Preparation and Corrosion Properties of TiO2-SiO2-Al2O3 Composite Coating on Q235 Carbon Steel
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Solution
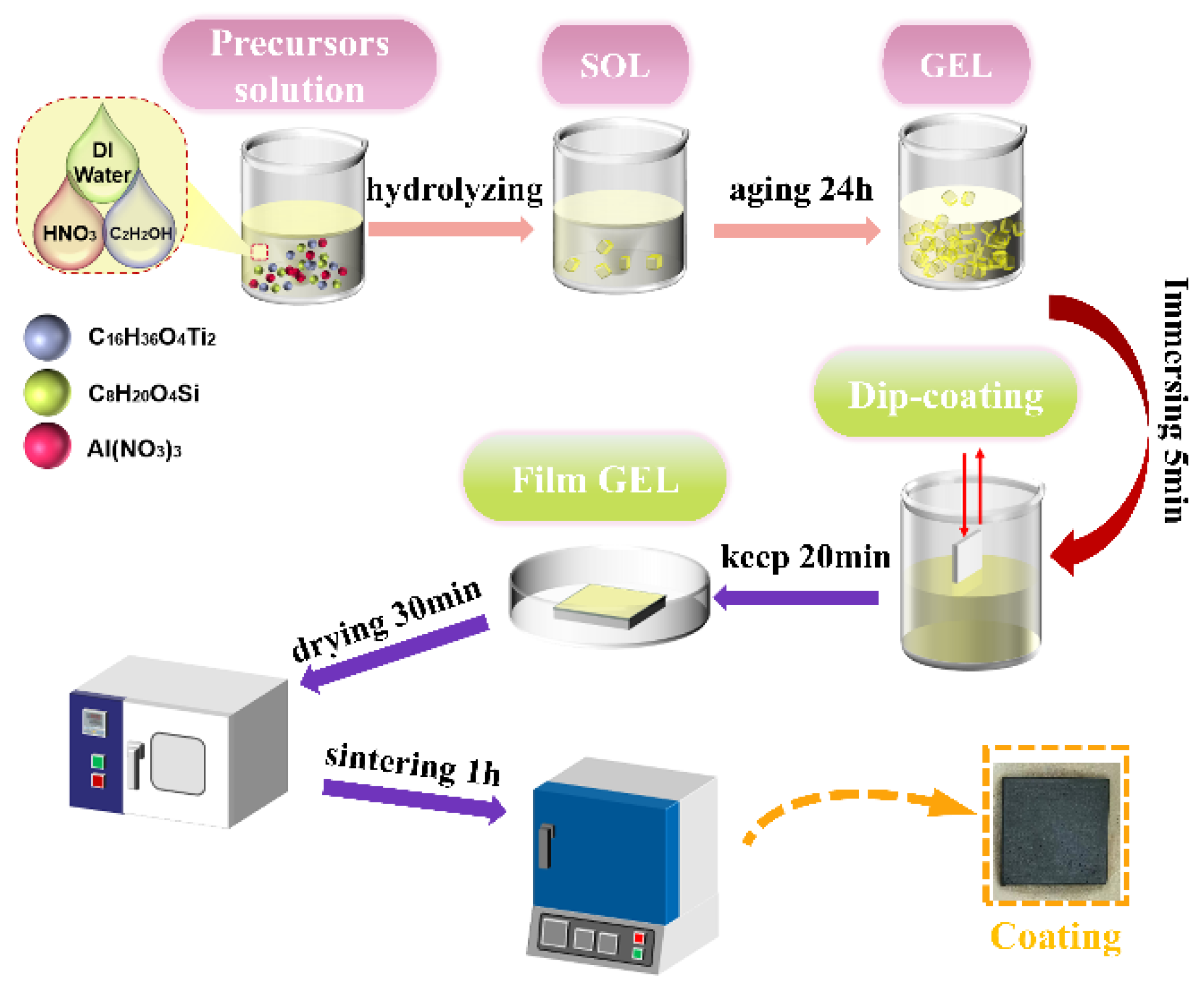
2.2. Experimental Materials and Preparation of Coating
2.3. Coating Characterization
2.4. Electrochemical Experiments
3. Results and Discussions
3.1. Surface Morphology
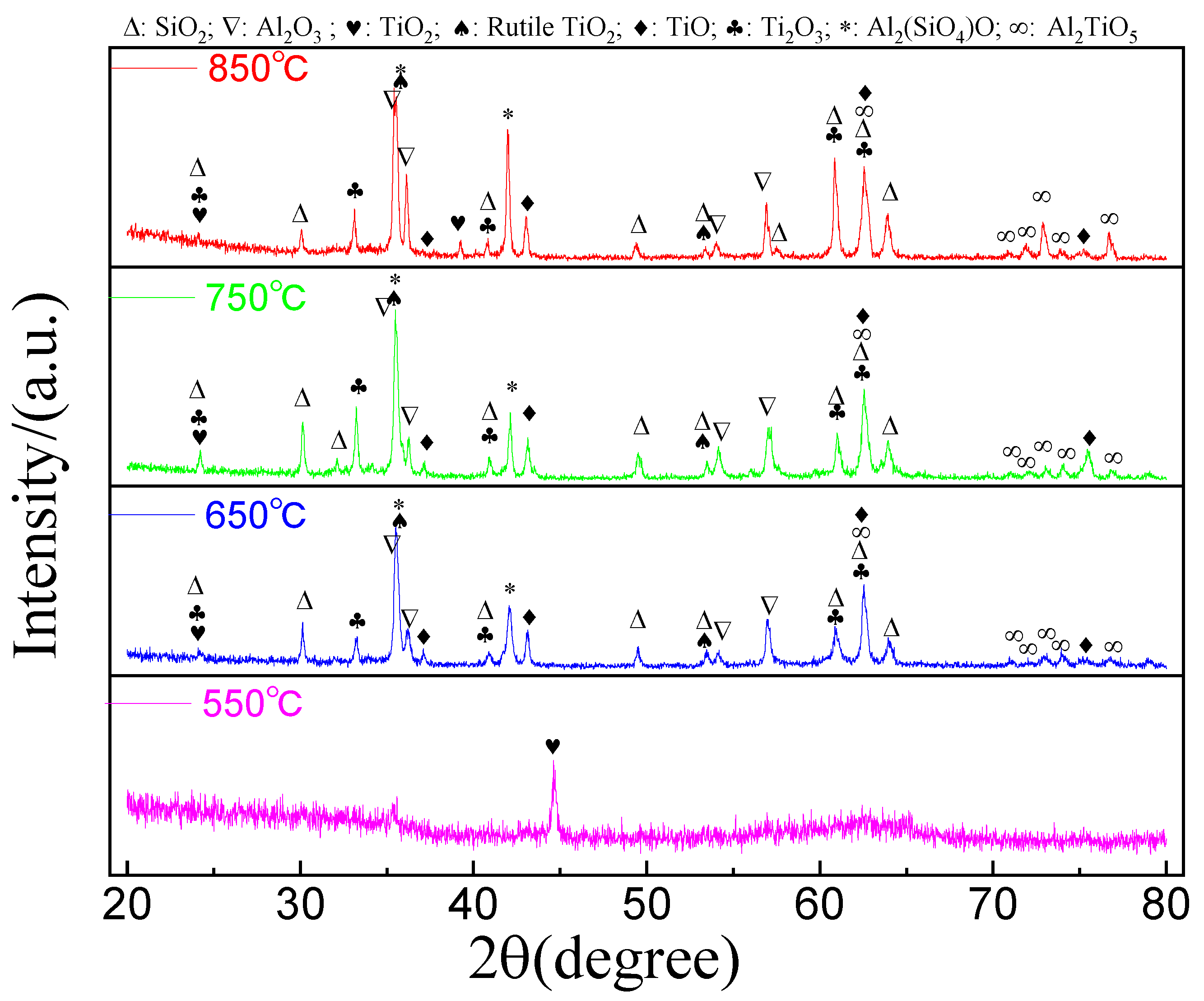
3.2. X-ray Diffraction Analysis
3.3. Electrochemical Corrosion Behavior
3.3.1. Linear Polarization
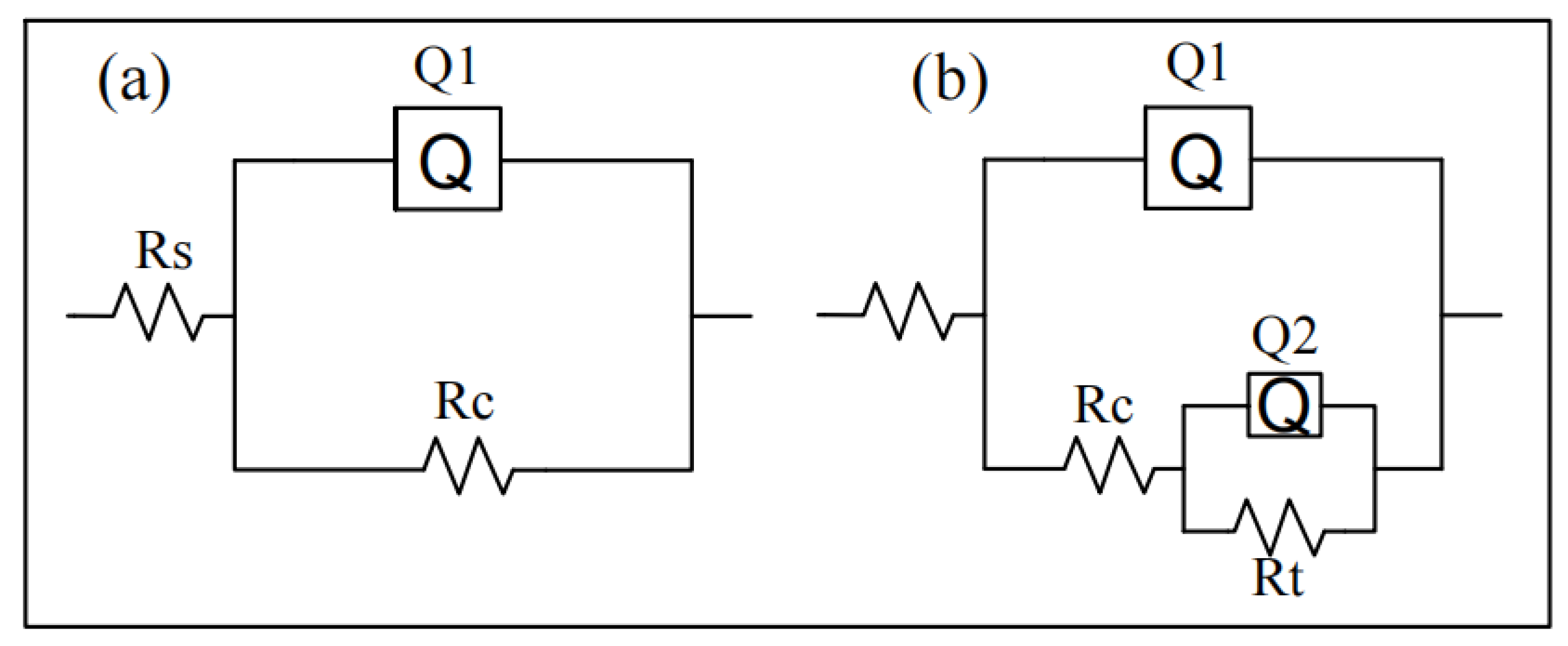
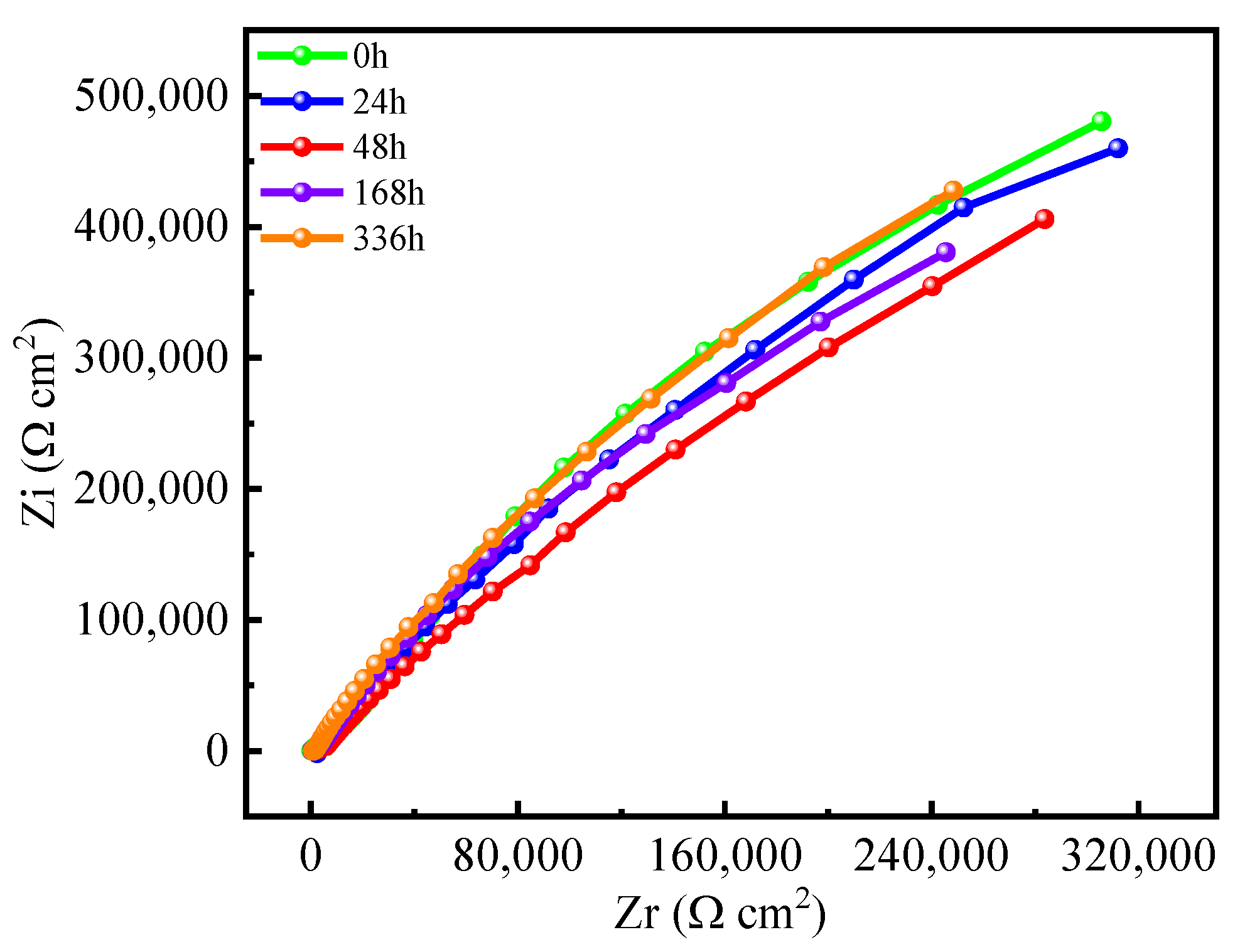
3.3.2. Electrochemical Impedance Spectroscopy
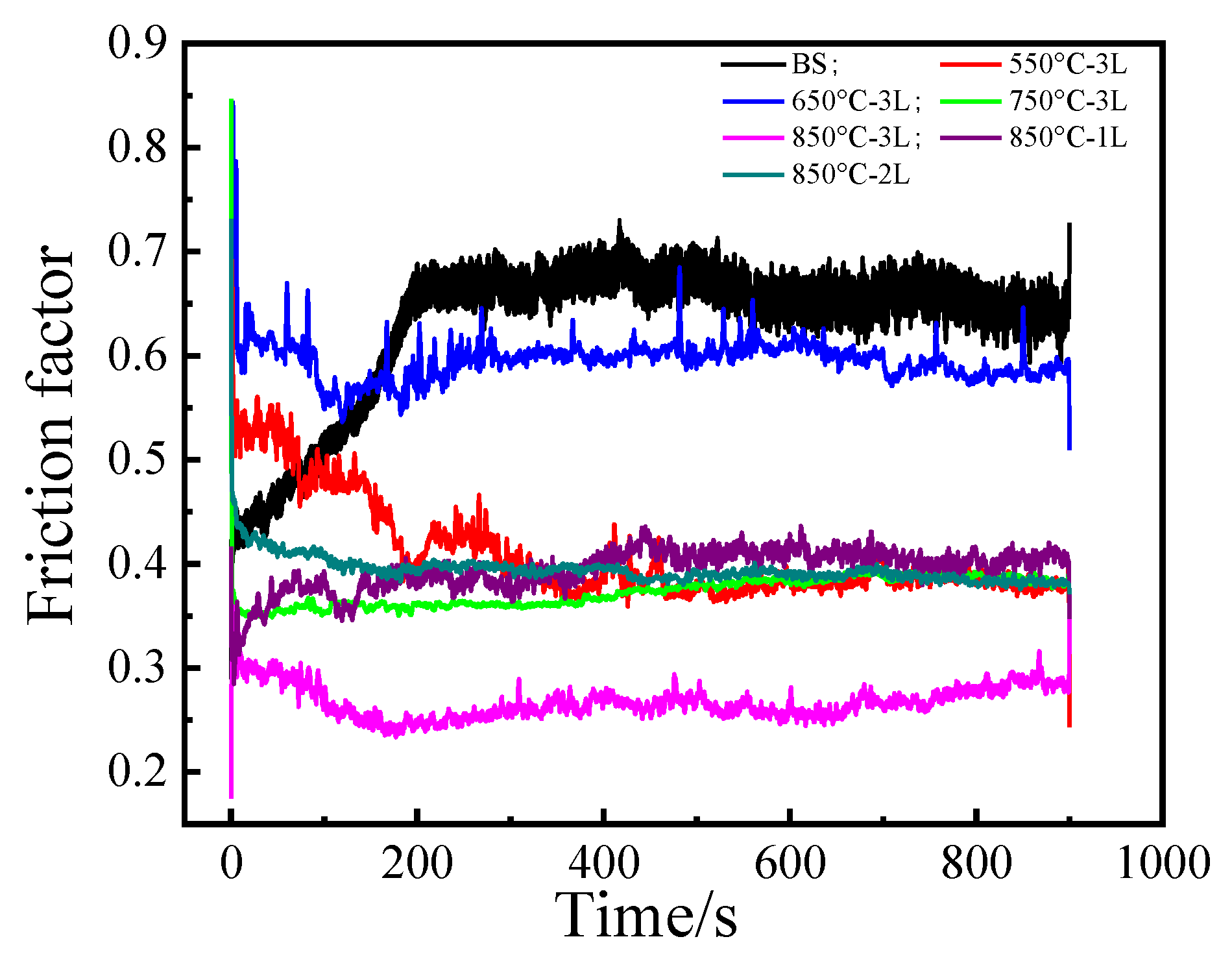
3.4. Friction Resistance Analysis
3.5. Immersion Experiment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vera, R.; Cruz, E.; Bagnara, M.; Araya, R.; Henríquez, R.; Díaz-Gómez, A.; Rojas, P. Evaluation of Anticorrosive Coatings on Carbon Steel in Marine Environments: Accelerated Corrosion Test and Field Exposure. Int. J. Electrochem. Sci. 2018, 13, 898–914. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Q.; Tu, S.; Wang, Y.; Li, Y.; Huang, Y. A study on time-variant corrosion model for immersed steel plate elements considering the effect of mechanical stress. Ocean Eng. 2016, 125, 134–146. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.; Liu, Z.; Li, Z.; Du, C.; Dong, C. Materials science: Share corrosion data. Nature 2015, 527, 441–442. [Google Scholar] [CrossRef] [PubMed]
- Fihri, A.; Bovero, E.; Al-Shahrani, A.; Al-Ghamdi, A.; Alabedi, G. Recent progress in superhydrophobic coatings used for steel protection: A review. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 378–390. [Google Scholar] [CrossRef]
- Brenna, A.; Ormellese, M.; Lazzari, L. A proposal of AC corrosion mechanism of carbon steel in cathodic protection condition. In Proceedings of the NACE Corrosion Conference, Orlando, FL, USA, 17–21 March 2013; pp. 2013–2457. [Google Scholar]
- Yu, Z.; Hu, J.; Meng, H. A review of recent developments in coating systems for hot-dip galvanized steel. Front. Mater. 2020, 7, 74. [Google Scholar] [CrossRef]
- Akande, I.G.; Fayomi, O.S.I.; Oluwole, O.O. Performance of composite coating on carbon steel—A Necessity. Energy Procedia 2019, 157, 375–383. [Google Scholar] [CrossRef]
- Iroh, J.O.; Su, W. Corrosion performance of polypyrrole coating applied to low carbon steel by an electrochemical process. Electrochim. Acta 2000, 46, 15–24. [Google Scholar] [CrossRef]
- Subramanian, V.; Van Ooij, W.J. Effect of the amine functional group on corrosion rate of iron coated with films of organofunctional silanes. Corrosion 1998, 54, 204–215. [Google Scholar] [CrossRef]
- Tabish, M.; Malik, M.U.; Khan, M.A.; Yasin, G.; Asif, H.M.; Anjum, M.J.; Khan, W.Q.; Ibraheem, S.; Nguyen, T.A.; Slimani, Y.; et al. Construction of NiCo/graphene nanocomposite coating with bulges-like morphology for enhanced mechanical properties and corrosion resistance performance. J. Alloys Compd. 2021, 867, 159138. [Google Scholar] [CrossRef]
- Nazari, M.H.; Zhang, Y.; Mahmoodi, A.; Xu, G.; Yu, J.; Wu, J.; Shi, X. Nanocomposite organic coatings for corrosion protection of metals: A review of recent advances. Prog. Org. Coat. 2022, 162, 106573. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Du, X.; Du, Z.; Cheng, X.; Wang, H. A self-healing polyurethane-based composite coating with high strength and anti-corrosion properties for metal protection. Compos. Part B Eng. 2021, 225, 109273. [Google Scholar] [CrossRef]
- Steffi, A.P.; Balaji, R.; Prakash, N.; Rajesh, T.P.; Ethiraj, S.; Samuel, M.S.; Nadda, A.K.; Chandrasekar, N. Incorporation of SiO2 functionalized gC3N4 sheets with TiO2 nanoparticles to enhance the anticorrosion performance of metal specimens in aggressive Cl− environment. Chemosphere 2022, 290, 133332. [Google Scholar] [CrossRef] [PubMed]
- Çomaklı, O.; Yazıcı, M.; Demir, M.; Yetim, A.F.; Çelik, A. Effect of bilayer numbers on structural, mechanical, tribological and corrosion properties of TiO2–SiO2 multilayer film-coated β-type Ti45Nb alloys. Ceram. Int. 2023, 4, 3007–3015. [Google Scholar] [CrossRef]
- Khosravi, H.S.; Veerapandiyan, V.K.; Vallant, R.; Reichmann, K. Effect of processing conditions on the structural properties and corrosion behavior of TiO2–SiO2 multilayer coatings derived via the sol-gel method. Ceram. Int. 2020, 46, 17741–17751. [Google Scholar] [CrossRef]
- Gutierrez, M.; Guerra, L.; Bermudez-Reyes, B.; Cabriales, R.; Reyes, L. Characterization of SiO2-TiO2 Coatings on 316L Stainless Steel Substrates. J. Adv. Mater. Process. 2018, 6, 3–13. [Google Scholar]
- Hayati, Z.; Hoomehr, B.; Khalesi, F.; Raeissi, K. Synthesis and electrophoretic deposition of TiO2-SiO2 composite nanoparticles on stainless steel substrate. J. Alloys Compd. 2023, 931, 167619. [Google Scholar] [CrossRef]
- Mazur, A.; Szczurek, A.; Chęcmanowski, J.G.; Szczygieł, B. Corrosion resistance and bioactivity of SiO2-Y2O3 coatings deposited on 316L steel. Surf. Coat. Technol. 2018, 350, 502–510. [Google Scholar] [CrossRef]
- Deyab, M.A.; Nada, A.A.; Hamdy, A. Comparative study on the corrosion and mechanical properties of nano-composite coatings incorporated with TiO2 nano-particles, TiO2 nano-tubes, and ZnO nano-flowers. Prog. Org. Coat. 2017, 105, 245–251. [Google Scholar] [CrossRef]
- Chęcmanowski, J.; Pelczarska, A.J.; Szczygieł, I.; Szczygieł, B. Influence of ceria and yttria on the protective properties of SiO2–Al2O3 coatings deposited by sol–gel method on FeCrAl alloy. J. Therm. Anal. Calorim. 2016, 126, 371–380. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, N.; Liu, L.; Liu, Y. Surface sedimentation and adherence of Nano-SiO2 to improve thermal stability and flame resistance of melamine-formaldehyde foam via sol-gel method. Fire Mater. 2018, 42, 183–189. [Google Scholar] [CrossRef]
- Dias, V.M.; Chiappim, W.; Fraga, M.A.; Maciel, H.S.; Marciano, F.R.; Pessoa, R.S. Atomic layer deposition of TiO2 and Al2O3 thin films for the electrochemical study of corrosion protection in aluminum alloy cans used in beverage. Mater. Res. Express 2020, 7, 076408. [Google Scholar] [CrossRef]
- Mthisi, A.; Popoola, P.I. Influence of Al2O3 addition on the hardness and in vitro corrosion behavior of laser synthesized Ti-Al2O3 coatings on Ti-6Al-4V. Int. J. Adv. Manuf. Technol. 2019, 100, 917–927. [Google Scholar] [CrossRef]
- Ruhi, G.; Modi, O.P.; Sinha, A.S.K.; Singh, I.B. Effect of sintering temperatures on corrosion and wear properties of sol–gel alumina coatings on surface pre-treated mild steel. Corros. Sci. 2008, 50, 639–649. [Google Scholar] [CrossRef]
- Díaz, B.; Härkönen, E.; Maurice, V.; Światowska, J.; Seyeux, A.; Ritala, M.; Marcus, P. Failure mechanism of thin Al2O3 coatings grown by atomic layer deposition for corrosion protection of carbon steel. Electrochim. Acta 2011, 56, 9609–9618. [Google Scholar] [CrossRef]
- Atik, M.; De Lima Neto, P.; Aegerter, M.A.; Avaca, L.A. Sol-gel TiO2-SiO2 films as protective coatings against corrosion of 316L stainless steel in H2SO4 solutions. J. Appl. Electrochem. 1995, 25, 142–148. [Google Scholar] [CrossRef]
- Forghani, S.M.; Ghazali, M.J.; Muchtar, A.; Daud, A.R. Mechanical properties of plasma sprayed nanostructured TiO2 coatings on mild steel. Ceram. Int. 2014, 40, 7049–7056. [Google Scholar] [CrossRef]
- Mora, L.V.; Naik, S.; Paul, S.; Dawson, R.; Neville, A.; Barker, R. Influence of silica nanoparticles on corrosion resistance of sol-gel based coatings on mild steel. Surf. Coat. Technol. 2017, 324, 368–375. [Google Scholar] [CrossRef]
- Khalil, T.; El-Nour, F.A.; El-Gammal, B.; Boccaccini, A.R. Determination of surface area and porosity of sol–gel derived ceramic powders in the system TiO2–SiO2–Al2O3. Powder Technol. 2001, 114, 106–111. [Google Scholar] [CrossRef]
- Izumi, K.; Minami, N.; Uchida, Y. Sol-Gel-Derived Coatings on Steel Sheets. Key Eng. Mater. 1998, 150, 77–88. [Google Scholar] [CrossRef]
- Yu, J.; Ji, G.; Shi, Z.; Wang, X. Corrosion resistance of ZrO2 films under different humidity coal gas conditions at high temperature. J. Alloys Compd. 2018, 783, 371–378. [Google Scholar] [CrossRef]
- John, S.; Salam, A.; Baby, A.M.; Joseph, A. Corrosion inhibition of mild steel using chitosan/TiO2 nanocomposite coatings. Prog. Org. Coat. Int. Rev. J. 2019, 129, 254–259. [Google Scholar] [CrossRef]
- Sahnesarayi, M.K.; Sarpoolaky, H.; Rastegari, S. Effect of heat treatment temperature on the performance of nano-TiO2 coating in protecting 316L stainless steel against corrosion under UV illumination and dark conditions. Surf. Coat. Technol. 2014, 258, 861–870. [Google Scholar] [CrossRef]
- Krishna, V.; Padmapreetha, R.; Chandrasekhar, S.B.; Murugan, K.; Johnson, R. Oxidation resistant TiO2–SiO2 coatings on mild steel by Sol–Gel. Surf. Coat. Technol. 2019, 378, 125041. [Google Scholar] [CrossRef]
- Xia, B.; Liu, H.; Fan, Y.; Zhu, W.; Geng, C. Preparation of robust CuO/TiO2 superamphiphobic steel surface through chemical deposition and sol–gel methods. Adv. Eng. Mater. 2017, 19, 1600572. [Google Scholar] [CrossRef]
- Johari, N.D.; Rosli, Z.M.; Juoi, J.M.; Yazid, S.A. Comparison on the TiO2 crystalline phases deposited via dip and spin coating using green sol–gel route. J. Mater. Res. Technol. 2019, 8, 2350–2358. [Google Scholar] [CrossRef]
- Huong, H.T.; Nhu, T.T.Q.; Nang, H.X.; Tuan, P.A.; Huy, P.T. High transmittance and excellent hardness TiO2-SiO2-Al2O3 nanocomposite thin film for anti-scratch surface applications. Polym. Compos. 2022, 43, 7473–7482. [Google Scholar] [CrossRef]
- Li, Y.L.; Huang, Z.R.; Zhong, Q.D. Microstructure and Corrosive Behavior of Enamel Coating Modified on Mild Steel. Surf. Rev. Lett. 2018, 25, 1850081. [Google Scholar] [CrossRef]
- Dai, J.; Yang, J.; Zhuge, L.; Wu, X. Al2O3–TiO2 composite coatings with enhanced anticorrosion properties for 316L stainless steel. Mater. Corros. 2020, 71, 1512–1520. [Google Scholar] [CrossRef]
- Sobolev, A.; Bograchev, D.; Borodianskiy, K.; Zinigrad, M. Kinetics and mechanism of corrosion of oxide coating fabricated on aluminum alloy by the plasma electrolytic oxidation in molten salt. Corros. Sci. 2022, 208, 110604. [Google Scholar] [CrossRef]
- Mouele, E.S.M.; Myo, T.Z.M.; Kyaw, H.H.; Tijani, J.O.; Dinu, M.; Parau, A.C.; Pana, I.; El Ouardi, Y.; Al-Sabahi, J.; Al-Belushi, M.; et al. Degradation of sulfamethoxazole by double cylindrical dielectric barrier discharge system combined with Ti/CN-TiO2 supported nanocatalyst. J. Hazard. Mater. Adv. 2022, 5, 100051. [Google Scholar] [CrossRef]
- Hajiyan Pour, F.; Behpour, M.; Shabani-Nooshabadi, M.; Jafari, Y. Investigation of corrosion protection properties of TiO2-CdO nanocomposite coating prepared by sol-gel method on copper. J. Nanostructures 2020, 10, 52–63. [Google Scholar]

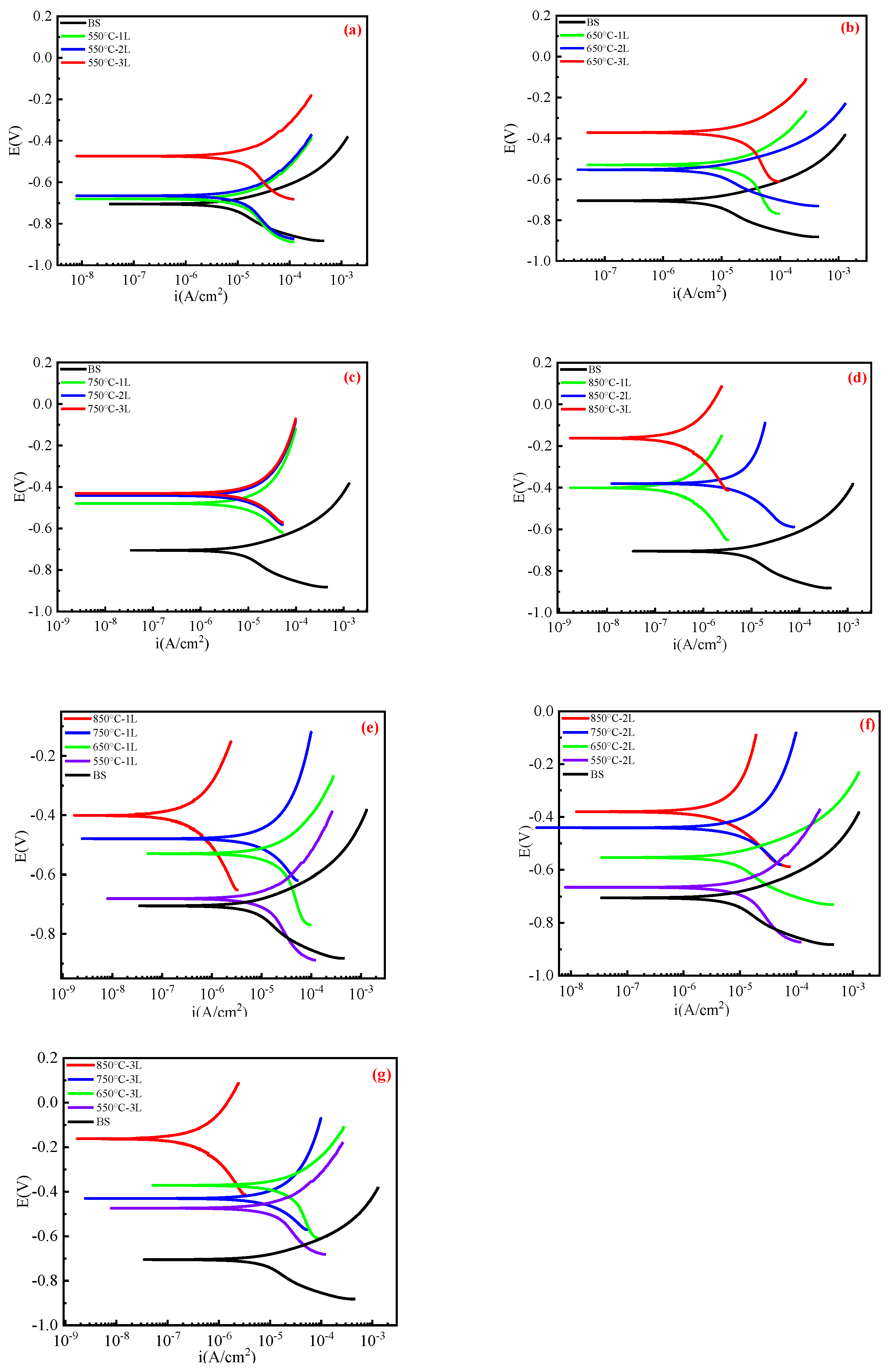

| Samples | Ecorr (V) | Icorr (A/cm2) | Rp (Ω·cm2) | |
|---|---|---|---|---|
| BS | −0.71 | 7.7 × 10−8 | 1028 | |
| 550 °C | 1 L | −0.68 | 6.3 × 10−8 | 1449 |
| 2 L | −0.67 | 6.2 × 10−8 | 2004 | |
| 3 L | −0.47 | 3.9 × 10−8 | 2380 | |
| 650 °C | 1 L | −0.53 | 5.1 × 10−8 | 1362 |
| 2 L | −0.55 | 5.2 × 10−8 | 3352 | |
| 3 L | −0.37 | 3.5 × 10−8 | 3358 | |
| 750 °C | 1 L | −0.48 | 2.5 × 10−9 | 1889 |
| 2 L | −0.44 | 5.6 × 10−8 | 7043 | |
| 3 L | −0.43 | 2.4 × 10−9 | 8399 | |
| 850 °C | 1 L | −0.40 | 6.9 × 10−9 | 8277 |
| 2 L | −0.34 | 1.7 × 10−9 | 21,213 | |
| 3 L | −0.16 | 1.3 × 10−9 | 114,000 |
| Samples | Rs (Ω·cm2) | n1 | Rc (Ω·cm2) | n2 | Rt (Ω·cm2) |
|---|---|---|---|---|---|
| RS | 42.25 | 0.6 | 589.9 | ||
| 550-1 L | 14.92 | 0.4 | 44.51 | 0.7 | 784.8 |
| 550-2 L | 39.86 | 0.8 | 72.68 | 0.8 | 599.2 |
| 550-3 L | 56.88 | 0.7 | 341.8 | 0.8 | 6023 |
| 650-1 L | 32.75 | 0.6 | 85.49 | 0.8 | 1257 |
| 650-2 L | 17.94 | 0.6 | 686.4 | 0.7 | 5398 |
| 650-3 L | 31.17 | 0.5 | 3868 | 0.7 | 4684 |
| 750-1 L | 22.82 | 0.6 | 106.9 | 0.8 | 916.7 |
| 750-2 L | 10.42 | 0.5 | 426.1 | 0.5 | 1178 |
| 750-3 L | 56.15 | 1 | 4005 | 0.7 | 41,510 |
| 850-1 L | 24.59 | 0.7 | 5505 | 0.9 | 97,840 |
| 850-2 L | 25.63 | 1 | 13,360 | 0.6 | 360,300 |
| 850-3 L | 28.28 | 0.7 | 15,580 | 0.9 | 1,874,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Wan, Y.; Zhang, X. Preparation and Corrosion Properties of TiO2-SiO2-Al2O3 Composite Coating on Q235 Carbon Steel. Coatings 2023, 13, 1994. https://doi.org/10.3390/coatings13121994
Liu X, Wan Y, Zhang X. Preparation and Corrosion Properties of TiO2-SiO2-Al2O3 Composite Coating on Q235 Carbon Steel. Coatings. 2023; 13(12):1994. https://doi.org/10.3390/coatings13121994
Chicago/Turabian StyleLiu, Xinyan, Ye Wan, and Xiang Zhang. 2023. "Preparation and Corrosion Properties of TiO2-SiO2-Al2O3 Composite Coating on Q235 Carbon Steel" Coatings 13, no. 12: 1994. https://doi.org/10.3390/coatings13121994
APA StyleLiu, X., Wan, Y., & Zhang, X. (2023). Preparation and Corrosion Properties of TiO2-SiO2-Al2O3 Composite Coating on Q235 Carbon Steel. Coatings, 13(12), 1994. https://doi.org/10.3390/coatings13121994





