Corrosion and Tribological Performance of Diamond-like Carbon-Coated ZK 60 Magnesium Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reference Substrate
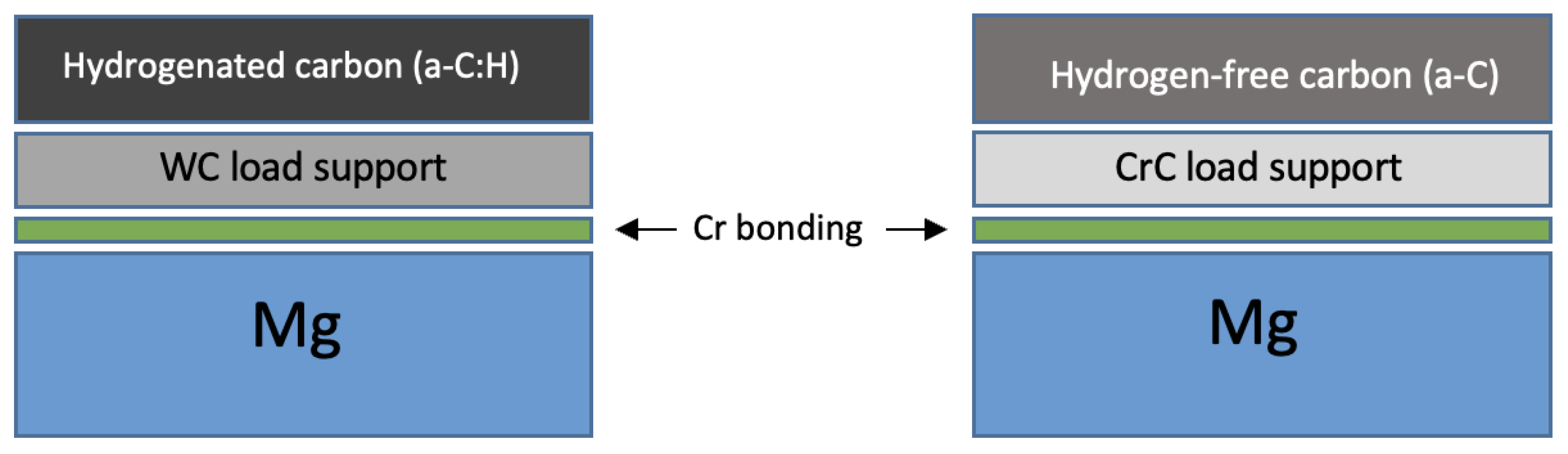
2.2. Film Deposition Technique
2.3. Thickness, Structural Properties, and Profile Composition
2.4. Mechanical and Tribological Tests
- Standard cut-off of the high-pass filter: λs = 2.50 µm.
- Standard cut-off of the low-pass filter: λc = 0.25 mm.
- First critical load (Lc1): The appearance of the first cohesive failure. Determined by plastic deformation failures, cracking for conformal type, laterals, or tensile ones, among others.
- Second critical load (Lc2): The appearance of the first adhesive failure. Determined by delamination failures, frontal deformation cracking, superficial lifts, lateral chipping, etc.
- Third critical load (Lc3): At least 50% delamination of the coating or the appearance of a critical defect.
2.5. Corrosion Tests
- Working electrode: test materials with an exposed area of 13.85 cm2.
- Reference electrode: silver/silver chloride (Ag/AgCl 3 M) electrode.
- Counter electrode: platinum electrode.
3. Results
3.1. Thickness, Structural Properties, and Profile Composition
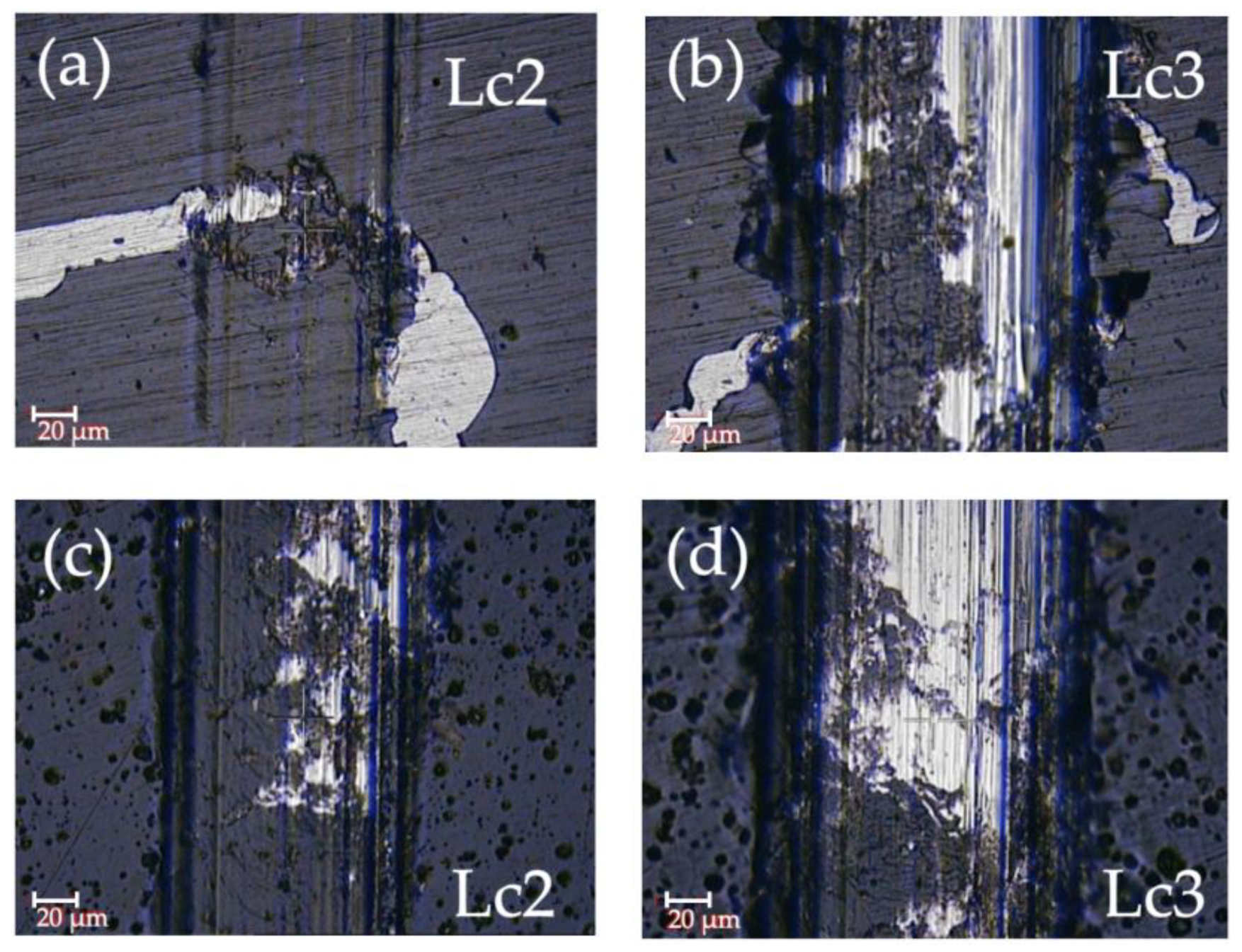
3.2. Roughness and Adhesion
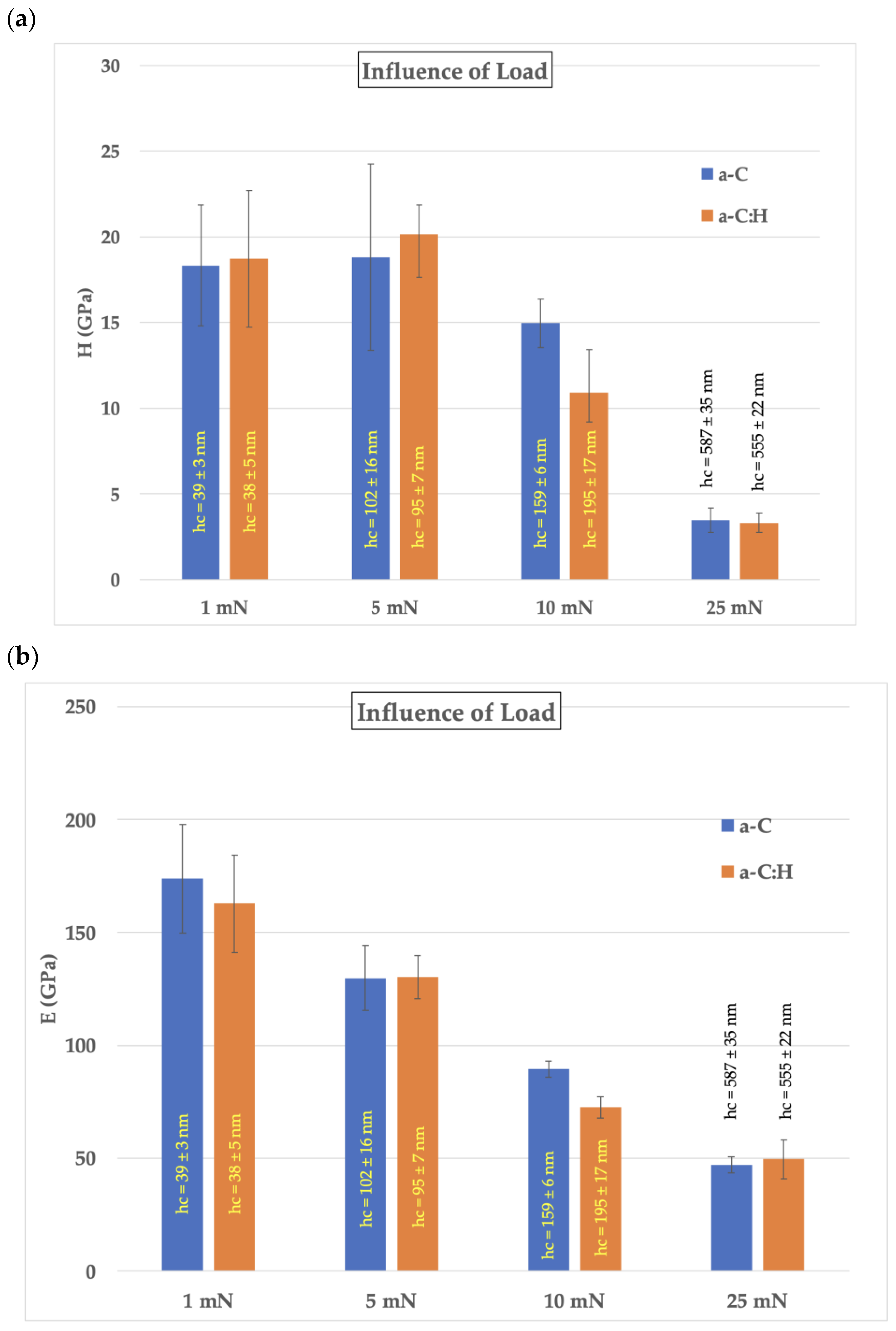
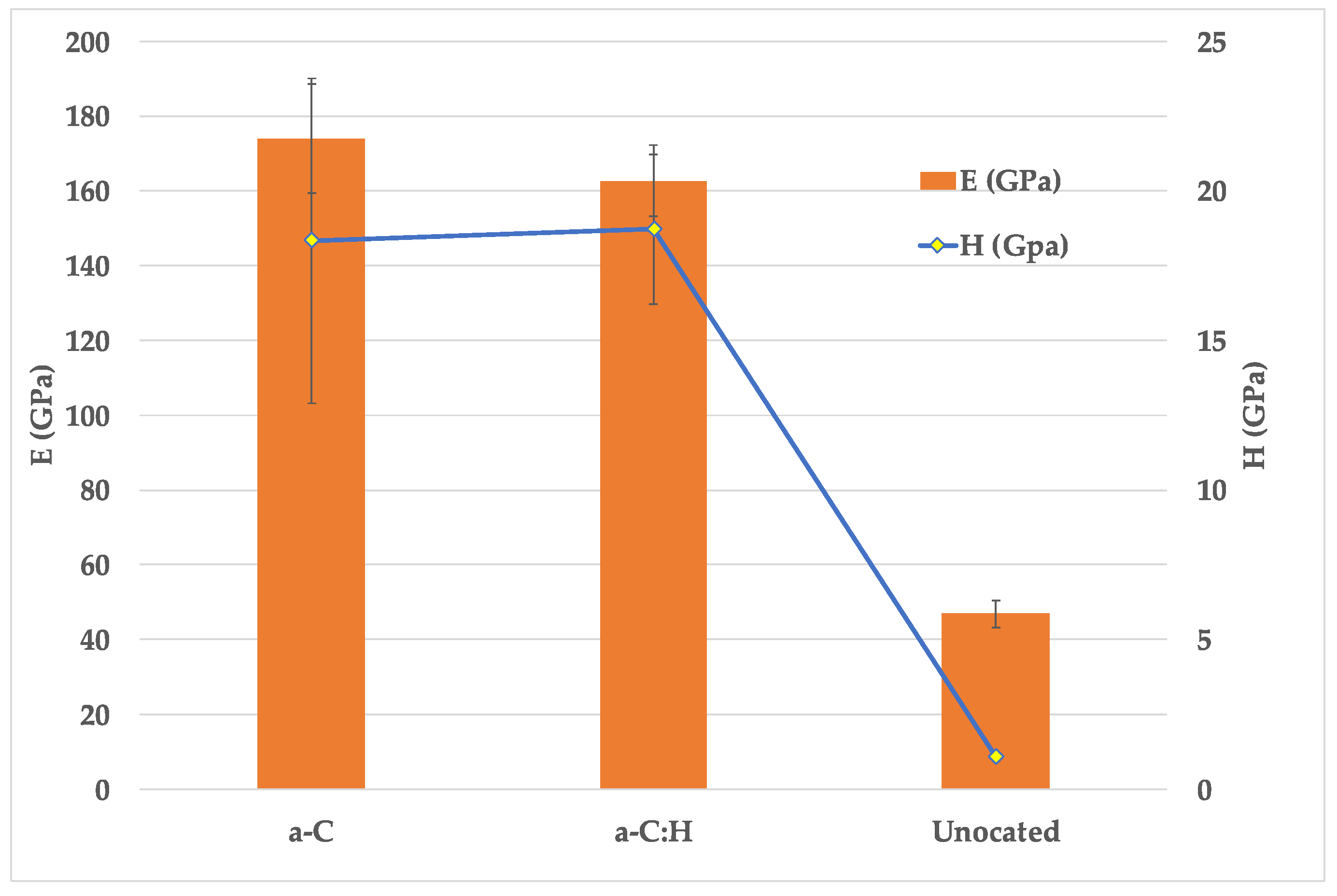
3.3. Nanoindentation
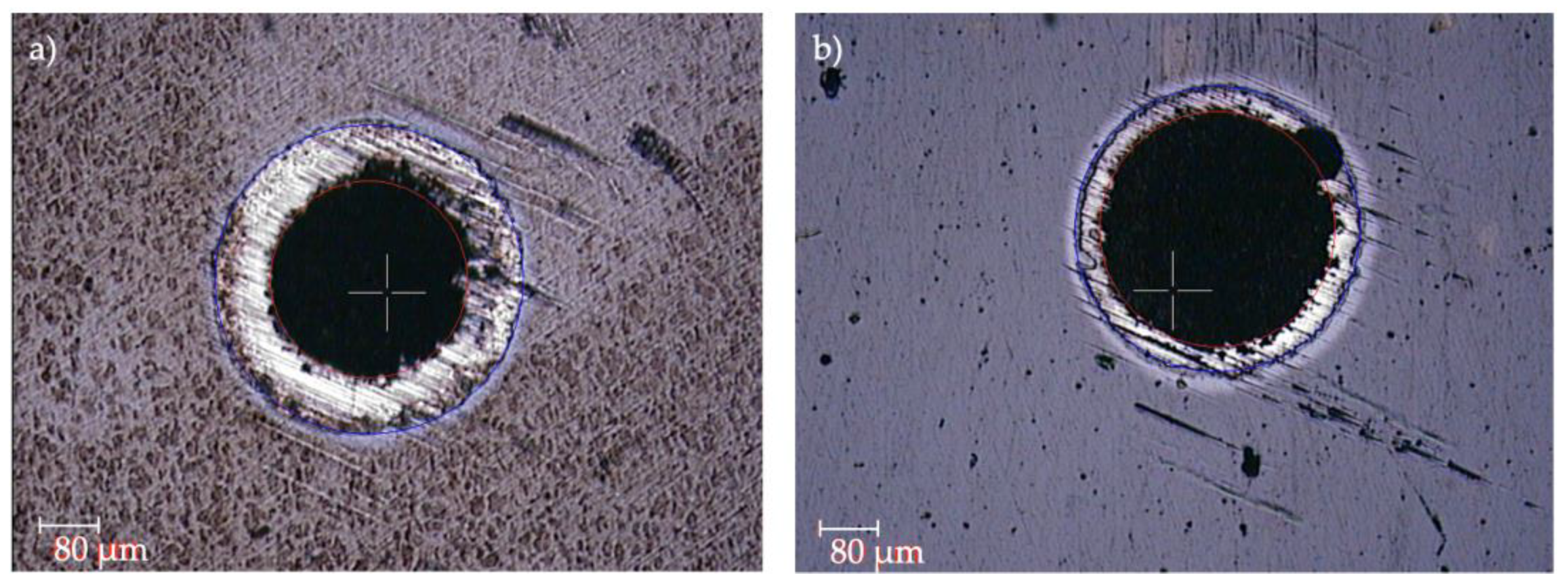
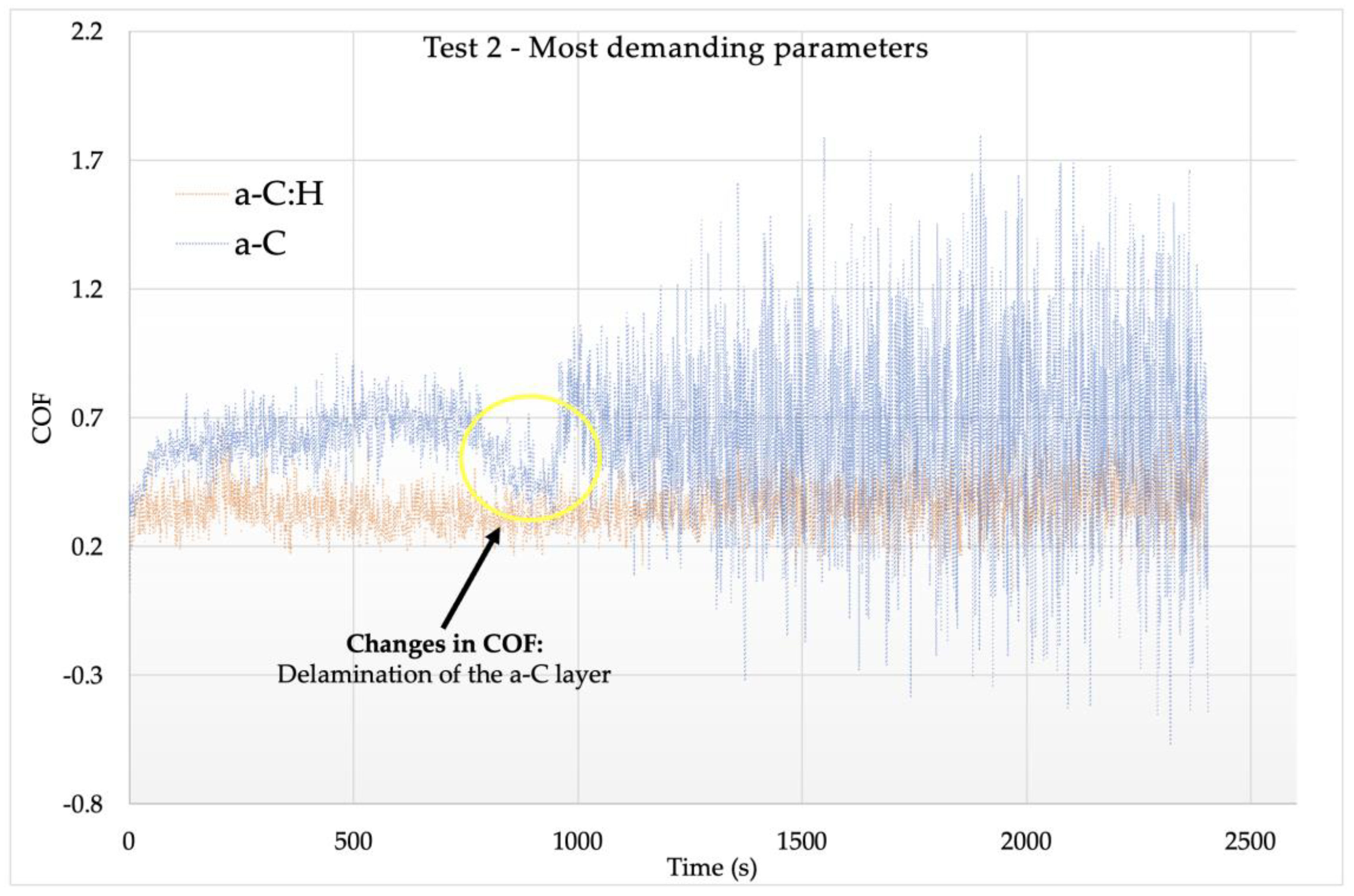
3.4. Tribological Tests
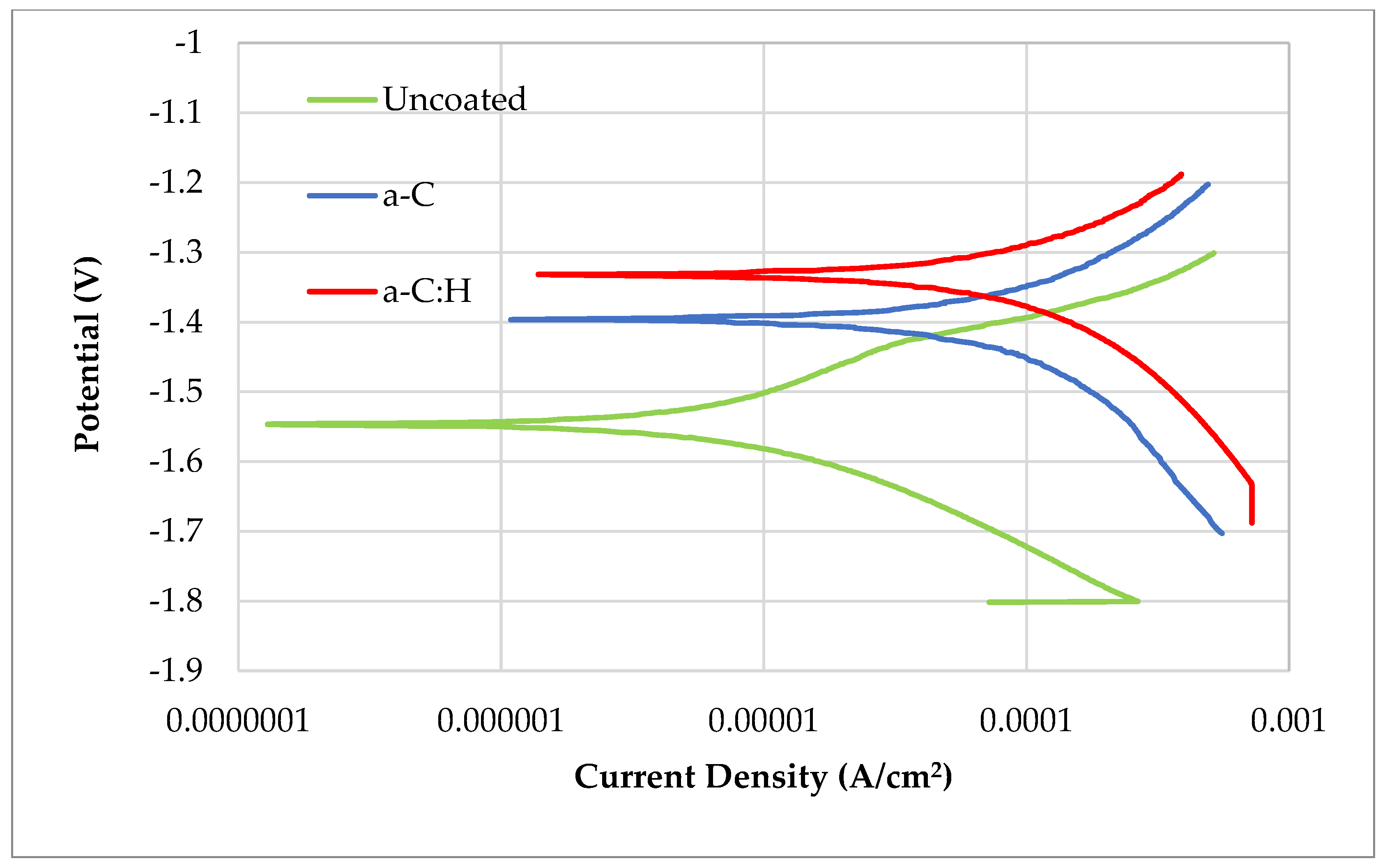
3.5. Corrosion Tests
4. Discussion
5. Conclusions
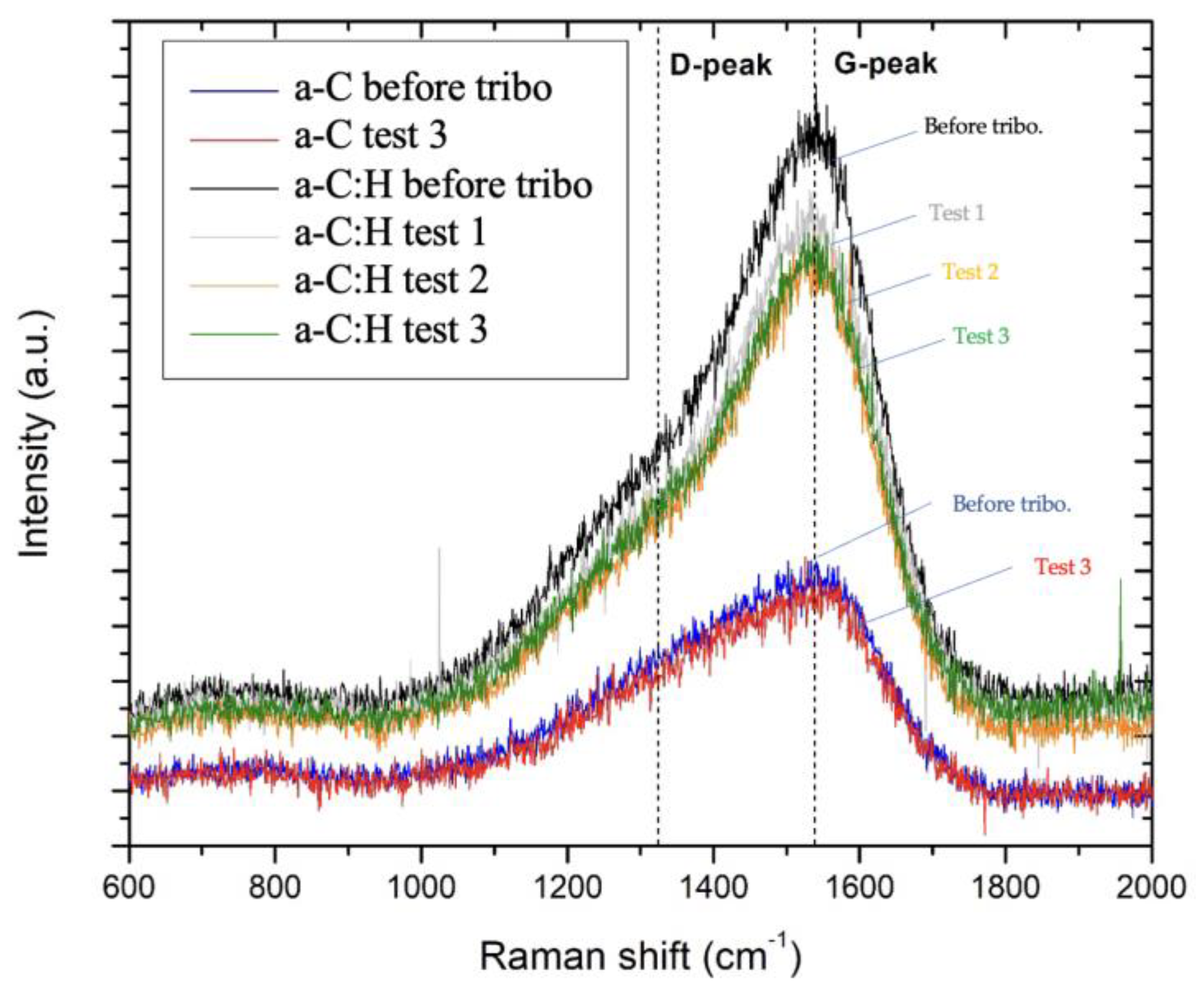
- The Raman spectra of all the films showed a disordered amorphous carbon structure. The G-peak was more predominant in a-C:H coatings in the Raman spectroscopy due to the higher order of the graphitic structures, as well as the higher hydrogen content in the samples. These characteristics also explain the reduced coefficient of friction found for this sample.
- The a-C:H coating (Lc2 and Lc3 of 7 and 12 N, respectively) showed better adhesion to the substrate than the a-C coating (Lc2 and Lc3 of 4 and 9 N, respectively).
- Nanoindentation results showed that both coatings improved H and E compared with the uncoated samples, with values of 18 GPa and 165 GPa, respectively. Furthermore, resistance to plastic deformation (H3/E2) was also improved.
- Mechanical properties, and load support shown by DLC thin films with both WC and CrC interlayers were similar, and the lower accumulation of internal stresses due to differences in thickness could be the reason for the enhanced adhesion strength of the a-C:H coating.
- The addition of H was shown to be beneficial for the tribological performance of the coating. The a-C:H coating presented lower COF (0.3–0.4) and k (up to 10−5) values, while the a-C layer suffered delamination during the tests. The lower COF value of the a-C:H samples was of great relevance to improving wear resistance.
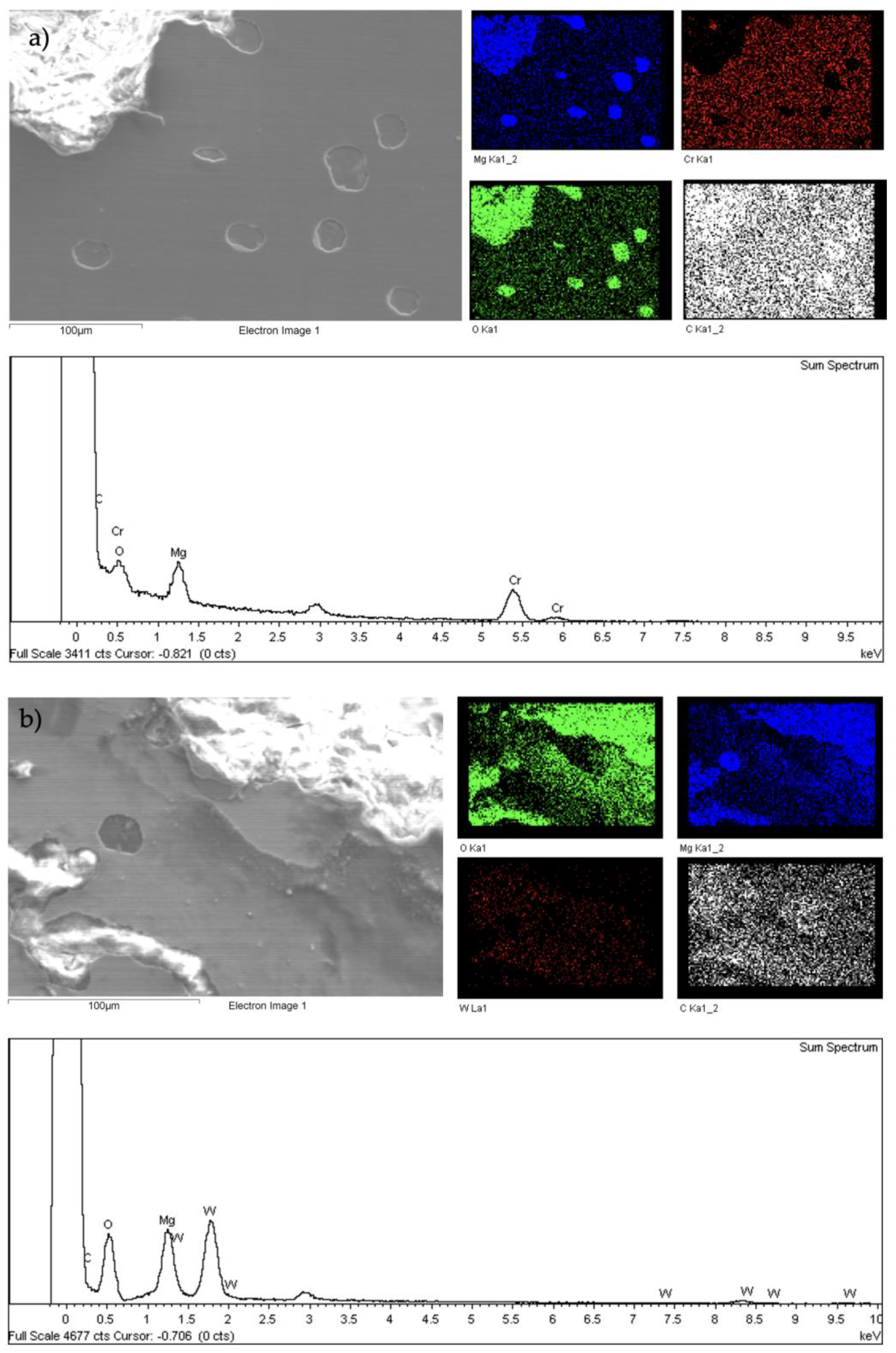
- Although the a-C:H coating showed better results than the a-C coating in the corrosion tests, both proved to be insufficient to protect Mg against corrosion. Both failed in the immersion tests, but the hydrogenated coating was shown to maintain elements of the coating while the hydrogen-free one did not.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In Vivo Corrosion of Four Magnesium Alloys and the Associated Bone Response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yang, J.; Zhang, X.; Yang, Q.; Zhang, J.; Li, X. Development and Application of Magnesium Alloy Parts for Automotive OEMs: A Review. J. Magnes. Alloys 2023, 11, 15–47. [Google Scholar] [CrossRef]
- Johnston, S.; Shi, Z.; Atrens, A. The Influence of PH on the Corrosion Rate of High-Purity Mg, AZ91 and ZE41 in Bicarbonate Buffered Hanks’ Solution. Corros. Sci. 2015, 101, 182–192. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, J.K.; Hopkins, C.; Chow, D.H.K.; Qin, L. Biodegradable Magnesium-Based Implants in Orthopedics—A General Review and Perspectives. Adv. Sci. 2020, 7, 1902443. [Google Scholar] [CrossRef] [PubMed]
- Sezer, N.; Evis, Z.; Kayhan, S.M.; Tahmasebifar, A.; Koç, M. Review of Magnesium-Based Biomaterials and Their Applications. J. Magnes. Alloys 2018, 6, 23–43. [Google Scholar] [CrossRef]
- Zhang, S.; Zou, X.; Liu, N.; Wang, H.; Xia, C.; Liang, C. In Situ Preparation of a Novel Ta2O5/MAO Composite Coating on Magnesium for Anti-Corrosion Protection. Surf. Coat. Technol. 2022, 430, 128003. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent Advances on the Development of Magnesium Alloys for Biodegradable Implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef]
- Yang, Y.; He, C.; Dianyu, E.; Yang, W.; Qi, F.; Xie, D.; Shen, L.; Peng, S.; Shuai, C. Mg Bone Implant: Features, Developments and Perspectives. Mater. Des. 2020, 185, 108259. [Google Scholar] [CrossRef]
- Chandra, G.; Pandey, A. Preparation Strategies for Mg-Alloys for Biodegradable Orthopaedic Implants and Other Biomedical Applications: A Review. IRBM 2020, 43, 229–249. [Google Scholar] [CrossRef]
- Bairagi, D.; Mandal, S. A Comprehensive Review on Biocompatible Mg-Based Alloys as Temporary Orthopaedic Implants: Current Status, Challenges, and Future Prospects. J. Magnes. Alloys 2021, 10, 627–669. [Google Scholar] [CrossRef]
- Merlo, J.L.; Detsch, R.; Ceré, S.; Boccaccini, A.R.; Ballarre, J. Degradable Magnesium Implants: Improving Bioactive and Antibacterial Performance by Designed Hybrid Coatings. J. Mater. Res. 2021, 36, 443–458. [Google Scholar] [CrossRef]
- Altun, H.; Sen, S. The Effect of PVD Coatings on the Corrosion Behaviour of AZ91 Magnesium Alloy. Mater. Des. 2006, 27, 1174–1179. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, P.; Wang, Q.; Wu, H.; Liu, Y.; Deng, Y.; Zhou, Y.; Shuai, C. The Enhancement of Mg Corrosion Resistance by Alloying Mn and Laser-Melting. Materials 2016, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Jian, S.-Y.; Ho, M.-L.; Shih, B.-C.; Wang, Y.-J.; Weng, L.-W.; Wang, M.-W.; Tseng, C.-C. Evaluation of the Corrosion Resistance and Cytocompatibility of a Bioactive Micro-Arc Oxidation Coating on AZ31 Mg Alloy. Coatings 2019, 9, 396. [Google Scholar] [CrossRef]
- Phani, A.R.; Gammel, F.J.; Hack, T.; Haefke, H. Enhanced Corrosioon Resistance by Sol-Gel-Based ZrO2-CeO2 Coatings on Magnesium Alloys. Mater. Corros. 2005, 56, 77–82. [Google Scholar] [CrossRef]
- Da Conceicao, T.F.; Scharnagl, N.; Blawert, C.; Dietzel, W.; Kainer, K.U. Surface Modification of Magnesium Alloy AZ31 by Hydrofluoric Acid Treatment and Its Effect on the Corrosion Behaviour. Thin Solid Films 2010, 518, 5209–5218. [Google Scholar] [CrossRef]
- Ning, C.-M.; Cui, X.-J.; Shang, L.-L.; Zhang, Y.-J.; Zhang, G.-A. Structure and Properties of Different Elements Doped Diamond-like Carbon on Micro-Arc Oxidation Coated AZ31B Mg Alloy. Diam. Relat. Mater. 2020, 106, 107832. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Dias, G.J.; Saud, S.N.; Yaghoubidoust, F.; Hadisi, Z. Fabrication and Characterisation of Novel ZnO/MWCNT Duplex Coating Deposited on Mg Alloy by PVD Coupled with Dip-Coating Techniques. J. Alloys Compd. 2017, 728, 159–168. [Google Scholar] [CrossRef]
- Hoche, H.; Blawert, C.; Broszeit, E.; Berger, C. General Corrosion and Galvanic Corrosion Properties of Differently PVD Treated Magnesium Die Cast Alloy AZ91. Adv. Eng. Mater. 2003, 5, 896–902. [Google Scholar] [CrossRef]
- Donnet, C.; Erdemir, A. New Horizon in the Tribology of Diamondlike Carbon Films. Surf. Eng. 2008, 24, 399–401. [Google Scholar] [CrossRef]
- Erdemir, A.; Donnet, C. Tribology of Diamond-like Carbon Films: Recent Progress and Future Prospects. J. Phys. D Appl. Phys. 2006, 39, R311. [Google Scholar] [CrossRef]
- Dai, M.; Zhou, K.; Yuan, Z.; Ding, Q.; Fu, Z. The Cutting Performance of Diamond and DLC-Coated Cutting Tools. Diam. Relat. Mater. 2000, 9, 1753–1757. [Google Scholar] [CrossRef]
- Hauert, R.; Thorwarth, K.; Thorwarth, G. An Overview on Diamond-like Carbon Coatings in Medical Applications. Surf. Coat. Technol. 2013, 233, 119–130. [Google Scholar] [CrossRef]
- Treutler, C.P.O. Industrial Use of Plasma-Deposited Coatings for Components of Automotive Fuel Injection Systems. Surf. Coat. Technol. 2005, 200, 1969–1975. [Google Scholar] [CrossRef]
- Bewilogua, K.; Hofmann, D. History of Diamond-like Carbon Films—From First Experiments to Worldwide Applications. Surf. Coat. Technol. 2014, 242, 214–225. [Google Scholar] [CrossRef]
- Robertson, J. Diamond-like Amorphous Carbon. Mater. Sci. Eng. R Rep. 2002, 37, 129–281. [Google Scholar] [CrossRef]
- Picas, J.A.; Menargues, S.; Martin, E.; Colominas, C.; Baile, M.T. Characterization of Duplex Coating System (HVOF + PVD) on Light Alloy Substrates. Surf. Coat. Technol. 2017, 318, 326–331. [Google Scholar] [CrossRef]
- Lin, Y.H.; Da Lin, H.; Liu, C.K.; Huang, M.W.; Chen, J.R.; Shih, H.C. Structure and Characterization of the Multilayered Ti-DLC Films by FCVA. Diam. Relat. Mater. 2010, 19, 1034–1039. [Google Scholar] [CrossRef]
- Dai, W.; Wu, G.; Wang, A. Preparation, Characterization and Properties of Cr-Incorporated DLC Films on Magnesium Alloy. Diam. Relat. Mater. 2010, 19, 1307–1315. [Google Scholar] [CrossRef]
- Wu, G.; Dai, W.; Zheng, H.; Wang, A. Improving Wear Resistance and Corrosion Resistance of AZ31 Magnesium Alloy by DLC/AlN/Al Coating. Surf. Coat. Technol. 2010, 205, 2067–2073. [Google Scholar] [CrossRef]
- Yang, W.; Ke, P.; Fang, Y.; Zheng, H.; Wang, A. Microstructure and Properties of Duplex (Ti:N)-DLC/MAO Coating on Magnesium Alloy. Appl. Surf. Sci. 2013, 270, 519–525. [Google Scholar] [CrossRef]
- Yang, W.; Deng, Z.; Zhang, D.; Ke, P.; Wang, A. Microstructure and Tribological Behavior of Self-Lubricating (Si:N)-DLC/MAO Coatings on AZ80 Magnesium Substrate. Acta Metall. Sin. 2013, 26, 693–698. [Google Scholar] [CrossRef]
- Cui, X.J.; Ning, C.M.; Shang, L.L.; Zhang, G.A.; Liu, X.Q. Structure and Anticorrosion, Friction, and Wear Characteristics of Pure Diamond-Like Carbon (DLC), Cr-DLC, and Cr-H-DLC Films on AZ91D Mg Alloy. J. Mater. Eng. Perform. 2019, 28, 1213–1225. [Google Scholar] [CrossRef]
- Wu, G.; Sun, L.; Dai, W.; Song, L.; Wang, A. Influence of Interlayers on Corrosion Resistance of Diamond-like Carbon Coating on Magnesium Alloy. Surf. Coat. Technol. 2009, 204, 2193–2196. [Google Scholar] [CrossRef]
- Masami, I.; Setsuo, N.; Tsutomu, S.; Junho, C. Improvement of Corrosion Protection Property of Mg-Alloy by DLC and Si–DLC Coatings with PBII Technique and Multi-Target DC–RF Magnetron Sputtering. Nucl. Instrum. Methods Phys. Res. B 2009, 267, 1675–1679. [Google Scholar] [CrossRef]
- Chen, W.; Huang, J.; Peng, J. Characterisation of TiAlN PVD Coatings on AZ31 Magnesium Alloy. Res. Chem. Intermed. 2013, 41, 1257–1266. [Google Scholar] [CrossRef]
- Çelik, A.; Bozkurt, Y.B. Improvement of Tribological Performance of AZ31 Biodegradable Alloy by TiN-Based PVD Coatings. Tribol. Int. 2022, 173, 107684. [Google Scholar] [CrossRef]
- Hoche, H.; Groß, S.; Troßmann, T.; Schmidt, J.; Oechsner, M. PVD Coating and Substrate Pretreatment Concepts for Magnesium Alloys by Multinary Coatings Based on Ti(X)N. Surf. Coat. Technol. 2013, 228, S336–S341. [Google Scholar] [CrossRef]
- Hoche, H.; Groß, S.; Oechsner, M. Development of New PVD Coatings for Magnesium Alloys with Improved Corrosion Properties. Surf. Coat. Technol. 2014, 259, 102–108. [Google Scholar] [CrossRef]
- Claver, A.; Jiménez-Piqué, E.; Palacio, J.F.; Almandoz, E.; Fernández de Ara, J.; Fernández, I.; Santiago, J.A.; Barba, E.; García, J.A. Comparative Study of Tribomechanical Properties of HiPIMS with Positive Pulses DLC Coatings on Different Tools Steels. Coatings 2020, 11, 28. [Google Scholar] [CrossRef]
- Li, G.; Sun, J.; Xu, Y.; Xu, Y.; Gu, J.; Wang, L.; Huang, K.; Liu, K.; Li, L. Microstructure, Mechanical Properties, and Cutting Performance of TiAlSiN Multilayer Coatings Prepared by HiPIMS. Surf. Coat. Technol. 2018, 353, 274–281. [Google Scholar] [CrossRef]
- Santiago, J.A.; Fernández-Martínez, I.; Sánchez-López, J.C.; Rojas, T.C.; Wennberg, A.; Bellido-González, V.; Molina-Aldareguia, J.M.; Monclús, M.A.; González-Arrabal, R. Tribomechanical Properties of Hard Cr-Doped DLC Coatings Deposited by Low-Frequency HiPIMS. Surf. Coat. Technol. 2020, 382, 124899. [Google Scholar] [CrossRef]
- Wei, X.; Ma, J.; Ma, S.; Liu, P.; Qing, H.; Zhao, Q. Enhanced Anti-Corrosion and Biocompatibility of a Functionalized Layer Formed on ZK60 Mg Alloy via Hydroxyl (OH-) Ion Implantation. Colloids Surf. B Biointerfaces 2022, 216, 112533. [Google Scholar] [CrossRef] [PubMed]
- Murni, N.S.; Dambatta, M.S.; Yeap, S.K.; Froemming, G.R.A.; Hermawan, H. Cytotoxicity Evaluation of Biodegradable Zn–3Mg Alloy toward Normal Human Osteoblast Cells. Mater. Sci. Eng. C 2015, 49, 560–566. [Google Scholar] [CrossRef]
- Li, Y.; Wen, C.; Mushahary, D.; Sravanthi, R.; Harishankar, N.; Pande, G.; Hodgson, P. Mg–Zr–Sr Alloys as Biodegradable Implant Materials. Acta Biomater. 2012, 8, 3177–3188. [Google Scholar] [CrossRef]
- UNE-EN ISO 25178-2:2023; Geometrical Product Specifications (GPS)—Surface Texture: Areal—Part 2: Terms, Definitions and Surface Texture Parameters. UNE: Madrid, Spain, 2023. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma/?c=N0071403 (accessed on 2 October 2023).
- Oliver, W.C.; Pharr, G.M. An Improved Technique for Determining Hardness and Elastic Modulus Using Load and Displacement Sensing Indentation Experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- ASTM G99—17; Standard Test Method for Wear Testing with a Pin-on-Disk Apparatus. ASTM International: West Conshohocken, PA, USA, 2017. Available online: https://www.astm.org (accessed on 2 October 2023).
- Kirkland, N.T.; Birbilis, N.; Staiger, M.P. Assessing the Corrosion of Biodegradable Magnesium Implants: A Critical Review of Current Methodologies and Their Limitations. Acta Biomater. 2012, 8, 925–936. [Google Scholar] [CrossRef]
- Witte, F. The History of Biodegradable Magnesium Implants: A Review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef]
- Schwan, J.; Ulrich, S.; Batori, V.; Ehrhardt, H.; Silva, S.R.P. Raman Spectroscopy on Amorphous Carbon Films. J. Appl. Phys. 1996, 80, 440–447. [Google Scholar] [CrossRef]
- Marchon, B. Photoluminescence and Raman Spectroscopy in Hydrogenated Carbon Films. IEEE Trans. Magn. 1997, 33, 3148–3150. [Google Scholar] [CrossRef]
- Marin, E.; Lanzutti, A.; Nakamura, M.; Zanocco, M.; Zhu, W.; Pezzotti, G.; Andreatta, F. Corrosion and Scratch Resistance of DLC Coatings Applied on Chromium Molybdenum Steel. Surf. Coat. Technol. 2019, 378, 124944. [Google Scholar] [CrossRef]
- Santiago, J.A.; Fernández-Martínez, I.; Wennberg, A.; Molina-Aldareguia, J.M.; Castillo-Rodríguez, M.; Rojas, T.C.; Sánchez-López, J.C.; González, M.U.; García-Martín, J.M.; Li, H.; et al. Adhesion Enhancement of DLC Hard Coatings by HiPIMS Metal Ion Etching Pretreatment. Surf. Coat. Technol. 2018, 349, 787–796. [Google Scholar] [CrossRef]
- Molak, R.M.; Topolski, K.; Spychalski, M.; Dulińska-Molak, I.; Morończyk, B.; Pakieła, Z.; Nieużyła, L.; Mazurkiewicz, M.; Wojucki, M.; Gebeshuber, A.; et al. Functional Properties of the Novel Hybrid Coatings Combined of the Oxide and DLC Layer as a Protective Coating for AZ91E Magnesium Alloy. Surf. Coat. Technol. 2019, 380, 125040. [Google Scholar] [CrossRef]
- Leyland, A.; Matthews, A. On the Significance of the H/E Ratio in Wear Control: A Nanocomposite Coating Approach to Optimised Tribological Behaviour. Wear 2000, 246, 1–11. [Google Scholar] [CrossRef]
- Charitidis, C.A. Nanomechanical and Nanotribological Properties of Carbon-Based Thin Films: A Review. Int. J. Refract. Met. Hard Mater. 2010, 28, 51–70. [Google Scholar] [CrossRef]
- Galvan, D.; Pei, Y.T.; De Hosson, J.T.M. Deformation and Failure Mechanism of Nano-Composite Coatings under Nano-Indentation. Surf. Coat. Technol. 2006, 200, 6718–6726. [Google Scholar] [CrossRef]
- Lopes, B.B.; Rangel, R.C.C.; Antonio, C.A.; Durrant, S.F.; Cruz, N.C.; Rangel, E.C. Mechanical and Tribological Properties of Plasma Deposited A-C:H:Si:O Films. In Nanoindentation in Materials Science; Nemecek, J., Ed.; IntechOpen: Rijeka, Croatia, 2012; Chapter 8. [Google Scholar]
- Mayrhofer, P.H.; Mitterer, C.; Musil, J. Structure-Property Relationships in Single-and Dual-Phase Nanocrystalline Hard Coatings. Surf. Coat. Technol. 2003, 174, 725–731. [Google Scholar] [CrossRef]
- Suh, C.M.; Hwang, B.W.; Murakami, R.I. Behaviors of Residual Stress and High-Temperature Fatigue Life in Ceramic Coatings Produced by PVD. Mater. Sci. Eng. A 2003, 343, 1–7. [Google Scholar] [CrossRef]
- Bai, Y.Y.; Gao, J.; Guo, T.; Gao, K.W.; Volinsky, A.A.; Pang, X.L. Review of the Fatigue Behavior of Hard Coating-Ductile Substrate Systems. Int. J. Miner. Metall. Mater. 2021, 28, 46–55. [Google Scholar] [CrossRef]
- Duminica, F.-D.; Belchi, R.; Libralesso, L.; Mercier, D. Investigation of Cr(N)/DLC Multilayer Coatings Elaborated by PVD for High Wear Resistance and Low Friction Applications. Surf. Coat. Technol. 2018, 337, 396–403. [Google Scholar] [CrossRef]
- Li, Y.; Meng, D.; Feng, Z.; She, D.; Yue, W. Effect of Different Working Conditions on Tribological Behaviors of PcBN/440c Stainless Steel in Vacuum Environment. Int. J. Refract. Met. Hard Mater. 2023, 116, 106366. [Google Scholar] [CrossRef]
- Sivaprakasam, P.; Hailu, T.; Elias, G. Experimental Investigation on Wear Behavior of Titanium Alloy (Grade 23) by Pin on Disc Tribometer. Results Mater. 2023, 19, 100422. [Google Scholar] [CrossRef]
- Wei, T.Z.; Shamsuri, S.R.B.; Chang, S.Y.; Rashid, M.W.A.; Ahsan, Q. Effect of Sliding Velocity on Wear Behavior of Magnesium Composite Reinforced with SiC and MWCNT. Procedia Eng. 2013, 68, 703–709. [Google Scholar] [CrossRef]
- Dixit, T.; Prasad, K.E. Effect of Temperature and Sliding Velocity on the Dry Sliding Wear Mechanisms of Boron Modified Ti-6Al-4V Alloys. Lubricants 2022, 10, 296. [Google Scholar] [CrossRef]
- Fisher, J.; Dowson, D.; Hamdzah, H.; Lee, H.L. The Effect of Sliding Velocity on the Friction and Wear of UHMWPE for Use in Total Artificial Joints. Wear 1994, 175, 219–225. [Google Scholar] [CrossRef]
- Zhu, W.; Yu, Z.; Yang, C.; Dong, F.; Ren, Z.; Zhang, K. Spatial Distribution of Corrosion Products Influenced by the Initial Defects and Corrosion-Induced Cracking of the Concrete. J. Test. Eval. 2023, 51, 2582–2597. [Google Scholar] [CrossRef]
- Zhu, W.; Yang, C.; Yu, Z.; Xiao, J.; Xu, Y. Impact of Defects in Steel-Concrete Interface on the Corrosion-Induced Cracking Propagation of the Reinforced Concrete. KSCE J. Civ. Eng. 2023, 27, 2621–2628. [Google Scholar] [CrossRef]
- Bolelli, G.; Lusvarghi, L.; Montecchi, M.; Mantini, F.P.; Pitacco, F.; Volz, H.; Barletta, M. HVOF-Sprayed WC-Co as Hard Interlayer for DLC Films. Surf. Coat. Technol. 2008, 203, 699–703. [Google Scholar] [CrossRef]
- Wahlström, J. A Pin-on-Disc Tribometer Study of Friction at Low Contact Pressures and Sliding Speeds for a Disc Brake Material Combination. Results Eng. 2019, 4, 100051. [Google Scholar] [CrossRef]





| Al | Zn | Mn | Fe | Cu | Zr | Ni | Si | Mg | |
|---|---|---|---|---|---|---|---|---|---|
| % | 0.0012 | 5.7 | 0.033 | 0.0025 | 0.0017 | 0.54 | 0.0012 | 0.0027 | Reminder |
| Mechanical Properties | Tensile Strength: 322 Mpa | Yield Strength: 256 MPa | Elongation: 10% | ||||||
| Step | Process | Material |
|---|---|---|
| 1 | Grounding | SiC emery paper: from 180 to 1200 grit size |
| 2 | Cleaning and rinsing | Distilled water and ethanol |
| 3 | Polishing | polishing cloths and diamond polycrystalline suspension (particle size 9 and 1 µm) |
| 4 | Cleaning | Ultrasonic cleaning with ethanol and drying in air |
| Step | Process | Description |
|---|---|---|
| 1 | Ar etching | Ar+ discharge established at the substrates: 15 min, DC-pulsed bias voltage of −500 V, and a frequency of 150 kHz. |
| 2 | Bonding layer | Cr target operated in HiPIMS:
|
| 3 | Interlayer | WC interlayer:
|
CrC interlayer:
| ||
| 4 | DLC coating | a-C:H coating:
|
| Fn (N) | Ω (rpm) | Duration (Cycles) | Radius (mm) | |
|---|---|---|---|---|
| Test 1 | 1 | 150 | 2000 | 16 |
| Test 2 | 1 | 150 | 6000 | 18 |
| Test 3 | 1 | 100 | 3000 | 22 |
| Sample | Roughness Sa (nm) |
|---|---|
| Uncoated ZK 60 | 19 ± 4 |
| a-C | 44 ± 8 |
| a-C:H | 18 ± 1 |
| Uncoated | a-C | a-C:H | |
|---|---|---|---|
| H3/E2 | 0.0006 ± 0.0001 | 0.22 ± 0.06 | 0.19 ± 0.03 |
| H/E | 0.02 ± 0.00 | 0.11 ± 0.01 | 0.12 ± 0.01 |
| COF Values | Test 1 | Test 2 | Test 3 |
|---|---|---|---|
| Uncoated | 0.4 | 1 | 1.2 |
| a-C | 0.8 | 0.6 | 0.5 |
| a-C:H | 0.5 | 0.4 | 0.3 |
| Sample | Ecorr (V) | jcorr (µA/cm2) |
|---|---|---|
| Uncoated ZK 60 | −1.55 ± 0.01 | 7.2 ± 0.7 |
| a-C | −1.40 ± 0.02 | 91.4 ± 5 |
| a-C:H | −1.33 ± 0.03 | 21.9 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Claver, A.; Fernández, I.; Santiago, J.A.; Díaz-Rodríguez, P.; Panizo-Laiz, M.; Esparza, J.; Palacio, J.F.; Fuentes, G.G.; Zalakain, I.; García, J.A. Corrosion and Tribological Performance of Diamond-like Carbon-Coated ZK 60 Magnesium Alloy. Coatings 2023, 13, 1871. https://doi.org/10.3390/coatings13111871
Claver A, Fernández I, Santiago JA, Díaz-Rodríguez P, Panizo-Laiz M, Esparza J, Palacio JF, Fuentes GG, Zalakain I, García JA. Corrosion and Tribological Performance of Diamond-like Carbon-Coated ZK 60 Magnesium Alloy. Coatings. 2023; 13(11):1871. https://doi.org/10.3390/coatings13111871
Chicago/Turabian StyleClaver, Adrián, Iván Fernández, José Antonio Santiago, Pablo Díaz-Rodríguez, Miguel Panizo-Laiz, Joseba Esparza, José F. Palacio, Gonzalo G. Fuentes, Iñaki Zalakain, and José Antonio García. 2023. "Corrosion and Tribological Performance of Diamond-like Carbon-Coated ZK 60 Magnesium Alloy" Coatings 13, no. 11: 1871. https://doi.org/10.3390/coatings13111871







