Effect of Microstructure on Electroless Ni Plating Behavior on Super Duplex Stainless Steel SAF2507 in Li-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
3. Results
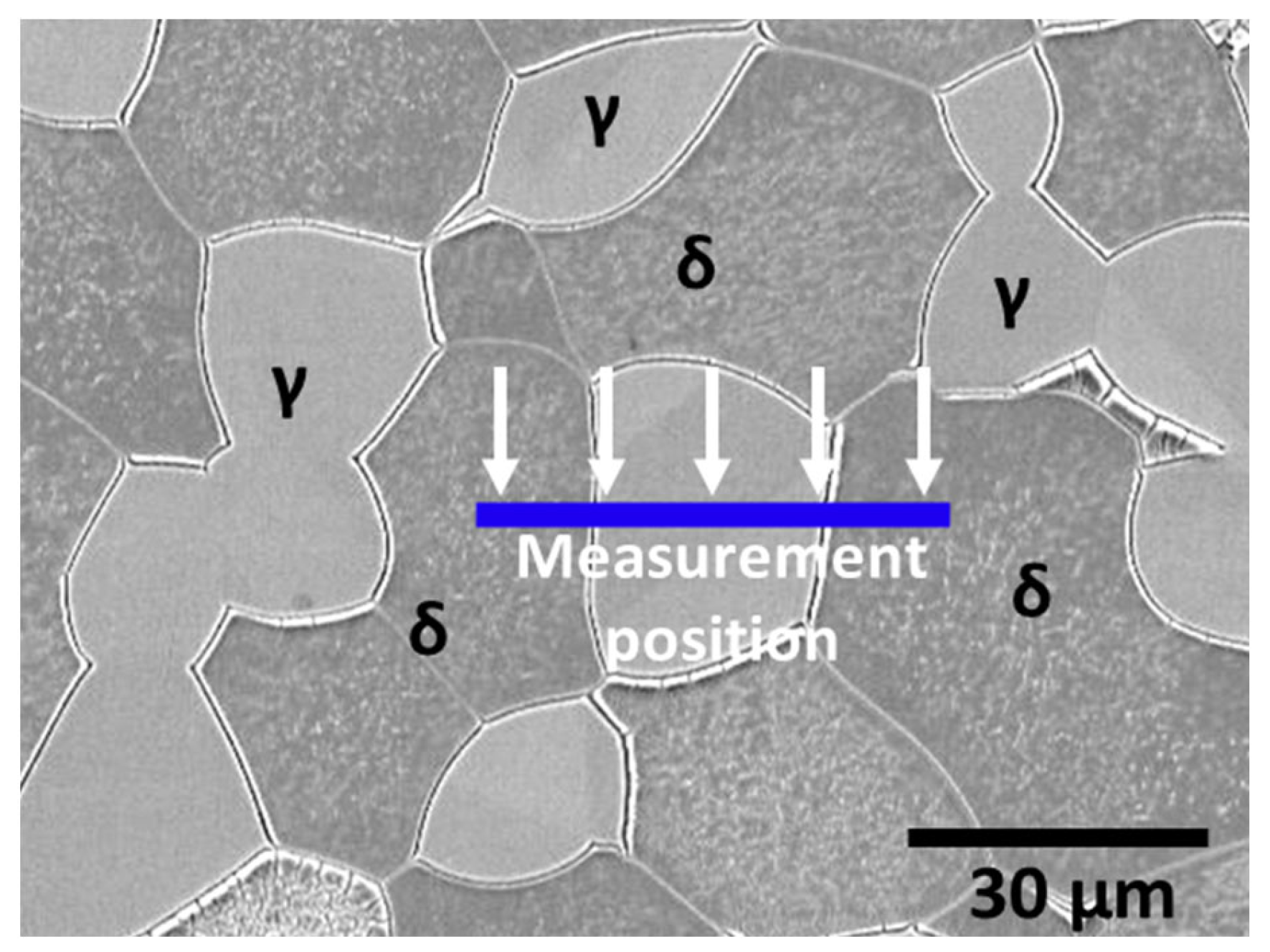
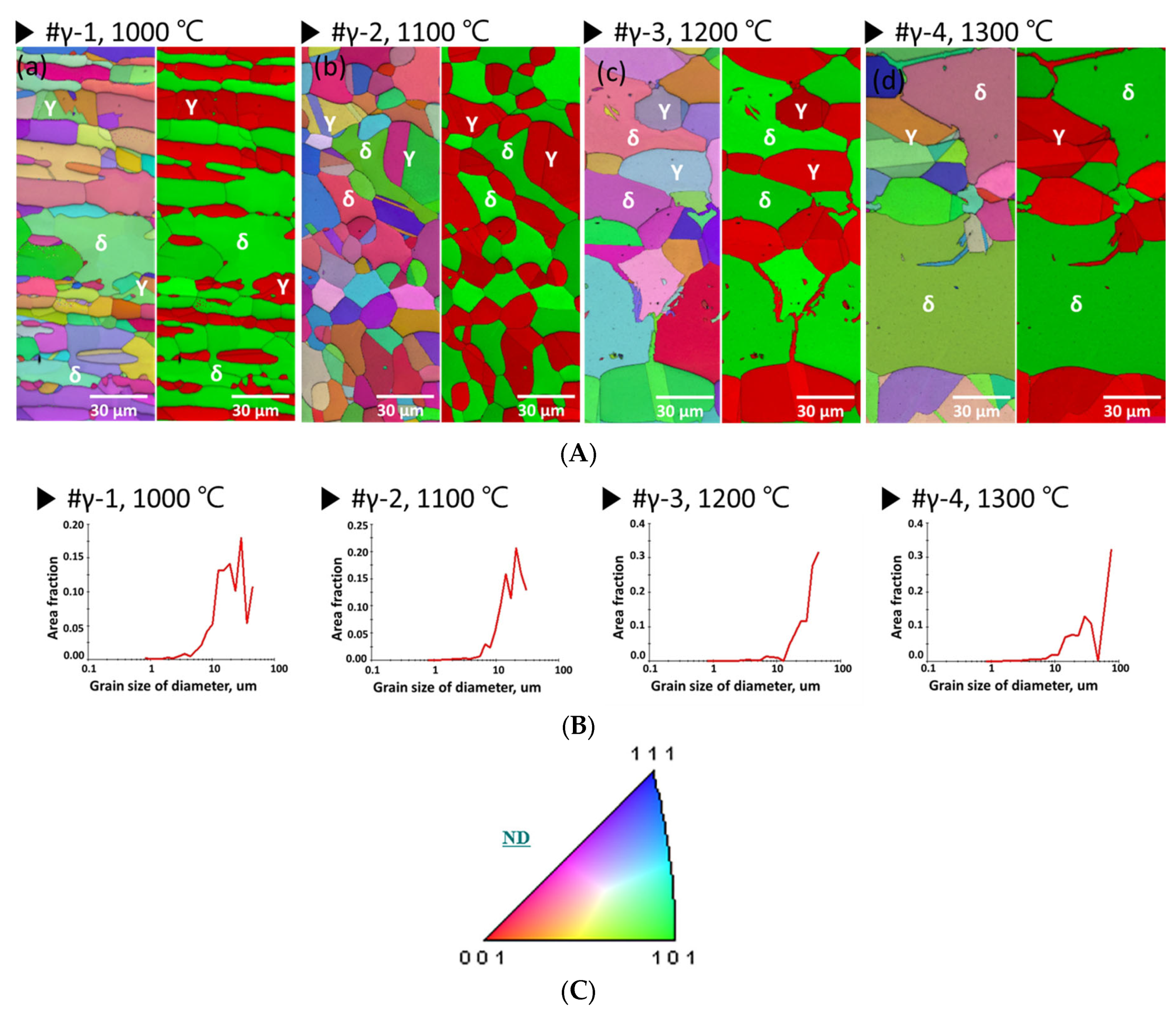
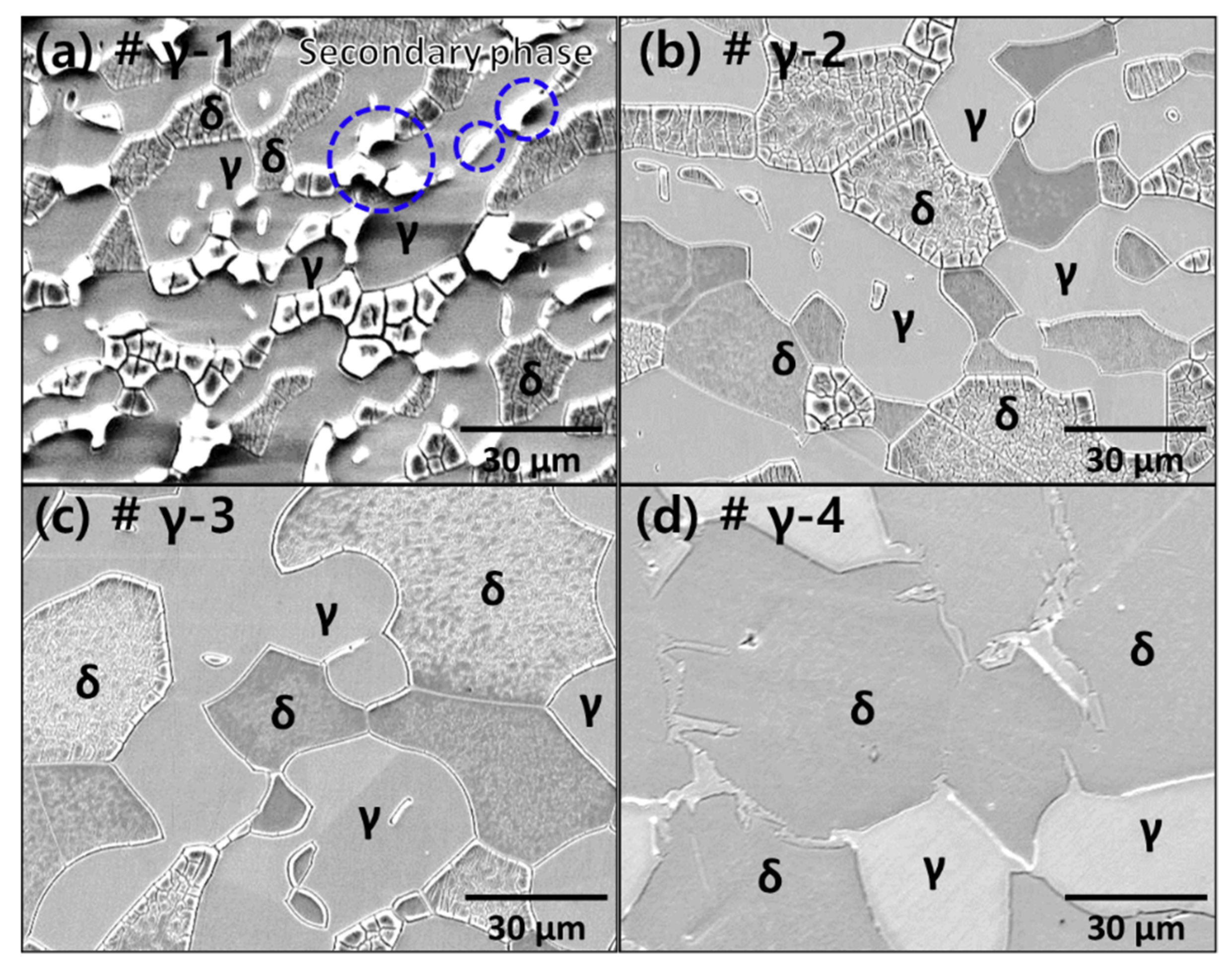
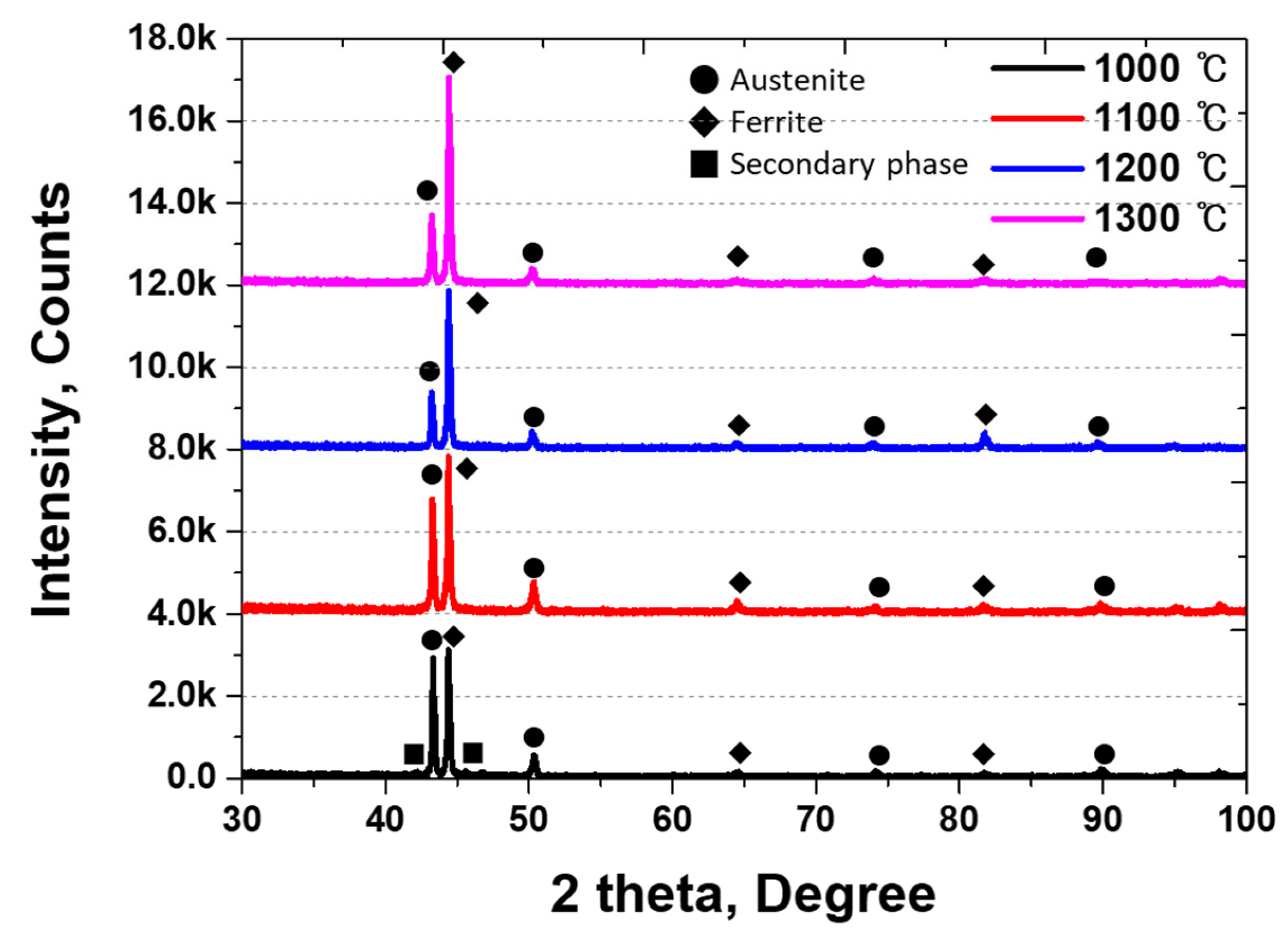
3.1. Microstructure of Ni-Deposited SDSS
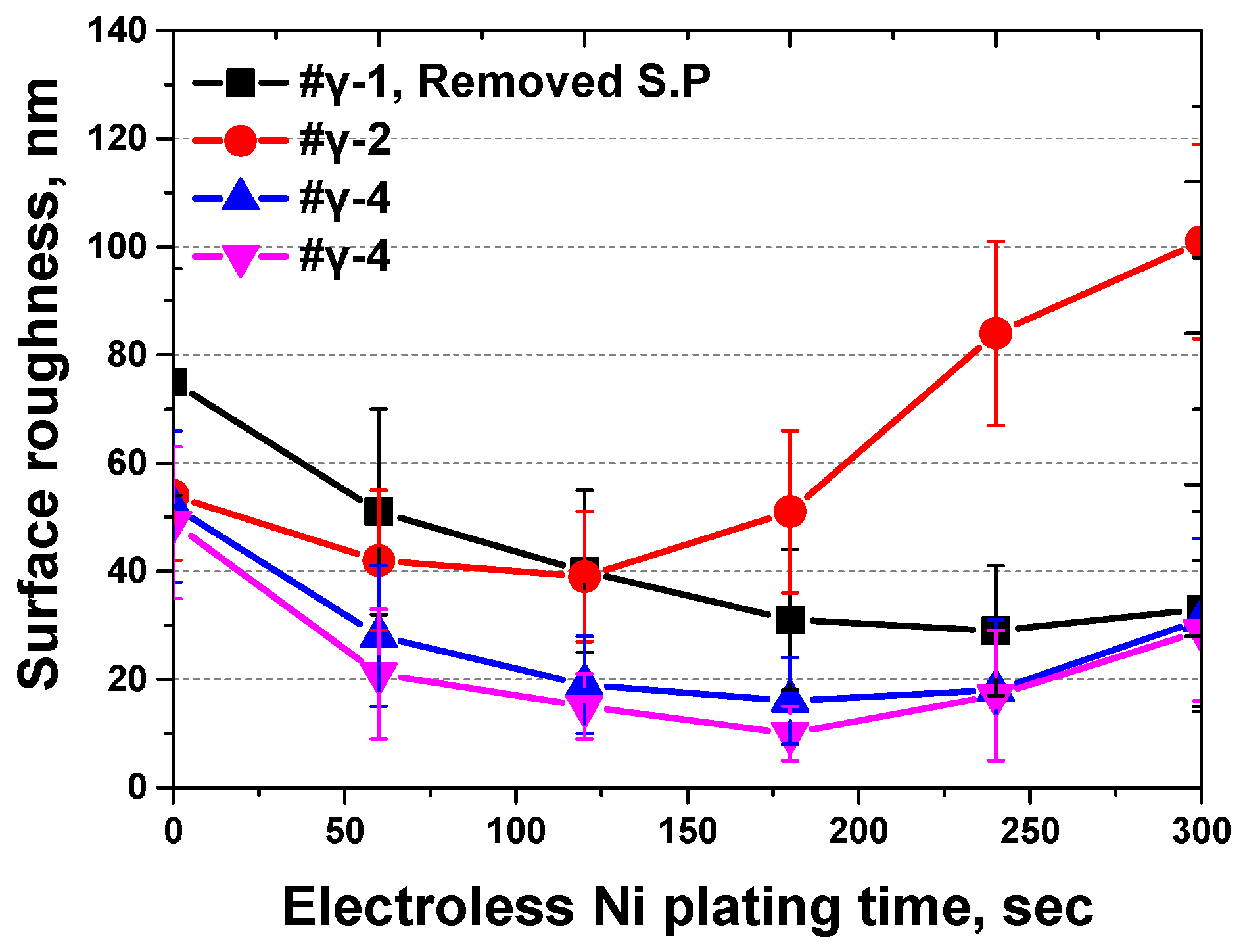
3.2. Electroless Ni Plating Behavior
3.3. Electrochemical Behavior
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahman, A.; Lin, X. Li-Ion Battery Individual Electrode State of Charge and Degradation Monitoring Using Battery Casing through Auto Curve Matching for Standard CCCV Charging Profile. Appl. Energy 2022, 321, 119367. [Google Scholar] [CrossRef]
- Tudoroiu, R.-E.; Zaheeruddin, M.; Tudoroiu, N.; Radu, S.M.; Chammas, H. Investigations of Different Approaches for Controlling the Speed of an Electric Motor with Nonlinear Dynamics Powered by a Li-Ion Battery-Case Study. In Electric Vehicles—Design, Modelling and Simulation; Intechopen: London, UK, 2023. [Google Scholar]
- Chang, W.-S.; Park, C.-M.; Kim, J.-H.; Kim, Y.-U.; Jeong, G.; Sohn, H.-J. Quartz (SiO2): A New Energy Storage Anode Material for Li-Ion Batteries. Energy Env. Sci. 2012, 5, 6895–6899. [Google Scholar] [CrossRef]
- Mayyas, A.; Steward, D.; Mann, M. The Case for Recycling: Overview and Challenges in the Material Supply Chain for Automotive Li-Ion Batteries. Sustain. Mater. Technol. 2019, 19, e00087. [Google Scholar] [CrossRef]
- Petit, M.; Prada, E.; Sauvant-Moynot, V. Development of an Empirical Aging Model for Li-Ion Batteries and Application to Assess the Impact of Vehicle-to-Grid Strategies on Battery Lifetime. Appl. Energy 2016, 172, 398–407. [Google Scholar] [CrossRef]
- Tobolsky, A.; Eyring, H. Mechanical Properties of Polymeric Materials. J. Chem. Phys. 1943, 11, 125–134. [Google Scholar] [CrossRef]
- Tjong, S.C. Structural and Mechanical Properties of Polymer Nanocomposites. Mater. Sci. Eng. R Rep. 2006, 53, 73–197. [Google Scholar] [CrossRef]
- Jacob, G.C.; Starbuck, J.M.; Fellers, J.F.; Simunovic, S.; Boeman, R.G. Strain Rate Effects on the Mechanical Properties of Polymer Composite Materials. J. Appl. Polym. Sci. 2004, 94, 296–301. [Google Scholar] [CrossRef]
- Rana, R.S.; Purohit, R.; Das, S. Reviews on the Influences of Alloying Elements on the Microstructure and Mechanical Properties of Aluminum Alloys and Aluminum Alloy Composites. Int. J. Sci. Res. Publ. 2012, 2, 1–7. [Google Scholar]
- Prasad, D.S.; Shoba, C.; Ramanaiah, N. Investigations on Mechanical Properties of Aluminum Hybrid Composites. J. Mater. Res. Technol. 2014, 3, 79–85. [Google Scholar] [CrossRef]
- Manesh, H.D.; Taheri, A.K. The Effect of Annealing Treatment on Mechanical Properties of Aluminum Clad Steel Sheet. Mater. Des. 2003, 24, 617–622. [Google Scholar] [CrossRef]
- Fredriksson, W.; Edström, K. XPS Study of Duplex Stainless Steel as a Possible Current Collector in a Li-Ion Battery. Electrochim. Acta 2012, 79, 82–94. [Google Scholar] [CrossRef]
- Masarapu, C.; Subramanian, V.; Zhu, H.; Wei, B. Long-Cycle Electrochemical Behavior of Multiwall Carbon Nanotubes Synthesized on Stainless Steel in Li Ion Batteries. Adv. Funct. Mater. 2009, 19, 1008–1014. [Google Scholar] [CrossRef]
- Pettersson, N.; Pettersson, R.F.A.; Wessman, S. Precipitation of Chromium Nitrides in the Super Duplex Stainless Steel 2507. Metall. Mater. Trans. A 2015, 46, 1062–1072. [Google Scholar] [CrossRef]
- Valeriano, L.d.C.; Correa, E.O.; Mariano, N.A.; Robin, A.L.M.; Machado, M.A.G. Influence of the Solution-Treatment Temperature and Short Aging Times on the Electrochemical Corrosion Behaviour of Uns S32520 Super Duplex Stainless Steel. Mater. Res. 2019, 22. [Google Scholar] [CrossRef]
- Nilsson, J.-O. Super Duplex Stainless Steels. Mater. Sci. Technol. 1992, 8, 685–700. [Google Scholar] [CrossRef]
- Topolska, S.; Łabanowski, J. Effect of Microstructure on Impact Toughness of Duplex and Superduplex Stainless Steels. J. Achiev. Mater. Manuf. Eng. 2009, 36, 142–149. [Google Scholar]
- Tehovnik, F.; Arzensek, B.; Arh, B.; Skobir, D.; Pirnar, B.; Zuzek, B. Microstructure Evolution in SAF 2507 Super Duplex Stainless Steel. Mater. Technol. 2011, 45, 339–345. [Google Scholar]
- Nilsson, J.O.; Wilson, A. Influence of Isothermal Phase Transformations on Toughness and Pitting Corrosion of Super Duplex Stainless Steel SAF 2507. Mater. Sci. Technol. 1993, 9, 545–554. [Google Scholar] [CrossRef]
- Paulraj, P.; Garg, R. Effect of Intermetallic Phases on Corrosion Behavior and Mechanical Properties of Duplex Stainless Steel and Super-Duplex Stainless Steel. Adv. Sci. Technol. Res. J. 2015, 9, 87–105. [Google Scholar]
- Valiente Bermejo, M.A.; Thalavai Pandian, K.; Axelsson, B.; Harati, E.; Kisielewicz, A.; Karlsson, L. Microstructure of Laser Metal Deposited Duplex Stainless Steel: Influence of Shielding Gas and Heat Treatment. Weld. World 2021, 65, 525–541. [Google Scholar] [CrossRef]
- Trinh, L.N.; Lee, D. The Characteristics of Laser Welding of a Thin Aluminum Tab and Steel Battery Case for Lithium-Ion Battery. Metals 2020, 10, 842. [Google Scholar] [CrossRef]
- Beziou, O.; Hamdi, I.; Boumerzoug, Z.; Brisset, F.; Baudin, T. Effect of Heat Treatment on the Welded Joint of X70 Steel Joined to Duplex Stainless Steel by Gas Tungsten Arc Welding. Int. J. Adv. Manuf. Technol. 2023, 127, 2799–2814. [Google Scholar] [CrossRef]
- Elhoud, A.M.; Renton, N.C.; Deans, W.F. Hydrogen Embrittlement of Super Duplex Stainless Steel in Acid Solution. Int. J. Hydrogen Energy 2010, 35, 6455–6464. [Google Scholar] [CrossRef]
- Kannan, A.R.; Shanmugam, N.S.; Rajkumar, V.; Vishnukumar, M. Insight into the Microstructural Features and Corrosion Properties of Wire Arc Additive Manufactured Super Duplex Stainless Steel (ER2594). Mater. Lett. 2020, 270, 127680. [Google Scholar] [CrossRef]
- Maurya, A.K.; Pandey, C.; Chhibber, R. Effect of Filler Metal Composition on Microstructural and Mechanical Characterization of Dissimilar Welded Joint of Nitronic Steel and Super Duplex Stainless Steel. Arch. Civ. Mech. Eng. 2022, 22, 90. [Google Scholar] [CrossRef]
- Lee, H.-B.; Sheu, H.-H.; Jian, J.-S.; Chang, S.-Y.; Yen, C.-H.; Lin, H.-E. Supercritical-CO2 Electroless Nickel Plating Enhanced Anti-Corrosion Properties of Micro-Arc Oxidized AZ31 Magnesium Alloy. Mater. Today Commun. 2022, 33, 104475. [Google Scholar] [CrossRef]
- Wang, D.; Li, F.; Liu, M.; Zhang, W.; Yu, X.; Da, W. Effect of Nanodiamond Content in the Plating Solution on the Corrosion Resistance of Nickel–Nanodiamond Composite Coatings Prepared on Annealed 45 Carbon Steel. Coatings 2022, 12, 1558. [Google Scholar] [CrossRef]
- Loto, C.A. Electroless Nickel Plating–A Review. Silicon 2016, 8, 177–186. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, D.; Son, K.; Lee, S.; Chung, W. Effect of Added Dispersants on Diamond Particles in Ni-Diamond Composites Fabricated with Electrodeposition. Met. Mater. Int. 2015, 21, 977–984. [Google Scholar] [CrossRef]
- Di Bari, G.A. Nickel Plating. Met. Finish. 1994, 9, 270–276. [Google Scholar]
- Baudrand, D.W. Electroless Nickel Plating; Cambridge Scientific Abstracts: Bethesda, ML, USA, 1994. [Google Scholar]
- Rybalka, K.V.; Beketaeva, L.A.; Davydov, A.D. Electrochemical Behavior of Stainless Steel in Aerated NaCl Solutions by Electrochemical Impedance and Rotating Disk Electrode Methods. Russ. J. Electrochem. 2006, 42, 370–374. [Google Scholar] [CrossRef]
- Baghdadchi, A.; Hosseini, V.A.; Valiente Bermejo, M.A.; Axelsson, B.; Harati, E.; Högström, M.; Karlsson, L. Wire Laser Metal Deposition of 22% Cr Duplex Stainless Steel: As-Deposited and Heat-Treated Microstructure and Mechanical Properties. J. Mater. Sci. 2022, 57, 9556–9575. [Google Scholar] [CrossRef]
- Ha, H.-Y.; Jang, M.-H.; Lee, T.-H.; Moon, J. Interpretation of the Relation between Ferrite Fraction and Pitting Corrosion Resistance of Commercial 2205 Duplex Stainless Steel. Corros. Sci. 2014, 89, 154–162. [Google Scholar] [CrossRef]
- Martins, M.; Casteletti, L.C. Sigma Phase Morphologies in Cast and Aged Super Duplex Stainless Steel. Mater. Charact. 2009, 60, 792–795. [Google Scholar] [CrossRef]
- Sung, C.; Shin, B.-H.; Chung, W. Effect of Solution Annealing on Austenite Morphology and Pitting Corrosion of Super Duplex Stainless Steel UNS S 32750. Int. J. Electrochem. Sci. 2021, 16, 210813. [Google Scholar] [CrossRef]
- Sung, C.; Kim, K.; Chung, W.; Shin, B.-H. Electrochemical Properties of UNS S 32750 and UNS S 32760 Annealed Super Duplex Stainless Steels. Int. J. Electrochem. Sci. 2022, 17, 220526. [Google Scholar] [CrossRef]
- Kim, D.; Kim, K.; Chung, W.; Shin, B.-H. Effect of the Plating Time on Nickel Electroless Coating Properties Deposited on the Super Duplex Stainless Steel UNS S 32750. Int. J. Electrochem. Sci. 2022, 17, 220630. [Google Scholar] [CrossRef]
- Goksu, E.I.; Vanegas, J.M.; Blanchette, C.D.; Lin, W.-C.; Longo, M.L. AFM for Structure and Dynamics of Biomembranes. Biochim. Et Biophys. Acta (BBA) Biomembr. 2009, 1788, 254–266. [Google Scholar] [CrossRef]
- Bogdan, D.; Grosu, I.-G.; Filip, C. How Thick, Uniform and Smooth Are the Polydopamine Coating Layers Obtained under Different Oxidation Conditions? An in-Depth AFM Study. Appl. Surf. Sci. 2022, 597, 153680. [Google Scholar] [CrossRef]
- Guerrini, E.; Cristiani, P.; Grattieri, M.; Santoro, C.; Li, B.; Trasatti, S. Electrochemical Behavior of Stainless Steel Anodes in Membraneless Microbial Fuel Cells. J. Electrochem. Soc. 2013, 161, H62. [Google Scholar] [CrossRef]
- Makhdoom, M.A.; Ahmad, A.; Kamran, M.; Abid, K.; Haider, W. Microstructural and Electrochemical Behavior of 2205 Duplex Stainless Steel Weldments. Surf. Interfaces 2017, 9, 189–195. [Google Scholar] [CrossRef]
- Korhonen, T.; Puska, M.J.; Nieminen, R.M. Vacancy-Formation Energies for FCC and Bcc Transition Metals. Phys. Rev. B 1995, 51, 9526. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Grover, A.K.; Dey, G.K.; Totlani, M.K. Nanocrystalline Ni–Cu Alloy Plating by Pulse Electrolysis. Surf. Coat. Technol. 2000, 126, 48–63. [Google Scholar] [CrossRef]
- Rehman, A.U.; Lee, S.H. Review of the Potential of the Ni/Cu Plating Technique for Crystalline Silicon Solar Cells. Materials 2014, 7, 1318–1341. [Google Scholar] [CrossRef]

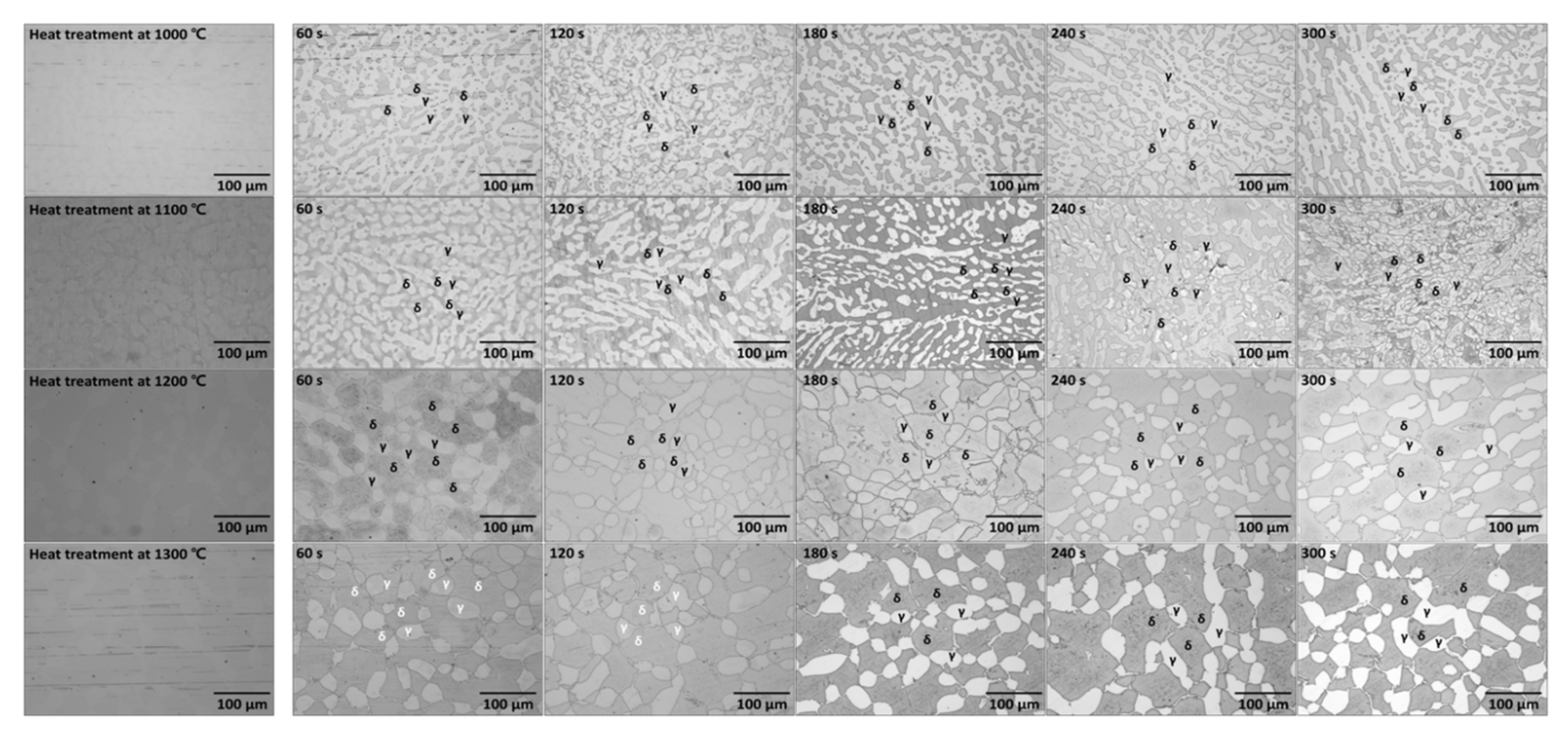
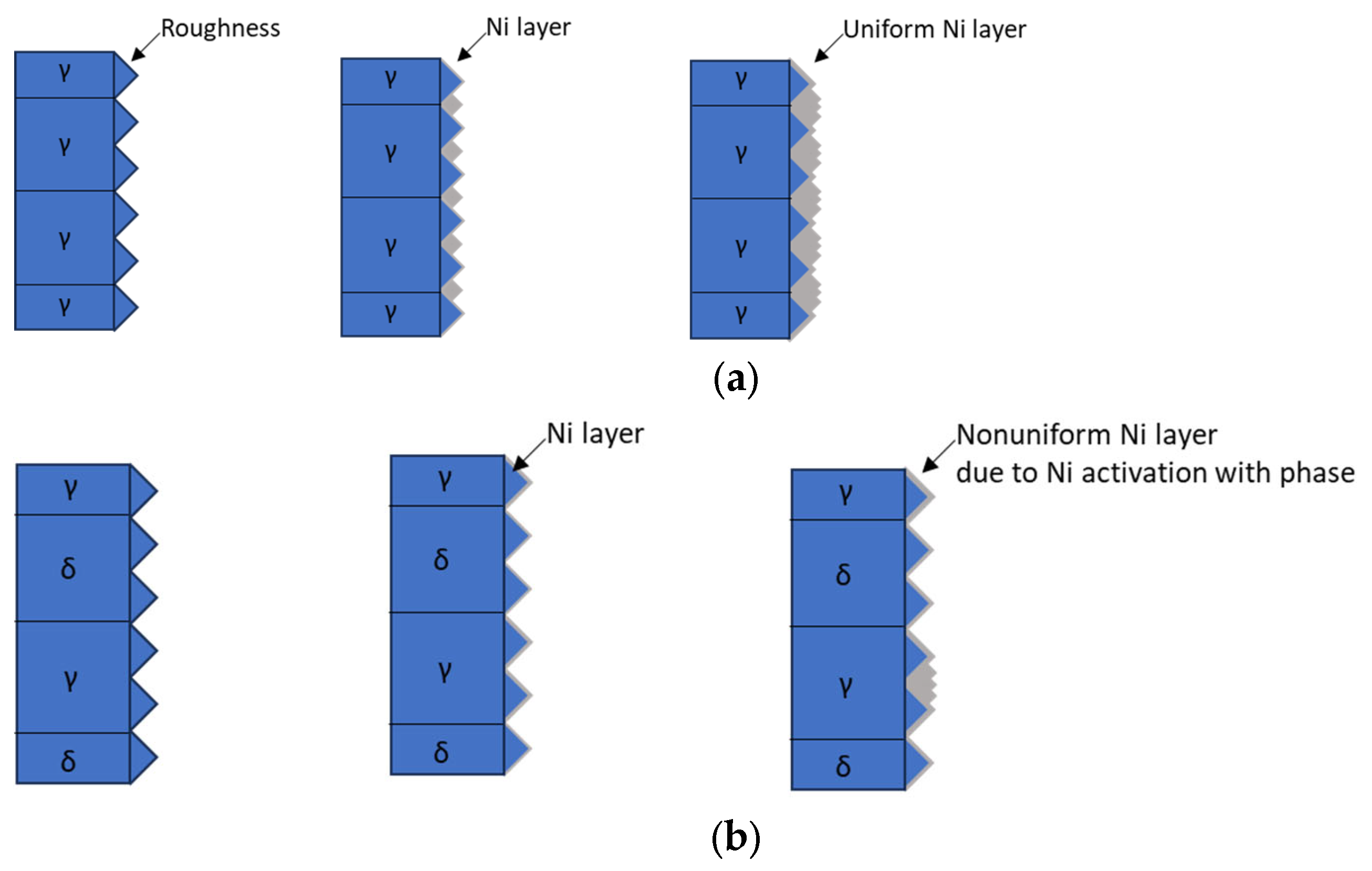
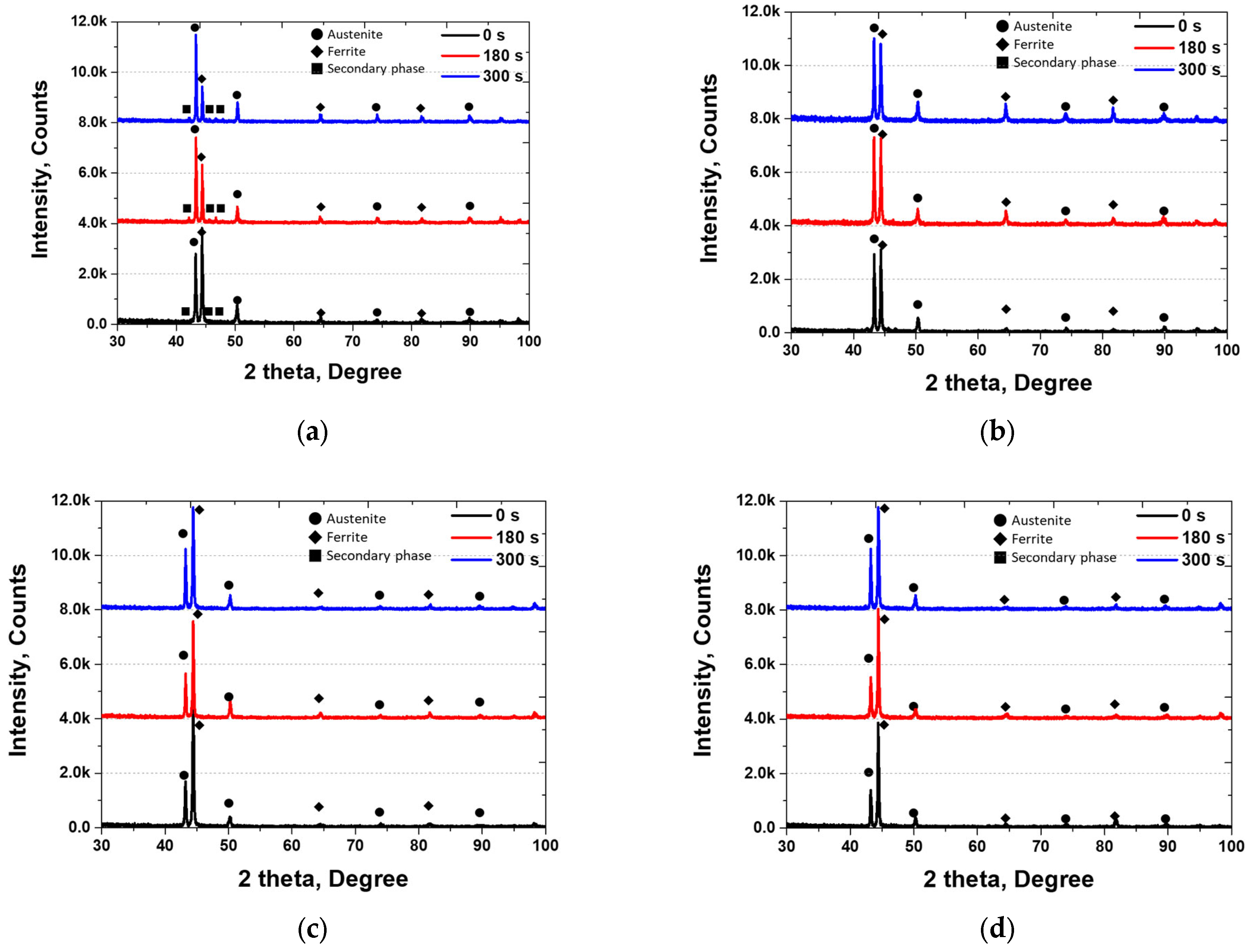

| C | N | Mn | Ni | Cr | Mo | Fe | |
|---|---|---|---|---|---|---|---|
| Total | 0.01 | 0.27 | 0.8 | 6.8 | 25.0 | 3.8 | Bal |
| Austenite | 0.01 | 0.51 | 0.9 | 5.5 | 23.3 | 3.2 | Bal |
| Ferrite | 0.01 | 0.05 | 0.8 | 7.9 | 26.6 | 4.4 | Bal |
| Condition | Properties | Volume Fraction | ||
|---|---|---|---|---|
| Austenite | Ferrite | Secondary Phase | ||
| (a) # γ-1, 1000 °C | Secondary phase | 56 ± 1% | 26 ± 1% | 18 ± 1% |
| (b) # γ-2, 1100 °C | Solution annealing | 50 ± 1% | 50 ± 1% | 0% |
| (c) # γ-3, 1200 °C | Over ferrite 60% | 39 ± 2% | 61 ± 2% | 0% |
| (d) # γ-4, 1300 °C | Over ferrite 70% | 28 ± 3% | 72 ± 3% | 0% |
| Ra, nm | 0 s | 60 s | 120 s | 180 s | 240 s | 300 s |
|---|---|---|---|---|---|---|
| # γ-1 | 1675 ± 221 | 1751 ± 219 | 1540 ± 215 | 1431 ± 213 | 1529 ± 212 | 1633 ± 218 |
| # γ-1 (Removed S.P) | 73 ± 21 | 49 ± 19 | 46 ± 15 | 36 ± 13 | 31 ± 12 | 32 ± 18 |
| # γ-2 | 54 ± 12 | 42 ± 13 | 39 ± 12 | 51 ± 15 | 84 ± 17 | 101 ± 18 |
| # γ-3 | 52 ± 14 | 28 ± 13 | 19 ± 9 | 16 ± 8 | 18 ± 13 | 31 ± 15 |
| # γ-4 | 49 ± 14 | 21 ± 12 | 15 ± 6 | 10 ± 5 | 17 ± 12 | 29 ± 13 |
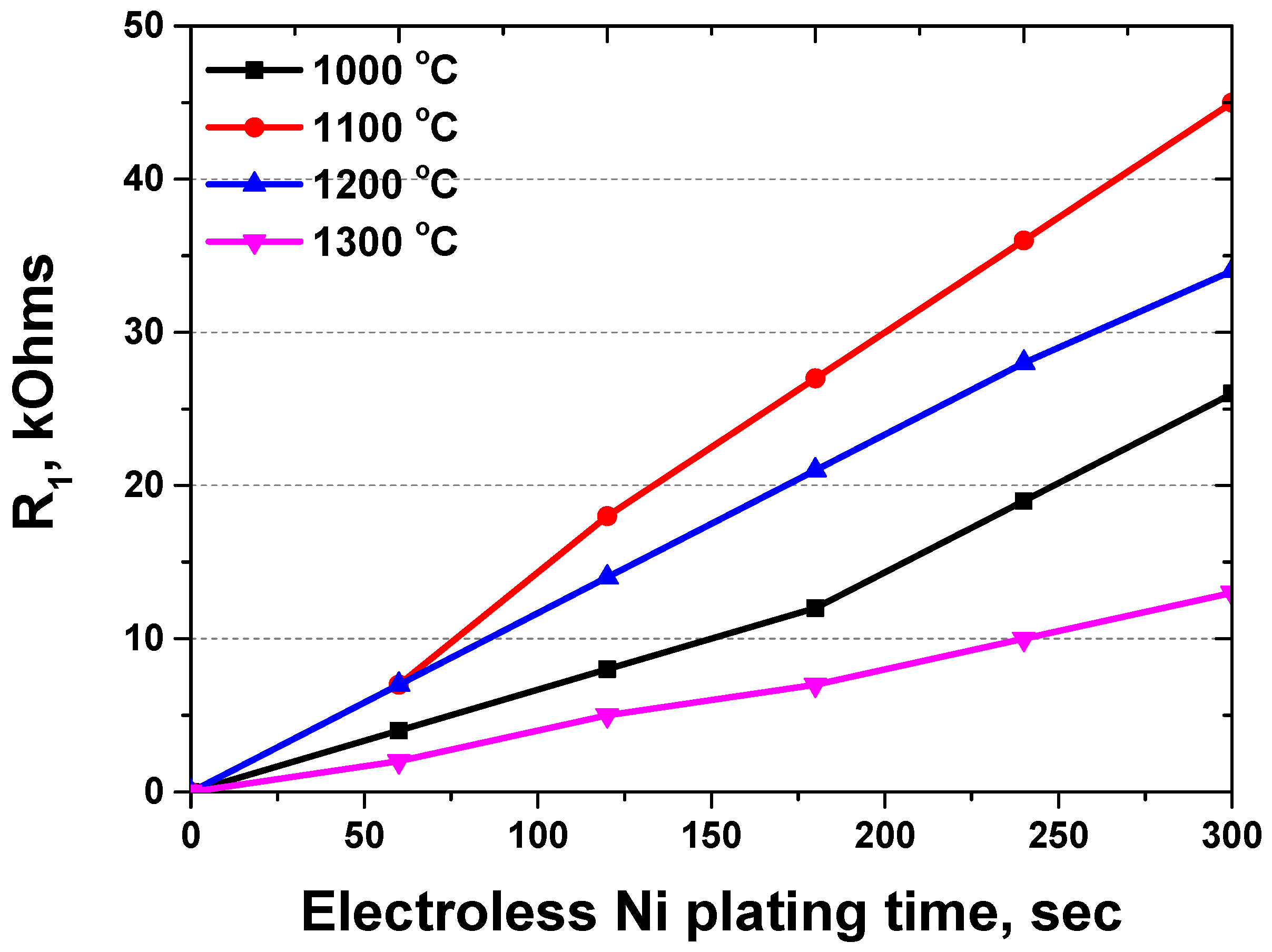
| Condition | Rs | CPE1 | R1 (kOhms) | CPE2 | R2 (kOhms) | |||
|---|---|---|---|---|---|---|---|---|
| C1 | N1 | C2 | N2 | |||||
| # γ-1 | 0 s | 0.1 | 0 | 0 | 0 | 6.5 × 10−5 | 0.5 | 326 |
| 60 s | 0.1 | 0.6 × 10−4 | 0.6 | 4 | 6.5 × 10−5 | 0.5 | 326 | |
| 120 s | 0.1 | 1.2 × 10−4 | 0.6 | 8 | 6.5 × 10−5 | 0.5 | 326 | |
| 180 s | 0.1 | 2.0 × 10−4 | 0.6 | 12 | 6.5 × 10−5 | 0.5 | 326 | |
| 240 s | 0.1 | 3.1 × 10−4 | 0.6 | 19 | 6.5 × 10−5 | 0.5 | 326 | |
| 300 s | 0.1 | 4.3 × 10−4 | 0.6 | 26 | 6.5 × 10−5 | 0.5 | 326 | |
| # γ-2 | 0 s | 0.1 | 0 | 0 | 0 | 6.8 × 10−5 | 0.5 | 340 |
| 60 s | 0.1 | 1.1 × 10−4 | 0.6 | 7 | 6.8 × 10−5 | 0.5 | 340 | |
| 120 s | 0.1 | 3.0 × 10−4 | 0.6 | 18 | 6.8 × 10−5 | 0.5 | 340 | |
| 180 s | 0.1 | 4.5 × 10−4 | 0.6 | 27 | 6.8 × 10−5 | 0.5 | 340 | |
| 240 s | 0.1 | 6.0 × 10−4 | 0.6 | 36 | 6.8 × 10−5 | 0.5 | 340 | |
| 300 s | 0.1 | 7.5 × 10−4 | 0.6 | 45 | 6.8 × 10−5 | 0.5 | 340 | |
| # γ-3 | 0 s | 0.1 | 0 | 0 | 0 | 6.7 × 10−5 | 0.5 | 335 |
| 60 s | 0.1 | 1.1 × 10−4 | 0.6 | 7 | 6.7 × 10−5 | 0.5 | 335 | |
| 120 s | 0.1 | 2.3 × 10−4 | 0.6 | 14 | 6.7 × 10−5 | 0.5 | 335 | |
| 180 s | 0.1 | 3.5 × 10−4 | 0.6 | 21 | 6.7 × 10−5 | 0.5 | 335 | |
| 240 s | 0.1 | 4.7 × 10−4 | 0.6 | 28 | 6.7 × 10−5 | 0.5 | 335 | |
| 300 s | 0.1 | 5.7 × 10−4 | 0.6 | 34 | 6.7 × 10−5 | 0.5 | 335 | |
| # γ-4 | 0 s | 0.1 | 0 | 0 | 0 | 6.6 × 10−5 | 0.5 | 325 |
| 60 s | 0.1 | 0.4 × 10−4 | 0.6 | 2 | 6.6 × 10−5 | 0.5 | 325 | |
| 120 s | 0.1 | 0.8 × 10−4 | 0.6 | 5 | 6.6 × 10−5 | 0.5 | 325 | |
| 180 s | 0.1 | 1.2 × 10−4 | 0.6 | 7 | 6.6 × 10−5 | 0.5 | 325 | |
| 240 s | 0.1 | 1.6 × 10−4 | 0.6 | 10 | 6.6 × 10−5 | 0.5 | 325 | |
| 300 s | 0.1 | 2.2 × 10−4 | 0.6 | 13 | 6.6 × 10−5 | 0.5 | 325 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, B.-H.; Kim, D.; Kim, D.-I.; Lee, W.; Kwon, S.-H. Effect of Microstructure on Electroless Ni Plating Behavior on Super Duplex Stainless Steel SAF2507 in Li-Ion Batteries. Coatings 2023, 13, 1807. https://doi.org/10.3390/coatings13101807
Shin B-H, Kim D, Kim D-I, Lee W, Kwon S-H. Effect of Microstructure on Electroless Ni Plating Behavior on Super Duplex Stainless Steel SAF2507 in Li-Ion Batteries. Coatings. 2023; 13(10):1807. https://doi.org/10.3390/coatings13101807
Chicago/Turabian StyleShin, Byung-Hyun, Dohyung Kim, Doo-In Kim, Wookjin Lee, and Se-Hun Kwon. 2023. "Effect of Microstructure on Electroless Ni Plating Behavior on Super Duplex Stainless Steel SAF2507 in Li-Ion Batteries" Coatings 13, no. 10: 1807. https://doi.org/10.3390/coatings13101807






