One-Step Synthesis of Nitrogen-Doped Porous Carbon Derived from Biomass for Lithium-Ion Battery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Bamboo Shoot Derived Carbon (BSC)
2.3. Material Characterization
2.4. Electrochemical Measurements
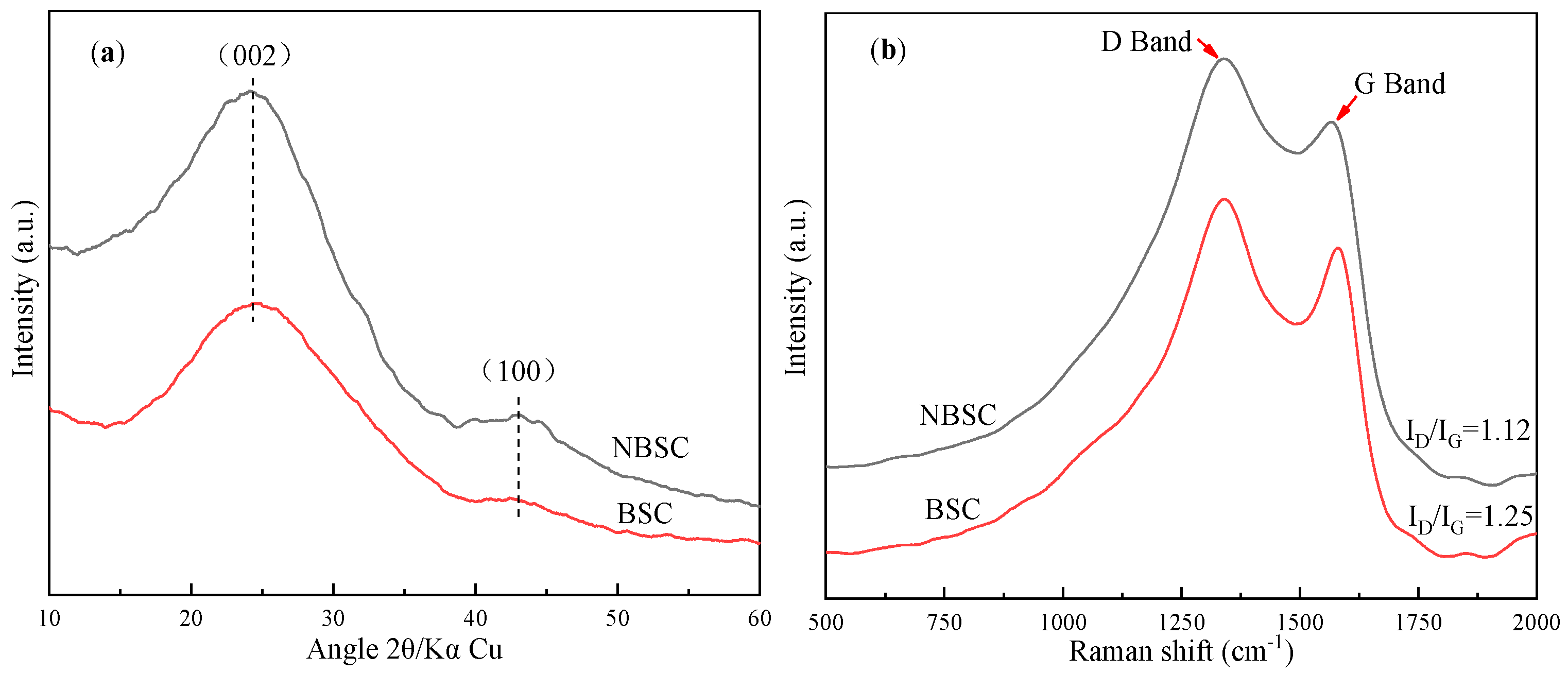
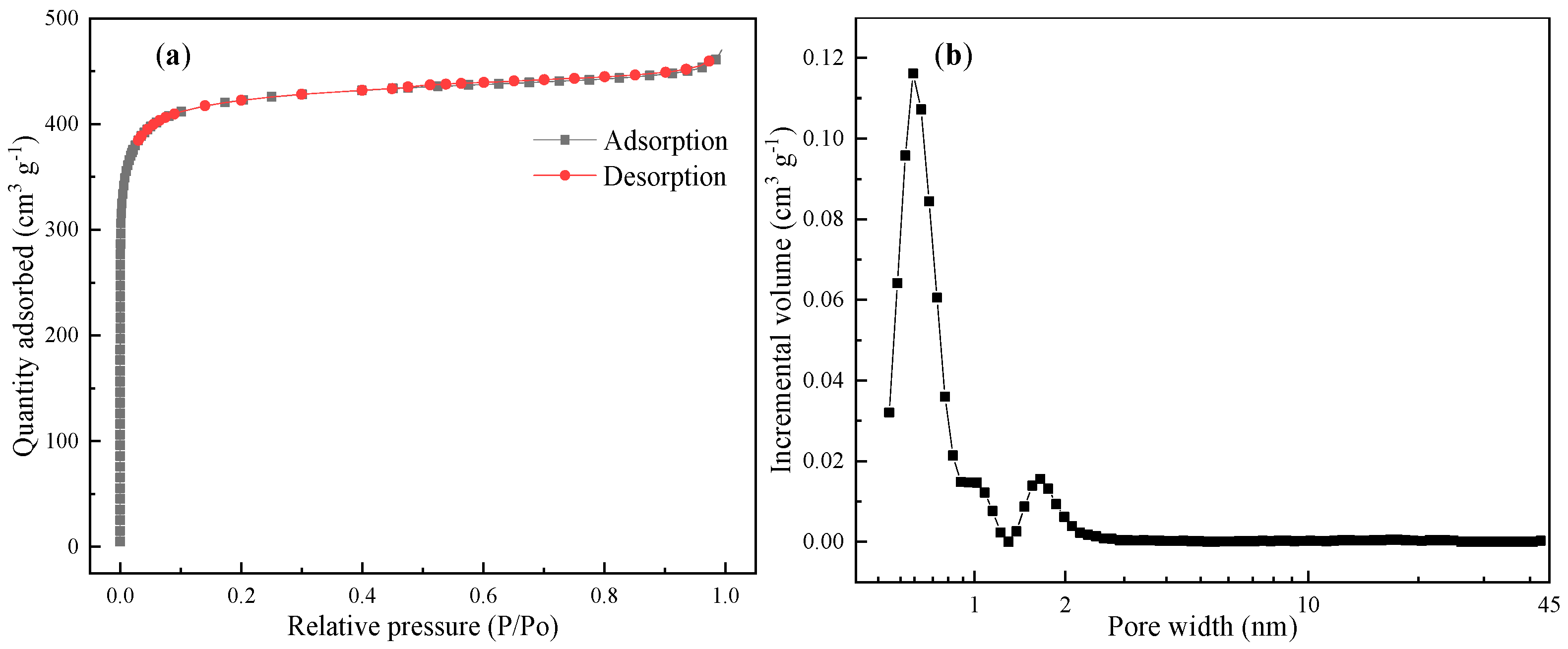
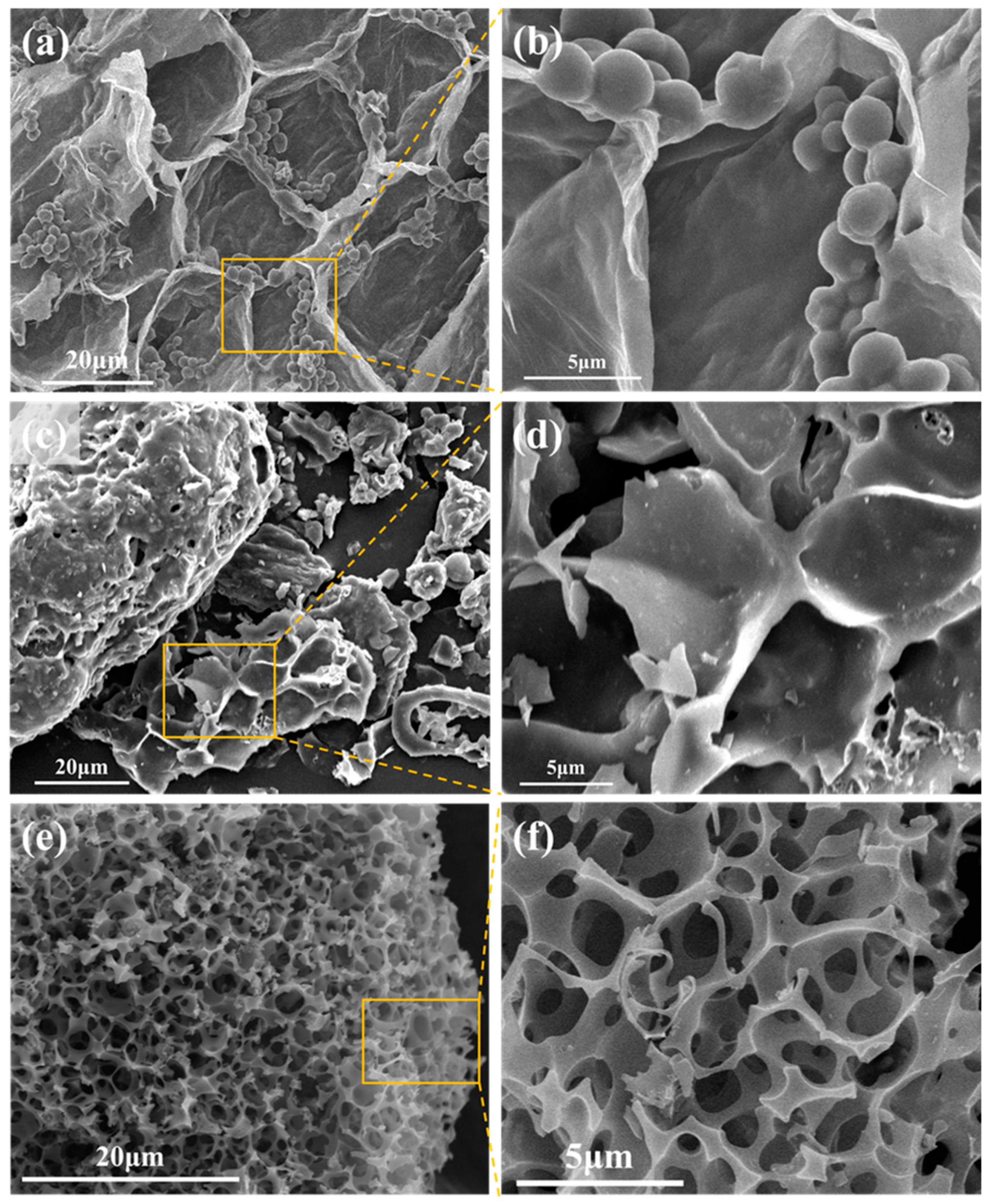
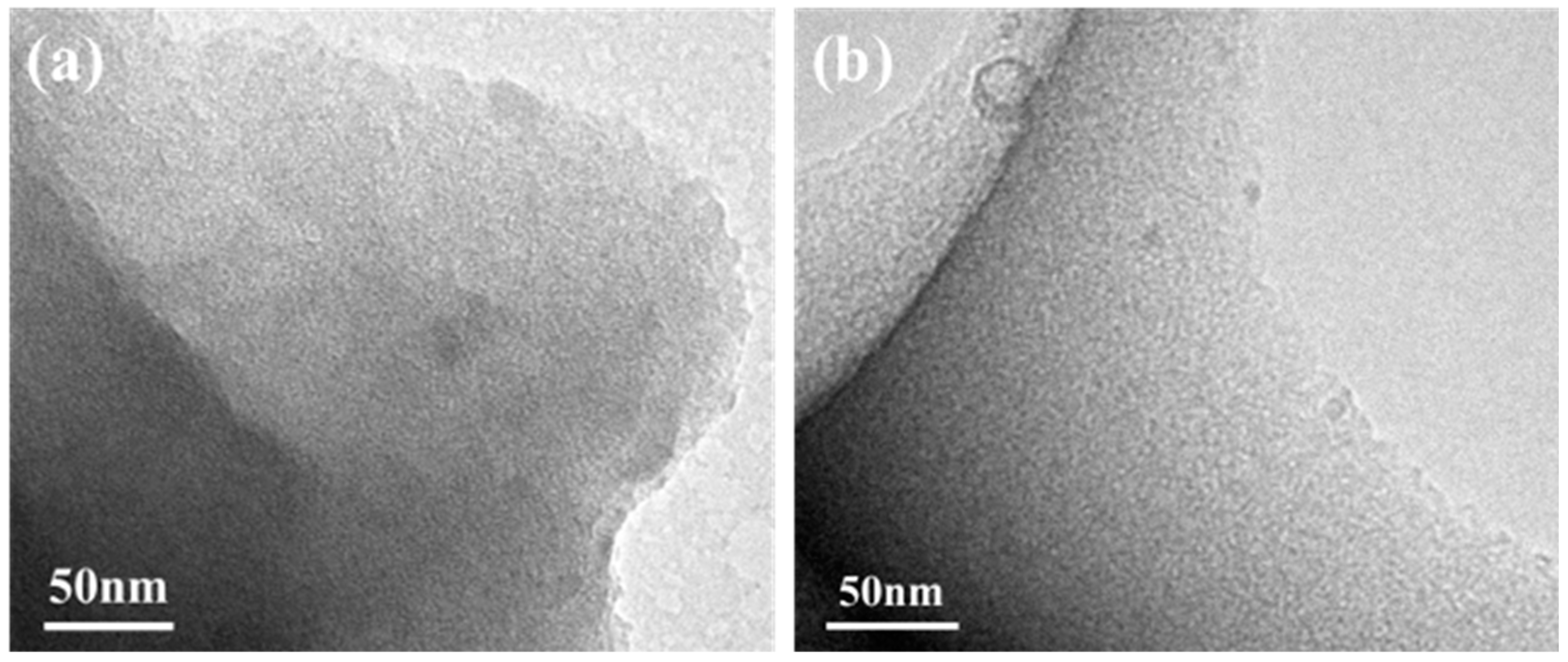
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, L.T.; Rui, X.H.; Chen, G.; Xu, W.C.; Zou, G.F.; Luo, H.M. Recent advances in nanostructured Nb-based oxides for electrochemical energy storage. Nanoscale 2016, 8, 8443–8465. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.V.; Rao, G.V.S.; Chowdari, B.V.R. Metal oxides and oxysalts as anode materials for Li ion batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [CrossRef] [PubMed]
- Rehmana, J.; Fana, X.F.; Zheng, W.T. Computational insight of monolayer SnS2 as anode material for potassium ion batteries. Appl. Surf. Sci. 2019, 496, 143625–143631. [Google Scholar] [CrossRef]
- Peng, Q.; Rehman, J.; Eid, K.; Alofi, A.S.; Laref, A.; Albaqami, M.D.; Alotabi, R.G.; Shibl, M.F. Vanadium Carbide (V4C3) MXene as an efficient anode for Li-ion and Na-ion batteries. Nanomaterials 2022, 12, 2825–2836. [Google Scholar] [CrossRef]
- Dahn, J.R.; Zheng, T.; Liu, Y.H.; Xue, J.S. Mechanisms for lithium insertion in carbonaceous materials. Science 1995, 270, 590–593. [Google Scholar] [CrossRef]
- Liu, X.X.; Chao, D.L.; Li, Y.; Hao, J.; Liu, X.S.; Zhao, J.P.; Lin, J.Y.; Fan, H.J.; Shen, Z.X. A low-cost and one-step synthesis of N-doped monolithic quasi-graphene films with porous carbon frameworks for Li-ion batteries. Nano Energy 2015, 17, 43–51. [Google Scholar] [CrossRef]
- Ren, M.M.; Yang, M.Z.; Liu, W.L.; Li, M.; Su, L.W.; Wu, X.B.; Wang, Y.H. Co-modification of nitrogen-doped graphene and carbon on Li3V2(PO4)3 particles with excellent long-term and high-rate performance for lithium storage. J. Power Sources 2016, 326, 313–321. [Google Scholar] [CrossRef]
- Ou, J.K.; Yang, L.; Zhang, Z.; Xi, X.H. Honeysuckle-derived hierarchical porous nitrogen, sulfur, dual-doped carbon for ultra-high rate lithium ion battery anodes. J. Power Sources 2016, 333, 193–202. [Google Scholar] [CrossRef]
- Yu, W.H.; Wang, H.L.; Liu, S.; Mao, N.; Liu, X.; Shi, J.; Liu, W.; Chen, S.G.; Wang, X. N, O-codoped hierarchical porous carbons derived from algae for high-capacity supercapacitors and battery anodes. J. Mater. Chem. A 2016, 4, 5973–5983. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.; Kishore, K.R.; Sharma, C.S. A hybrid flexible N-doped candle-soot carbon nanofibers for binder-free lithium-ion battery anode. Mater. Lett. 2023, 349, 134873–134876. [Google Scholar] [CrossRef]
- Selvamani, V.; Ravikumar, R.; Suryanarayanan, V.; Velayutham, D.; Gopukumar, S. Fish scale derived from nitrogen doped hierarchical porous carbon-a high rate performing anode for lithium ion cell. Electrochim. Acta 2015, 182, 1–10. [Google Scholar] [CrossRef]
- Hou, J.H.; Cao, C.B.; Idrees, F.; Ma, X.L. Hierarchical porous nitrogen-doped carbon nanosheets derived from silk for ultrahigh-capacity battery anodes and supercapacitors. ACS Nano 2015, 9, 2556–2564. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, P.L.; Zhang, Y.G.; Wang, L.L.; Zhang, L.T.; Zhang, L.; Xu, L.; Liu, L. Biomass based nitrogen-doped structure-tunable versatile porous carbon materials. J. Mater. Chem. A 2017, 5, 12958–12968. [Google Scholar] [CrossRef]
- Hu, C.G.; Xiao, Y.; Zhao, Y.; Chen, N.; Zhang, Z.P.; Cao, M.H.; Qu, L.T. Highly nitrogen-doped carbon capsules: Scalable preparation and high-performance applications in fuel cells and lithium ion batteries. Nanoscale 2013, 5, 2726–2733. [Google Scholar] [CrossRef] [PubMed]
- Kan-nari, N.; Okamura, S.; Fujita, S.; Ozaki, J.; Arai, M. Nitrogen-doped carbon materials prepared by ammoxidation as solid base catalysts for Knoevenagel condensation and transesterification reactions. Adv. Synth. Catal. 2010, 352, 1476–1484. [Google Scholar] [CrossRef]
- Zhao, W.M.; Wen, J.J.; Zhao, Y.M.; Wang, Z.F.; Shi, Y.R.; Zhao, Y. Hierarchically porous carbon derived from biomass reed flowers as highly stable Li-ion battery anode. Nanomaterials 2020, 10, 346–356. [Google Scholar] [CrossRef]
- Li, X.N.; Liang, J.W.; Hou, Z.G.; Zhu, Y.C.; Qian, Y.T. Recycling chicken eggshell membranes for high-capacity sodium battery anodes. RSC Adv. 2014, 4, 50950–50954. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Chen, L.; Meng, Y.; Xie, J.; Guo, Y.; Xiao, D. Lithium and sodium storage in highly ordered mesoporous nitrogen-doped carbons derived from honey. J. Power Sources 2016, 335, 20–30. [Google Scholar] [CrossRef]
- Ou, J.K.; Zhang, Y.Z.; Chen, L.; Zhao, Q.; Meng, Y.; Guo, Y.; Xiao, D. Nitrogen-rich porous carbon derived from biomass as a high performance anode material for lithium ion batteries. J. Mater. Chem. A 2015, 3, 6534–6541. [Google Scholar] [CrossRef]
- Selvamani, V.; Ravikumar, R.; Suryanarayanan, V.; Velayutham, D.; Gopukumar, S. Garlic peel derived high capacity hierarchical N-doped porous carbon anode for sodium/lithium ion cell. Electrochim. Acta 2016, 190, 337–345. [Google Scholar] [CrossRef]
- Elizabeth, I.; Singh, B.P.; Trikha, S.; Gopukumar, S. Bio-derived hierarchically macro-meso-micro porous carbon anode for lithium/sodium ion batteries. J. Power Sources 2016, 329, 412–421. [Google Scholar] [CrossRef]
- Lv, W.M.; Zhao, J.; Wen, F.S.; Xiang, J.Y.; Li, L.; Wang, L.M.; Liu, Z.Y.; Tian, Y.J. Carbonaceous photonic crystals as ultralong cycling anodes for lithium and sodium batteries. J. Mater. Chem. A 2015, 3, 13786–13793. [Google Scholar] [CrossRef]
- Mondal, A.K.; Kretschmer, K.; Zhao, Y.F.; Liu, H.; Fan, H.B.; Wang, G.X. Naturally nitrogen doped porous carbon derived from waste shrimp shells for high-performance lithium ion batteries and supercapacitors. Micropor. Mesopor. Mat. 2017, 246, 72–80. [Google Scholar] [CrossRef]
- Hu, X.D.; Sun, X.H.; Yoo, S.J.; Evanko, B.; Fan, F.R.; Cai, C.; Zheng, C.M.; Hu, W.B.; Stucky, G.D. Nitrogen-rich hierarchically porous carbon as a high-rate anode material with ultra-stable cyclability and high capacity for capacitive sodium-ion batteries. Nano Energy 2019, 56, 828–839. [Google Scholar] [CrossRef]
- Mao, Y.; Duan, H.; Xu, B.; Zhang, L.; Hu, Y.S.; Zhao, C.C.; Wang, Z.X.; Chen, L.Q.; Yang, Y.S. Lithium storage in nitrogen-rich mesoporous carbon materials. Energy Environ. Sci. 2012, 5, 7950–7955. [Google Scholar] [CrossRef]
- Xing, R.H.; Zhou, T.S.; Zhou, Y.; Ma, R.G.; Liu, Q.; Luo, J.; Wang, J.C. Creation of triple hierarchical micro-meso-macroporous N-doped carbon shells with hollow cores toward the electrocatalytic oxygen reduction reaction. Nano-Micro Lett. 2018, 10, 3. [Google Scholar] [CrossRef]
- Xiang, J.Y.; Lv, W.M.; Mu, C.P.; Zhao, J.; Wang, B.C. Activated hard carbon from orange peel for lithium/sodium ion battery anode with long cycle life. J. Alloys Compd. 2017, 701, 870–874. [Google Scholar] [CrossRef]
- Xu, G.Y.; Han, J.P.; Ding, B.; Nie, P.; Pan, J.; Dou, H.; Li, H.S.; Zhang, X.G. Biomass-derived porous carbon materials with sulfur and nitrogen dual-doping for energy storage. Green Chem. 2015, 3, 1668–1674. [Google Scholar] [CrossRef]
- Chongtham, N.; Elangbam, D.; Manohar, L.S. Changes in nutrient components during ageing of emerging juvenile bamboo shoots. Int. J. Food Sci. Nutr. 2007, 58, 612–618. [Google Scholar]
- Chongtham, N.; Bisht, M.S.; Haorongbam, S. Nutritional properties of bamboo shoots: Potential and prospects for utilization as a health food. Compr. Rev. Food Sci. Food Saf. 2011, 10, 153–168. [Google Scholar] [CrossRef]
- Mi, B.B.; Chen, X.F.; Jiang, C.L.; Wang, J.X.; Chen, X.J.; Zhang, B.; Liu, X.M.; Liu, Z.J.; Fei, B.H. Nitrogen-doped porous carbon derived from bamboo shoot as solid base catalyst for Knoevenagel condensation and transesterification reactions. Catalysts 2018, 8, 232–241. [Google Scholar] [CrossRef]
- Ding, J.; Wang, H.L.; Li, Z.; Cui, K.; Karpuzov, D.; Tan, X.H.; Kohandehghan, A.; Mitlin, D. Peanut shell hybrid sodium ion capacitor with extreme energy-power rivals lithium ion capacitors. Energy Environ. Sci. 2015, 8, 941–955. [Google Scholar] [CrossRef]
- Pan, D.Y.; Wang, S.; Zhao, B.; Wu, M.H.; Zhang, H.J.; Wang, Y.; Jiao, Z. Li storage properties of disordered graphene nanosheets. Chem. Mater. 2009, 21, 3136–3142. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.Z.; Lin, C.H.; Yang, W.; Meng, Y.; Guo, Y.; Li, M.L.; Xiao, D. Hierarchically porous nitrogen-rich carbon derived from wheat straw as an ultra-high-rate anode for lithium ion batteries. J. Mater. Chem. A 2014, 2, 9684–9690. [Google Scholar] [CrossRef]
- Yan, Y.; Yin, Y.X.; Guo, Y.G.; Wan, L.J. A sandwich-like hierarchically porous carbon/graphene composite as a high-performance anode material for sodium-ion batteries. Adv. Energy Mater. 2014, 4, 1079–1098. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, D.M.; Han, W.B.; Cheng, Y.; Sun, B.Q.; Hou, C.L.; Zhao, G.D.; Liu, D.Z.; Chen, G.Q.; Han, J.C.; et al. Nature-inspired self-activation method for the controllable synthesis of highly porous carbons for high-performance supercapacitors. Carbon 2023, 205, 1–9. [Google Scholar] [CrossRef]
- Wang, Q.H.; Lai, Z.Y.; Mu, J.; Chu, D.; Zang, X.R. Converting industrial waste cork to biochar as Cu (II) adsorbent via slow pyrolysis. Waste Manag. 2020, 105, 102–109. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Z.W.; Tan, X.H.; Wang, H.L.; Holt, C.M.; Stephenson, T.; Olsen, B.C.; Mitlin, D. Mesoporous nitrogen-rich carbons derived from protein for ultrahigh capacity battery anodes and supercapacitors. Energy Environ. Sci. 2013, 6, 871–878. [Google Scholar] [CrossRef]
- Ma, C.C.; Shao, X.H.; Cao, D.P. Nitrogen-doped graphene nanosheets as anode materials for lithium ion batteries: A first-principles study. J. Mater. Chem. 2012, 22, 8911–8915. [Google Scholar] [CrossRef]
- Liu, J.; Kopold, P.; van Aken, P.A.; Maier, J.; Yu, Y. Energy storage materials from nature through nanotechnology: A sustainable route from reed plants to a silicon anode for lithium-ion batteries. Angew. Chem. 2015, 127, 9768–9772. [Google Scholar] [CrossRef]
- Pels, J.R.; Kapteijn, F.; Moulijn, J.A.; Zhu, Q.; Thomas, K.M. Evolution of nitrogen functionalities in carbonaceous materials during pyrolysis. Carbon 1995, 33, 1641–1653. [Google Scholar] [CrossRef]
- Sevilla, M.; Parra, J.B.; Fuertes, A.B. Assessment of the role of micropore size and N-doping in CO2 capture by porous carbons. ACS Appl. Mater. Interfaces 2013, 5, 6360–6368. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjya, D.; Park, H.Y.; Kim, M.S.; Choi, H.S.; Inamdar, S.N.; Yu, J.S. Nitrogen-doped carbon nanoparticles by flame synthesis as anode material for rechargeable lithium-ion batteries. Langmuir 2014, 30, 318–324. [Google Scholar] [CrossRef]
- Qie, L.; Chen, W.M.; Wang, Z.H.; Shao, Q.G.; Li, X.; Yuan, L.X.; Hu, X.L.; Zhang, W.X.; Huang, Y.H. Nitrogen-doped porous carbon nanofiber webs as anodes for lithium ion batteries with a superhigh capacity and rate capability. Adv. Mater. 2012, 24, 2047–2050. [Google Scholar] [CrossRef]
- Zheng, F.C.; He, M.N.; Yang, Y.; Chen, Q.W. Nano electrochemical reactors of Fe2O3 nanoparticles embedded in shells of nitrogen-doped hollow carbon spheres as high-performance anodes for lithium-ion batteries. Nanoscale 2015, 7, 3410–3417. [Google Scholar] [CrossRef]
- Mullaivananathan, V.; Sathish, R.; Kalaiselvi, N. Coir pith derived bio-carbon: Demonstration of potential anode behavior in lithium-ion batteries. Electrochim. Acta 2017, 225, 143–150. [Google Scholar] [CrossRef]
- Tao, L.; Huang, Y.B.; Zheng, Y.W.; Yang, X.Q.; Liu, C.; Di, M.W.; Larpkiattaworn, S.; Nimlos, M.R.; Zheng, Z.F. Porous carbon nanofiber derived from a waste biomass as anode material in lithium-ion batteries. J. Taiwan Inst. Chem. Eng. 2019, 95, 217–226. [Google Scholar] [CrossRef]
- Sekar, S.; Ahmed, A.A.; Inamdar, A.I.; Lee, Y.; Im, H.; Kim, D.Y.; Lee, S. Activated carbon-decorated spherical silicon nanocrystal composites synchronously-derived from rice husks for anodic source of lithium-ion battery. Nanomaterials 2019, 9, 1055–1067. [Google Scholar] [CrossRef]
- Zhu, C.Y.; Akiyama, T. Cotton derived porous carbon via an MgO template method for high performance lithium ion battery anodes. Green Chem. 2016, 18, 2106–2114. [Google Scholar] [CrossRef]
- Stephan, A.M.; Kumar, T.P.; Ramesh, R.; Thomas, S.; Jeong, A.K.; Nahm, K.S. Pyrolitic carbon from biomass precursors as anode materials for lithium batteries. Mater. Sci. Eng. A 2006, 430, 132–137. [Google Scholar] [CrossRef]



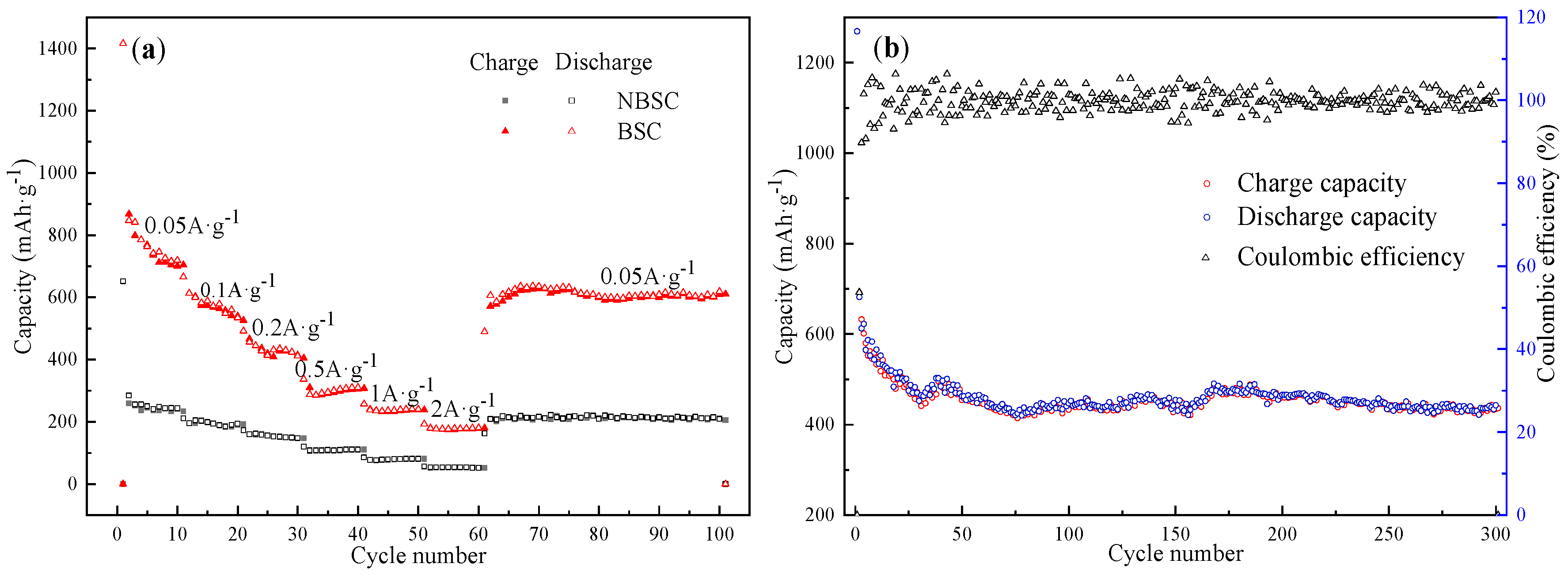
| Current Density (A g−1) | Charge Capacity (mA h g−1) | Discharge Capacity (mA h g−1) | Coulombic Efficiency (%) |
|---|---|---|---|
| 0.05 | 868.4 | 1416.0 | 61.3 |
| 0.1 | 613.7 | 666.0 | 92.1 |
| 0.2 | 466.6 | 491.7 | 94.9 |
| 0.5 | 310.3 | 337.2 | 92.0 |
| 1.0 | 240.7 | 257.7 | 93.4 |
| 2.0 | 180.0 | 192.8 | 93.4 |
| 0.05 | 611.3 | 619.1 | 98.7 |
| Carbon Source | Current Density (mA g−1) | Cycle Number | Reversible Capacity (mA h g−1) | Reference |
|---|---|---|---|---|
| Reed flowers | 100 | 100 | 581.2 | [16] |
| Coir pith waste | 100 | 50 | 837 | [46] |
| Walnut shell | 100 | 200 | 280 | [47] |
| Rice husks | 100 | 100 | 429 | [48] |
| Orange peel | 1000 | 100 | 301 | [25] |
| Bamboo shoot | 100 | 100 | 449.9 | This work |
| Bamboo shoot | 100 | 300 | 436.1 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mi, B.; Yuan, J.; Li, H.; Hu, W.; Jiang, C.; Liu, X.; Lei, Y.; Liu, Z. One-Step Synthesis of Nitrogen-Doped Porous Carbon Derived from Biomass for Lithium-Ion Battery. Coatings 2023, 13, 1960. https://doi.org/10.3390/coatings13111960
Mi B, Yuan J, Li H, Hu W, Jiang C, Liu X, Lei Y, Liu Z. One-Step Synthesis of Nitrogen-Doped Porous Carbon Derived from Biomass for Lithium-Ion Battery. Coatings. 2023; 13(11):1960. https://doi.org/10.3390/coatings13111960
Chicago/Turabian StyleMi, Bingbing, Jing Yuan, Hecheng Li, Wanhe Hu, Changle Jiang, Xianmiao Liu, Yafang Lei, and Zhijia Liu. 2023. "One-Step Synthesis of Nitrogen-Doped Porous Carbon Derived from Biomass for Lithium-Ion Battery" Coatings 13, no. 11: 1960. https://doi.org/10.3390/coatings13111960




