Research on Simulation of Coating Fusion and Solidification Process in Electro-Spark Deposition
Abstract
:1. Introduction
2. Coating Fusion and Solidification Process Model of Single-Pulse ESD
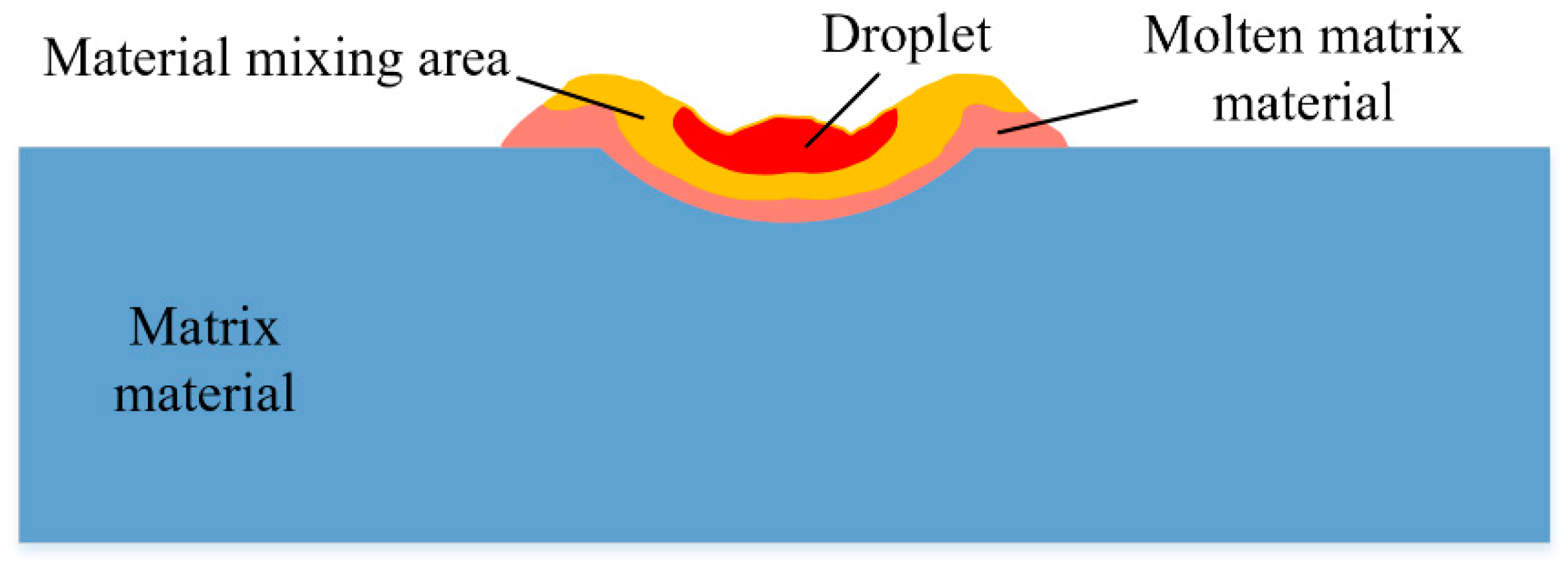
2.1. Analysis of Coating Fusion and Solidification Process
2.2. Theoretical Model
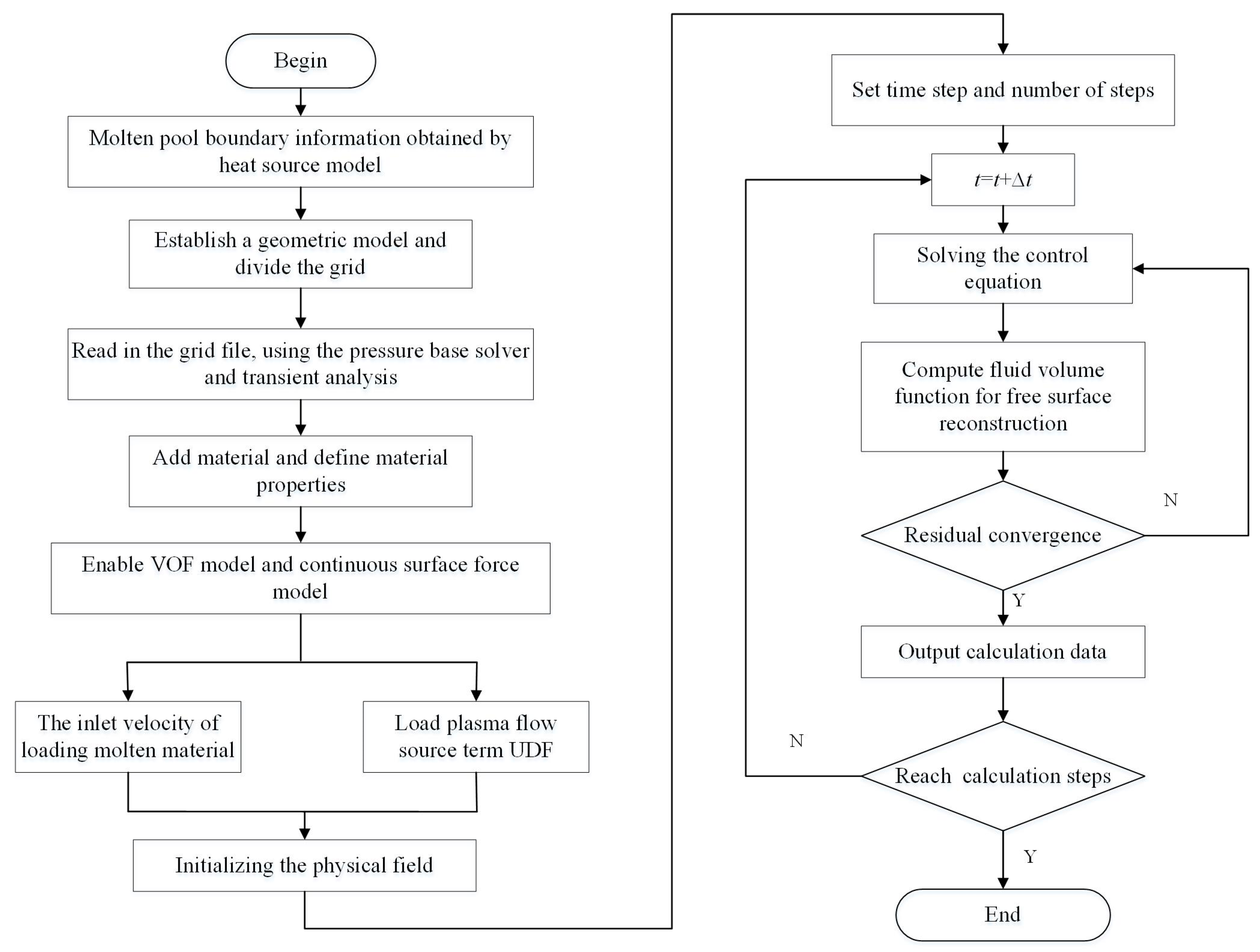
2.3. Simulation Model and Boundary Conditions
3. Analysis of Simulation Results
3.1. Material Distribution during Fusion Process
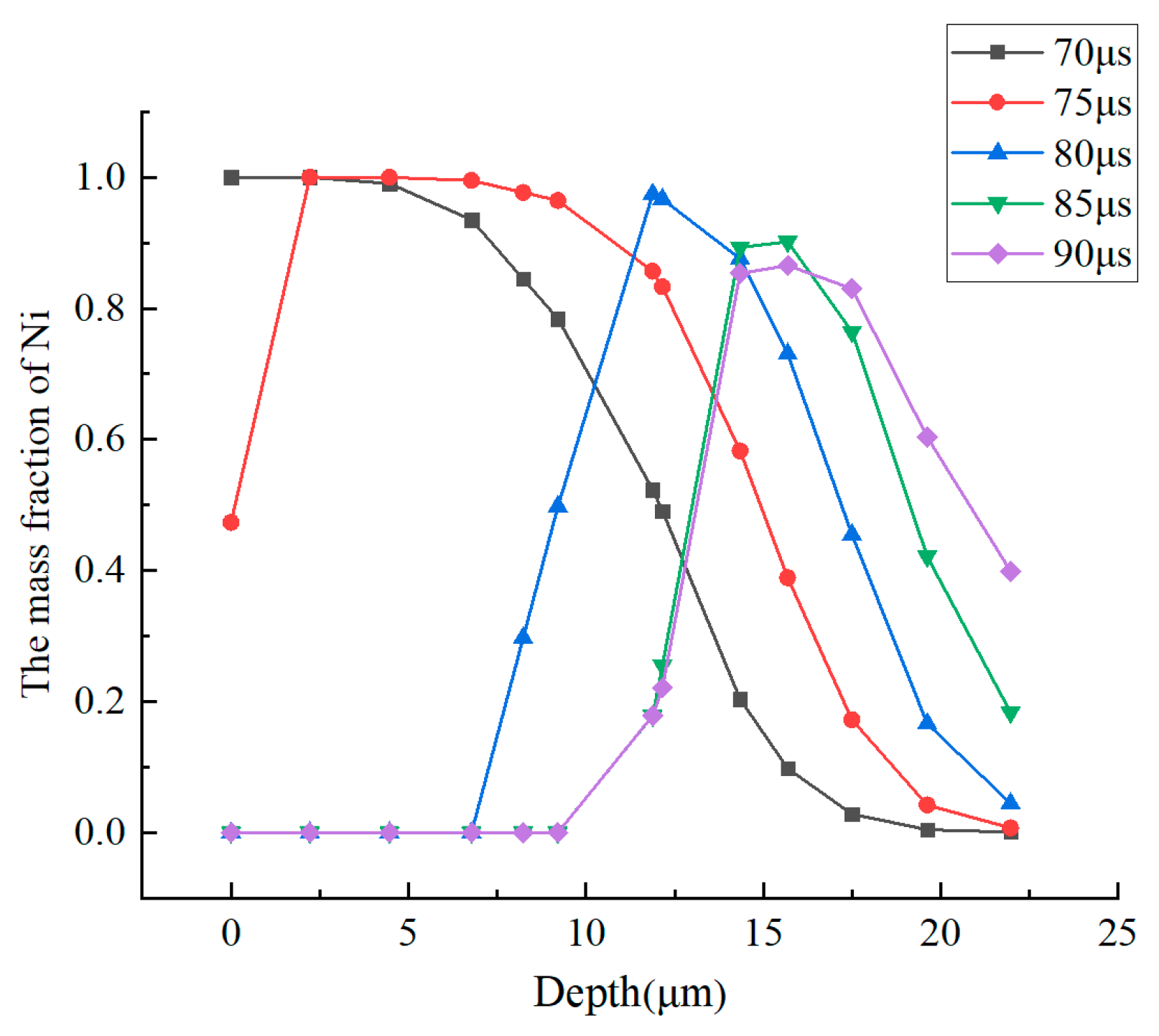
3.2. Distribution of Electrode Materials in the Molten Pool
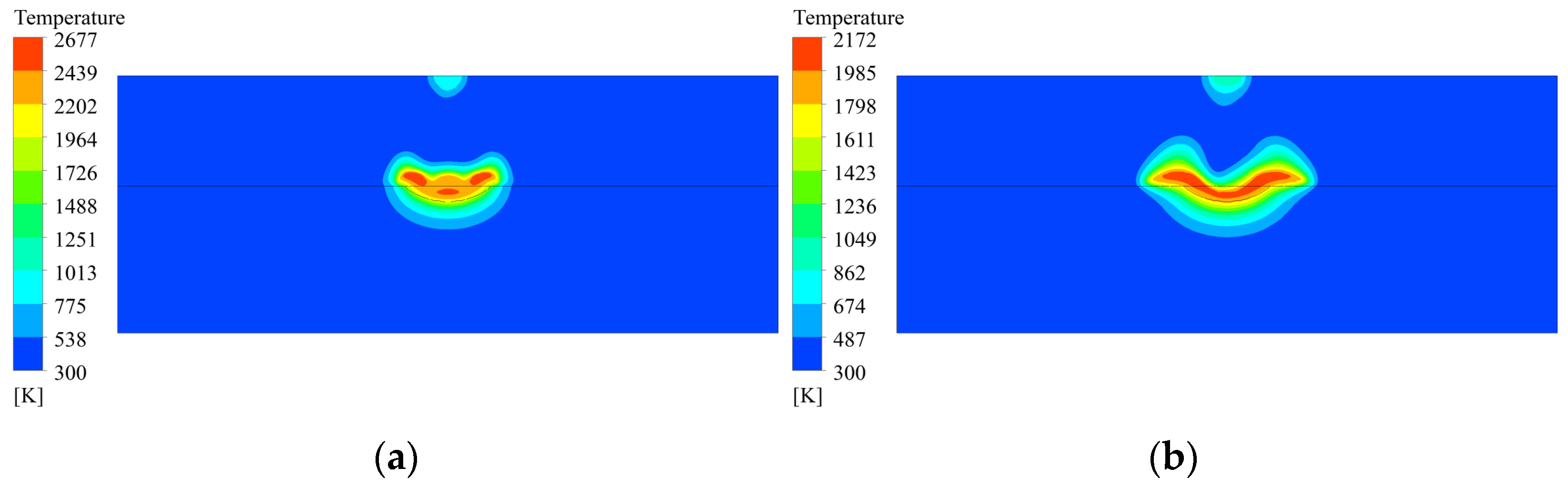
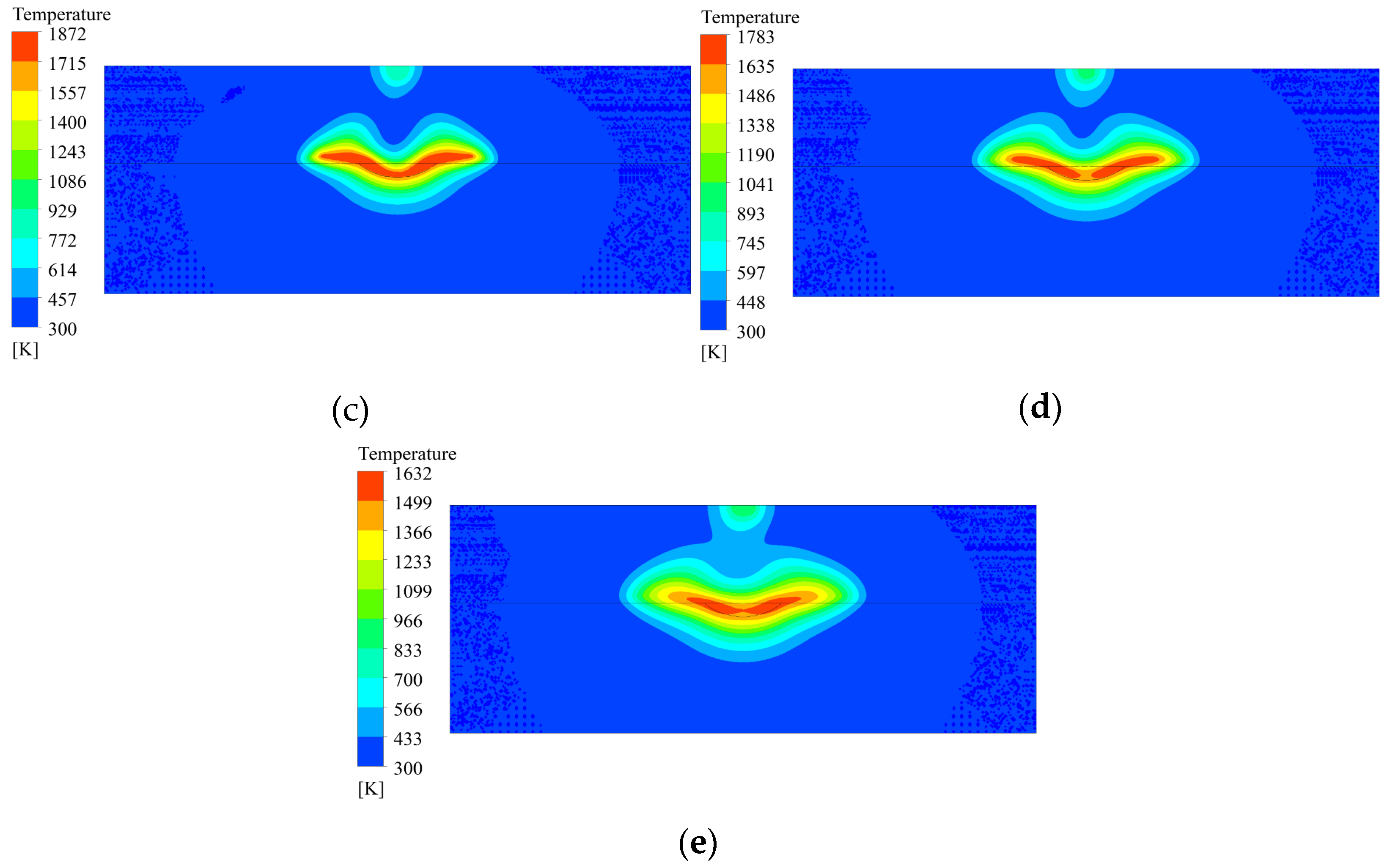
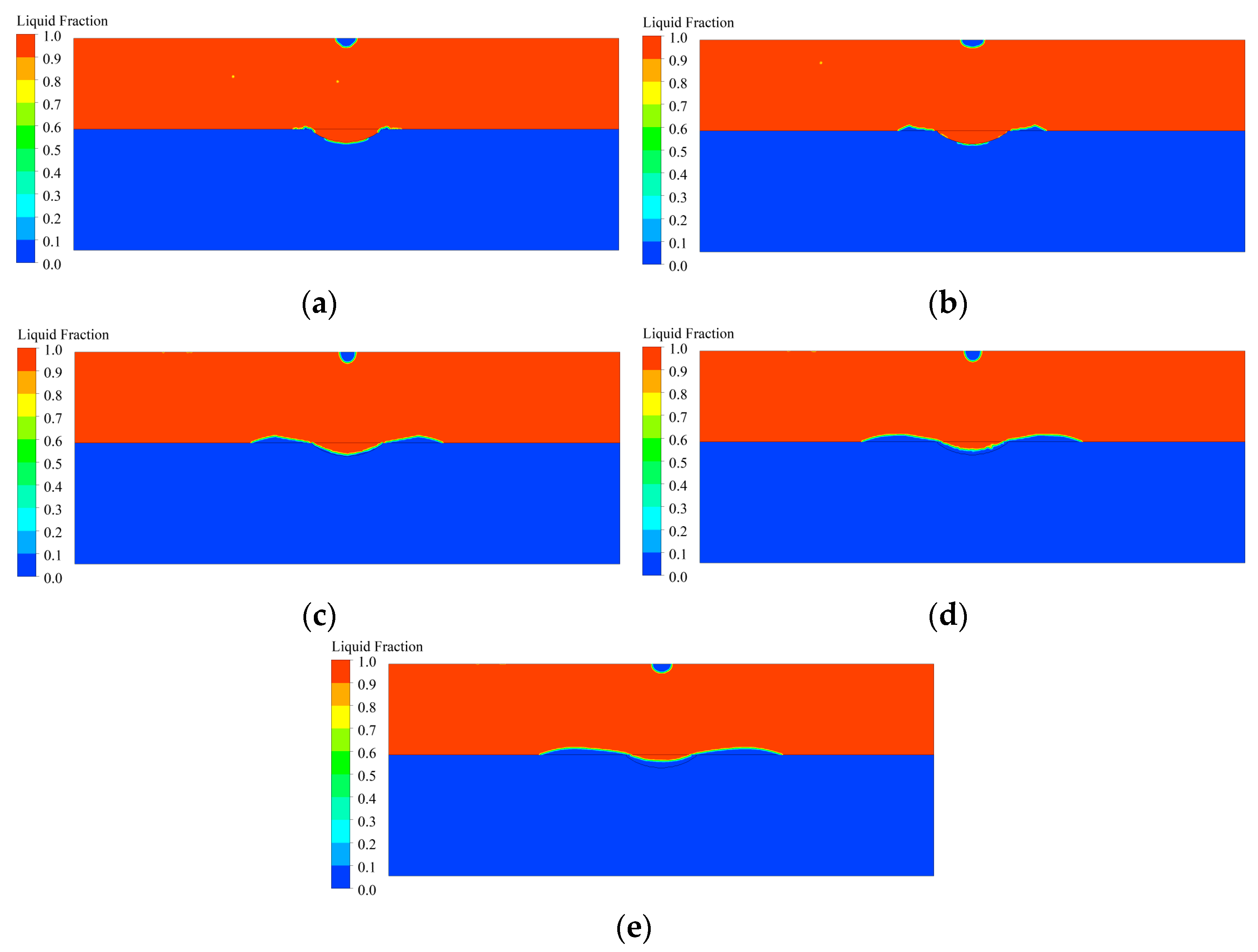
3.3. Temperature Distribution of Coating Solidification Process
3.4. Analysis of the Coating Solidification Process
4. Experimental Verification
4.1. Experimental Conditions
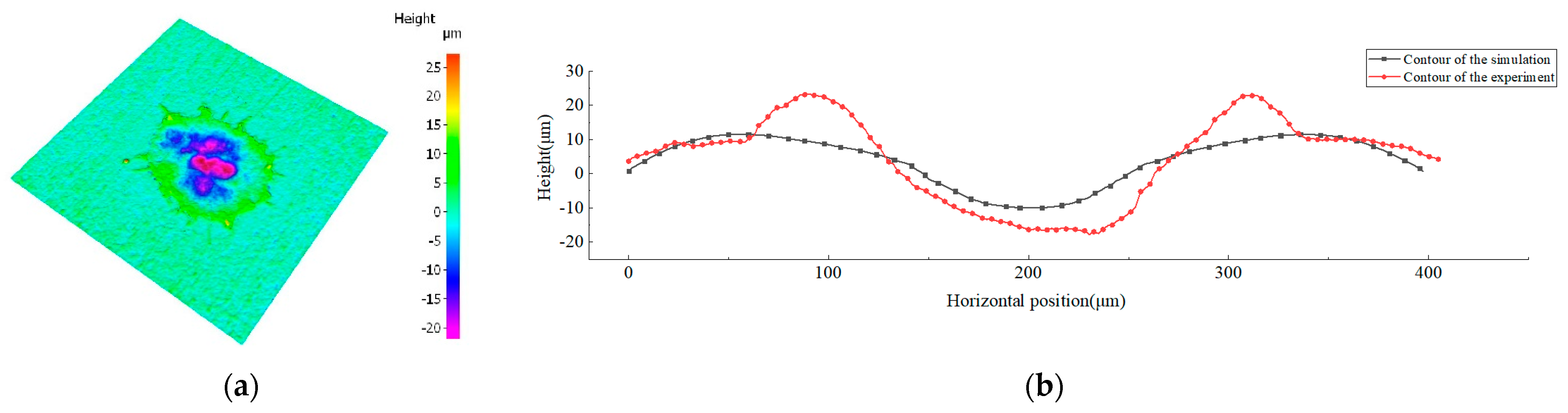
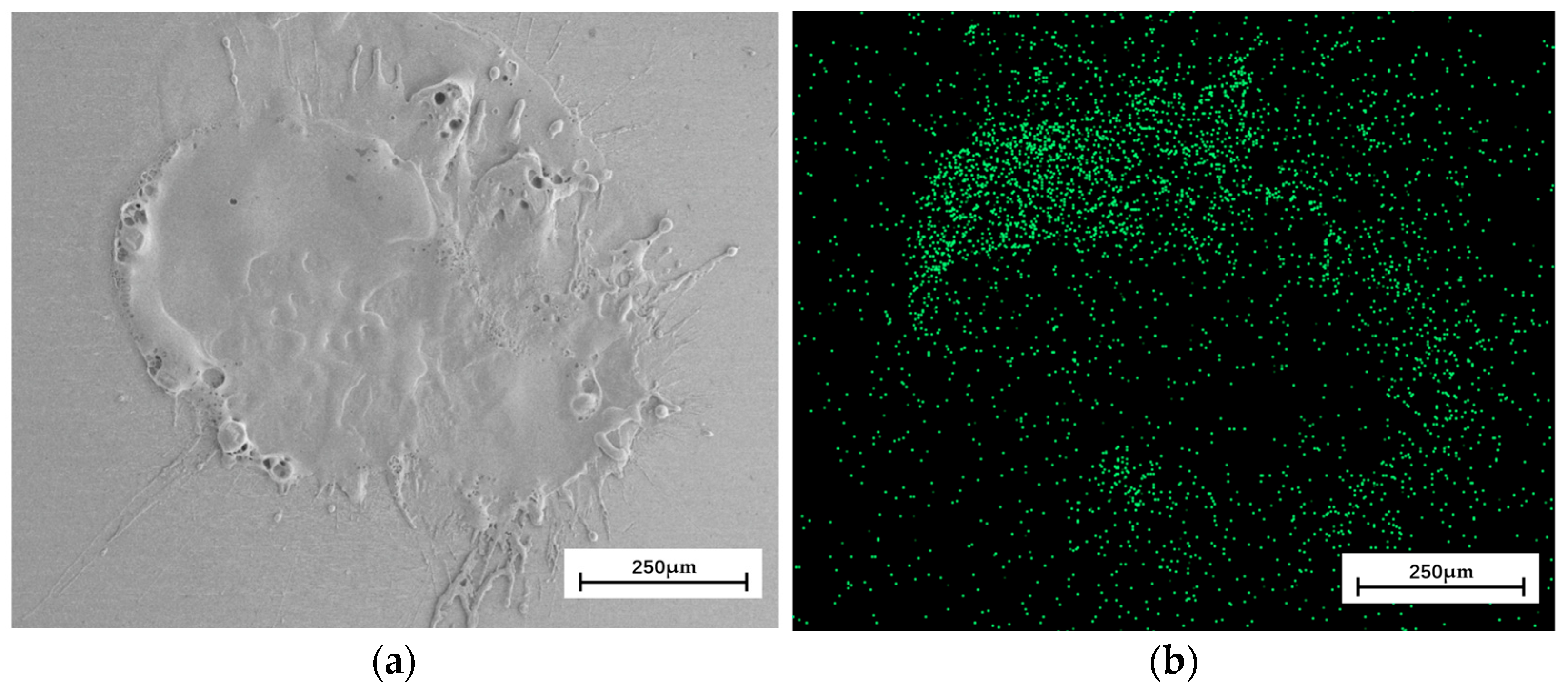
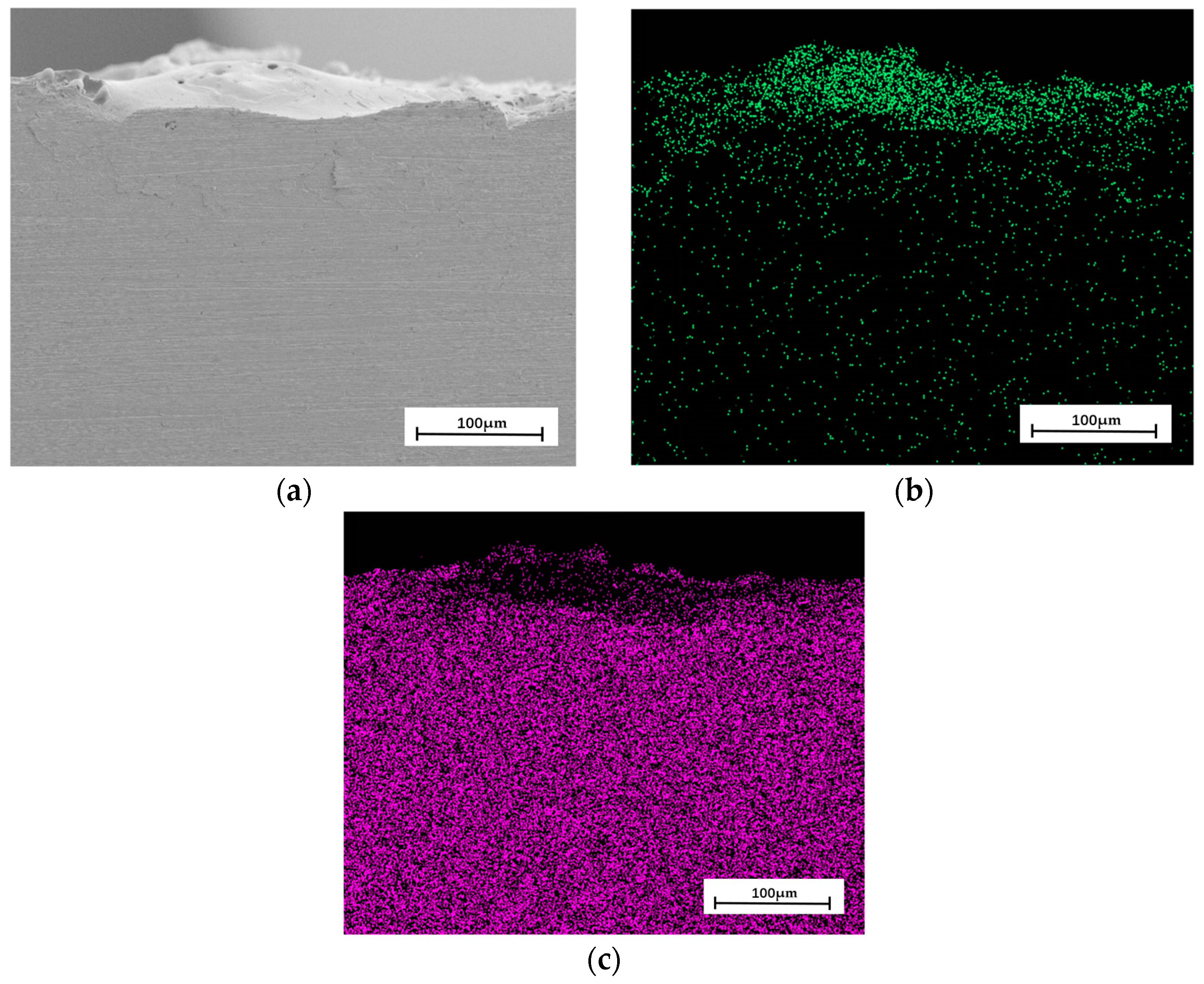
4.2. Analysis of Deposition Experimental Results
5. Conclusions
- (1)
- In the deposition area, the content of electrode material decreases rapidly from the droplet to the bottom of the molten pool. When the deposition time reaches 80 μs, the content of electrode material at the bottom of the molten pool reaches 4.5%. In the material gushing out of the molten pool, the content of electrode material in the upper region is higher than that in the bottom region.
- (2)
- In the deposition area, the high-temperature area moves from the center of the molten pool to the edges as the material flows, and the temperature of the molten material decreases as a whole. The material outside the molten pool solidifies first, and in the molten pool, the material gradually solidifies from the bottom upwards to the surface. The shape of the solidification interface is similar to that of the molten pool boundary, and the molten material at the surface finally solidifies.
- (3)
- Comparing the simulation results and experiment results, the fluctuation degree of the central part of the simulated contour curve is smaller than that of the experimented one. The average error in the depth direction is 4 μm, the diameter error is about 4%, and the element comparison analysis shows that the element diffusion distance in the depth direction is 4 μm, while the lateral diffusion distance reaches 364 μm, with an average error of 7.6%. The cross-section structure comparison analysis shows that the surface profile shape obtained from the simulation is basically consistent with the experimental results, and the error in diameter is about 11%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sychev, A.P.; Kolesnikov, I.V.; Sycheva, M.A. Extending the life of frictional components in freight cars by hybrid electrospark deposition. Russ. Eng. Res. 2022, 42, 49–52. [Google Scholar] [CrossRef]
- Enrique, P.D.; Peterkin, S.; Zhou, N.Y. Parametric Study of Automated Electrospark Deposition for Ni-Based Superalloys. Weld. J. 2021, 100, 239–248. [Google Scholar] [CrossRef]
- Senin, P.V.; Velichko, S.A.; Martynov, A.V.; Martynova, E.G. Increasing the wear resistance of forged tools by electrospark deposition. Russ. Eng. Res. 2020, 40, 427–430. [Google Scholar] [CrossRef]
- Tarelnyk, V.B.; Gaponova, O.P.; Myslyvchenko, O.M.; Sarzhanov, B.O. Electrospark deposition of multilayer coatings. Powder Metall. Met. Ceram. 2020, 59, 76–88. [Google Scholar] [CrossRef]
- Holubets, V.M.; Pashechko, M.I.; Borc, J.; Tisov, O.V.; Shpuliar, Y.S. Wear resistance of electrospark-deposited coatings in dry sliding friction conditions. Powder Metall. Met. Ceram. 2021, 60, 90–96. [Google Scholar] [CrossRef]
- Burkov, A.A. Improvement of Ti6Al4V-alloy wear resistance by electric-spark hafnium carbide coatings. Frict. Wear 2020, 41, 543–548. [Google Scholar] [CrossRef]
- Salmaliyan, M.; Ghaeni, F.M.; Ebrahimnia, M. Effect of electro spark deposition process parameters on WC-Co coating on H13 steel. Surf. Coat. Technol. 2017, 321, 81–89. [Google Scholar] [CrossRef]
- Feng, X.R.; Cui, X.F.; Zheng, W.; Lu, B.W.; Dong, M.L.; Wen, X.; Zhao, Y.; Jin, G. Effect of the protective materials and water on the repairing quality of nickel aluminum bronze during underwater wet laser repairing. Opt. Laser Technol. 2019, 114, 140–145. [Google Scholar] [CrossRef]
- Theimer, S.; Graunitz, M.; Schulze, M.; Gaertner, F.; Klassen, T. Optimization adhesion in cold spraying onto hard substrates: A case study for brass coatings. J. Therm. Spray Technol. 2019, 28, 124–134. [Google Scholar] [CrossRef]
- Samsonov, G.V.; Verkhoturov, A.D.; Sychev, V.S.; Bezykornov, A.I. Investigation of some of thelaws governing the formation of a hardened layer during the process of electro-spark alloying. Fiz.-Khimicheskaya Mekhanika Mater. 1974, 7, 3–6. [Google Scholar]
- Johnson, R.N.; Sheldon, G.L. Advances in the electrospark deposition coating process. J. Vac. Sci. Technol. A Vac. Surf. Film. 1986, 4, 2740–2746. [Google Scholar] [CrossRef]
- Lešnjak, A.; Tušek, J. Some characteristics of electrospark deposition. Int. J. Mater. Res. 2003, 94, 1260–1266. [Google Scholar] [CrossRef]
- Tušek, J.; Kosec, L.; Lešnjak, A.; Muhič, T. Electrospark deposition for die repair. Metalurgija 2012, 51, 17–20. [Google Scholar]
- Liu, J.; Wang, R.; Qian, Y. The formation of a single-pulse electrospark deposition spot. Surf. Coat. Technol. 2005, 200, 2433–2437. [Google Scholar] [CrossRef]
- Chen, C.; Wang, M.; Liu, Y.; Wang, D.; Jin, R. Mass transfer trends and the formation of a single deposition spot during high-energy micro-arc alloying of AZ31 Mg alloy. J. Mater. Process. Technol. 2008, 198, 275–280. [Google Scholar]
- Renna, G.; Leo, P.; Casavola, C. Effect of electrospark process parameters on the WE43 Magnesium Alloy Deposition Quality. Appl. Sci. 2019, 9, 4383. [Google Scholar] [CrossRef]
- Galinov, I.V.; Luban, R.B. Mass transfer trends during electrospark alloying. Surf. Coat. Technol. 1996, 79, 9–18. [Google Scholar] [CrossRef]
- Thamer, A.D.; Hafiz, M.H.; Mahdi, B.S. Mechanism of building-up deposited layer during electro-spark deposition. J. Surf. Eng. Mater. Adv. Technol. 2012, 2, 258–263. [Google Scholar] [CrossRef]
- Ebrahimnia, M.; Malek Ghaini, F.; Xie, Y.J.; Shahverdi, H. Microstructural characteristics of the built up layer of a precipitation hardened nickel based superalloy by electrospark deposition. Surf. Coat. Technol. 2014, 258, 515–523. [Google Scholar] [CrossRef]
- Kovacik, J.; Baksa, P.; Emmer, Š. Electro spark deposition of TiB2 layers on Ti6Al4V alloy. Acta Metall. Slovaca 2016, 22, 52–59. [Google Scholar] [CrossRef]
- Chandrakant; Reddy, N.S.; Panigrahi, B.B. Electro spark coating of AlCoCrFeNi high entropy alloy on AISI410 stainless steel. Mater. Lett. 2021, 304, 130580. [Google Scholar] [CrossRef]
- Anderson, J.D. Fundamentals of Aerodynamics; McGraw-Hill Education: New York, NY, USA, 2017. [Google Scholar]
- Yunus, A.C.; Michael, A.B. Thermodynamics: An Engineering Approach, 8th ed.; McGraw-Hill Education: New York, NY, USA, 1989. [Google Scholar]
- DiGiovanni, C.; Kalashami, A.G.; Biro, E.; Zhou, N.Y. Liquid metal embrittlement transport mechanism in the Fe/Zn system: Stress-assisted diffusion. Materialia 2021, 18, 101153. [Google Scholar] [CrossRef]
- DiBitonto, D.D.; Eubank, P.T.; Patel, M.R.; Barrufet, M.A. Theoretical models of the electrical discharge machining process. I. A simple cathode erosion model. J. Appl. Phys. 1989, 66, 4095–4103. [Google Scholar] [CrossRef]
- Lou, L. Computer Simulation of EDM. Ph.D. Thesis, Shanghai Jiao Tong University, Shanghai, China, 2000. [Google Scholar]
- Kiyoshi, I. The Principle of Electric Discharge Machining—Mold Processing Technology; National Defense Industry Press: Beijing, China, 1983; pp. 72–83. [Google Scholar]
- Kim, Y.S.; Eagar, T.W. Analysis of metal transfer in gas metal arc welding. Weld. J. 1993, 72, 269–278. [Google Scholar]
- Chhabra, R.; Shankar, V. Coulson and Richardson’s Chemical Engineering, 7th ed.; Butterworth-Heinemann: Oxford, UK, 2017. [Google Scholar]
- Fran, C. ASM Ready Reference: Thermal Properties of Metals; ASM International: Materials Park, OH, USA, 2002. [Google Scholar]
- Zhang, J.R.; Zhao, T.Y. Manual of Thermophysical Properties of Substances Commonly Used in Engineering; National Defense Industry Press: Beijing, China, 1987. [Google Scholar]





| No. | Boundary | Temperature | Velocity |
|---|---|---|---|
| 1 | MN | 300 K | 2 m/s |
| 2 | AM, BN | 300 K | 0 |
| 3 | AF, BC | ||
| 4 | EF, CD | Equation (8) | 0 |
| 5 | DE | Equation (8) | 0 |
| 6 | FG, CH Arc GH | Equation (8) Equation (24) | 0 0 |
| Inlet Speed v/(m·s−1) | Electrode Material Loading Time t/μs | Gap Voltage U/(V) | Peak Current I/(A) | Acceleration due to Gravity g/(m·s−2) | Surface Tension Coefficient σ/(N·m−1) |
|---|---|---|---|---|---|
| 2 | 20 | 45 | 5 | 9.81 | 1 |
| Temperature (K) | Specific Heat Capacity (J·kg−1·K−1) | Thermal Conductivity (W·m−1·K−1) |
|---|---|---|
| 293 | 456.89 | 92.28 |
| 373 | 469.86 | 82.52 |
| 573 | 502.08 | 63.92 |
| 773 | 529.28 | 61.6 |
| 973 | 550.2 | 58.11 |
| 1273 | 571.12 | 56.95 |
| 1573 | 585.76 | 55.21 |
| Temperature (K) | Diffusion Coefficient (cm2/S) | Specific Heat Capacity (J·kg−1·K−1) | Thermal Conductivity (W·m−1·K−1) |
|---|---|---|---|
| 293 | 0.120 | 472 | 47.68 |
| 373 | 0.113 | 480 | 43.53 |
| 473 | 0.104 | 498 | 40.44 |
| 573 | 0.095 | 524 | 38.13 |
| 673 | 0.086 | 560 | 36.02 |
| 773 | 0.077 | 615 | 34.16 |
| 873 | 0.068 | 700 | 31.96 |
| 973 | 0.062 | 854 | 28.66 |
| 1028 | 0.060 | 1064 | 25.14 |
| 1073 | 0.059 | 806 | 26.49 |
| 1173 | 0.061 | 637 | 25.92 |
| 1273 | 0.063 | 602 | 24.02 |
| Nomenclature | Symbol | Unit | Steel | Argon |
|---|---|---|---|---|
| Specific heat | Cp | (J·kg−1·K−1) | Cp (T) | 510 |
| Thermal conductivity | k | (W·m−1·K−1) | k (T) | 0.08 |
| Density | (kg·m−3) | 7200 | 1.78 | |
| Dynamic viscosity | (Kg·m−1·s−1) | 0.006 | 0.1 | |
| Surface tension | (N·m−1) | 1 | ||
| Latent heat of fusion | (J·kg−1) | 2.47 × 105 | ||
| Solidus temperature | Ts | (K) | 1750 | |
| Liquidus temperature | Tl | (K) | 1800 | |
| Melting temperature | Tavg | (K) | 1775 | |
| Enthalpy | H | (J) | Equation (17) | |
| Liquidus slope | mi | K·(wt%)−1 | −7.6 | |
| Thermal diffusion coefficient | Dm | (cm2·s−1) | 0.063 |
| Ingredients | Content |
|---|---|
| C | ≤0.15 |
| Mn | ≤1.0 |
| Fe | ≤1.0 |
| Si | ≤0.7 |
| Cu | ≤0.2 |
| Ni | ≥92.0 |
| Al | ≤1.5 |
| Ti | 2.0~3.5 |
| Parameters | Description |
|---|---|
| Electrode material | ERNi-1 |
| Workpiece material | 45 steel |
| Environment | Air |
| Protective gas | Argon |
| Discharge voltage | 45 V |
| Pulse width percentage | 40% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Su, Q.; Zhang, S.; Qu, J.; Zhang, S. Research on Simulation of Coating Fusion and Solidification Process in Electro-Spark Deposition. Coatings 2023, 13, 1865. https://doi.org/10.3390/coatings13111865
Liu Y, Su Q, Zhang S, Qu J, Zhang S. Research on Simulation of Coating Fusion and Solidification Process in Electro-Spark Deposition. Coatings. 2023; 13(11):1865. https://doi.org/10.3390/coatings13111865
Chicago/Turabian StyleLiu, Yu, Quanning Su, Shiqi Zhang, Jiawei Qu, and Shengfang Zhang. 2023. "Research on Simulation of Coating Fusion and Solidification Process in Electro-Spark Deposition" Coatings 13, no. 11: 1865. https://doi.org/10.3390/coatings13111865






