A Comparative Thermodynamic Study of AlF3, ScF3, Al0.5Sc0.5F3, and In0.5Sc0.5F3 for Optical Coatings: A Computational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Crystallographic Inputs
2.2. Calculation of Thermal Properties
3. Results and Discussion
3.1. Structural Properties
3.2. Thermal Properties
| Material | |||
|---|---|---|---|
| 80.73 (75.1) [50] | 79.71 | (−4.38)–(−5.15) | |
| 82.21 (83.5) [51] | 81.15 | (−19.2)–(−28.67) | |
| 120.40 | 119.35 | (−2.33)–(−3.58) | |
| 56.21 | 55.23 | (−0.684)–(−1.34) | |
| 955.6 (980) [8] | 951.1 | - |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Piegari, A.; Flory, F. Optical Thin Films and Coatings: From Materials to Applications; Series in Electronic and Optical Materials; Woodhead Publishing: Cambridge, UK, 2008. [Google Scholar]
- Thelen, A. Design strategies for thin film optical coatings. In Thin Films on Glass; Bach, H., Krause, D., Eds.; Schott Series on Glass and Glass Ceramics; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar] [CrossRef]
- Stenzel, O. Optical Coatings: Material Aspects in Theory and Practice; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Ristau, D.; Ehlers, H. Thin Film Optical Coatings. In Springer Handbook of Lasers and Optics; Träger, F., Ed.; Springer: New York, NY, USA, 2007. [Google Scholar] [CrossRef]
- Lisitsyn, V.M.; Lisitsyna, L.A.; Popov, A.I.; Kotomin, E.A.; Abuova, F.U.; Akilbekov, A.; Maier, J. Stabilization of primary mobile radiation defects in MgF2 crystals. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2016, 374, 24–28. [Google Scholar] [CrossRef]
- Pankratova, V.; Purans, J.; Pankratov, V. Low-temperature luminescence of ScF3 single crystals under excitation by VUV synchrotron radiation. Low Temp. Phys. 2020, 46, 1196–1200. [Google Scholar] [CrossRef]
- Fritz, C.; Scholz, G.; Feista, M.; Kemnitz, E. Preparation and stabilization of aluminium trifluoroacetate fluoride sols for optical coatings. Dalton Trans. 2007, 41, 11351–11360. [Google Scholar] [CrossRef] [PubMed]
- Cotter, T.M.; Thomas, M.E.; Tropf, W.J. Magnesium Fluoride (MgF2). In Handbook of Optical Constants of Solids; Palik, E.D., Ed.; Academic Press: London, UK, 1997; pp. 899–918. [Google Scholar] [CrossRef]
- Golota, A.F.; Khoroshilova, S.E.; Tarala, L.V.; Evtushenko, E.A. Compacted magnesium fluoride: Preparation, characterization, and optics. Russ. J. Inorg. Chem. 2019, 64, 705–709. [Google Scholar] [CrossRef]
- Uy, A. Atomic Layer Deposition of Aluminum Fluoride for Use in Optical Devices. Ph.D. Thesis, University of Maryland, College Park, MD, USA, 2022. [Google Scholar] [CrossRef]
- Hennessy, J.; Jewell, A.D.; Balasubramanian, K.; Nikzad, S. Ultraviolet optical properties of aluminum fluoride thin films deposited by atomic layer deposition. J. Vac. Sci. Technol. A 2016, 34, 01A120. [Google Scholar] [CrossRef]
- Chernoburova, O.; Chagnes, A. The future of scandium recovery from wastes. Mater. Proc. 2021, 5, 55. [Google Scholar] [CrossRef]
- Smith, W.J. Modern Optical Engineering, 3rd ed.; McGraw-Hill: New York, NY, USA, 2000. [Google Scholar]
- Paschotta, R. Optical Materials. RP Photonics Encyclopedia. Available online: https://www.rp-photonics.com/optical_materials.html (accessed on 11 August 2023).
- Hołyńska, M.; Tighe, A.; Semprimoschnig, C. Coatings and thin films for spacecraft thermo-optical and related functional applications. Adv. Mater. Interfaces 2018, 5, 1701644. [Google Scholar] [CrossRef]
- Materials Project Database. Available online: https://next-gen.materialsproject.org/materials (accessed on 11 August 2023).
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. Quantum espresso: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin zone integration. Phys. Rev. B. 1976, 13, 1592–5188. [Google Scholar] [CrossRef]
- Taylor, R.E. Thermal Expansion of Solids; ASM International: Detroit, MI, USA, 1998. [Google Scholar]
- Drebushchak, V.A. Thermal expansion of solids: Review on theories. J. Therm. Anal. Calorim. 2020, 142, 1097–1113. [Google Scholar] [CrossRef]
- Allen, P. Theory of thermal expansion: Quasi-harmonic approximation and corrections from quasi-particle renormalization. Mod. Phys. Lett. B 2020, 34, 2050025. [Google Scholar] [CrossRef]
- Kittel, C. Introduction to Solid State Physics; John Wiley and Sons: New York, NY, USA, 2005. [Google Scholar]
- Degheidy, A.R.; Elkenany, E.B.; Madkour, M.; AbuAli, A.M. Temperature dependence of phonons and related crystal properties in InAs, InP and InSb zinc-blende binary compounds. Comput. Condens. Matter. 2018, 16, e00308. [Google Scholar] [CrossRef]
- Baroni, S.; Giannozzi, P.; Isaev, E. Density-functional perturbation theory for quasi-harmonic calculations. Rev. Mineral. Geochem. 2010, 71, 39–57. [Google Scholar] [CrossRef]
- Togo, A.; Tanaka, I. First principles phonon calculations in materials science. Scr. Mater. 2015, 108, 1–5. [Google Scholar] [CrossRef]
- Barron, R.F.; Barron, B.R. Design for Thermal Stresses; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Alonso, C.; Morato, A.; Medina, F.; Guirado, F.; Cesteros, Y.; Salagre, P.; Sueiras, J.E. Preparation and characterization of different phases of aluminum trifluoride. Chem. Mater. 2000, 12, 1148–1155. [Google Scholar] [CrossRef]
- Sifuna, J.; Manyali, G.S.; Sakwa, T.; Kitui, M.M. Structural and mechanical properties of bulk scandium trifluoride investigated by first-principles calculations. J. Multidiscip. Eng. Sci. Technol. 2017, 4, 6667–6668. [Google Scholar]
- Bocharov, D.; Žguns, P.; Piskunov, S.; Kuzmin, A.; Purans, J. Electronic structure of cubic ScF3 from first-principles calculations. Fiz. Nizk. Temp. 2017, 42, 710–715. [Google Scholar] [CrossRef]
- Morelock, C.R.; Gallington, L.C.; Angus, W.P. Evolution of negative thermal expansion and phase transitions in Sc1−xTixF3. Chem. Mater. 2014, 26, 1936–1940. [Google Scholar] [CrossRef]
- Haines, J.; Gorelli, F.A.; Klung, D.; Tse, J. X-ray diffraction and theoretical studies of the high-pressure structures and phase transitions in magnesium fluoride. Phys. Rev. Lett. 2001, 64, 134110. [Google Scholar] [CrossRef]
- Babu, R.K.; Lingam, C.B.; Auluck, S.; Tewari, S.P. Structural, thermodynamic and optical properties of MgF2 studied from first-principles theory. J. Solid State Chem. 2011, 184, 343–350. [Google Scholar] [CrossRef]
- U.S. Coast Guard. Chemical Hazard Response Information System (CHRIS)—Hazardous Chemical Data; Government Printing Office: Washington, DC, USA, 1999. [Google Scholar]
- Patnaik, P. Handbook of Inorganic Chemicals; McGraw-Hill: New York, NY, USA, 2002. [Google Scholar]
- Busk, R.S. Effect of temperature on the lattice parameters of magnesium alloys. JOM 1952, 4, 207–209. [Google Scholar] [CrossRef]
- Gulina, L.B.; Tolstoy, V.P.; Kasatkin, I.A.; Murin, I.V. Facile synthesis of scandium fluoride oriented single-crystalline rods and urchin-like structures by a gas–solution interface technique. CrystEngComm 2017, 19, 5412–5416. [Google Scholar] [CrossRef]
- Sun, X.W.; Song, T.; Wei, X.P.; Quan, W.L.; Liu, X.B.; Su, W.F. Thermal stability and thermal expansion studies of cubic fluorite-type MgF2 up to 135GPa. Mater. Res. Bull. 2014, 52, 151–157. [Google Scholar] [CrossRef]
- Er-Jun, L.; Sun, Q.; Yuan, H.; Wang, J.; Zeng, G.; Gao, Q. Negative thermal expansion: Mechanisms and materials. Front. Phys. 2021, 16, 53302. [Google Scholar] [CrossRef]
- Wang, J.; Xu, P.; Yuan, H.; Gao, Q.; Sun, Q.; Er-Jun, L. Negative thermal expansion driven by acoustic phonon modes in rhombohedral Zn2GeO4. Results Phys. 2020, 19, 103531. [Google Scholar] [CrossRef]
- Ravindra, N.M.; Marthi, S.R.; Bañobre, A. Optical and thermal properties. In Radiative Properties of Semiconductors; Morgan & Claypool Publishers: San Rafael, CA, USA, 2017. [Google Scholar]
- Lunkenheimer, P.; Loidl, A.; Riechers, B.; Zaccone, A.; Samwer, K. Thermal expansion and the glass transition. Nat. Phys. 2023, 19, 694–699. [Google Scholar] [CrossRef]
- Haynes, W.M. (Ed.) CRC Handbook of Chemistry and Physics, 92nd ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar] [CrossRef]
- Greve, B.K.; Martin, K.L.; Lee, P.L.; Chupas, P.J.; Chapman, K.W.; Wilkinson, A.P. Pronounced negative thermal expansion from a simple structure: Cubic ScF3. J. Am. Chem. Soc. 2010, 132, 15496–15498. [Google Scholar] [CrossRef]
- Rao, K.V.; Naidu, S.V.; Setty, P.L.N. Thermal expansion of magnesium fluoride. Acta Cryst. 1962, 15, 528–530. [Google Scholar] [CrossRef]
- Abe, H. National standard and new reference material for specific heat capacity measurements. Anal. Sci. 2021, 37, 201–210. [Google Scholar] [CrossRef]
- Krastev, R.K. Measuring of heat capacity. Int. J. Heat Mass Transf. 2010, 53, 3847–3854. [Google Scholar] [CrossRef]
- Trevisan, S.; Wang, W.; Laumert, B. A high-temperature thermal stability and optical property study of inorganic coatings on ceramic particles for potential thermal energy storage applications. Sol. Energy Mater. Sol. Cells 2022, 239, 111679. [Google Scholar] [CrossRef]
- Redondo, A.; Beery, J.G. Thermal conductivity of optical coatings. J. Appl. Phys. 1986, 60, 3882–3885. [Google Scholar] [CrossRef]
- Smith, N.O. The difference between Cp and Cv for liquids and solids. J. Chem. Educ. 1954, 42, 654. [Google Scholar] [CrossRef]
- Lide, R.D. (Ed.) CRC Handbook of Chemistry and Physics, 81st ed.; CRC Press LLC.: Boca Raton, FL, USA, 2000. [Google Scholar]
- Aristovaa, N.M.; Belov, G.V. Thermodynamic parameters of scandium trifluoride and triiodide in the condensed state. Russ. J. Phys. Chem. 2015, 89, 947–951. [Google Scholar] [CrossRef]
- Nakajima, Y.; Tanaka, H.; Mochizuki, K.; Fuse, K.; Arashitani, Y.; Nishimoto, T.; Seno, A.; Okada, M. A study for estimating thermal strain and thermal stress in optical fiber coatings. Furukawa Rev. 2008, 34, 8–12. [Google Scholar]
- Kodur, V.R.; Sultan, M.A. Effect of temperature on thermal properties of high-strength concrete. J. Mater. 2003, 15, 101–107. [Google Scholar] [CrossRef]
- Ren, H.; Zou, G.; Jia, Q.; Deng, Z.; Du, C.; Wang, W.; Liu, L. Thermal stress reduction strategy for high-temperature power electronics with Ag sintering. Microelectron. Reliab. 2021, 127, 114379. [Google Scholar] [CrossRef]

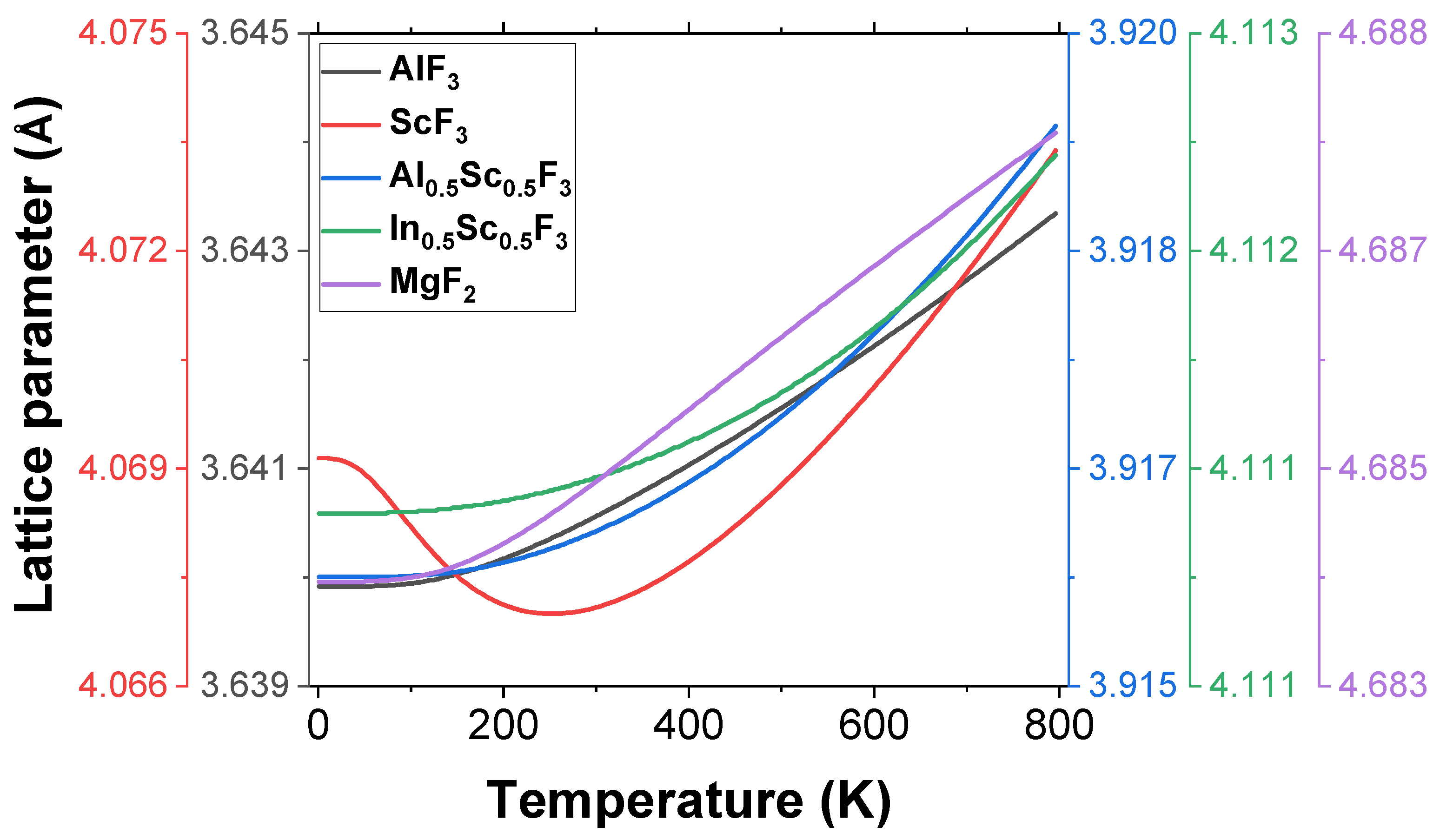
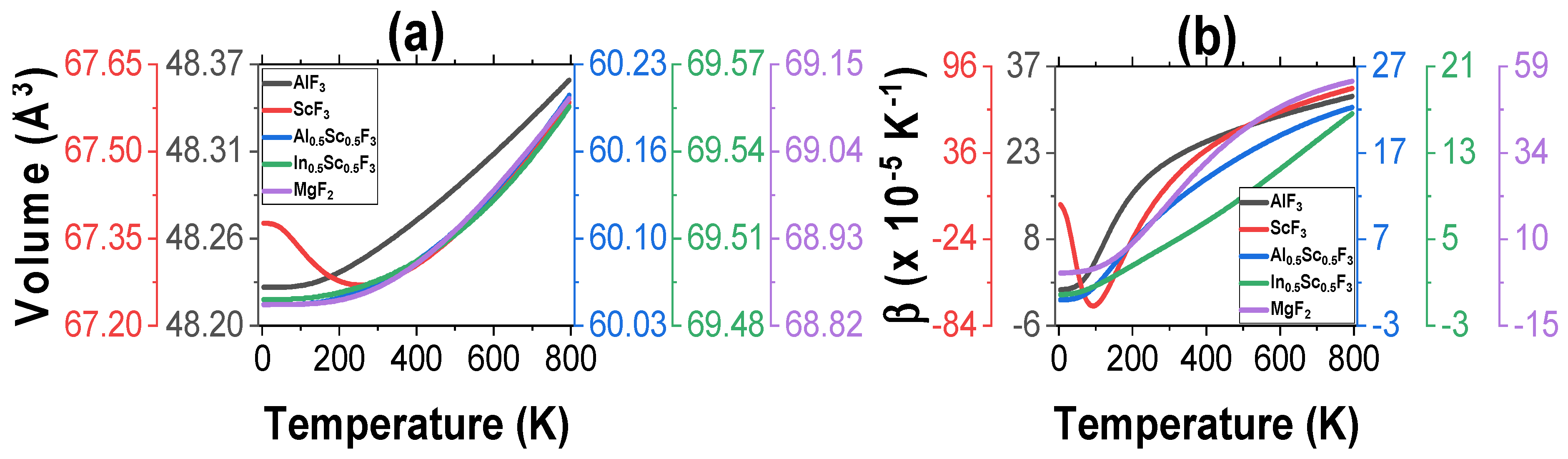
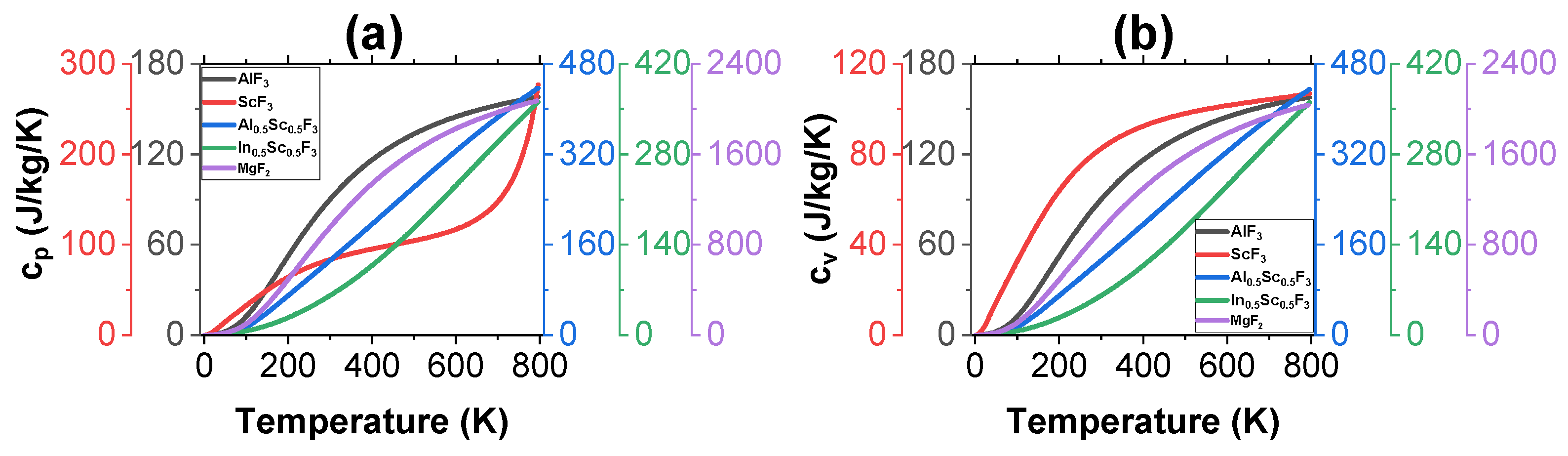
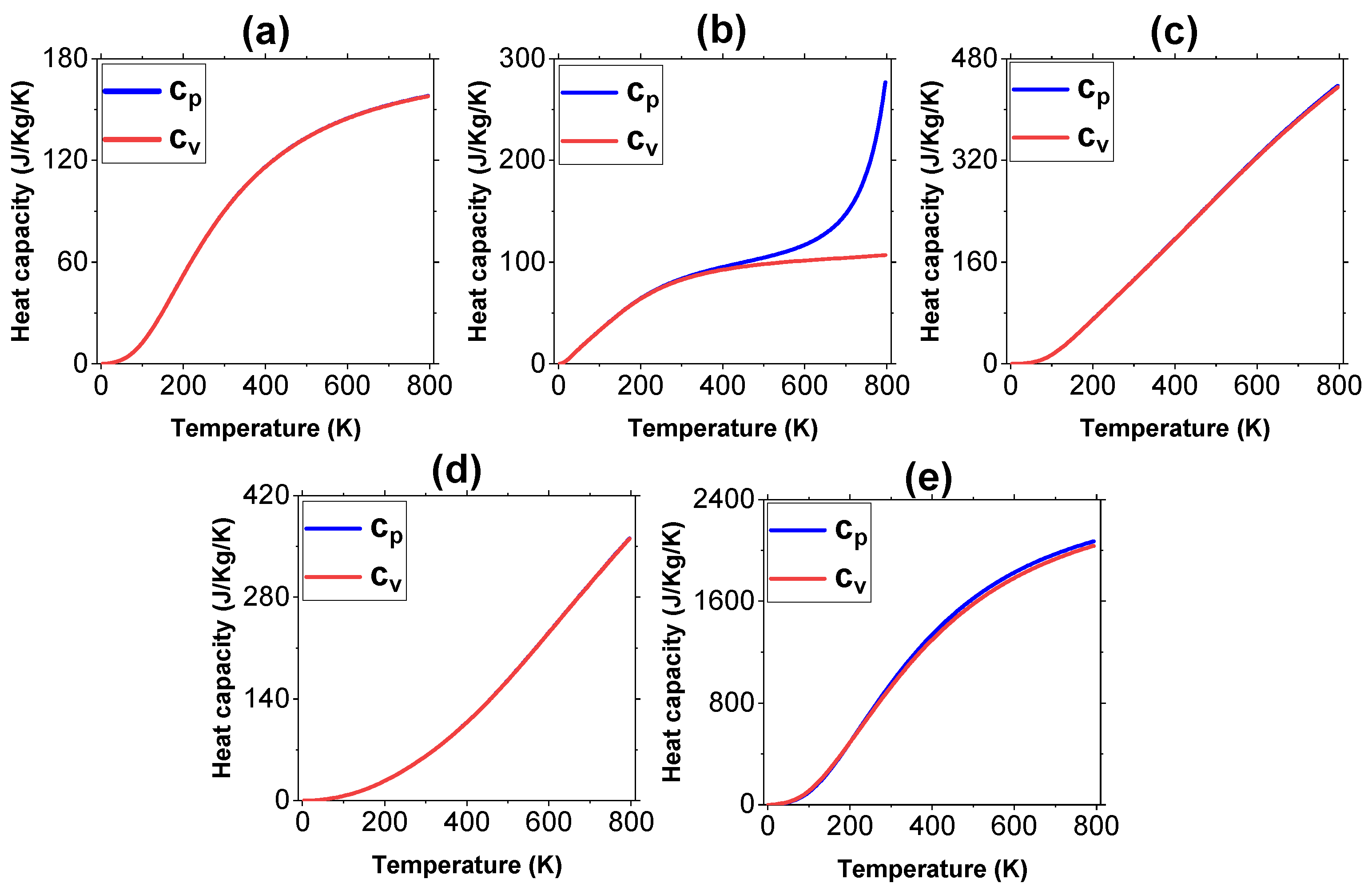

| Sample | |||||
|---|---|---|---|---|---|
| 3.6051 (3.528) [27] | 4.0379 (4.07, 4.02–4.07, 4.03) [28,29,30] | 3.8943 | 4.0867 | 4.673 (4.625, 4.735) [31,32] | |
| - | - | - | - | 3.082 (3.052, 3.124) [29,30] | |
| 2.925 (2.88) [33] | 2.546 (2.44–2.61) [28] | 2.674 | 3.115 | 3.070 (3.15) [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alruqi, A.B.; Ongwen, N.O. A Comparative Thermodynamic Study of AlF3, ScF3, Al0.5Sc0.5F3, and In0.5Sc0.5F3 for Optical Coatings: A Computational Study. Coatings 2023, 13, 1840. https://doi.org/10.3390/coatings13111840
Alruqi AB, Ongwen NO. A Comparative Thermodynamic Study of AlF3, ScF3, Al0.5Sc0.5F3, and In0.5Sc0.5F3 for Optical Coatings: A Computational Study. Coatings. 2023; 13(11):1840. https://doi.org/10.3390/coatings13111840
Chicago/Turabian StyleAlruqi, Adel Bandar, and Nicholas O. Ongwen. 2023. "A Comparative Thermodynamic Study of AlF3, ScF3, Al0.5Sc0.5F3, and In0.5Sc0.5F3 for Optical Coatings: A Computational Study" Coatings 13, no. 11: 1840. https://doi.org/10.3390/coatings13111840





