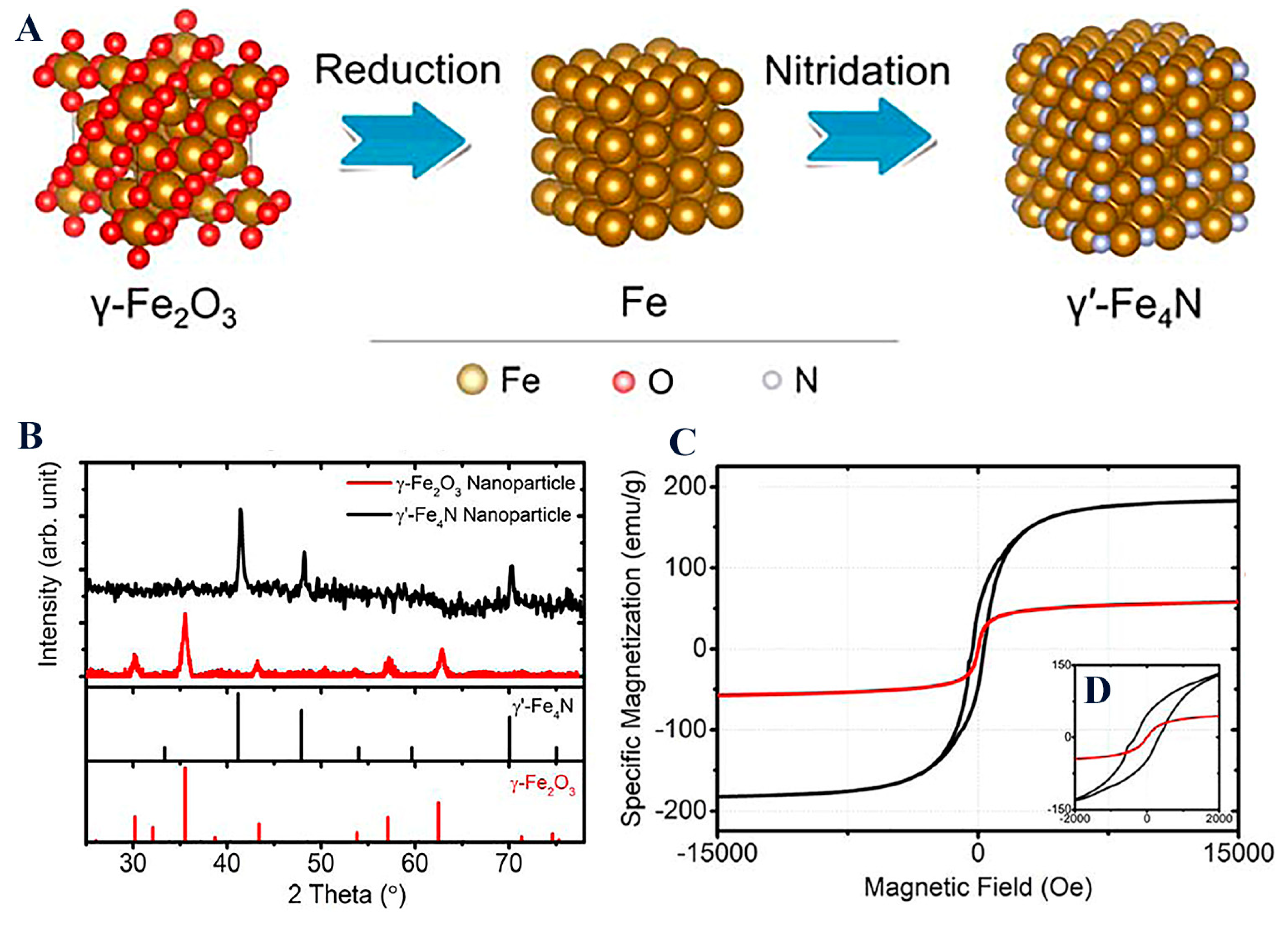
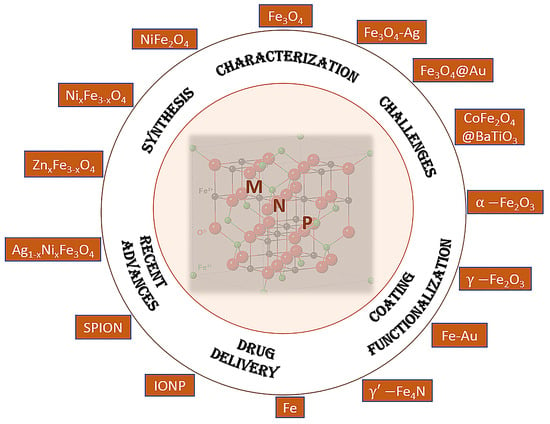
| Synthesis Method | Description | Characterization Tools |
|---|---|---|
| Co-Precipitation | In this classic method, metal salts (FeCl3, FeCl2) are dissolved in a solvent (water, ethanol) followed by the addition of reducing agents (NaBH4, NH3, NH2NH2). The reduction of metal ions yields MNPs. | Transmission electron microscopy (TEM) and X-ray diffraction (XRD) elucidate the core structure of MNPs. |
| Thermal Decomposition | Organic metal precursors (iron pentacarbonyl) are decomposed at elevated temperatures (200–300 °C) in organic solvents (oleic acid, oleyl amine) to generate MNPs. | TEM and XRD reveal their size and crystallinity. |
| Microemulsion | Water-in-oil microemulsions, containing surfactants (CTAB) and co-surfactants (butanol, hexanol), are employed to control MNP nucleation and growth. Iron salts (FeSO4) in the aqueous phase react with reducing agents (NaBH4, hydrazine, etc.) to form MNPs. | Dynamic light scattering (DLS) and UV-Vis spectroscopy monitor the reaction progress. |
| Sol-Gel | Silica-coated MNPs are synthesized via hydrolysis and condensation of silane precursors (TEOS) in the presence of MNPs. The resulting silica shell stabilizes the MNPs and provides functional groups for subsequent modifications. | FTIR spectroscopy can attest for shell formation. |
| Microfluidics | Continuous-flow microreactors facilitate controlled nucleation and growth of MNPs by mixing iron precursors (Fe(acac)3) with reducing agents (hydrazine) under controlled flow conditions. | In-line spectroscopy monitors the reaction kinetics. |
| Hydrothermal | Iron precursors (FeCl3, Fe(acac)3) are hydrothermally treated at elevated temperatures (150–300 °C) and pressures (10–100 atm) in a solvent (water, ethylene glycol, benzyl ether) to yield crystalline MNPs. | Synthesis Scanning electron microscopy (SEM) and XRD confirm particle morphology and crystallinity. |
| Electrochemical Synthesis | Electrodeposition involves the reduction of iron ions onto an electrode surface. Precursors (iron salts) are dissolved in an electrolyte solution, and an electric current is applied. | Electrochemical techniques monitor the deposition process, while SEM reveals surface morphology. |
| Sonolysis | Ultrasound irradiation of metal salt solutions (FeCl3) generates reactive species that reduce metal ions to MNPs. | The process is monitored by UV-Vis spectroscopy, while TEM elucidates their morphology and size. |
| Radiolysis | Irradiation of metal salt solutions (FeCl3) with ionizing radiation (gamma rays, electron beams) induces the reduction of metal ions to MNPs. | Size and composition are determined by TEM and inductively coupled plasma mass spectrometry (ICP-MS). |
| Biogenic Synthesis | Microorganisms (bacteria, fungi) or plant extracts reduce metal ions (Fe3+) to MNPs. | Energy-dispersive X-ray spectroscopy (EDS) and Fourier-transform infrared spectroscopy (FTIR) confirm the presence of biomolecules on the MNP surface. |
| Characterization Methods | Description |
|---|---|
| Transmission Electron Microscopy (TEM) | TEM offers high-resolution imaging, revealing MNP morphology, size, and core–shell structures. Contrast variations highlight surface coatings and confirm successful functionalization. Energy-dispersive X-ray spectroscopy (EDS) coupled with TEM maps elemental distribution across MNPs. |
| X-ray Photoelectron Spectroscopy (XPS) | XPS provides elemental composition information and oxidation states of surface-functionalized MNPs. Binding energy shifts indicate surface chemical interactions, validating ligand attachment or shell formation. |
| Fourier-Transform Infrared Spectroscopy (FTIR) | FTIR identifies functional groups on MNPs’ surfaces through vibrational spectra. Shifts in or the appearance of peaks confirm ligand exchange or coating formation. Attenuated total reflection (ATR) FTIR enables the analysis of solid MNPs. |
| Dynamic Light Scattering (DLS) | DLS evaluates the hydrodynamic size and dispersity of surface-functionalized MNPs in solution. Changes in size or dispersity after functionalization reflect coating stability and influence on hydrodynamic behavior. |
| Zeta Potential Analysis | Zeta potential quantifies surface charge of MNPs, revealing electrostatic interactions between functional coatings and the surrounding medium. Zeta potential changes indicate successful charge modification. |
| Nuclear Magnetic Resonance (NMR) | Solution-state NMR elucidates surface functional groups and molecular dynamics. Ligand exchange or bioconjugation is confirmed by chemical shift changes or the appearance of new peaks. |
| UV-Visible Spectroscopy: | UV-Vis spectroscopy reveals ligand-specific absorbance, confirming functionalization. Shifts in absorption bands indicate changes in the electronic environment due to surface engineering. |
| Scanning Electron Microscopy (SEM) | SEM provides topographical information on MNP surfaces. Microstructural changes due to functionalization, such as shell formation or aggregation, are discerned. |
| Magnetic Measurements | Superconducting quantum interference device (SQUID) magnetometry quantifies magnetic behavior, confirming core–shell architecture and magnetic moments of surface-functionalized MNPs. |
| X-ray Diffraction (XRD) | XRD verifies MNP crystallinity and phase changes due to functionalization. Shifts in or broadening of diffraction peaks indicate surface modifications or encapsulation. |
| Type of Functionalization | Precursors/Materials and Short Description of Method | Observations | Characterization Techniques |
|---|---|---|---|
| Organic Coatings | Organic ligands, such as citrate, polyethylene glycol (PEG), or polymers, are grafted onto MNPs’ surfaces through covalent or non-covalent interactions. | This imparts colloidal stability, biocompatibility, and modulates surface charge. The enhanced dispersibility of MNPs in biological media facilitates their utilization in drug delivery and targeted imaging. | Dynamic light scattering (DLS) and zeta potential measurements verify the stability and charge modification. |
| Inorganic Shells | MNPs are encapsulated within inorganic materials like silica, gold, or metal oxides. These shells enhance stability, protect MNPs from degradation, and enable bioconjugation. For instance, core–shell Fe3O4/Pt MNPs have been obtained via silylation/polymerization [26]. | Silica-coated MNPs, for instance, offer a platform for versatile surface functionalization and controlled drug release. | Transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS) confirm shell formation. |
| Ligand Exchange | The exchange of native surface ligands with functional molecules (amines, thiols) allows for precise control over MNP properties. | This strategy enables targeted drug delivery through the conjugation of targeting ligands or the attachment of therapeutic payloads. | Fourier-transform infrared spectroscopy (FTIR) confirms successful ligand exchange. |
| Self-Assembly | MNP surface functionalization can exploit the self-assembly of molecules (DNA, peptides) onto the MNP surface. | DNA-functionalized MNPs, for instance, enable programmable interactions, leading to controlled aggregation or dispersal. | Gel electrophoresis and fluorescence assays validate self-assembly. |
| Bioconjugation | MNPs are conjugated with biomolecules (antibodies, peptides) through affinity interactions, yielding specific targeting capabilities. | Antibody-conjugated MNPs offer exquisite cellular or molecular targeting in imaging and drug delivery. | Enzyme-linked immunosorbent assays (ELISA) confirm successful bioconjugation. |
| Click Chemistry | Bioorthogonal reactions (click chemistry) facilitate specific and robust surface functionalization. | Azide-terminated MNPs react with alkyne-modified molecules, yielding stable and versatile conjugates. | Copper-catalyzed or copper-free click reactions validate successful coupling. |
| Layer-by-Layer Assembly | Sequential deposition of polyelectrolyte layers onto MNPs yields multifunctional coatings. | This approach enables the controlled release and modulated surface charges. | Quartz crystal microbalance (QCM) and UV-Vis spectroscopy monitor layer-by-layer assembly. |
| Responsive Polymers | Stimuli-responsive polymers (pH, temperature) can be grafted onto MNP surfaces, facilitating controlled drug release in specific microenvironments [27]. | Suitable for controlled drug release | The swelling behavior of polymers is probed using dynamic light scattering (DLS) and turbidimetry. |
| Host-Guest Systems | Cyclodextrins or cucurbiturils form host–guest complexes with molecules on MNP surfaces, enhancing stability and enabling controlled release. | Suitable for controlled drug release | Nuclear magnetic resonance (NMR) and UV-Vis spectroscopy |
| NP Type | Coating Agent | Active Agent | Characterization Methods | Activity | Obs. | Ref. |
|---|---|---|---|---|---|---|
| NiFe2O4, Fe3O4 of ~5 nm | Oleic acid coating (2 nm) | Conjugated polymer poly(9,9-dioctylfluorene-alt-benzothiadiazole, F8BT) or polystyrene grafted with ethylene oxide functionalized with carboxyl groups (PS-EG-COOH) | UV-VIS, DLS, TEM, MRI evaluation, cytotoxicity in vitro (U-87 MG and T98G cells, MTT and Live/Dead cell viability assays), fluorescence imaging | Glioblastoma (GBM); MR imaging (MRI) | Theranostic agents with prospects for multifunctionality in imaging and treatment; preclinical MRI studies | [42] |
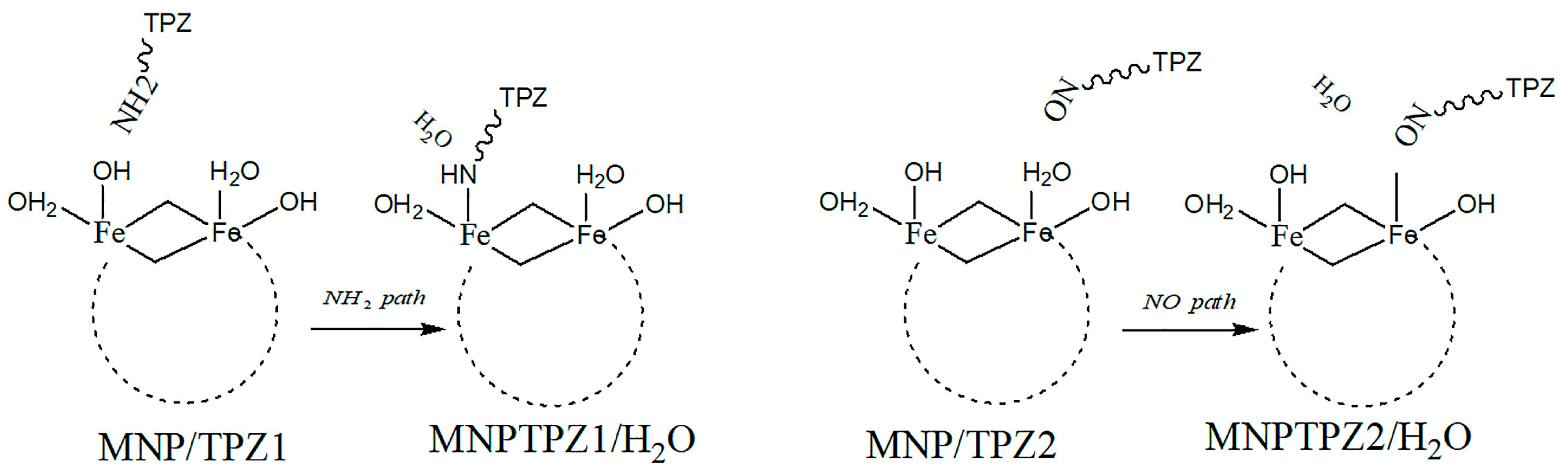
| magnetic nanoparticle Fe6(OH)18(H2O)6 | – | Tirapazamine (TPZ,) | DFT study | Anticancer (not evaluated) | Drug binding via intraring N-atom, -NH2 | [43] |
| Fe3O4@MSN-PDMAEMA-FA (~180 nm) | MSN-PDMAEMA-FA | DOX (doxorubicin) | SEM, TEM, FTIR, surface area, TGA, XPS, UV, DLS | Anticancer (MCF-7, and MCF-7 ADR cells) | Excellent biocompatibility, minimally toxicity | [44] |
| Au/Fe3O4 NPs (15 nm diameter) | L-cysteine (Cys); dithiol-terminated polyethylene glycol (PEG(SH)2), | Luciferase (enzyme) | FTIR, FESEM, luminescence, bioluminescence | Enzymatic activity, luminescence activity | 48% activity preservation in case of CysAuNPMag | [46] |
| Superparamagnetic Iron Oxide Nanoparticles (SPIONs) | Citrate coating | SPIONs@citrate (52–58 nm hydrodynamic Z size) loaded into human T cells (27 or 80 µg/mL) | TEM, Magnetic susceptibility, hydrodynamic Z-average size, zeta potential | Anticancer; infiltration of SPIONs into primary human CD3+ T cells (1,4 pg Fe/cell)) | Magnetic delivery of immune cells (dynamic regime)—potential future application | [47] |
| maghemite (γ-Fe2O3), magnetite (Fe3O4) 10.9 ± 1.6 nm | 0olyol synthesis from Fe(acac)3 and diethylene glycol (DEG) | IONPs@DEG obtained with continuous growth method | TEM, XPS, XRD, magnetization | MRI; T1-imaging, T2-imaging (high relaxivities r2:163.4 mM−1s−1, r1:135.0 mM−1s−1) | Water-dispersible NPs obtained when T = 190°, 220° and 235 °C (higher T leads to agglomeration); high wt% C (XPS) | [48] |
| biological magnetite nanoparticles (BMs), by magnetotactic bacteria Magnetovibrio blakemorei strain MV-1T | Glutaraldehyde GA, poly-L-lysine PLL (linking reagents) | Amphotericin B, AmB (to yield BM–PLL–AmB and BM–PLL–GA–AmB conjugates) | TEM, FTIR | Controlled drug release, magnetic hyperthermia (in PBS media) | Magnetosomes with high encapsulation efficiencies and drug loadings (0.1‰ PLL: 52.7%, and 25.3 mg per 100 mg; while 0.1‰ PLL–GA 12.5%: 45.0%, 21.6 mg per 100 mg) | [49] |
| Magnetic nanoparticles (MNPs) of Fe3O4 | Chitosan (CS) | Telmisartan (TEL), yielding MNP–CS–TEL TEL is an angiotensin II receptor blocker (ARB), treating high blood pressure, heart failure, diabetic kidney disease, and cancer | FTIR, TGA, XRD, FE-SEM (field emission scanning electron microscope), TEM, VSM (vibrating sample magnetometer), BET surface area analyzer | Anticancer drug therapy, as carriers of Telmisartan (TEL); tested against PC-3 human prostate cancer | Chitosan CS coating validated by FTIR and TGA data. Grafting TEL, a poorly soluble drug, on the surface-coated of MNPs (MNP-CS) by amide bond between amino groups of chitosan CS and carboxylic groups of TEL | [50] |
| Magnetic nanoparticles (maghemite NPs) on anodic alumina nanotubes, to give magnetic anodic alumina nanotubes (MAANTs) | Silanization by means of (3-aminopropyl)triethoxysilane (3-APTES, 99%) | Protein padding of albumin-fluorescein isothiocyanate conjugate (FITC-BSA) | Environmental scanning electron microscopy (ESEM), energy dispersive X-ray (EDX); FTIR (ATR), ζ-potential, dynamic light scattering (DLS); TEM, fluorescence | Drug delivery and biosensing applications | Proteolytic hydrolysis (amide bond breaking) in presence of cathepsin B- protease (growth and initial stages of tumor metastasis), releasing fluorescent fragments of the protein | [51] |
| Cobalt Nanoparticles | Carbon-coating, yields CCo nanoparticles | Functionalization with Sulfonated Arene Derivatives via aqueous in situ diazotization reaction | FTIR, SEM, elemental analysis | Catalysis (anionic ROP of glycidol), or recyclable anticoagulant | Covalent linkage of an in situ generated diazonium on the graphene-like surface | [52] |
| Fe3O4 nanoparticles (MNPs) | Mesoporous silica (SBA-15), To yield PEI grafted Fe3O4@SiO2@SBA-15 labeled FA | Doxorubicin (DOX) | FTIR, TGA, XRD, VSM, SEM, EDX, UV-Vis | Targeted delivery to MCF-7 cell line (breast cancer) | pH-sensitive mesoporous magnetic and biocompatible nanocarrier, high internalization | [53] |
| Fe3O4 | SiO2-NH2 coating, alkyne surface functionalization to yield magnetic nanoparticle Fe3O4@SiO2-NH2-alkyne | Azide-functionalized E. Coli | XRD, FTIR, TEM, SEM, immobilization yield (Y), activity recovery (E), | Biomedicine and catalysis –conversion of glycerol into DHA; Tested against recombinant E. coli harboring glycerol dehydrogenase | Click chemistry affords covalent bonding between azide and alkyne groups; immobilization yield 83%, activity recovery 94% | [54] |
| Au/Fe | Glutathione (GSH)-capped on hybrid gold-magnetic-iron-oxide NPs (Au-Mag-GSH) | – | Colloidal stability, DLS: Z-potential, HR–TEM, (S)TEM, SEM, EDX, FTIR, Photoluminescence (PL) excitation and emission spectra, cytotoxicity | Biomedical applications | NPs were found to not be toxic at typically used concentrations (1.5 μg/mL) | [55] |
| Superparamagnetic iron oxide nano-rods (IONRs) based on magnetite | Branched polyethyleneimine (BPEI) to yield PEI-coated Fe3O4 nano-rods; cyclohexane layer prevents NPs oxidation during synthesis | Carnosine dipeptide (β-alanine and L-histidine) | SEM-EDS, TEM, XRD, FT-IR, TGA, GC-MS, DLS, zeta potential, magnetic properties (by whole body 1.5 T MRI system) | Cancer treatment: glioblastoma brain tumors (GBM); inhibition of post-surgery metastasis; MRI monitoring | Superparamagnetic nano-rods tested against U87 human glioblastoma astrocytoma cell line. Carnosine was fully released by mild hyperthermia (40 °C) | [56] |
| Ag(1-X)NiXFe2O4 | Polyethylene glycol (PEG) | Curcumin | XRD, FTIR, SEM, TEM, VSM, UV-Vis | Drug delivery | Synthesis of Ag- doped Ni ferrite nanoparticle; PEG was used as a solvent during synthesis; curcumin loading was pH-dependent | [57] |
| NiFe2O4 (~5 nm) | No pre-functionalization required | Serum albumin (BSA) | DLS, FT-IR, TEM, SAED | Immobilized metal affinity chromatography of proteins (IMAC) | BSA binding fitted Langmuir isotherm; high capacity 916 mg BSA/g dried NPs | [58] |
| SPIONs | Polymers: Poly(2-ethyl-2- oxazoline) (PEtOZ); Poly(2-ethyl-2-oxazoline-co-2-isopropyl-2-oxazoline) (PEtIOZ) | Opsonins and Albumin | TEM, TGA/DSC, DLS, Isothermal Titration Calorimetry, ProtParam tool (Computation of Protein Properties) | Biomedical applications | Protein corona formation on poly(2-alkyl-2-oxazoline)-grafted SPIONs depends on protein size, flexibility, and charge | [59] |
| MNPs based on commercial 75%–80% (w/w) Fe3O4 (diameter 100 nm) | Streptavidin-functionalized, encapsulation with hydroxyethyl starch | Oligonucleotide-functionalized (stability test 92% after 3 months at 4 °C) | Fluorescence, AC Susceptibility | Circle-to-circle amplification (C2CA)’ diagnostic | Newcastle disease virus and Salmonella as target sequences | [60] |
| γ-Fe2O3/CeO2 Maghemite(seeds)/cerium oxide MNPs | PEG/neridronate with PEG of 2000 or 5000 Da, producing γ-Fe2O3/CeO2@- PEG2k and γ-Fe2O3/CeO2@PEG5k | – | TEM, SAED, EDX, EELS, DLS, relaxometry, fluorescence | Antioxidant, MRI tracing (high r2 relaxivity); theranostic platform | Improved colloidal stability in PBS, enhanced biocompatibility; CeO2—radical scavenger | [61] |
| iron oxide Fe3O4 MNPs | Oleic acid (OA), silica (TEOS), cationic polymer P poly[N-isopropylacrylamide-co-(3-acrylamidopropyl) trimethylammonium chloride], P(NIPAm-co-AMPTMA) | Vancomycin (Van) | TEM, XRD, FTIR, TGA, SEM, DLS, magnetization curves | Antibacterial (Shigella boydii, Bacillus cereus, Staphylococcus aureus and Escherichia coli) | MNPs synthesized by co-precipitation/microemulsion method; Fe3O4/SiO2/P(NIPAM-coAMPTMA). Vancomycin (Van) creates stronger H–bonding between Van and C-Terminal L-lysyl-D-alanyl-D-alanine of bacteria | [62] |
| Superparamagnetic Iron Oxide Nanoparticle SPIONs (Fe3O4, 40 nm size) | HAD/OA ratio changes MNPs shape | – | TEM, XRD, Electrophoretic mobility measurements, magnetization, SAR/hyperthermia | Hyperthermia (MH, MFH) | SPIONs with spherical, cuboidal (SARmax) or rod-like shape; efficient MFH (rt to 45 °C, in 60 s, 20 kA/m, 136–205 kHz) | [63] |
| iron oxide nanoparticle (ION) | – | 5-aminolevulinic acid (ALA) | Computational study/quantum chemistry | Anticancer therapy | Configurations optimized at optimized at B3LYP/6-31G(d,p) in aq. solution; H-bonding plays a central role | [64] |
| MNPs manganese ferrite (MnFe2O4), spinel structure | Citrate-stabilized (14.4 ± 2.6 nm), lipid-coated (8.9 ± 2.1 nm) | Doxorubicin (Dox) | Fluorescence, Förster resonance energy transfer (FRET), STEM, XRD, Raman, SQUID, rheology, UV-Vis, hyperthermia | Drug delivery/release; theranostic | Dehydropeptide-based supramolecular magnetogels; improved drug release of lipid-coated vs. citrate-coated MNPs | [65] |
| γ′-Fe4N (prepared by gas nitridation from commercial γ-Fe2O3, 20 nm) | The first report of surface-modified iron nitrides; α″-Fe16NxZ2−x, and α′-Fe8NxZ1−x, by wet ball milling | – | PPMS (Ms, Hc), XRD, TEM, DLS, FTIR, seta-potential | Envisioned biomedical applications (DNA, protein or drug delivery) | γ′-Fe4N have 3 times higher saturation magnetizations than IONPs (HC = 310 Oe, MS (15 kOe) = 182.7 emu/g; MR = 45 emu/g) | [66] |
| Fe3O4 | Chitosan (Cs), with/without silica; Cs-f-SiO2@Fe3O4, Cs-f-Fe3O4 | Silymarin (SIL) | Cytotoxicity tests (MCF-7, MTT assay) | Drug delivery, anticancer, antioxidant, | 99–120 mg SIL/g functionalized MNPs (Folin–Ciocalteu method) | [67] |
| SPION | APTES Modification (SPION@APTES) | Toxin: dianthin-epidermal growth factor (DiaEGF) or endosomal escape enhancers (EEE), glycosylated triterpenoids SO1861 | Enzymatic Activity, TEM, DLS, DSC, In Vitro Cytotoxicity, Relaxivity | Targeted tumor therapy, drug delivery | 2000-fold enhancement in tumor cell cytotoxicity, 6.7-fold gain in specificity; steric stabilization inhibits agglomeration | [68] |
| MNPs | Functionalized graphene oxide (acylated, G-COCl), polylactic acid, polyvinyl alcohol, polyethylene glycol, and nilotinib (second layer), sodium alginate, polyethylene glycol, poly (lactic-co-glycolic acid), polylactic acid and nilotinib gel (third layer) | Nilotinib (TasignaTM, medication for chronic myelogenous leukemia) | UV-Vis, FTIR, FT-NMR, VSM, SEM, TEM, TGA | Drug delivery | Nilotinib 400 mg, super paramagnetic particles 0.01 g and total MFGO mass (0.1 g) were kept constant in all samples. Faster drug release at acidic pH 3 (24 h) vs. slightly basic pH 7.4 (48 h). | [69] |
| SPIONs, 166Ho doped iron oxide | Au layer coating | Monoclonal antibody trastuzumab (Tmab) | TEM, TGA, cytotoxicity studies | Multimodal cancer therapy | [166Ho] Fe3O4@Au NPs (150 nm) conjugated with Tmab targets HER2+ receptors. Cytotoxic effect toward SKOV-3 ovarian cancer cells. | [70] |
| MNPs (Fe2O3, ~15 nm, 29 emu/g) | Silica coating, a multistep synthesis, activated NP couples a triethylene glycol spaced glycosyl imidazole; silyl propyl-H-imidazole functionalization, glycosylation and deacetylation to NpFeSiImSugar NPs. | – | TLC (thin-layer chromatography), ATR FTIR, TG-DTA, TEM, SEM, EDX, XRD, VSM, BET, 1H and 13C NMR | Nucleic acid (NA) extraction | hydrogen bonding between the surface bonded carbohydrate and nucleic acid targets (NpFeSiImSugar/DNA complex) to ensure nucleic acid selectivity and avoid protein contamination. high DNA particle loading ratio of 30–45 wt% (MNP/DNA ratio) | [71] |
| SPIONs | Caffeic acid (Caf-SPIONs); citrate-stabilized (Cit-SPION) | Bovine serum albumin (BSA) | Hydrodynamic size (Z-Average), polydispersity index (PDI), zeta potential at pH 7.3, volumetric susceptibility, and iron content Atomic Emission Spectroscopy (AES); HPLC-UV; fluorescence; magnetic field simulations (COMSOL) | Anticancer | Caf-BSA-SPIONs; tested against A375M melanoma cells, fibroblasts; | [72] |
| SPION (“SPIO”) | Glycyrrhizin-chitosan coating (SPIO@Chitosan-GL) | Glycyrrhizin (anti-inflammatory, anti-ulcer, anti-allergic, antioxidant, anti-tumor, anti-diabetic, hepatoprotective) | MRI, immunofluorescence | Anti–inflammatory | Monitoring of pancreatic islets and mesenchymal stem cell (MSC) spheroids; inhibition of inflammatory damage-associated molecular pattern (DAMP) protein in mice | [73] |
| Fe3O4 MNP | Silica-coating, Polyamide 6 (PA6) by in situ polymerization, to yield self-healable magnetic nanoparticle polymer (SHMNP) composite | – | TEM, XRD, XRD (small/wide angle, SAXS/WAXS), DSC, magnetic properties (SQUID, 100 K, 400 K), ZFC-FC | Self-healable polymer nanocomposites | Multiferroic-polyamide 6 (PA6) nanocomposite | [74] |
| hematite (α-Fe2O3) | Polylactic acid (PLA) | – | FTIR, TGA, DSC, VSM | Biomedical applications (3D printing, cardiovascular stents) | Stimuli-responsive PLA/α-Fe2O3 nanocomposites | [75] |
| MNPs | MNP-CA-PEI nanoparticles 290.74 ± 63.84 nm: citric acid (CA)-modified MNP cross-linked with polyethyleneimine (PEI) (carbonyldiimidazole as the crosslinker) | -(GFP plasmid; MNP/nucleic acid polyplexes) | DLS, TGA, SQUID, FTIR, zeta-potential, surface characterization (Langmuir, Freundlich), fluorescence | Multifunctional magnetic nanocarriers (siRNA, shRNA), gene delivery | Nucleic acid delivery by caveolae-mediated endocytosis; adsorption isotherm follows pseudo-first order kinetics; HEK 293 cells were used. | [76] |
| Fe3O4–Ag | In situ reduction of Ag with gallic acid (reducing agent), silica shell | – | FE-SEM, FTIR | Antibacterial, antitumor | Nanoflower-like or nanodumbbell (NP/gallic acid ratio of 10:1) multifunctional nanocomposites | [77] |
| SPIONPs (10 nm, toluene), cubosomes. CD44 and CD221) | Hyaluronic acid (HA, ligand for CD44) and antibodies (Abs) against CD221 coupled to cubosomes via electrostatic attraction and thiol-Michael reaction | Helenalin | Cryo-EM, SAXS, | Anticancer | Tested on rhabdomyosarcoma cells (RMS) and control (fibroblast) cells | [78] |
| CoFe2O4@BaTiO3 (CFO@BTO), by solvothermal synthesis (38 nm, MS = 47.4 emu/g) | Surface functionalization with amphiphilic polymer, polyisobutylene-alt-maleic anhydride (PMA) | Doxorubicin (DOX) and methotrexate (MTX) | XRD, HRSEM, HRTEM, SAED, MH measurements, ZFC–FC, DLS < zeta-potential, drug release kinetics | Drug delivery; cancer treatment, suitable for chemo-resistant cancers | Core–shell magnetoelectric NPs obtained as nanorods; 98% drug release in 20 min (MF = 4 mT). Tested on HepG2 and HT144 cells and 3D spheroid models (p < 0.05). | [79] |
| IONPs | Thiolated β-cyclodextrin (β-CD-SH), through Fe–S bonding (TβCD-IONPs) | Doxorubicin (DOX), to yield DOX-TβCD-IONPs | XPS, drug release (modelled by Higuchi model) | (Targeted) cancer treatment | Ellipsoidal shape, ~14 nm. Cellular response dependent on IONPs’ functionalization. Tested against breast cancer cell line MCF-07. | [80] |
| MNP (Fe3O4) | Citrate coating | – | XRD, TEM, magnetic measurements (VSM) | Biomedical applications; drug delivery, imaging diagnostic (especially in liver, where iron accumulates) | In vivo models showed increased iron content in liver. No viability issues against different cell lines (HaCaT and HepG2). | [81] |
| Luminescent, LMNPs Fe3O4@BaMoO4:Eu3+ (LMNPs) | 3-aminopropyl-triethoxysilane (APTES). β-cyclodextrin (β-CD) | Triazole derivatives | XRD, FTIR, PL, TEM, VSM | Drug carrier | Hybrid nanoparticle system LMNPs@APES-CD had high drug loading of 61.69 mg/g | [82] |
| Fe3O4 | Polyethyleneimine, gold, silica, and graphene derivatives | dsDNA | STEM, ICP-MS/MS, UV-Vis, TGA, FTIR, charge (ζ-potential). ICP-MS/MS | DNA isolation | Fe3O4@PEI MNPs adsorbed DNA efficiently | [83] |
| Fe3O4 | –COOH coating | rtPA | SEM, TEM, XRD, EDX, VSM, FTIR | Thrombolytic nanomedicine | Efficient thrombolysis (rabbit carotid artery occlusion model) | [84] |
| Magnetic beads (MBs), ZnxFe3−xO4 nanoparticles (ZnFeNPs), 13 ± 3 nm, MS = 81 emu/g | Polymeric matrix of poly(lactic-co-glycolic) acid (PLGA), generating polymeric MBs that were covered with polyethyleneimine (PEI) (MB@PEI), obtaining particles of 96 ± 16 nm | Affinity protein neutravidin (NAV), by glutaraldehyde crosslinking | TEM, HRTEM, DLS, XRD, FTIR, ICP-MS | Biosensing (detection of Tau protein) | MB@NAV has high MS (higher than commercial NAV formulation). Alzheimer’s disease biomarker (Tau protein) could be detected using MB@NAV at very low 63 mg/mL | [85] |
| Fe3O4 | Silica, double-chain surfactant, ionic liquids (ILs) | Epirubicin hydrochloride (EPI) | XRD, FT-IR TG, TEM. liquid chromatography–fluorescence detection (LC-FL) | Analytical extraction methods | didodecyldimethylammonium bromide was the most effective surfactant for adsorption tests on MNPs | [86] |
| NixFe3−xO4 NPs (x = 0.0, 0.2, 0.4, 0.6, 0.8, and 1.0) | Aminosilane coating | – | XRD, Raman, EDX, FTIR, DLS | Cancer treatment (MCF7 and HeLa cell lines); drug delivery, hyperthermia | Fe2+ substitution by Ni2+, inverse spinel structure; hydrodynamic radii 10 nm (DLS). Coating decreases agglomeration and cell viability | [87] |
| IONPs | Oleic acid (OA) and tetraethylene glycol (TEG) | Dexamethasone (Dexa) | NMR, HR-MS, TEM, DLS, HPLC, | Biological applications, drug delivery | Novel encapsulation system based on triazole-derived micelle precursor | [88] |
| MNP (Fe3O4) | Hyperbranched polyglycerol and carboxymethyl cellulose, to Fe3O4@PG and Fe3O4@PG/CMC-PEG@DOX | Doxorubicin (DOX) | IR, NMR, TG, VSM, XRD, DLS, HR-TEM and UV–Vis | Drug delivery, anticancer, contrast agent in magnetic resonance imaging MRI | biocompatibility toward normal cells (HEK-293), high toxicity against cancerous cells (HeLa). | [89] |
| SPIONs (Fe3O4) | Copolyester, poly (globalide-co-ε-caprolactone) (PGlCL), modified with amino acid cysteine (Cys) via a thiol-ene reaction (PGlCLCys); folic acid (FA) | Methotrexate (MTX); conjugation achieved via -NH2 group of cysteine | 1H NMR, Gel Permeation Chromatography (GPC), HPLC, FTIR, DSC, TEM/SAED, XRD, magnetic properties (VSM), TGA, DLS | Enzymatic release, antitumor nanoplatform (tested on tumor cells MDA-MB 231) | SPIONS (SPION@PGlCLCys) enable further conjugation with active biomolecules; drug loaded SPION@PGlCLCys_MTX obtained by carbodiimide-mediated coupling (amide bond) | [90] |
| Fe, MnFe, CoFe | Oleic acid, allyl amine (synthesis), polypropylene sulphide PPS-coating to produce PPS-MNPs (80 ± 15 nm) | DOX and CUR loaded PPS–MNPs | DLS, TEM, FTIR, XRD, VSM, TGA, Calorimetric magnetic fluid hyperthermia, UV-Vis (drug encapsulation efficiency), drug loading (HPLC) | Hyperthermia, Cancer treatment (tested on human epithelial cells HEK293) | MNPs of 8 nm, 12 nm and 16 nm prepared by seed-mediated method from M-acetylacetonates; high breast cancer cell death (95%) | [91] |
| MNP (Fe3O4) | 3-amino propyl triethoxy silane (APTES) coating to MNPs@SiO2/CMT (carboxymethyl tragacanth) | Doxorubicin (Dox) | FT-IR, SEM, EDX, TEM, XRD, VSM, TGA, and zeta potential, fluorescence microscopy | Anti-cancer | Effect studied on MCF7 human breast cancer cells | [92] |
| Fe3O4 | PEI-modified | Dopamine (DA), self-polymerized | FTIR, AFM, SEM, agarose gel electrophoresis, fluorescence microscopy, flow cytometry | Gene vector (DNA delivery) | Fe3O4@PDA@PEI modified NPs are stable hydrophilic NPs (50–150 nm) | [93] |
| MNPs (iron oxide, 18 nm diameter) | Mesoporous silica shell (93 nm diameter) | trans-resveratrol (post-silanization) | DLS, zeta potential, NMR, IR, magnetism (SQUID, ZFC-FC), fluorescence resonance energy transfer (FRET) | Biomedical (protein–ligand, immunoassay design) | Superparamagnetic behavior useful for magnetic bioseparation; high zeta-potential kept the mesoporous NPs in alkaline solution | [94] |
| magnetic nanocomposites (MNCs), nanocapsules (NCs) based on Fe3O4 | Oleic-acid-modified, Nylon-6 coated | Doxorubicin (Dox) | DLS, ζ-potential, FTIR, TEM, drug loading/release (UV-Vis), cytotoxicity studies | Anticancer; drug delivery (against A549 and HEK 293FT cell lines) | High DOX loading: 732 µg/mg (DOX/MNC) and 943 µg/mg (DOX/NC); pH-sensitive drug release | [95] |
| Fe3O4 nanoclusters | Stabilizers: 3,4-dihydroxybenzhydrazide (DHBH) and poly [3,4-dihydroxybenzhydrazide] (PDHBH) | – | TEM, XPS, FTIR, VSM (magnetization) | Anticancer, hyperthermia (human normal dermal fibroblasts-BJ, colon adenocarcinoma-CACO2, and melanoma-A375) | In situ solvothermal process of MNPs-magnetite nanoclusters, MNC of 50 emu/g and 60 emu/g | [96] |
| Cobalt-Iron Ferrite Nanoparticles CoxFe1−xFe2O4 (x = 0.0, 0.2,0.4, 0.6, 0.8, and 1.0) | Surface modification with amino-silane (AEPTMS) | – | SEM, EDX, XRD, FTIR | Cytocompatibility, biomedical applications | MTT assay on fibroblast cells (cytotoxicity tests); Co/Fe ratio influences cytotoxicity | [97] |
| Fe3O4 | L-Cysteine (L-Cys)-coating | Doxorubicin (Dox) | EDS, SAED, XRD, FTIR, TEM. XPS, Mössbauer spectroscopy, SQUID | Drug delivery, anticancer (anti-melanoma) | Fe3O4-L-Cys-Dox NPs showed anti-melanoma activity on mouse (B16F10) and human (A375) metastatic melanoma | [98] |
| Application | Recent Advances | Market Solutions |
|---|---|---|
| Surface Functionalization Strategies | Advanced strategies like click chemistry, polymer brushes via ATRP, multifunctional coatings. | Established strategies like silane coupling agents and antibody immobilization; advanced strategies emerging in commercial products, like click chemistry-based functionalization kits. |
| Applications in Drug Delivery | Novel surface coatings and functional groups—enhanced drug loading, controlled release. | MNPs functionalized with specific ligands for targeted drug delivery. Some companies provide customizable options for specific drug payloads. |
| Magnetic Resonance Imaging (MRI) | Coatings with superior magnetic properties and responsive materials for improved contrast in MRI. | High-quality coatings, ensuring stability and improved imaging performance; some products integrate advanced surface modifications to enhance contrast. |
| Hyperthermia Treatment | Surface functionalization strategies to optimize heating efficiency for hyperthermia applications. | Commercial options with tailored surface properties to achieve precise heating of targeted tissues within physiologically relevant limits. |
| Diagnostic Applications | Improved diagnostic capabilities through enhanced binding specificity and signal amplification. | Surface functionalizations optimized for specific diagnostic assays, that may include pre-conjugated antibodies or customizable options for specific biomarker detection. |
| Multifunctionality | Recent research focuses on integrating multiple functionalities within a single NP platform. | Multifunctional MNPs that combine features like drug delivery, imaging, and targeting within a single particle, designed to streamline complex applications. |
| Environmental Remediation and Catalysis | Functionalized MNPs are being explored in catalysis and environmental remediation. | An emerging interest in functionalized MNPs for non-biomedical uses, with customizable options for research in these fields. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Comanescu, C. Recent Advances in Surface Functionalization of Magnetic Nanoparticles. Coatings 2023, 13, 1772. https://doi.org/10.3390/coatings13101772
Comanescu C. Recent Advances in Surface Functionalization of Magnetic Nanoparticles. Coatings. 2023; 13(10):1772. https://doi.org/10.3390/coatings13101772
Chicago/Turabian StyleComanescu, Cezar. 2023. "Recent Advances in Surface Functionalization of Magnetic Nanoparticles" Coatings 13, no. 10: 1772. https://doi.org/10.3390/coatings13101772
APA StyleComanescu, C. (2023). Recent Advances in Surface Functionalization of Magnetic Nanoparticles. Coatings, 13(10), 1772. https://doi.org/10.3390/coatings13101772