Oxygen Plasma-Induced Conversion of Silver Complex Ink into Conductive Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of AgNO3-PVA and the Plasma Process
2.3. Measurements and Instruments
3. Results
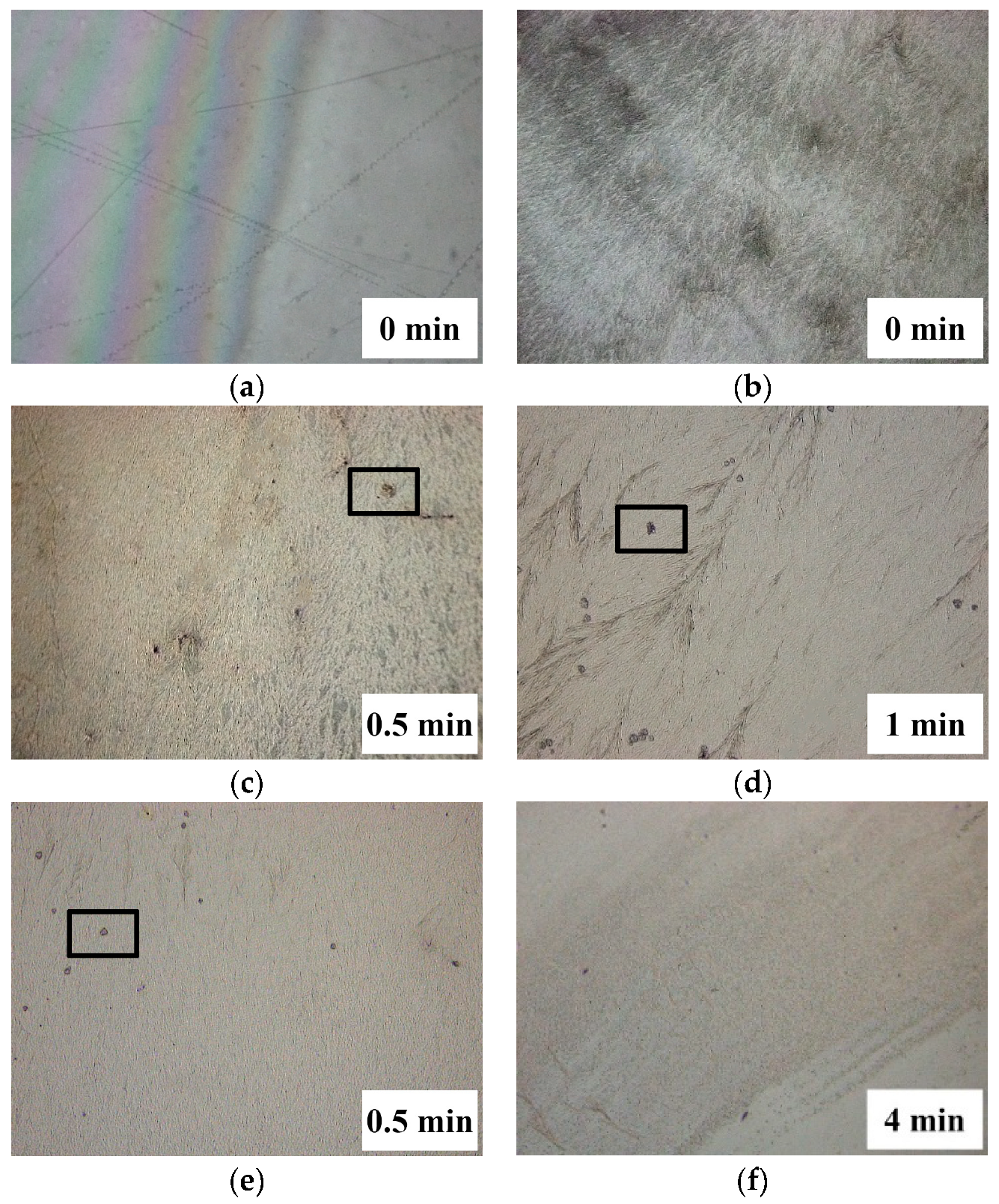
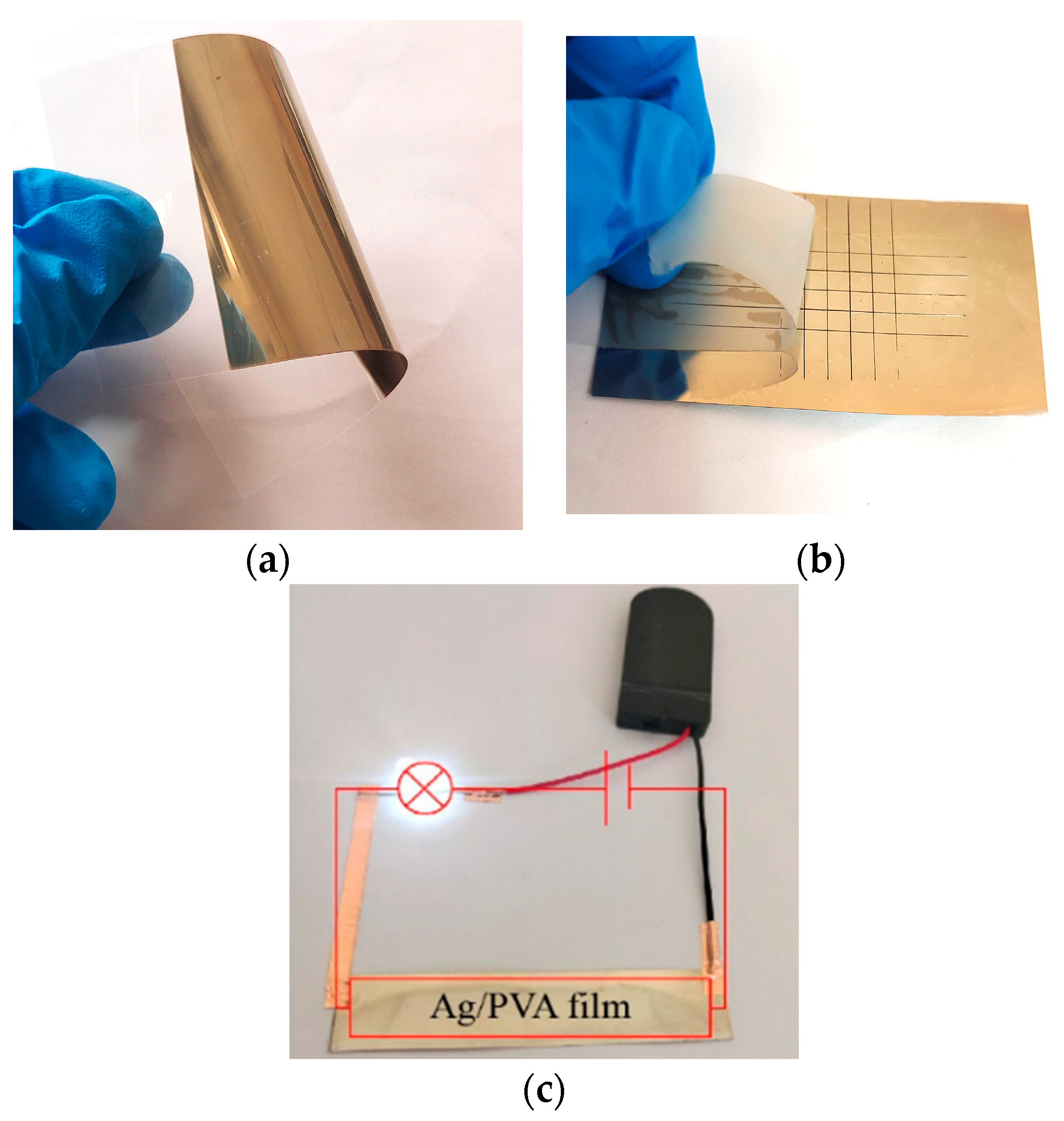
3.1. Preparation of Conductive Coating
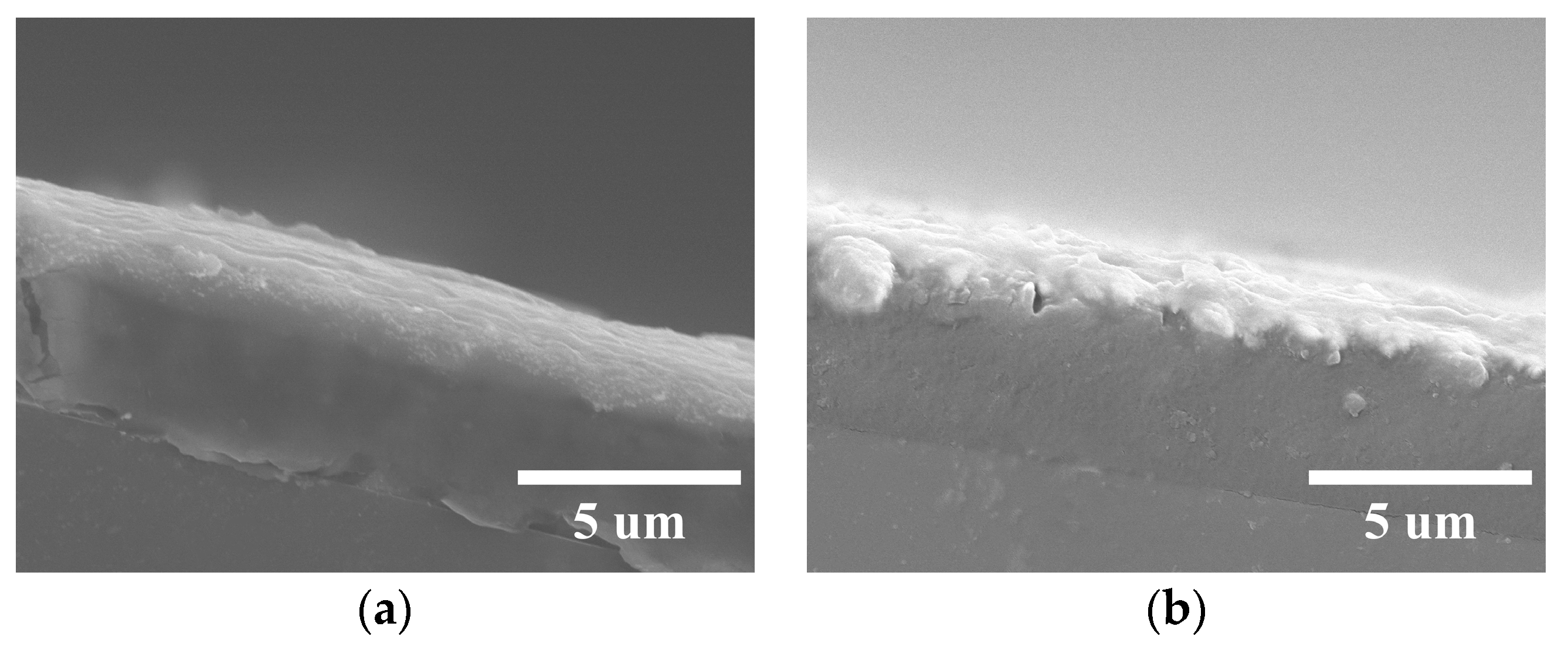
3.2. XPS of Conductive Coatings and Surface Morphology
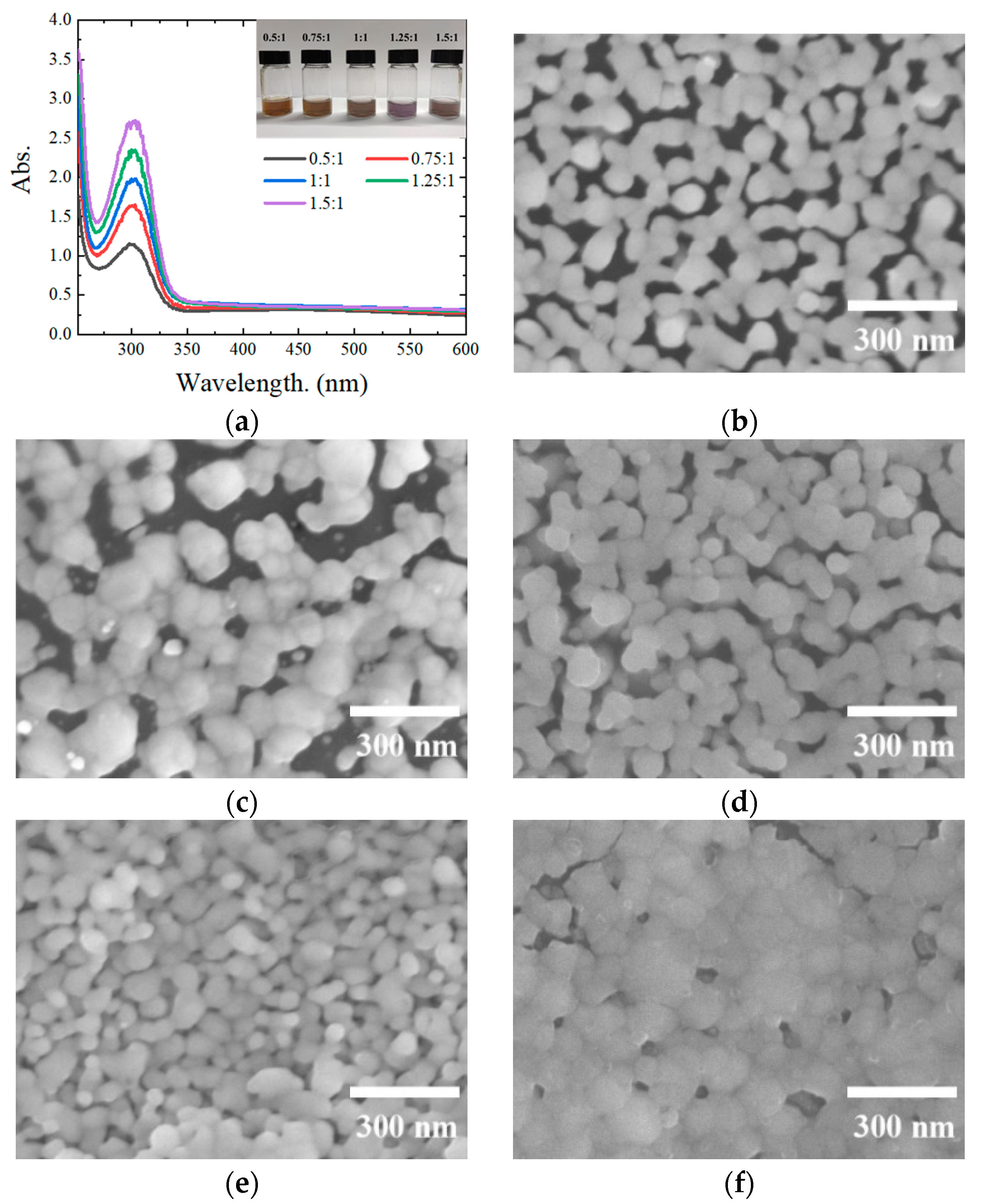
3.3. Effect of the AgNO3 Ratio on the Conductive Coating
3.4. Growth Mechanisms
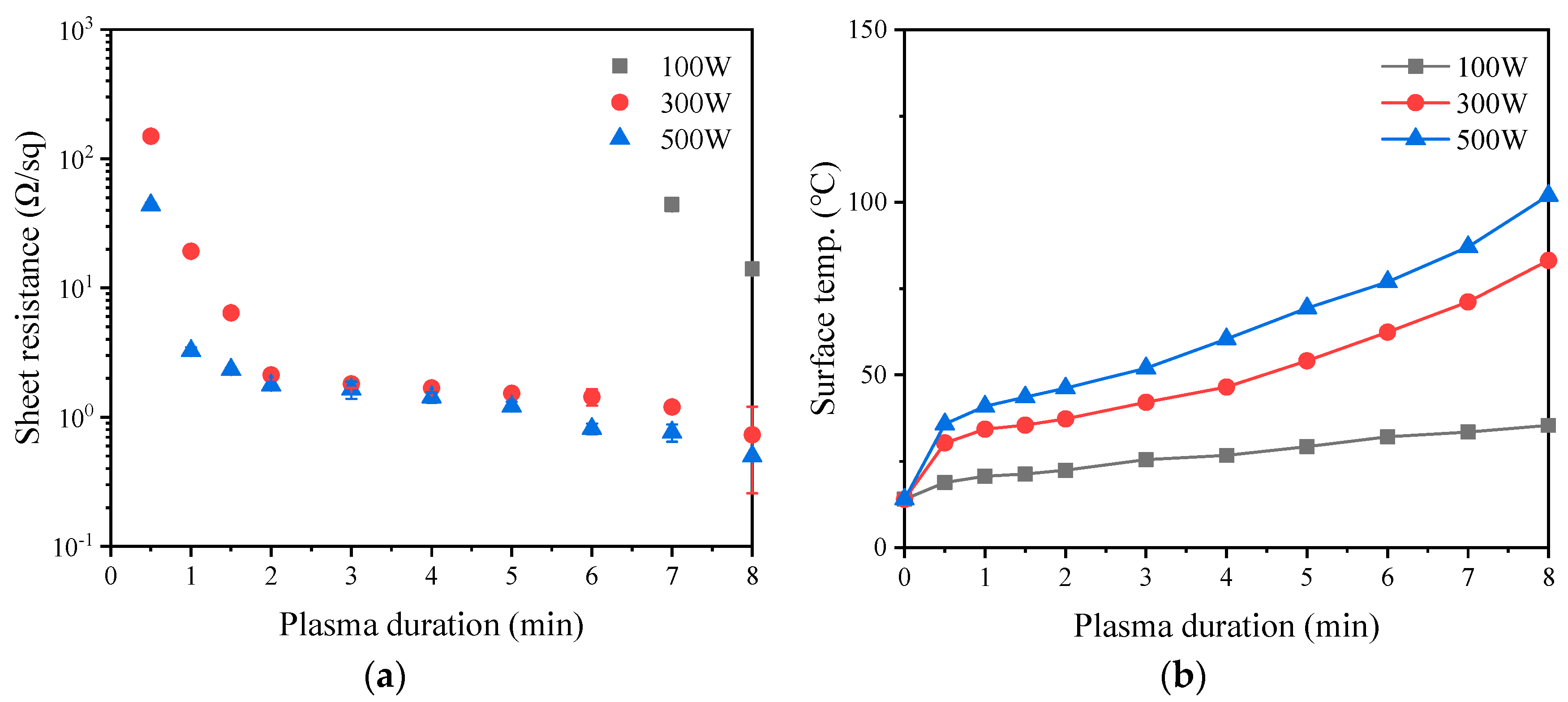
3.5. Conductive Properties of Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gipperich, M.; Riepe, J.; Day, R.; Bergs, T. Express wire coil cladding as an advanced technology to accelerate Additive Manufacturing and coating. J. Eng. Gas Turbines Power 2022, 144, 021003. [Google Scholar]
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. On coating techniques for surface protection: A review. J. Manuf. Mater. Process. 2019, 3, 28. [Google Scholar]
- Mohanty, S.; Gokuldoss Prashanth, K. Metallic coatings through additive manufacturing: A review. Materials 2023, 16, 2325. [Google Scholar] [PubMed]
- Bromberg, V.; Ma, S.; Egitto, F.D.; Singler, T.J. Highly conductive lines by plasma-induced conversion of inkjet-printed silver nitrate traces. J. Mater. Chem. C 2013, 1, 6842. [Google Scholar]
- Farraj, Y.; Smooha, A.; Kamyshny, A.; Magdassi, S. Plasma-induced decomposition of copper complex ink for the formation of highly conductive copper tracks on heat-sensitive substrates. ACS Appl. Mater. Interfaces 2017, 9, 8766–8773. [Google Scholar] [CrossRef]
- Farraj, Y.; Grouchko, M.; Magdassi, S. Self-reduction of a copper complex MOD ink for inkjet printing conductive patterns on plastics. Chem. Commun. 2015, 51, 1587–1590. [Google Scholar]
- Douglas, S.P.; Knapp, C.E. Low-temperature deposition of highly conductive aluminum metal films on flexible substrates using liquid alane MOD precursors. ACS Appl. Mater. Interfaces 2020, 12, 26193–26199. [Google Scholar] [CrossRef]
- Cano-Raya, C.; Denchev, Z.Z.; Cruz, S.F.; Viana, J.C. Chemistry of solid metal-based inks and pastes for printed electronics—A review. Appl. Mater. Today 2019, 15, 416–430. [Google Scholar]
- Hu, G.; Kang, J.; Ng, L.W.; Zhu, X.; Howe, R.C.; Jones, C.G.; Hersam, M.C.; Hasan, T. Functional inks and printing of two-dimensional materials. Chem. Soc. Rev. 2018, 47, 3265–3300. [Google Scholar]
- Wang, Y.; Zhu, C.; Pfattner, R.; Yan, H.; Jin, L.; Chen, S.; Molina-Lopez, F.; Lissel, F.; Liu, J.; Rabiah, N.I.; et al. A highly stretchable, transparent, and conductive polymer. Sci. Adv. 2017, 3, e1602076. [Google Scholar]
- Xie, Y.; Ouyang, S.; Wang, D.; Lee, W.-Y.; Fong, H.H. Highly smooth and conductive silver film with metallo-organic decomposition ink for all-solution-processed flexible organic thin-film transistors. J. Mater. Sci. 2020, 55, 15908–15918. [Google Scholar] [CrossRef]
- Zhao, P.; Huang, J.; Nan, J.; Liu, D.; Meng, F. Laser sintering process optimization of microstrip antenna fabricated by inkjet printing with silver-based MOD ink. J. Mater. Process. Technol. 2020, 275, 116347. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, D.; Zhao, P.; Huang, J. Effect of processing parameters on laser sintering of silver MOD inks for inkjet printing. IET Collab. Intell. Manuf. 2019, 1, 10–19. [Google Scholar] [CrossRef]
- Choi, Y.; Seong, K.; Piao, Y. Metal–organic decomposition ink for printed electronics. Adv. Mater. Interfaces 2019, 6, 1901002. [Google Scholar] [CrossRef]
- Zope, K.R.; Cormier, D.; Williams, S.A. Reactive silver oxalate ink composition with enhanced curing conditions for flexible substrates. ACS Appl. Mater. Interfaces 2018, 10, 3830–3837. [Google Scholar] [CrossRef]
- Bai, Y.; Williams, C.B. Binder jetting additive manufacturing with a particle-free metal ink as a binder precursor. Mater. Des. 2018, 147, 146–156. [Google Scholar] [CrossRef]
- Yang, W.; Wang, C.; Arrighi, V. Silver oxalate ink with low sintering temperature and good electrical property. J. Electron. Mater. 2018, 47, 2824–2835. [Google Scholar] [CrossRef]
- Rosker, E.S.; Barako, M.T.; Nguyen, E.; DiMarzio, D.; Kisslinger, K.; Duan, D.-W.; Sandhu, R.; Goorsky, M.S.; Tice, J. Approaching the practical conductivity limits of aerosol jet printed silver. ACS Appl. Mater. Interfaces 2020, 12, 29684–29691. [Google Scholar] [CrossRef]
- Zhong, T.; Jin, N.; Yuan, W.; Zhou, C.; Gu, W.; Cui, Z. Printable stretchable silver ink and application to printed RFID tags for wearable electronics. Materials 2019, 12, 3036. [Google Scholar] [CrossRef]
- Vaseem, M.; McKerricher, G.; Shamim, A. Robust Design of a Particle-Free Silver-Organo-Complex Ink with High Conductivity and Inkjet Stability for Flexible Electronics. ACS Appl. Mater. Interfaces 2016, 8, 177–186. [Google Scholar] [CrossRef]
- Dong, Y.; Li, X.; Liu, S.; Zhu, Q.; Li, J.G.; Sun, X. Facile synthesis of high silver content MOD ink by using silver oxalate precursor for inkjet printing applications. Thin Solid Film. 2015, 589, 381–387. [Google Scholar] [CrossRef]
- Marchal, W.; Vandevenne, G.; D’Haen, J.; de Andrade Almeida, A.C.; Sola, M.D.; Van Den Ham, E.J.; Drijkoningen, J.; Elen, K.; Deferme, W.; Van Bael, M.K.; et al. Ultrasonically spray coated silver layers from designed precursor inks for flexible electronics. Nanotechnology 2017, 28, 215202. [Google Scholar] [CrossRef]
- Machala, Z.; Graves, D.B. Frugal biotech applications of low-temperature plasma. Trends Biotechnol. 2018, 36, 579–581. [Google Scholar] [CrossRef]
- Akayeti, A.; Zhang, W.; Yan, X.; Wang, Q. Low-temperature oxygen plasma treatment of single-layer molybdenum disulfide. Mater. Res. Express 2019, 6, 055007. [Google Scholar] [CrossRef]
- Adusei, P.K.; Gbordzoe, S.; Kanakaraj, S.N.; Hsieh, Y.Y.; Alvarez, N.T.; Fang, Y.; Johnson, K.; McConnell, C.; Shanov, V. Fabrication and study of supercapacitor electrodes based on oxygen plasma functionalized carbon nanotube fibers. J. Energy Chem. 2020, 40, 120–131. [Google Scholar] [CrossRef]
- Turan, N.; Saeidi-Javash, M.; Chen, J.; Zeng, M.; Zhang, Y.; Go, D.B. Atmospheric pressure and ambient temperature plasma jet sintering of aerosol jet printed silver nanoparticles. ACS Appl. Mater. Interfaces 2021, 13, 47244–47251. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.-T.; Lee, Y.-I.; Kim, S.; Lee, K.-J.; Choa, Y.-H. Full densification of inkjet-printed copper conductive tracks on a flexible substrate utilizing a hydrogen plasma sintering. Appl. Surf. Sci. 2017, 396, 1239–1244. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, Q.; Li, M.; Jin, S.; Fan, J.; Zhao, L.; Yao, Z. Surface modification of activated carbon fiber by low-temperature oxygen plasma: Textural property, surface chemistry, and the effect of water vapor adsorption. Chem. Eng. J. 2021, 418, 129474. [Google Scholar] [CrossRef]
- Shi, Q.; Vitchuli, N.; Nowak, J.; Caldwell, J.M.; Breidt, F.; Bourham, M.; Zhang, X.; McCord, M. Durable antibacterial Ag/polyacrylonitrile (Ag/PAN) hybrid nanofibers prepared by atmospheric plasma treatment and electrospinning. Eur. Polym. J. 2011, 47, 1402–1409. [Google Scholar] [CrossRef]
- Jelil, R.A. A review of low-temperature plasma treatment of textile materials. J. Mater. Sci. 2015, 50, 5913–5943. [Google Scholar]
- Wang, L.; Wang, W.; Zhao, H.; Liu, Y.; Liu, J.; Bai, N. Bioactive effects of low-temperature argon–oxygen plasma on a titanium implant surface. ACS Omega 2020, 5, 3996–4003. [Google Scholar] [CrossRef] [PubMed]
- Knapp, C.E.; Chemin, J.B.; Douglas, S.P.; Ondo, D.A.; Guillot, J.; Choquet, P.; Boscher, N.D. Room-Temperature Plasma-Assisted Inkjet Printing of Highly Conductive Silver on Paper. Adv. Mater. Technol. 2018, 3, 1700326. [Google Scholar] [CrossRef]
- Contreras-Arzate, D.; Islas-Espinoza, M.; Fall, C.; Alcántara-Díaz, D.; Olguin, M.T.; López-Callejas, R.; Peña-Eguiluz, R. Microbial mortality behavior promoted by silver (Ag+/Ago)-modified zeolite-rich tuffs for water disinfection. J. Environ. Health Sci. Eng. 2020, 18, 755–768. [Google Scholar] [CrossRef]
- Bolli, E.; Mezzi, A.; Burratti, L.; Prosposito, P.; Casciardi, S.; Kaciulis, S. X-ray and UV photoelectron spectroscopy of Ag nanoclusters. Surf. Interface Anal. SIA 2020, 52, 1017–1022. [Google Scholar] [CrossRef]
- Ferraria, A.M.; Carapeto, A.P.; do Rego, A.M.B. X-ray photoelectron spectroscopy: Silver salts revisited. Vacuum 2012, 86, 1988–1991. [Google Scholar] [CrossRef]
- Li, S.; Cai, K.; Li, Y.; Liu, S.; Yu, M.; Wang, Y.; Ma, X.; Huang, S. Identifying the active silver species in carbonylation of dimethyl ether over Ag−HMOR. ChemCatChem 2020, 12, 3290–3297. [Google Scholar] [CrossRef]
- Idriss, H. On the wrong assignment of the XPS O1s signal at 531–532 eV attributed to oxygen vacancies in photo- and electro-catalysts for water splitting and other materials applications. Surf. Sci. 2021, 712, 121894. [Google Scholar] [CrossRef]
- Khelifa, F.; Ershov, S.; Habibi, Y.; Snyders, R.; Dubois, P. Free-radical-induced grafting from plasma polymer surfaces. Chem. Rev. 2016, 116, 3975–4005. [Google Scholar] [CrossRef]
- Elashry, S.; ELsaeed, H.; El-Siragy, N.M. Microwave plasma discharge-assisted surface modification of PVA films: Coatings and food packaging. Eur. Phys. J. Plus 2022, 137, 1252. [Google Scholar] [CrossRef]
- Liang, K.-L.; Wang, Y.-C.; Lin, W.-L.; Lin, J.-J. Polymer-assisted self-assembly of silver nanoparticles into interconnected morphology and enhanced surface electric conductivity. RSC Adv. 2014, 4, 15098. [Google Scholar] [CrossRef]
- Huang, X.; Wang, S.; Wu, J.; Dai, L.; Li, L.; Guo, Y.; Zhang, J.; Shi, J. Characterization of atmospheric-pressure helium–oxygen dual-frequency glow discharges using optical emission spectroscopy. IEEE Transactions on Plasma Science. IEEE Nucl. Plasma Sci. Soc. 2019, 47, 1788–1792. [Google Scholar] [CrossRef]
- Xiao, D.; Bao, D.L.; Liang, X.; Wang, Y.; Shen, J.; Cheng, C.; Chu, P.K. Experimental and theoretical investigation of the control and balance of active sites on oxygen plasma-functionalized MoSe2 nanosheets for efficient hydrogen evolution reaction. Appl. Catal. B Environ. 2021, 288, 119983. [Google Scholar] [CrossRef]
- Wu, J.-T.; Hsu, S.L.-C.; Tsai, M.-H.; Hwang, W.-S. Inkjet printing of low-temperature cured silver patterns by using AgNO3/1-dimethylamino-2-propanol inks on polymer substrates. J. Phys. Chem. C Nanomater. Interfaces 2011, 115, 10940–10945. [Google Scholar] [CrossRef]
- Nie, X.; Wang, H.; Zou, J. Inkjet printing of silver citrate conductive ink on PET substrate. Appl. Surf. Sci. 2012, 261, 554–560. [Google Scholar] [CrossRef]



| Silver Precursor | Substrate | Temperature (°C) | Time (min) | Resistivity (μΩ·cm) | Source |
|---|---|---|---|---|---|
| Bulk silver | - | 961 | - | 1.61 | - |
| AgNO3 | PET | 37.3 | 2 | 166.4 | This study |
| AgNO3 | PET | 100 | 60 | 13.7 | Ref. [43] |
| Silver citrate | PET | 150 | 50 | 17 | Ref. [44] |
| Silver oxalate | PI | 150 | 30 | 8.6 | Ref. [21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Cao, M.; Yang, J.; Guo, X.; Sun, X.; Wang, T.; Qi, Y.; Li, L.; Zeng, H.; Sun, M. Oxygen Plasma-Induced Conversion of Silver Complex Ink into Conductive Coatings. Coatings 2023, 13, 1977. https://doi.org/10.3390/coatings13121977
Li S, Cao M, Yang J, Guo X, Sun X, Wang T, Qi Y, Li L, Zeng H, Sun M. Oxygen Plasma-Induced Conversion of Silver Complex Ink into Conductive Coatings. Coatings. 2023; 13(12):1977. https://doi.org/10.3390/coatings13121977
Chicago/Turabian StyleLi, Shasha, Meijuan Cao, Ji Yang, Xiangjun Guo, Xinfeng Sun, Tao Wang, Yuansheng Qi, Luhai Li, Huabin Zeng, and Meng Sun. 2023. "Oxygen Plasma-Induced Conversion of Silver Complex Ink into Conductive Coatings" Coatings 13, no. 12: 1977. https://doi.org/10.3390/coatings13121977
APA StyleLi, S., Cao, M., Yang, J., Guo, X., Sun, X., Wang, T., Qi, Y., Li, L., Zeng, H., & Sun, M. (2023). Oxygen Plasma-Induced Conversion of Silver Complex Ink into Conductive Coatings. Coatings, 13(12), 1977. https://doi.org/10.3390/coatings13121977







