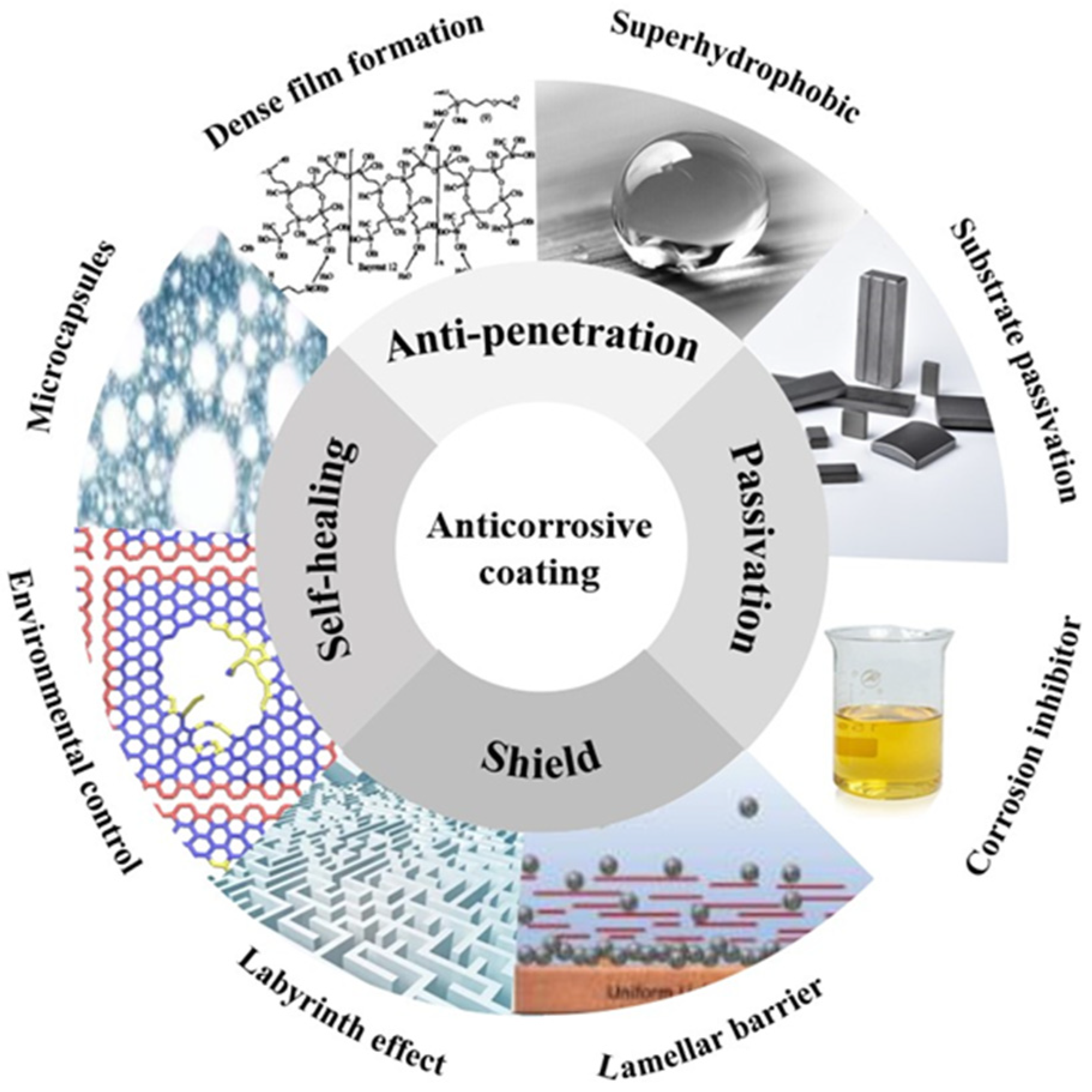
Abstract
Corrosion problems are widespread in nature. As one of the most convenient and efficient methods for metal anticorrosion protection, anticorrosive coatings have received increasing attention. With the continuous advancement of science and technology, more novel functional anticorrosion coatings are being extensively researched. This review provides an overview of recent research progress in anticorrosion coatings and functionalized modified materials. Recent methods for performance optimization can be categorized into three main sections: modification by nanoparticles, modification by carbon-based materials, and specific functionalization (barrier effect, passivation, shielding effect, resistivity, self-repair). Through modification, the anticorrosion performance of coatings is significantly enhanced, with impedance levels improving by up to three orders of magnitude. Furthermore, modification imparts additional outstanding features to the coatings, such as high-temperature resistance, thermal conductivity, self-healing, and hydrophobicity. Finally, the future development trend of anticorrosion coatings is proposed, and several reasonable suggestions are put forward for the challenges faced.
1. Introduction
As a product applied to the surface of an object to protect, modify, and conceal flaws, paint has been widely used in various fields [1,2]. With the continual advancements in scientific and technological levels, metal materials provide the necessary guarantee for various large-scale projects and infrastructure construction. However, the susceptibility of metal materials to corrosion and rust poses a significant challenge if not adequately protected [3,4,5]. In alignment with the country’s strategy to build a “resource-saving and environment-friendly society”, reducing resource waste and energy consumption has become the main trend in social development [6]. The prevalence of metal corrosion issues has caused considerable resource wastage and equipment losses.
The most straightforward solution to solve the problem of metal corrosion is to apply the surface with a protective coating, which effectively prevents the metal substrate from making contact with oxygen, water, and various ions in the atmosphere, thereby prolonging the lifespan of the metal [7,8,9]. Although anticorrosion coating products have been widely used, products’ performance and anticorrosion properties still have much room for improvement. Therefore, anticorrosive coating products can be modified and upgraded by designing functionalized resin products, introducing nanoparticles, functionalized modified materials, and adding functionalized additives to prepare novel functionalized anticorrosive coating products [10].
There are four ways to improve the anticorrosion performance of coatings, as shown in Figure 1:
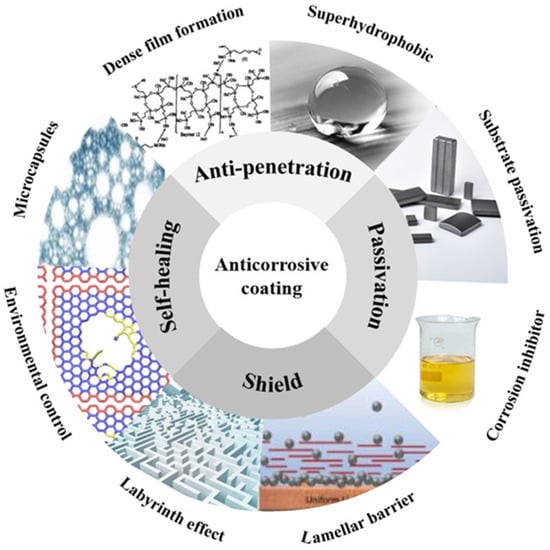
Figure 1.
Schematic Diagram of Action Methods of Metal Anticorrosive Coating.
- Increase the cross-linking density of the paint film to enhance the water, oxygen, and ion-blocking performance, making it more difficult for corrosive media to contact the surface of the base metal, thereby slowing down the metal corrosion. To increase cross-linking density, adopting a proper curing agent, adjusting a fitting curing period and temperature, and using a cross-linking auxiliary are often prevalent methods [11,12].
- Improve the bonding between the coating and the metal substrate to prevent further corrosion once the corrosive medium penetrates the coating. Improving the permeability and wettability of the coating to the substrate by using a substrate-wetting agent or penetrant could enhance the bonding. Using a coupling agent can increase the amino group, hydroxyl group, and carboxyl group between substrate and coating to further increase the chemical bonding density [13,14].
- Introduce functional materials or additives to protect the metal substrate through cathodic protection, blocking effects, or passivation effects. An active metal powder or corrosion inhibitor can be introduced to prevent the corrosion of the substrate metal through electrochemical principles, or through the introduction of graphene, polyaniline, and other conductive materials for corrosion protection [15,16,17].
- Improve the self-repairing ability of the coating. Most of the reasons leading to further metal corrosion are the damage of the paint film due to the corrosion of corrosive media or long-term collisions. The improvement of the self-repairing ability can occur in the micro or small range of the coating. The coating should recover quickly when damaged to prevent further corrosion [18,19,20].
This review delves into the relevant anticorrosion mechanisms of anticorrosion coatings and the latest research progress. It discusses the performance methods and modification methods of anticorrosion coatings, encompassing nanoparticle modification, functional carbon-based materials, and new specific functionalization, which are summarized in Table 1. It can be seen that when nanomaterials are employed to enhance anticorrosion performance, they are typically modified or functionalized with coupling agents or surfactants to improve dispersibility. The influence of chemical material modification methods on the protective ability of paint film was studied, and the future development direction of anticorrosive coatings was proposed.

Table 1.
A brief overview of the modification methods to improve anticorrosion performance.
2. Research Progress of Functional Anticorrosive Coatings
2.1. Research on Nanoparticle-Modified Anticorrosive Coatings
Nanomaterials, characterized by surface effects [21], size effects [22], and quantum effects [23], can be used as additives or modifiers in the field of coatings, conferring specialized properties to the coatings. Nanomodified coatings use nanoparticles as modifiers, which are introduced into both new and traditional coating products to improve the mechanical ability and corrosion resistance [24,25,26]. However, due to the small size effect of nanoparticles, achieving homogeneous dispersion within the resin system can be challenging. To address this issue, surface treatments, including the use of silicone coupling agents and surfactants can be applied to ensure the uniform dispersion of nanoparticles [27]. Alternatively, modified nanoparticles can be engineered with a core-shell structure through methods such as organic coating, thereby altering the surface structure of the nanoparticles to achieve the desired surface modification. At the same time, the introduction of nanoparticles can also improve the mechanical properties and shielding performance of the coating. In the crosslinking process of the coating, tiny pores and gaps may form. The inclusion of nanoparticles can effectively fill these defects, making it more difficult for corrosive agents to penetrate the substrate and preventing the diffusion of corrosive agents onto the substrate surface. This, in turn, leads to highly effective corrosion protection [28,29,30].
2.1.1. Silicon-Based Nanoparticle Modification
Dai et al. [31] studied the effect of silicon-based composite particles on the performance of epoxy resin. The three different silicon-based nanoceramic particles of Si3N4, SiO2, and SiC were selected to modify the epoxy resin, and their corrosion resistance and stability performance were investigated. The research results show that epoxy resin exhibits good compatibility with nanoparticles. Among them, the SiC-modified resin showed a strong corrosion resistance, with a corrosion current density of 1.525 × 10−6 A/cm2 and a maximum coating resistance of 9.405 × 106 Ω.
Xu et al. [32] conducted a study on the modification of epoxy coatings using hydrophobic nano-SiO2. They prepared modified epoxy anticorrosion coatings by mixing triethoxypropane tricyanide, diethyltriamine, and nano-SiO2, and Q235 carbon steel was employed to test its corrosion resistance. The results reveal that the modified epoxy coating exhibits excellent hydrophobicity and good thermal stability at 250 °C with only a weight loss of 3%. With a thickness of 10 μm, the electrochemical impedance of the material is 5.25 × 105 Ω·cm2. The addition of the hydrophobic SiO2 nanoparticles effectively extends the diffusion path of the water-soluble corrosive media, making the penetration of corrosive media more difficult, and leading to enhanced corrosion protection.
2.1.2. Titanium-Based Nanoparticle Modification
Shafaamri et al. [33] studied a preparation method of polydimethylsiloxane (PDMS)-modified nano-TiO2-modified epoxy resin. The research results show that PDMS has an extremely low surface energy. As a modifier, it can improve the hydrophobicity of the paint film without changing the degree of curing and dispersion of the coating. For the EP-PDMS-TiO2 mixed modified resin, the contact angle reached 103.2°. At the same time, the introduction of nano-TiO2 significantly improved the blocking performance of the coating to the corrosive medium. Nano-TiO2 changes the network structure of the epoxy matrix, and the nanoparticles and the polymer matrix are reorganized to close the voids in the epoxy matrix network to prevent corrosion by medium infiltration. When the amount of nano-TiO2 introduced is 0.1 wt.%, the barrier properties of the paint film are significantly improved.
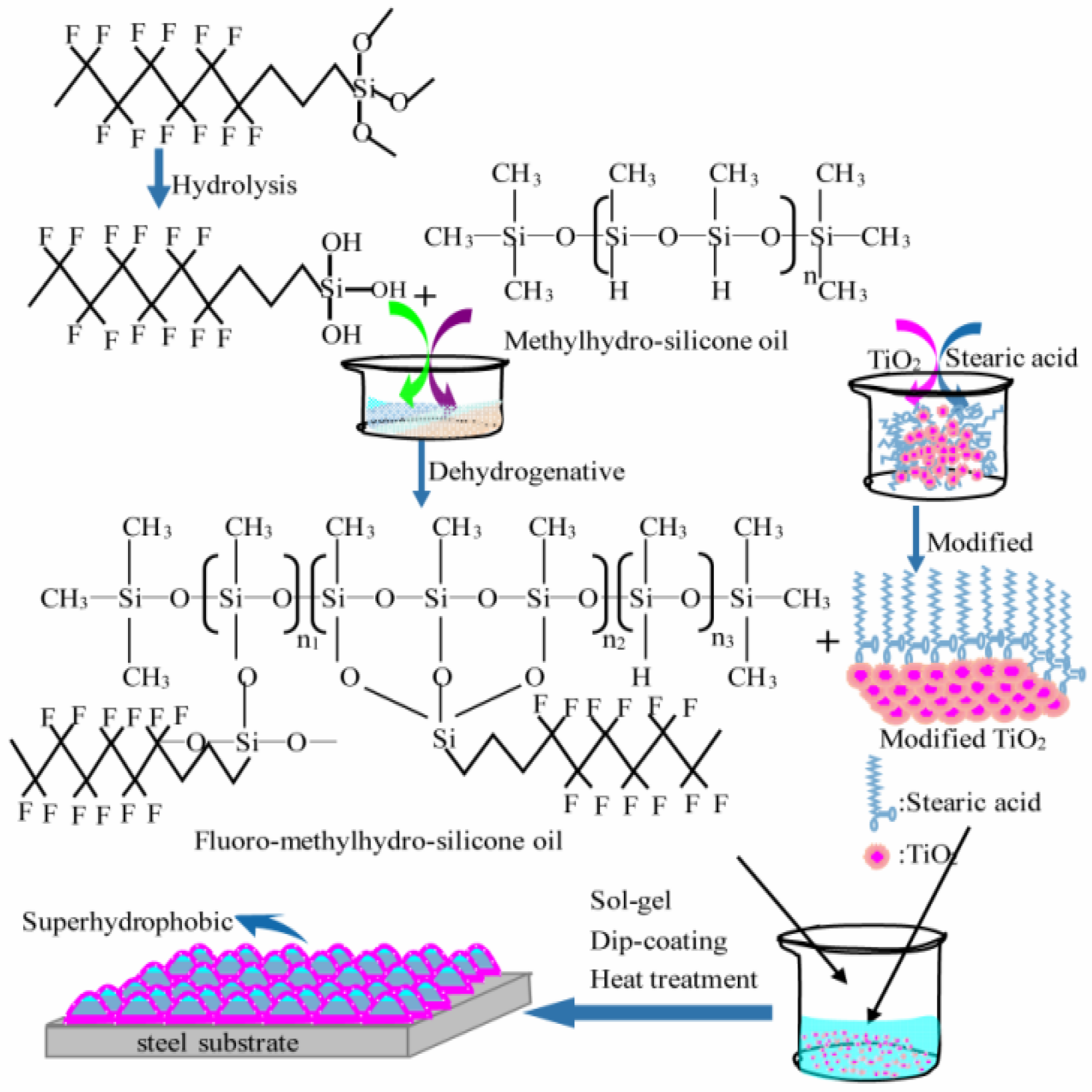
Mo et al. [34] used fluoro-methylhydro-silicone oil, which was blended with nano TiO2 particle after it was prepared by methylhydro-silicone oil and dodecafluoroheptyl-propyl-trimethoxysilane, as raw materials. The Organic/TiO2 composite coating was fabricated on a steel substrate using a sol-gel method. The preparation process of the composite coating is shown in Figure 2, and they tested the hydrophobic and corrosion resistance of the coating. The test results show that the corrosion potential of bare steel is increased from −1.15V to −0.91V, −0.98V, and −0.93V, respectively, by modifying the TiO2, methyl hydrosilicone oil, and fluoromethyl hydrosilicone oil coatings, while the best corrosion potential of TiO2/fluoromethyl hydrosilicone oil is −0.85V, and the water contact angle can reach 151.5 ± 1°.
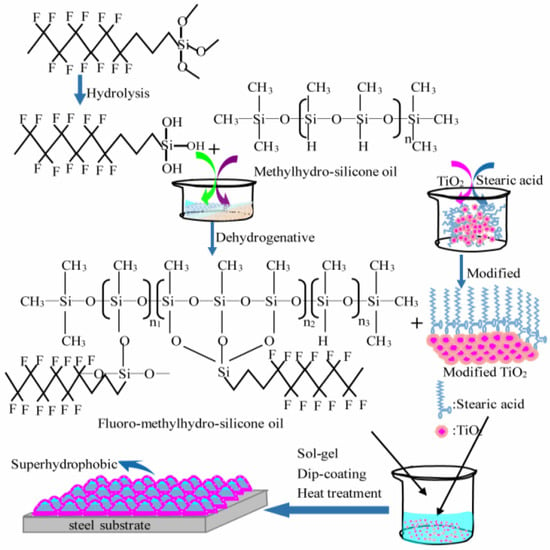
Figure 2.
Schematic diagram of the preparation process of the composite coating [34]. Fluoro-methylhydro-silicon oil is prepared via dehydrogenation coupling reaction of dodecafluoroheptyl-propyl-trimethoxysilane and methylhydro-silicone oil. The superhydrophobic coating was fabricated using a sol-gel method (With permission from Ref. [34] under a Creative Commons license, ESG (www.electrochemsci.org, accessed on 11 October 2023), open access).
2.1.3. Composite Nanoparticle Modification
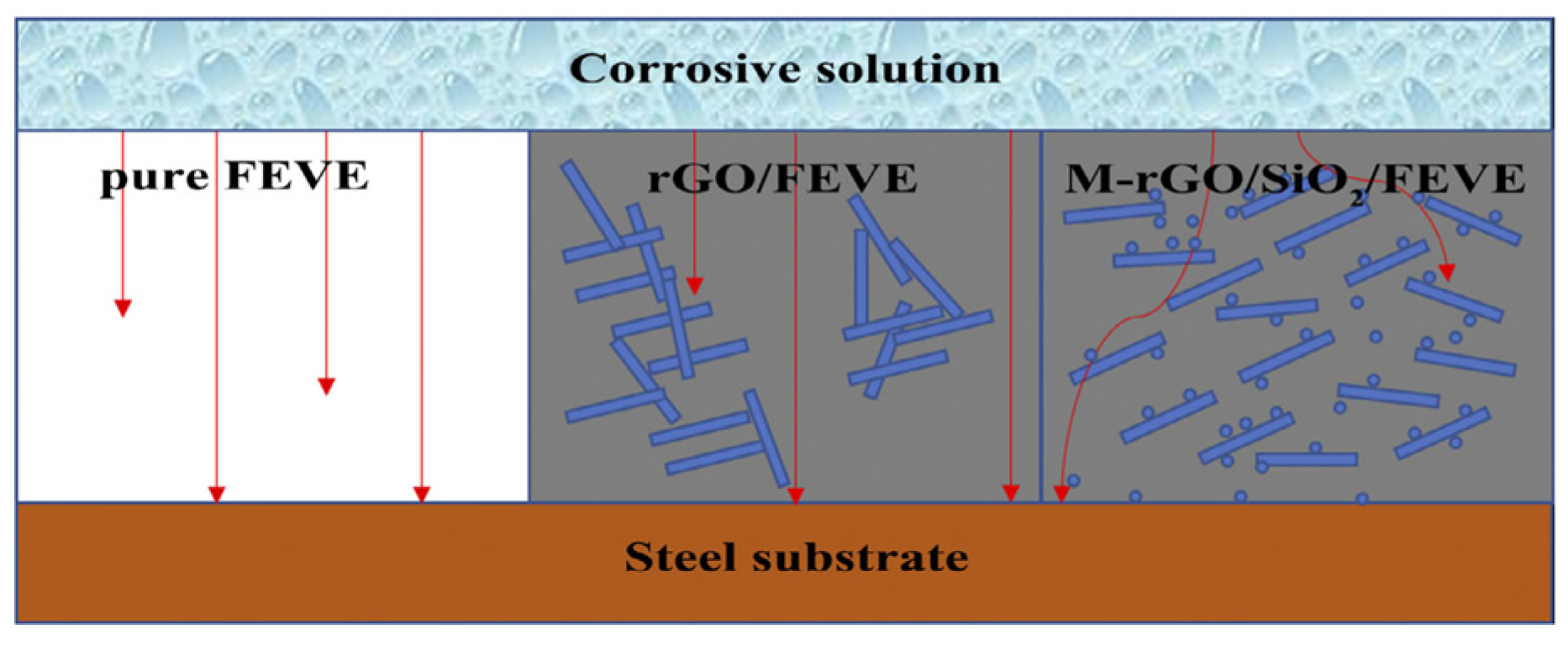
Zhong et al. [35] prepared reduced graphene oxide-silica nanocomposite particles (rGO-SiO2) using ball milling and modified the nanoparticles using a coupling agent of NH2C3H6Si(OC2H5)3 (KH550) so that they could be used in the fluoroethylene vinyl ether (FEVE) coating. A better dispersion performance is obtained, and the modified FEVE performance is tested. The experimental results show that the KH550-modified rGO-SiO2 is distributed in the coating and effectively extends the corrosion path, which improves the protective properties of FEVE. In the 30-day test, the electrochemical impedance of the rGO-SiO2-reinforced FEVE coating was maintained at 1010 Ω·cm2, and the protection rate reached 95.18%. The diffusion path model diagram of the corrosive medium is shown in Figure 3.
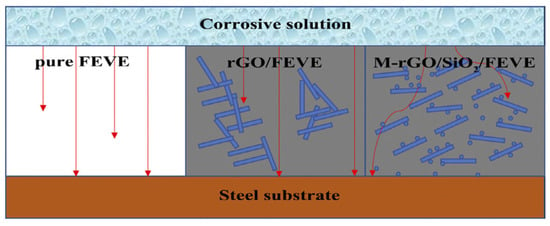
Figure 3.
Diffusion path model diagram of the corrosive medium [35]. In the pure FEVE coating, the corrosive media can directly pass through different kinds of defects in the coating to corrode the metal substrate. In the rGO/FEVE coating, although the sheet structure of rGO causes obstacles, the defects resulting from the agglomeration of rGO allow the corrosive media to pass through. In the M-rGO/SiO2/FEVE coating, the well-dispersed SiO2 nanoparticles and the rGO sheets form a three-dimensional structure with the FEVE matrix to effectively minimize the intrusion of the corrosive media, resulting in the good anticorrosion performance of the M-rGO/SiO2/FEVE coating (With permission from Ref. [35], License number: 5645920566068, 2023, Wiley).
Jiang et al. [36] used acrylic monomers and silicone prepolymers for copolymerization to obtain silicone-acrylic resin (SAR) and added methacryloxy propyl trimethoxyl silane (KH570)-modified SiO2 and TiO2 nanoparticles to SAR to prepare their nanocomposite coatings. The research results show that the introduction of silicone prepolymer improves the thermal stability and hydrophobicity of the coating, and the addition of nanoparticles further enhances the hydrophobicity. When the amount of modified SiO2 and TiO2 mixed nanoparticles introduced was 3 wt%, the contact angle of the coating reached 108.4°. In a 3.5% NaCl electrolyte solution, the resistance of the coating is greater than 109 Ω·cm2, and the dielectric stability reaches 1800 h.
2.1.4. Other Nanoparticles Modification
Osipchik et al. [37] modified epoxy resin ED-20 with a polyvinyl monomer to obtain a polyvinyl-epoxy composite material, and improved the corrosion resistance of the coating by introducing nano-ZnO particles. The research results show that the composite material has high adhesion, stability, dielectric properties, etc. The introduction of nano-ZnO particles reduces the residual stress, and when the introduction amount is 2%, the glass transition temperature of the composite is reduced, and the coating has better anticorrosion performance.
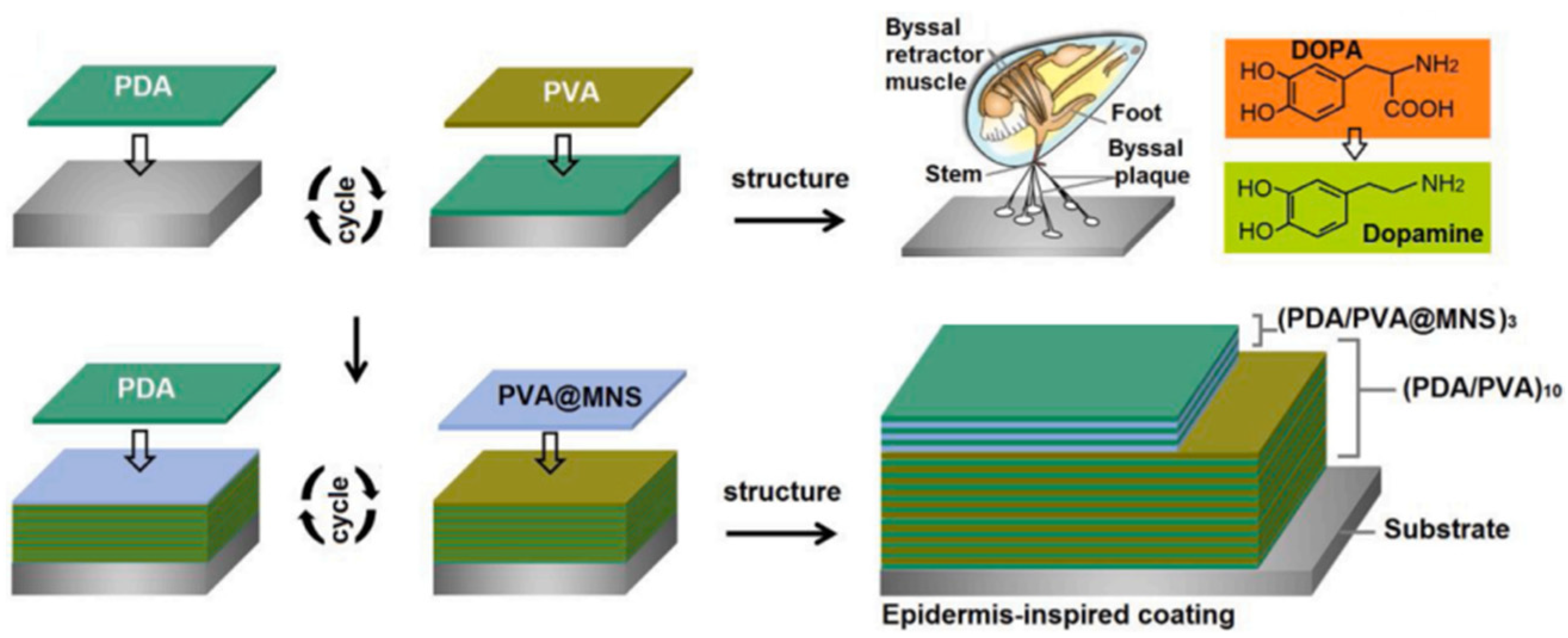
Inspired by the microstructure of the epidermis, Ding et al. [38] combined the excellent adhesion of polydopamine (PDA) and the shielding effect of mica nanosheets (MNSs) to prepare a composite coating product with independent repair ability. The coating is composed of PDA and PVA, which can completely repair the defects of the water-based coating. The design concept of the PDA/PVA@MNS coating is shown in Figure 4. The introduction of MNSs can not only improve the barrier properties of the coating but also be repaired by polymer materials. The corrosion voltages of the PDA/PVA and PDA/PVA@MNS coatings are −0.684 V and −0.557 V, respectively, and the corrosion current densities are 8.59 × 10−6 A·cm2 and 2.02 × 10−8 A·cm2, respectively. The PDA/PVA@MNS coating is 102 kΩ·cm2 lower than the PDA/PVA coating, the resistance of the PDA/PVA@MNS coating is 553.1 kΩ·cm2, and the PDA/PVA@MNS coating has a protection efficiency of 99.8%.
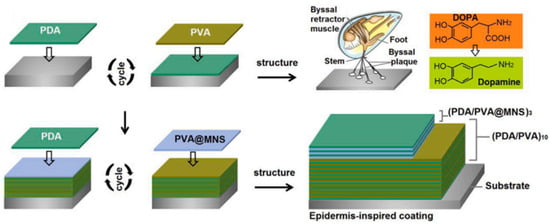
Figure 4.
Design concept diagram of the PDA/PVA@MNS coating [38]. It was noted that the stable PVA@MNS dispersion provided a satisfactory opportunity to efficiently integrate them into the coating systems. Interestingly, the PDA was selected as a component to improve the interface binding forces between the coating system and the steel surface, which was inspired by the mussel that can attach to a wide spectrum of solid substrates via byssal threads and adhesion plaques consisting of levodopa (With permission from Ref. [38], License number: 5645921159935, 2023, Elsevier).
Pan et al. [39] used tannic acid (TA) to modify boron nitride nanosheets (BNNSs) to obtain TA/BNNS materials (M-BNNSs) and compared the prepared nanosheets mixed with epoxy resin, and an anticorrosion coating with excellent anticorrosion, thermal conductivity, and mechanical properties was obtained. The thermal conductivity of the coating is 0.45 W/(m·K) and the tensile strength is 15.10 MPa. The Tafel curve and EIS analysis show that after immersion in a 3.5 wt.% NaCl aqueous solution for 30 min, the corrosion current density of the 10% M-BNNSs@EP coating was 8.05 × 10−7 A·cm2. After being immersed for 120 h, the coating still had good corrosion resistance.
2.2. Research on Functionalized Carbon-Based Material-Modified Anticorrosive Coatings
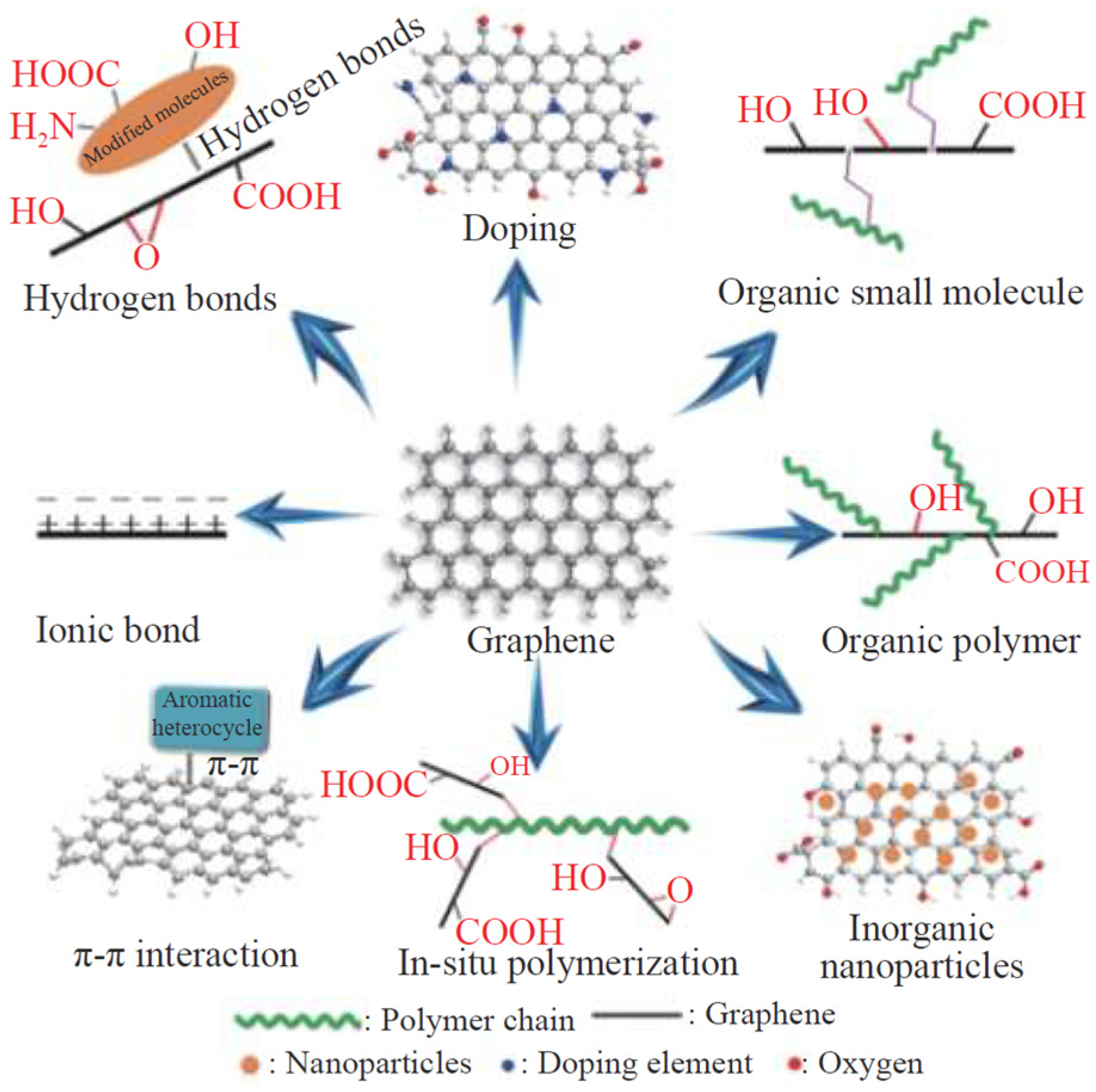
Carbon-based materials such as graphene and CNTs have excellent mechanical and electrical properties. Graphene-based functional materials such as graphene, graphene oxide, and reduced graphene have a layered structure [40], providing ultrahigh isolation properties that can reduced porosity and improved shielding effect of the coating [41,42]. However, due to the high specific surface area and van der Waals forces between graphene layers, graphene tends to aggregate easily. This issue can be addressed by increasing steric hindrance between graphene layers through surface modification, in situ polymerization, doping, etc. This improves its compatibility with the coating system, thereby facilitating its uniform dispersion within the coating. The modification method of graphene and its derivatives is shown in Figure 5 [43]. Moreover, carbon-based functional materials like graphene and its derivatives have excellent conductivity, which allows the electrons lost by the metal materials to be transferred to the surface of the coating. This process forms a passivation layer on the metal substrate, effectively preventing the occurrence of metal corrosion and providing electrochemical protection to metal materials [44].
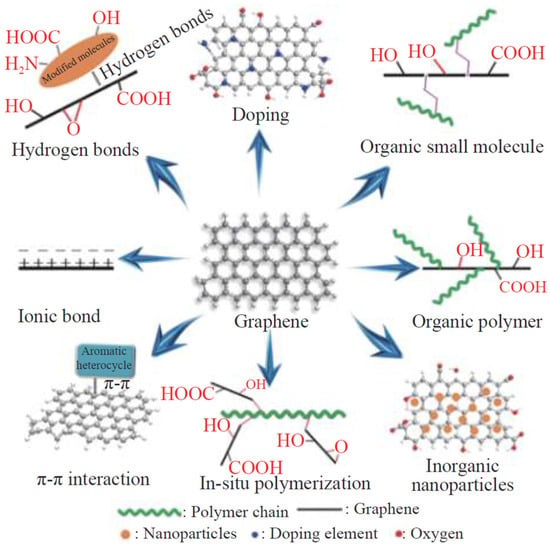
Figure 5.
Summary diagram of the modification methods of graphene and its derivatives [43].
2.2.1. Graphene-Based Material Modification
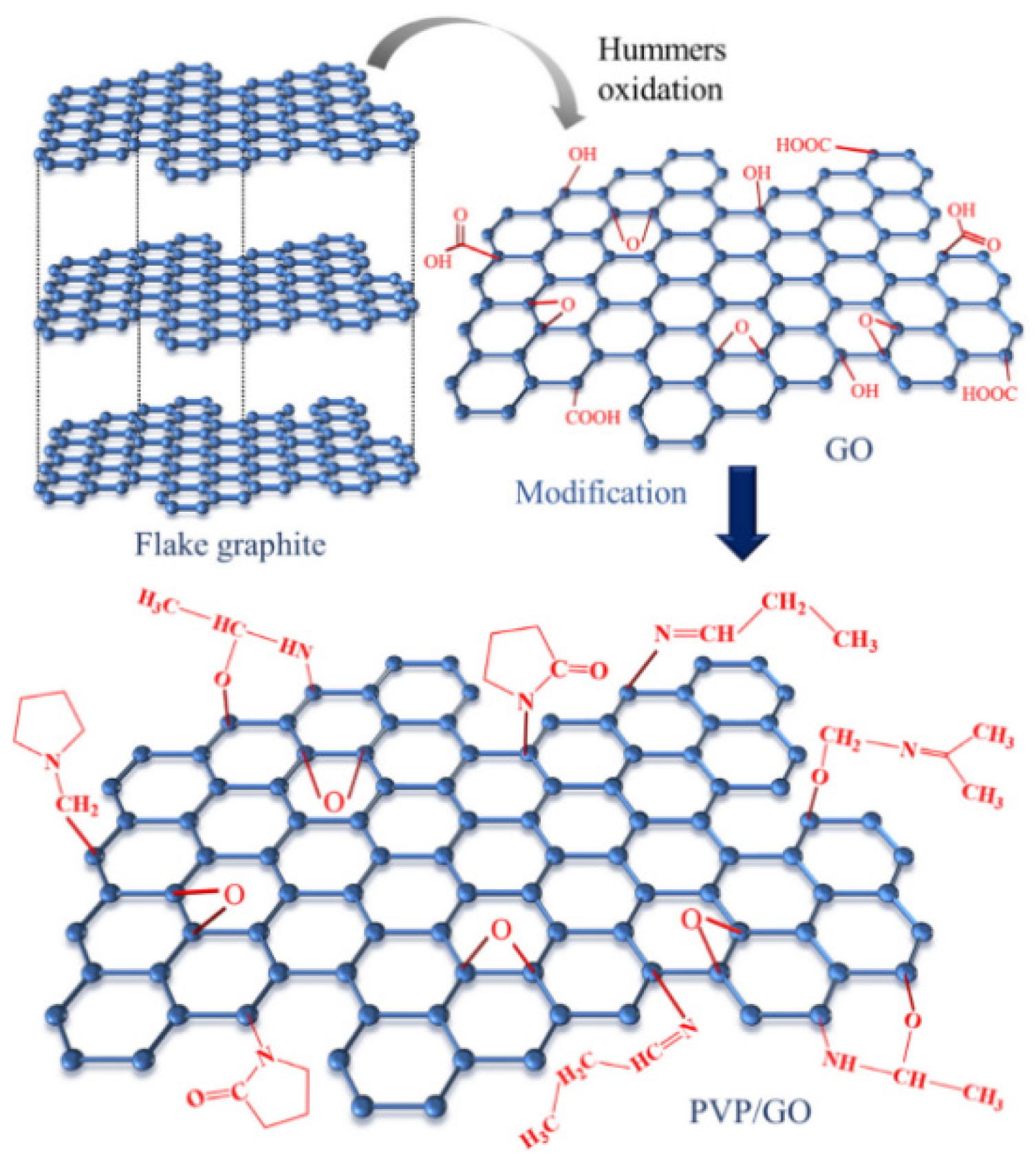
Zhang et al. [45] doped polyvinylpyrrolidone (pvp) and graphene oxide (GO) into EP to improve the shielding of EP coatings. The structure of the PVP/GO composite is shown in Figure 6. The research results show that in the PVP/GO composite, the spatial structure of the GO changes, and it is combined in the form of covalent bonds, which reduces the impact of the GO structural defects. The modification of the GO by PVP also improves the compatibility of GO. To avoid the agglomeration of GO, the PVP/GO composite material fills the holes and gaps in the resin layer. After testing, the corrosion voltage of the PVP/GO/EP coating is −0.571 V, the corrosion current density is 7.795 × 10−7 A·cm−2, the protection rate of the coating reaches 94.69%, and it has good corrosion resistance.
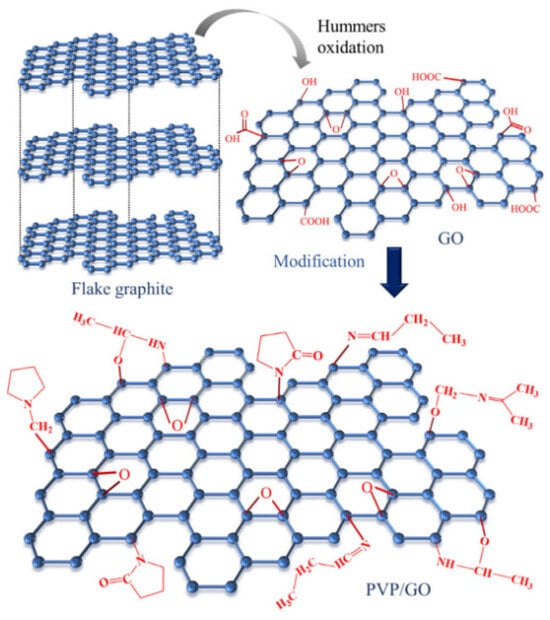
Figure 6.
Schematic diagram of the PVP/GO composite structure [45]. The graphite oxide was prepared using the improved hummers method. And then, through solution blending, the PVP-modified GO was prepared (With permission from Ref. [45], License number: 5645930153779, 2023, Wiley).
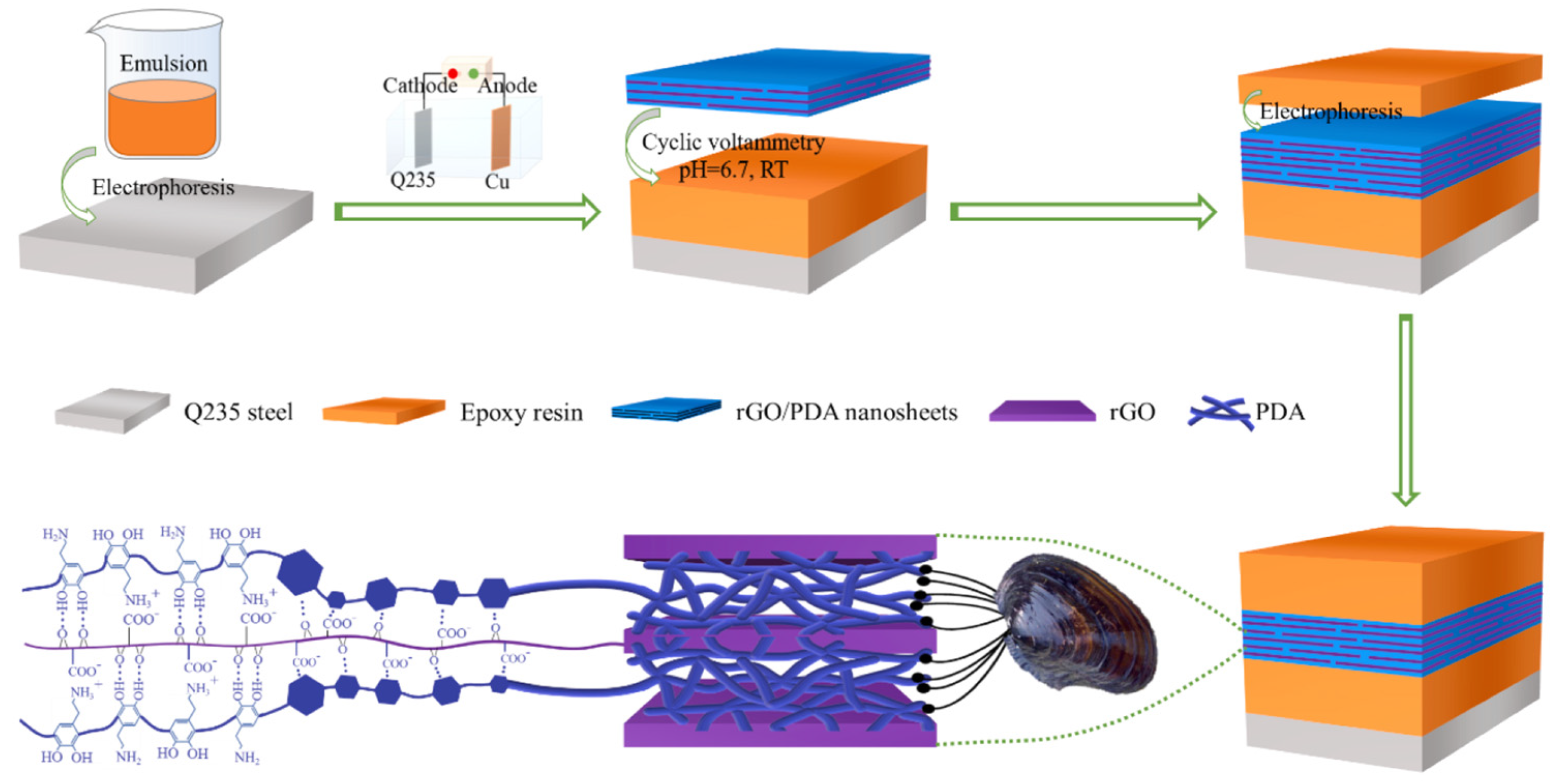
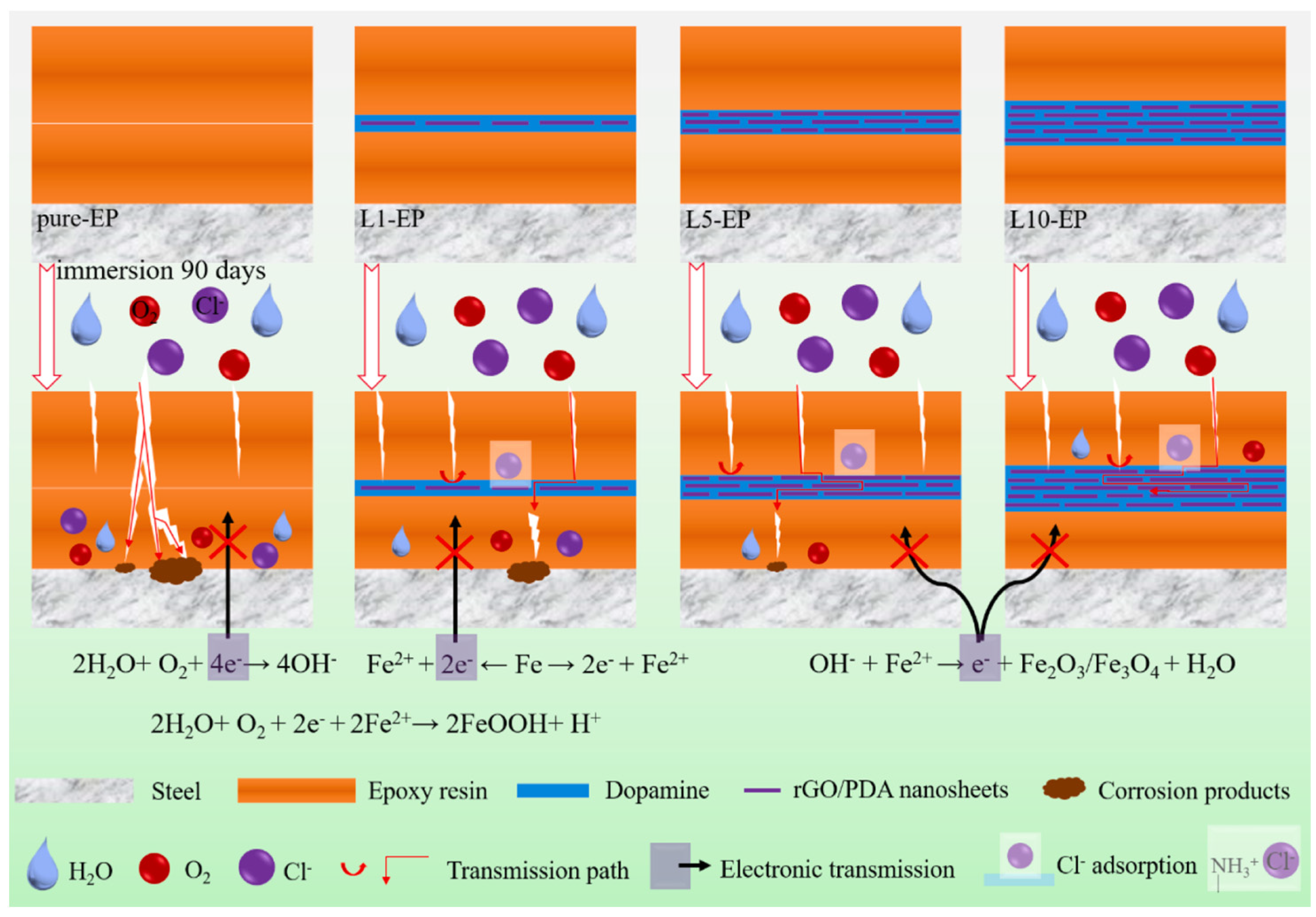
Inspired by mussels and natural corn, Zhu et al. [46] prepared a bionic graphene nanosheet and introduced it into an epoxy resin layer. The EP-(GR-DA)n-EP sandwich coating was prepared using electrodeposition. The production method is shown in Figure 7. Among the graphene and epoxy resin, the presence of dopamine can not only improve the compatibility between the graphene and the epoxy resin but also fully enhance the shielding effect of graphene. The research results show that after the functionalized dopamine-modified graphene oxide is added and the coating is immersed in a 3.5 wt.% NaCl solution for 90 d, the electrochemical impedance is 1.3 × 109 Ω·cm2, which is three orders of magnitude higher, indicating that the coating can have a long-term protection ability. The mechanism of the coating is shown in Figure 8.
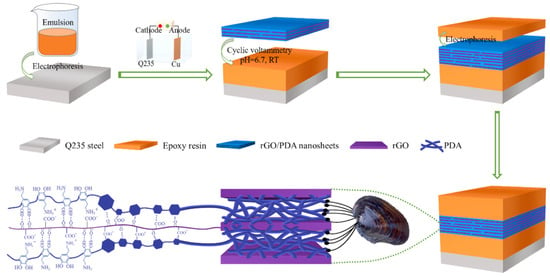
Figure 7.
Preparation process diagram of the EP-(GR-DA)n-EP sandwich coating [46]. The cleaned Q235 steel and Cu are used as the cathode and anode of the electrophoretic deposition system, respectively. The electrophoretic emulsion of the water-based cathode epoxy resin with a 25% solid content was used as the electroplating solution, and a layer of epoxy resin was deposited on the surface of the steel using a direct-current (DC) power at ambient temperature. Then, a graphene interlayer with controlled thickness was prepared by the deposition of graphene oxide/dopamine electrolyte onto the surface of the pre-deposited epoxy resin using cyclic voltammetry (CV). Finally, the upper epoxy layer coating was formed using electrodeposition and cured at 160 °C for 15 min to acquire the bionic sandwich composite epoxy coating (With permission from Ref. [46], License number: 5645931039170, 2023, Elsevier).
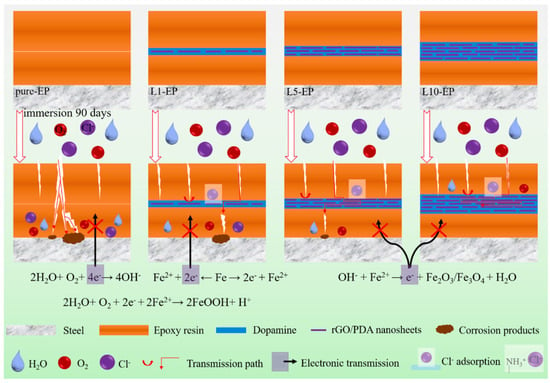
Figure 8.
Schematic diagram of the anticorrosion mechanism of the EP-(GR-DA)n-EP sandwich coating [46]. The graphene arranged parallel to the substrate not only gives full play to the interaction between its high surface area and electrolyte to achieve the barrier limit of graphene, but the arrangement also effectively prevents the penetration of the corrosive medium and prolongs the diffusion path of the corrosive substances. Secondly, the bionic sandwich composite coating can shield from galvanic corrosion by avoiding direct contact with the substrate. Finally, the presence of dopamine enhances the interfacial compatibility between the graphene and the epoxy matrix, and gives graphene a bionic nacre structure to achieve long-term protection of the substrate (With permission from Ref. [46], License number: 5645931039170, 2023, Elsevier).
Yang et al. [47] prepared aminopropyltriethoxysilane (ATPES)-modified functionalized graphene nanosheets (f-GNPs), grafted amino-terminated polyether to the surface of the graphene nanosheets, and compounded F-GNPs and water-based acrylic resins to prepare water-based acrylic coatings with high-anticorrosion performance. The experimental results show that when the number of GNPs introduced is 3.6 wt.%, it is optimal. GNPs act as physical isolation. When the amount of modified material introduced is 2.8 wt.%, the salt resistance time of the coating reaches 600 h. Through research, f-GNPs have more advantages in interface dispersion and physical isolation, and the prepared coating has better corrosion resistance.
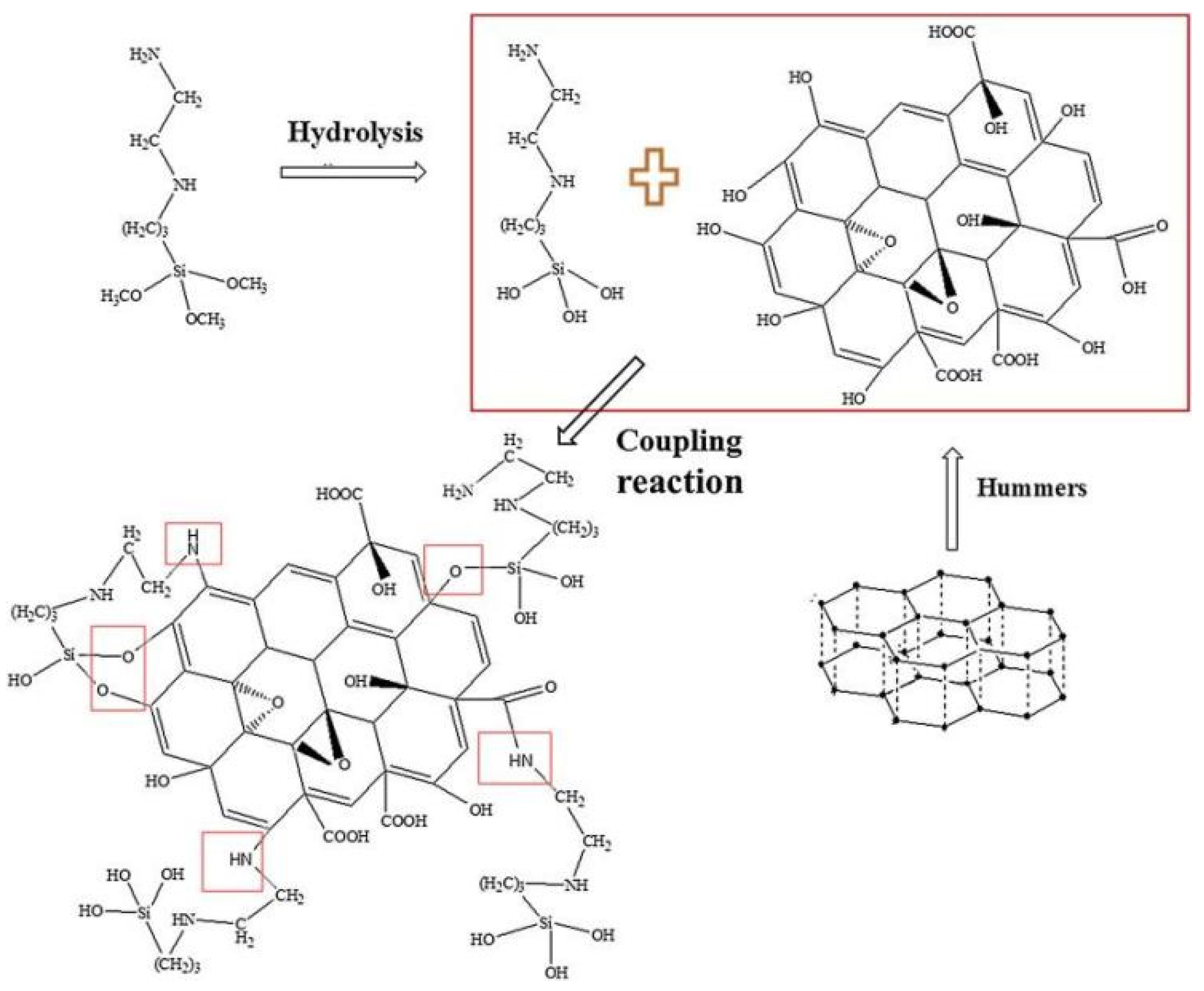
Li et al. [48] researched a coating with a “labyrinth structure” using [3-(2-aminoethyl) aminopropyl] trimethoxyl silane (KH792)-modified graphene oxide to obtain a modified composite material (MGO). The MGO has excellent dispersibility and corrosion resistance, and the layered GO structure does not change. The increase in the interlayer spacing can be compared with that of resin. Cross-linking occurs, forming an anticorrosion path similar to a “labyrinth structure”, which reduces the conductivity of the GO. The research results show that when the amount of MGO introduced is 0.5 wt%, the protection efficiency of the material reaches 99.7%. A schematic diagram of the KH792-modified GO is shown in Figure 9.
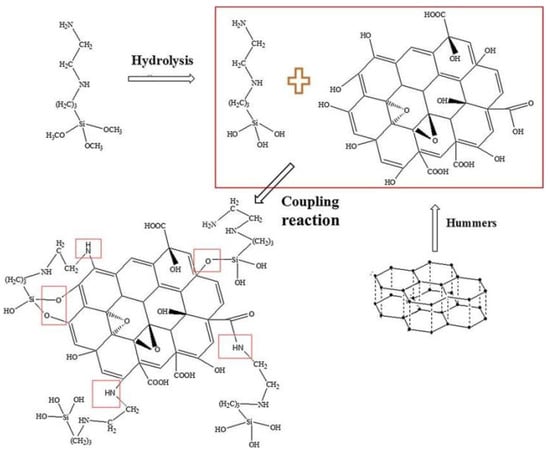
Figure 9.
Schematic diagram of GO modified by (2-aminoethylamino)propyltrimethoxysilane [48]. GO was prepared using an improved Hummers method at medium temperature. The OH groups were produced by the hydrolysis of [3-(2-aminoethyl) aminopropyl] trimethoxysilane, which reacts with the carboxyl group of GO along with the amino group (With permission from Ref. [48], License number: 5645931510105, 2023, Elsevier).
Shen et al. [49] prepared a coating that combined the common advantages of zinc-rich epoxy (ZRC) and graphene and analyzed the mechanism. A liquid (IL) was used to modify the GO to improve its dispersibility. The research results show that the Rc value of EP + GO/ZRC + GO gradually decreases from 7.76 × 107 Ω·cm2 to 2.47 × 106 Ω·cm2. The barrier performance and cathodic protection are provided by the special double-layer structure of EP + GO/ZRC + GO. The synergistic effect gives the coating excellent protective properties.
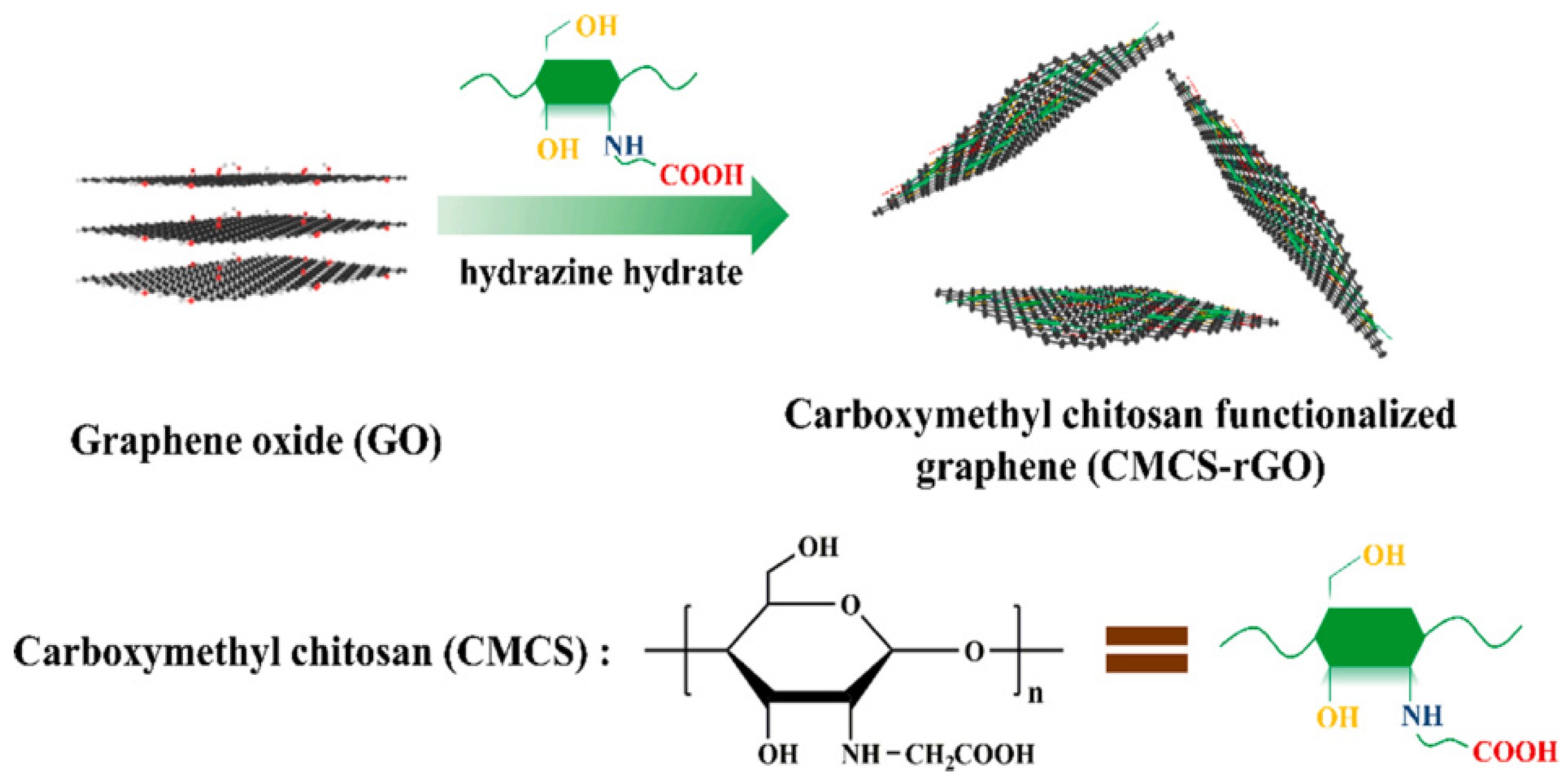
Shi et al. [50] prepared carboxymethyl chitosan-functionalized graphene (CMCS-rGO) nanomaterials using noncovalent functionalization methods and introduced them into waterborne epoxy (w-EP) coatings as nano-anticorrosive additives. The preparation of CMCS-rGO is shown in Figure 10. The research results show that the introduction of CMCS-rGO into the w-EP system can improve the corrosion resistance of the coating. After immersion for 180 d, the Rc value of the EP and rGO/EP coatings decreased from 8.45 × 107 Ω·cm2 and 1.03 × 108 Ω·cm2 to 1.97 × 105 Ω·cm2 and 1.67 × 106 Ω·cm2, respectively, and that of the 0.2% CMCS-rGO/EP coating was 9.98 × 107 Ω·cm2, indicating that the CMCS-rGO nanomaterial can significantly improve the corrosion resistance of the EP coating.
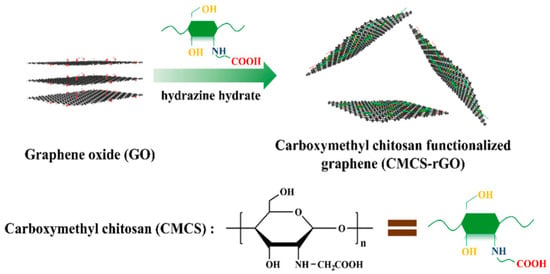
Figure 10.
Production process chart of CMCS-rGO [50]. Loading carboxymethyl chitosan onto the surface of graphene can prevent the direct connection of graphene–graphene and graphene–steel, thus inhibiting the occurrence of galvanic corrosion and leading to excellent corrosion resistance (With permission from Ref. [50], License number: 5645940209125, 2023, Elsevier).
2.2.2. Carbon Nanotube-Based Material Modification
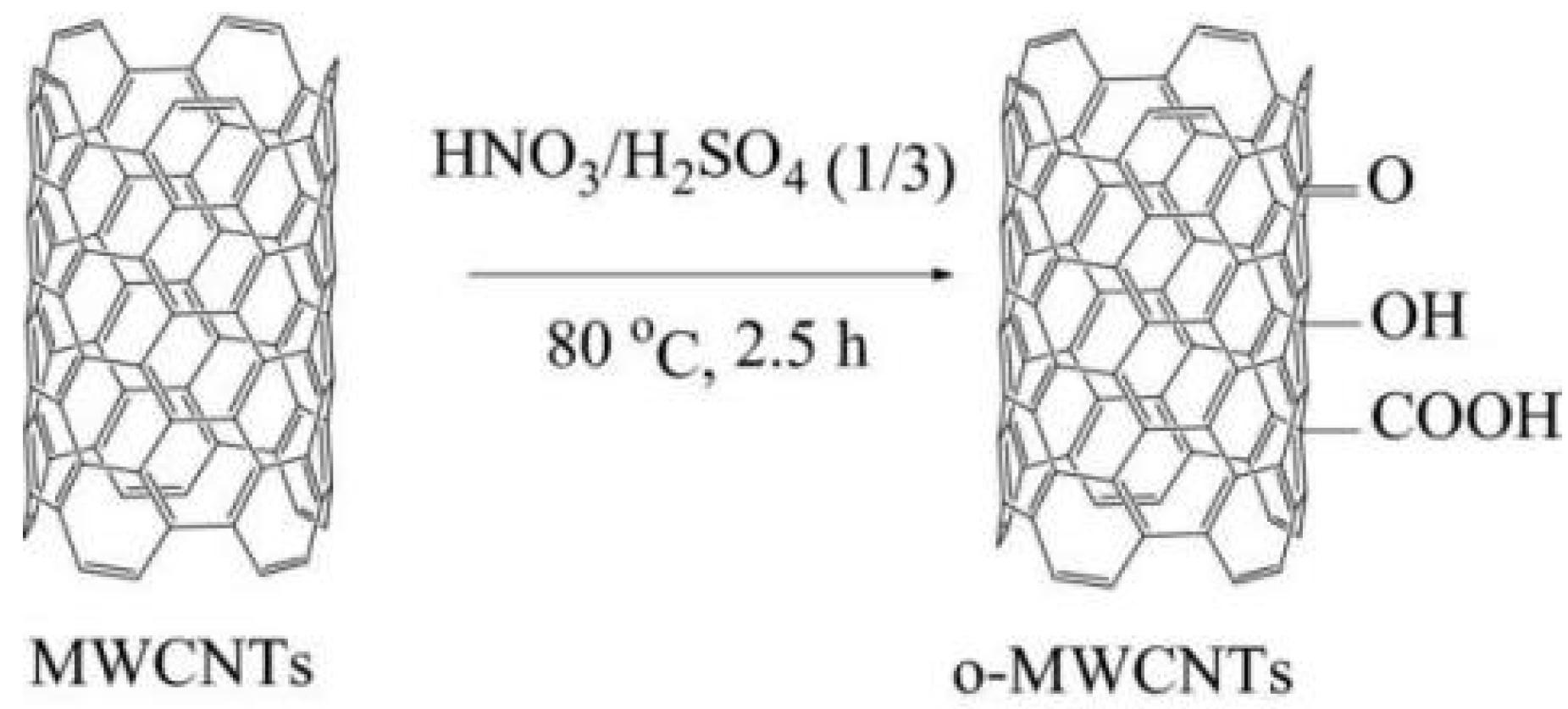
Vu et al. [51] used a concentrated mixed acid of HNO3:H2SO4 = 1:3 to process multiwalled carbon nanotubes (MWCNTs) to obtain oxidized multiwalled carbon nanotubes (o-MWCNTs). The preparation process of o-MWCNTs is shown in Figure 11. The o-MWCNTs can be added as a preservative to an epoxy coating. The functional group formed by the oxidation process can improve the adhesion characteristics of the coating, and it can also form a connection with the substrate surface through the charge change. The research results show that the resistance value and capacitance value of the pure EP coating are 124 KΩ·cm2 and 36.2 × 10−9 F·cm−2, respectively, the resistance value of the EP/MWCNT coating increases to 1376 KΩ·cm2, and the capacitance value decreases to 5.4 × 10−9 F·cm−2. The resistance value of the EP/o-MWCNT coating increased to 2566 KΩ·cm2, and the capacitance value dropped significantly to 1.2 × 10−9 F·cm−2. The EP/o-MWCNT coating exhibits the highest corrosion resistance.
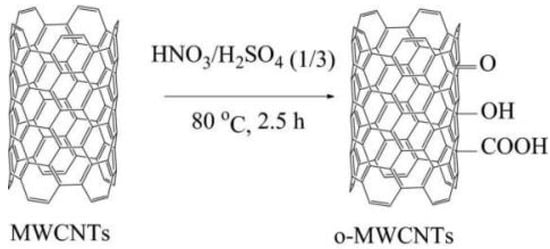
Figure 11.
Preparation process diagram of o-MWCNT [51]. The pristine MWCNTs were immersed in a blended concentrated acid of HNO3 and H2SO4 (with a ratio of 1/3) for 2.5 h at 80 °C (With permission from Ref. [51], License number: 5646210958621, 2023, Springer).
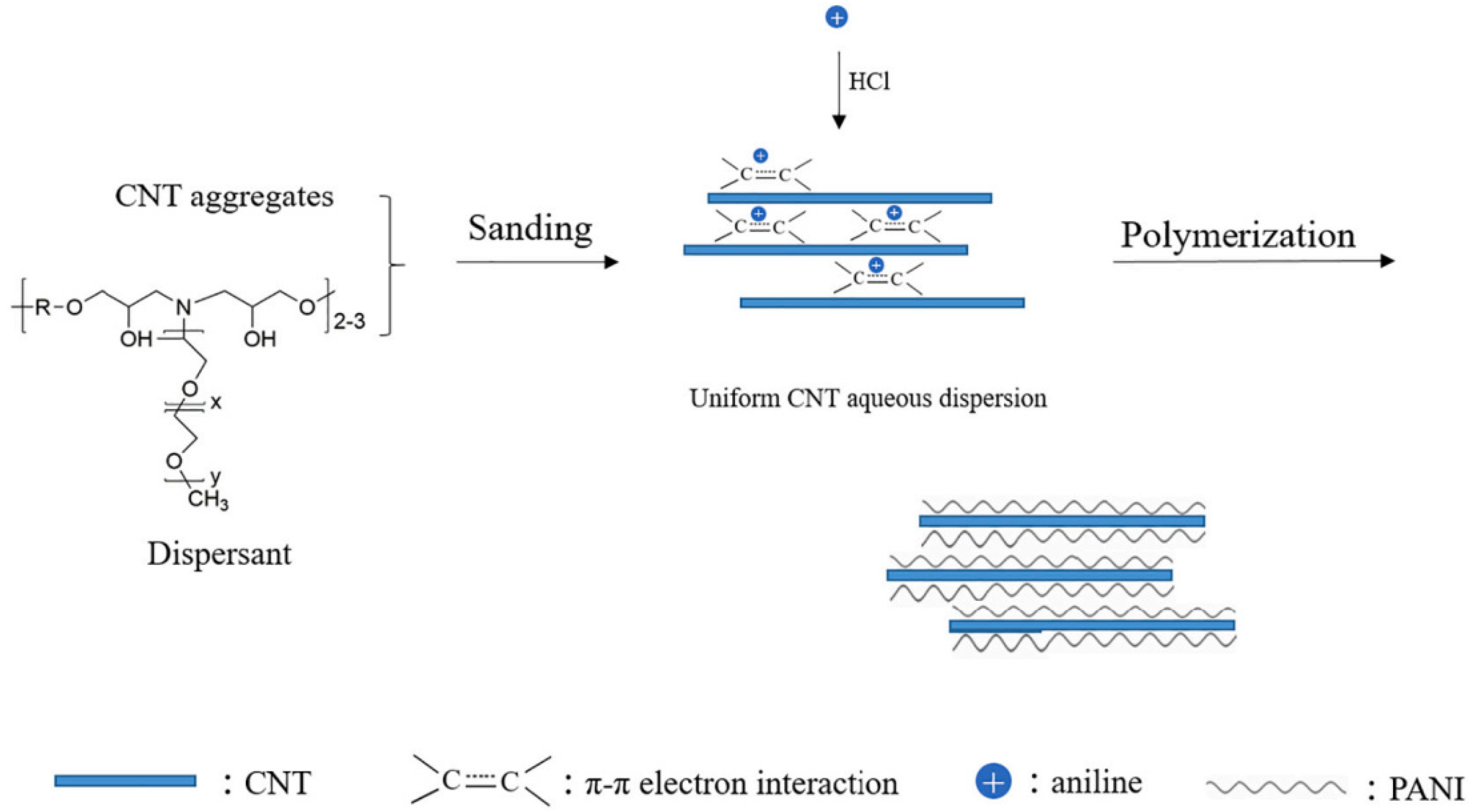
The experimental results of Zhang et al. [52] show that the synthesized PANI/CNT nanocomposites have reversible redox activity in both acidic and neutral media. When the coating is damaged, the anodic protection of the passive film formed on the metal surface plays a decisive role. The PANI/CNT (8:1) nanocomposites have the best metal corrosion resistance. The anticorrosion mechanism of the PANI/CNY nanocomposite coating is composed of the synergistic structure–activity relationship of the nano shielding effect, cathodic inhibition effect, and anodic protection effect. The preparation scheme of the PANI/CNT nanocomposites is shown in Figure 12.
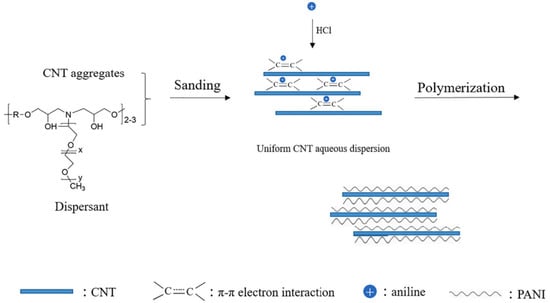
Figure 12.
Preparation of PANI/CNT nanocomposites [52], which was prepared via aniline polymerization in CNT aqueous dispersion (With permission from Ref. [52], License number: 5645940431352, 2023, Elsevier).
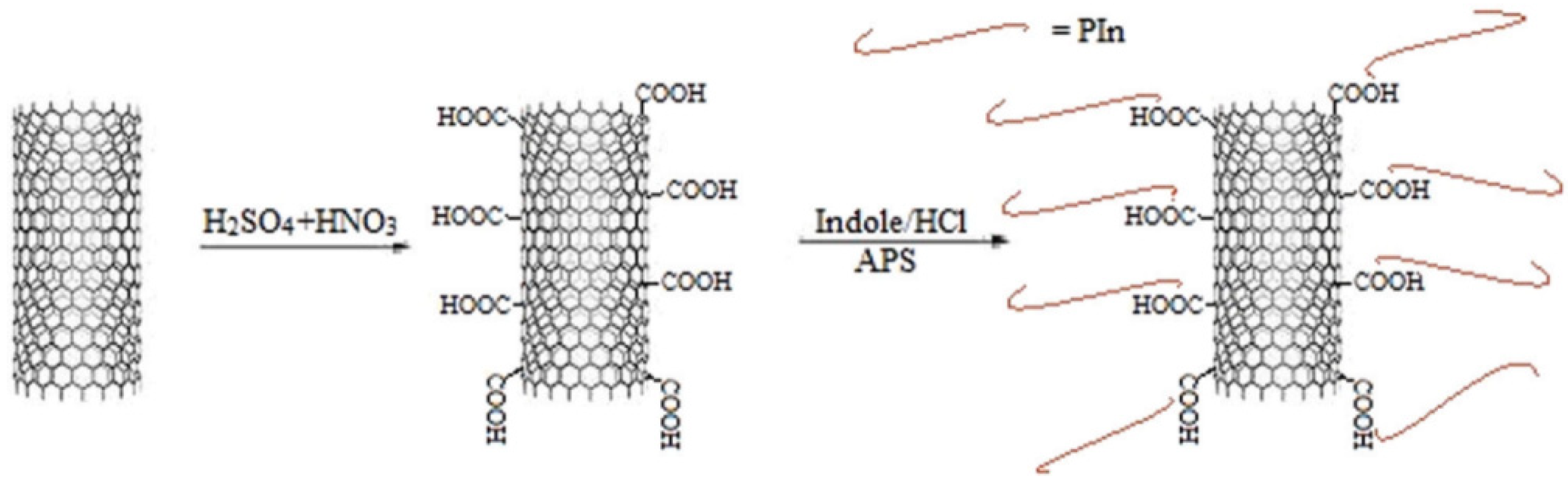
Saurav et al. [53] introduced functionalized multiwall carbon nanotubes/polyindole (MWCNT/PIn) composites into epoxy resin to prepare a low-carbon steel anticorrosion coating with excellent performance. As a conductive polymer, PIn can show supercapacitor performance when combined with carbon nanotubes. The carboxyl functionalized MWCNT and PIn are combined through noncovalent bonds to form a nanohybrid. The preparation of the MWCNT/PIn nanohybrid is shown in Figure 13. The MWCNT/PIn nanocomposites were uniformly dispersed in the epoxy resin to improve the anticorrosion and barrier properties of the coating. The experimental results showed that when the MWCNT/PIn content was 0.25 wt%, the impedance of the coating was 107 Ω/cm2. When the MWCNT/PIn is introduced into the coating, the micropores and small defects in the coating are blocked, which extends the path of the corrosion medium to the interface between the coating and metal, reduces the possibility of the oxidation-reduction reaction at the interface, and effectively inhibits the formation of corrosion products.
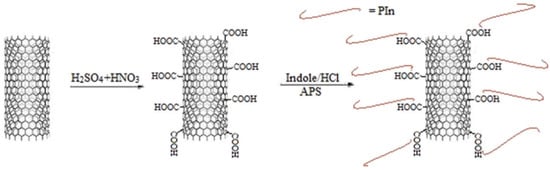
Figure 13.
Preparation Diagram of the MWCNT/PIn Nanohybrid [53]. First, the MWCNTs were functionalized in an H2SO4 /HNO3 aqueous solution. Then, they were added into an indole monomer solution, through APS initiate indole polymerization, to prepare the MWCNT/Pin nanohybrid (With permission from Ref. [53], License number: 5645940709521, 2023, Elsevier).
2.3. Research on New Functional Material-Modified Anticorrosion Coating
As the performance requirements of coating products in various fields are increasing, coating products are required to have certain special functions in some special fields so that an increasing number of new composite materials and functional materials are widely used in the field of coatings [54]. Phosphoric acid-based materials, polyaniline, organic silicon, fluoride, and other functional composite materials with excellent anticorrosion properties, according to their different anticorrosion mechanisms, play a very important protective role in different types of anticorrosion coatings [55,56,57,58]. At present, the method of modifying anticorrosive coatings with functional materials is generally based on blending modification. First, materials with special functions are synthesized, and then surface modification or coating is performed to make them uniformly dispersed in the coating, thereby obtaining a functional anticorrosion coating [59]. At the same time, new functional materials such as microcapsules can also be introduced to give the coating a certain self-repairing function. When the coating surface is damaged macroscopically or microscopically, the microcapsules can release the repairing components to reattach the damaged part of the coating. Joint film formation prevents coating failure, thereby prolonging the use time of the coating and effectively slowing down the corrosion rate of the metal substrate [60,61,62].
2.3.1. Prevent Corrosion Medium from Penetrating
Parineeta et al. [63] extracted neem seed oil from neem seeds and reacted it with maleic anhydride and terephthalic anhydride using an alcoholysis-polyester vulcanization method to prepare a neem seed oil-based alkyd resin. The research results show that during the resin synthesis process, as the proportion of maleic anhydride increases to enhance the degree of densification of the coating and gradually form a three-dimensional structure, it is more difficult for corrosive media to enter the inside of the coating, and the maleic anhydride coating contains 75% of water. The contact angle is 97.9°, indicating that the coating has better hydrophobicity and thus has better anticorrosion performance.
MoS2 has good anticorrosion properties and can be used as an anticorrosion additive for coatings. However, MoS2 has poor dispersibility and compatibility with resin systems and needs to be modified by wetting and dispersing to improve its dispersion properties. Liu et al. [64] used 3-aminopropyltriethoxysilane (APTES) to modify MoS2 so that an organic thin layer appeared on the surface of MoS2, giving it better compatibility. The process flow of the APTES-modified MoS2 is shown in Figure 14. Through the experimental analysis of the waterborne epoxy coating introduced with the MoS2-APTES, after being immersed in 3.5 wt.% NaCl solution for 50 d, the electrochemical impedance of the coating was 3.647 × 107 Ω·cm2. After the introduction of the MoS2-APTES with good dispersion properties, the diffusion path of the corrosive medium is prolonged, thereby delaying the time for the corrosive medium to contact the substrate and improving the corrosion resistance of the coating.
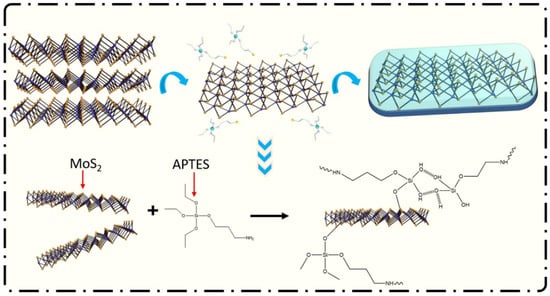
Figure 14.
Synthesis process diagram of the APTES-modified MoS2 [64]. APTES hydrolyzed first and then reacted with MoS2 in an ethanol solution (With permission from Ref. [64] under a Creative Commons license, MDPI, open access).
2.3.2. Base Surface Passivation
Sumi et al. [65] prepared a polyaniline-Fe2O3 (PANI-Fe2O3) composite material via in situ synthesis and blended the material with alkyd resin, giving a high-anticorrosion performance “conductive polymer-metal oxide-alkyd resin” hybrid coating. The research results show that the material has good metal passivation ability and can be used as an anticorrosion filler in the coating compounding process. When the introduction amount was 2 g/L, the paint film exhibited the best anticorrosion performance.
Iman et al. [66] prepared a zinc-aluminum layered composite oxide coating (LDH-CC) modified with sodium diethyldithiocarbamate (DEDTC) and conducted experiments on the surface of an AA2024-T3 alloy to study its anticorrosion properties. The experimental results show that the DEDTC modifies LDH-CC through an ion exchange process. Due to the existence of ion exchange capacity, the LDH-CC coating inserted into tDEDTC can induce the AA2024-T3 alloy to indicate passivation and exhibit an active anticorrosion effect.
Wang et al. [67] used polyethersulfone and epoxy resin as the matrix resin, a double-layer coating was prepared, and a primer with high-corrosion resistance was prepared by introducing different contents of polyaniline (PANI), polytetrafluoroethylene (PTFE), and topcoat. Because PTFE has low surface energy, it can have excellent surface-shielding performance in the topcoat; in the primer, PANI can quickly form a passivation layer after contacting the corrosive medium and the metal substrate to protect the metal substrate for a second time. The results show that when the amount of PANI introduced is 40 wt% the overall performance of the coating is the best, and the use of a double-layer coating composed of 40 wt% PANI primer and PTFE topcoat is effective in adhesion and electrochemical corrosion resistance. Excellent performance in terms of salt spray performance and other aspects, with extremely high corrosion resistance, is observed.
2.3.3. Shielding Effect
Yin et al. [68] studied an environmentally friendly inorganic magnesium phosphate coating (MPC), which can effectively prevent the formation of pores between the coating and the substrate. The research results show that the coating has no obvious corrosion in a neutral NaCl salt spray for 2400 h. After immersing the MPC-modified coating in a 3.5 wt.% NaCl solution for 30 min, the electrochemical impedance is (1.8 ± 0.6) × 104 Ω·cm2. After soaking for 24 h, it drops to the lowest value of (8.2 ± 1.7) × 103 Ω·cm2, and then finally increases gradually. After soaking for 14 d, it rises to (1.9 ± 0.2) × 104 Ω·cm2. The MPC coating did not corrode after being immersed for 14 d. According to the analysis, the passivation of the MgO in the coating is an important factor affecting the corrosion performance of the coating, and the shielding effect and dissolved phosphate ions further enhance the protective effect.
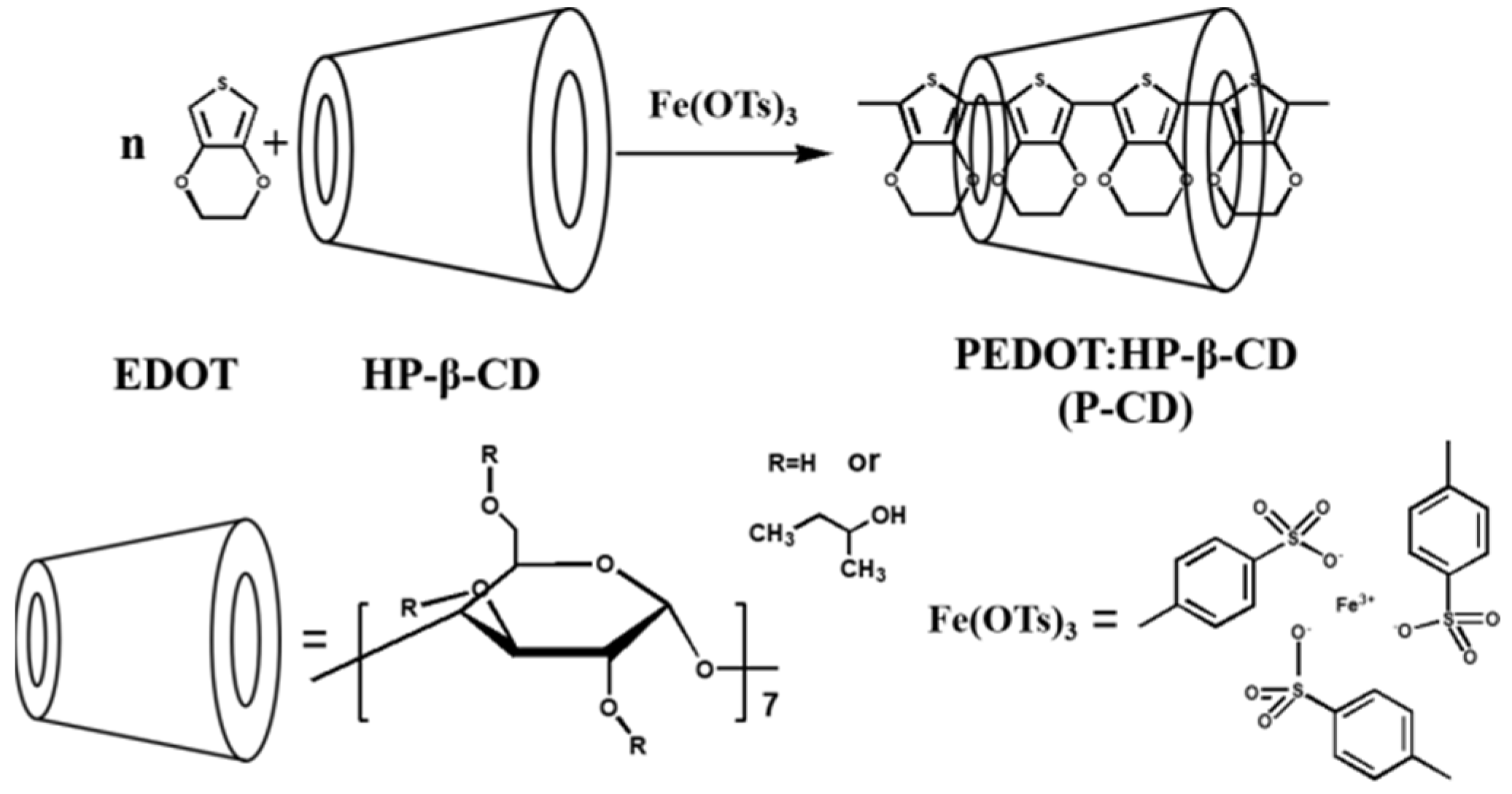
Zhang et al. [69] prepared a PEDOT-hydroxypropyl-β-cyclodextrin (P-CD)-coated composite material. PEDOT has good steric hindrance and ion exchange properties but has relatively poor compatibility. The compatibility can be improved through coating modification and it can be added as a preservative to anticorrosive coatings. The preparation process of the P-CD is shown in Figure 15. The research results show that the P-CD can be fully fused with epoxy resin, and the coating has a better anticorrosion effect by its using shielding and passivation effects. According to EIS analysis, the electrochemical impedance of the 0.5 wt.% P-CD coating is 2.20 × 109 Ω·cm2, which drops to 4.84 × 108 Ω·cm2 after immersion for 80 days, which is significantly higher than that of pure epoxy resin. P-CD with good dispersion performance can greatly improve the shielding ability of epoxy coating.
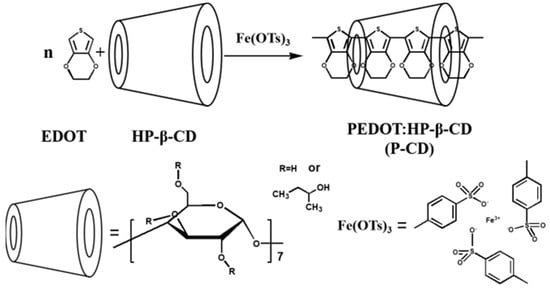
Figure 15.
Schematic diagram of the P-CD preparation process [69]. In the mixed solution of EDOT and HP-β-CD, an Fe(OTs)3 solution was dropped in to initiate the EDOT to polymerize (With permission from Ref. [69] under a Creative Commons license, ESG (www.electrochemsci.org, accessed on 11 October 2023), open access).
2.3.4. Improve Resistivity
He et al. [70] studied and prepared a siloxane-based epoxy resin (SiEP) containing benzoxazine (Ba) by blending. The study results show that SiEP resin can provide a large number of Si-O-Si bonds that increase the water contact angle, in which the optimal water contact angle is 108°. At the same time, it also solves the problem of the high brittleness and high adhesion of PBa. The corrosion current density of the modified coating decreased by 104 A·cm−2, and the resistance value increased from 955 Ω to 32,285 Ω after modifying the protective properties of the coating up to 99%.
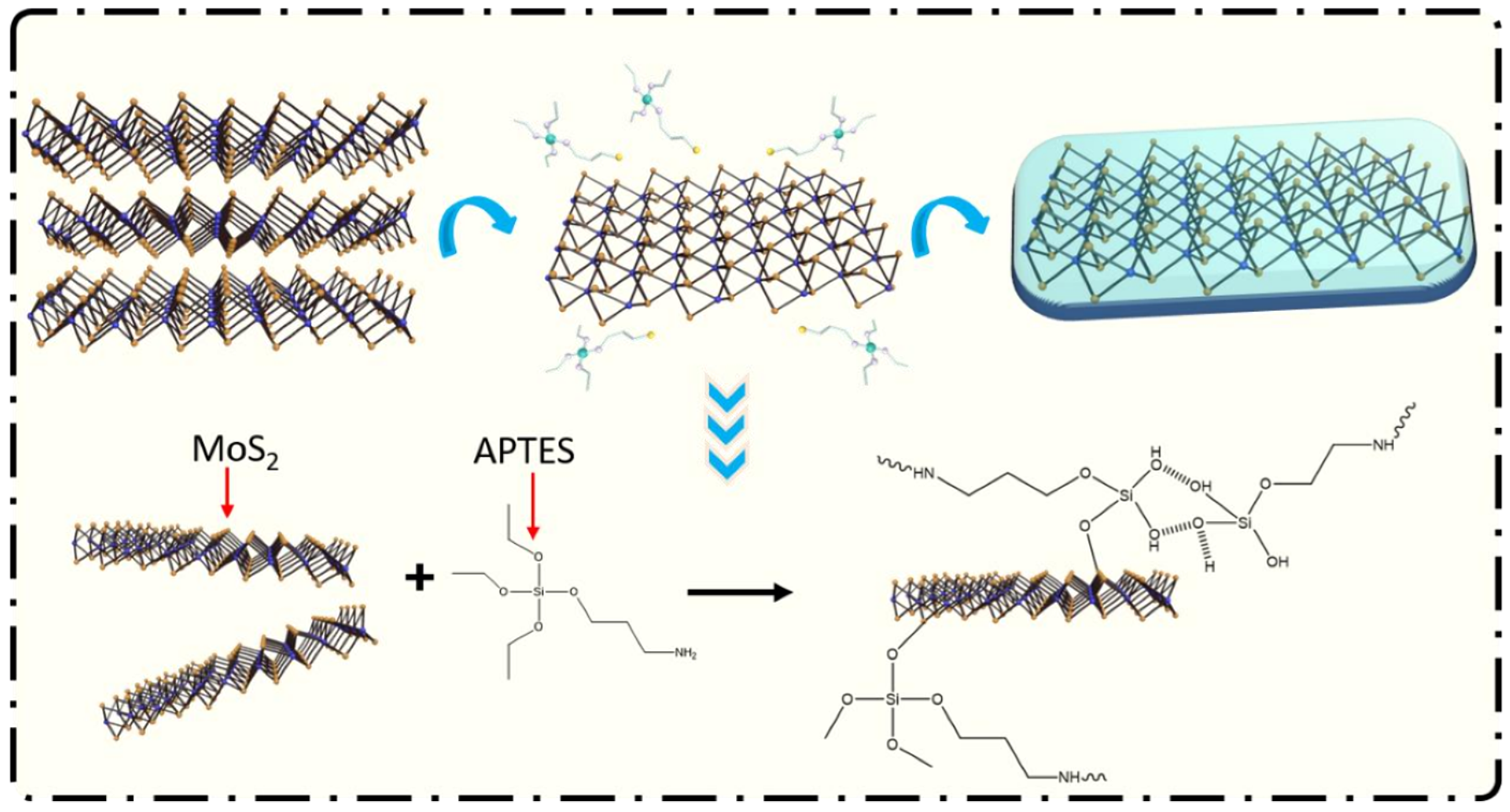
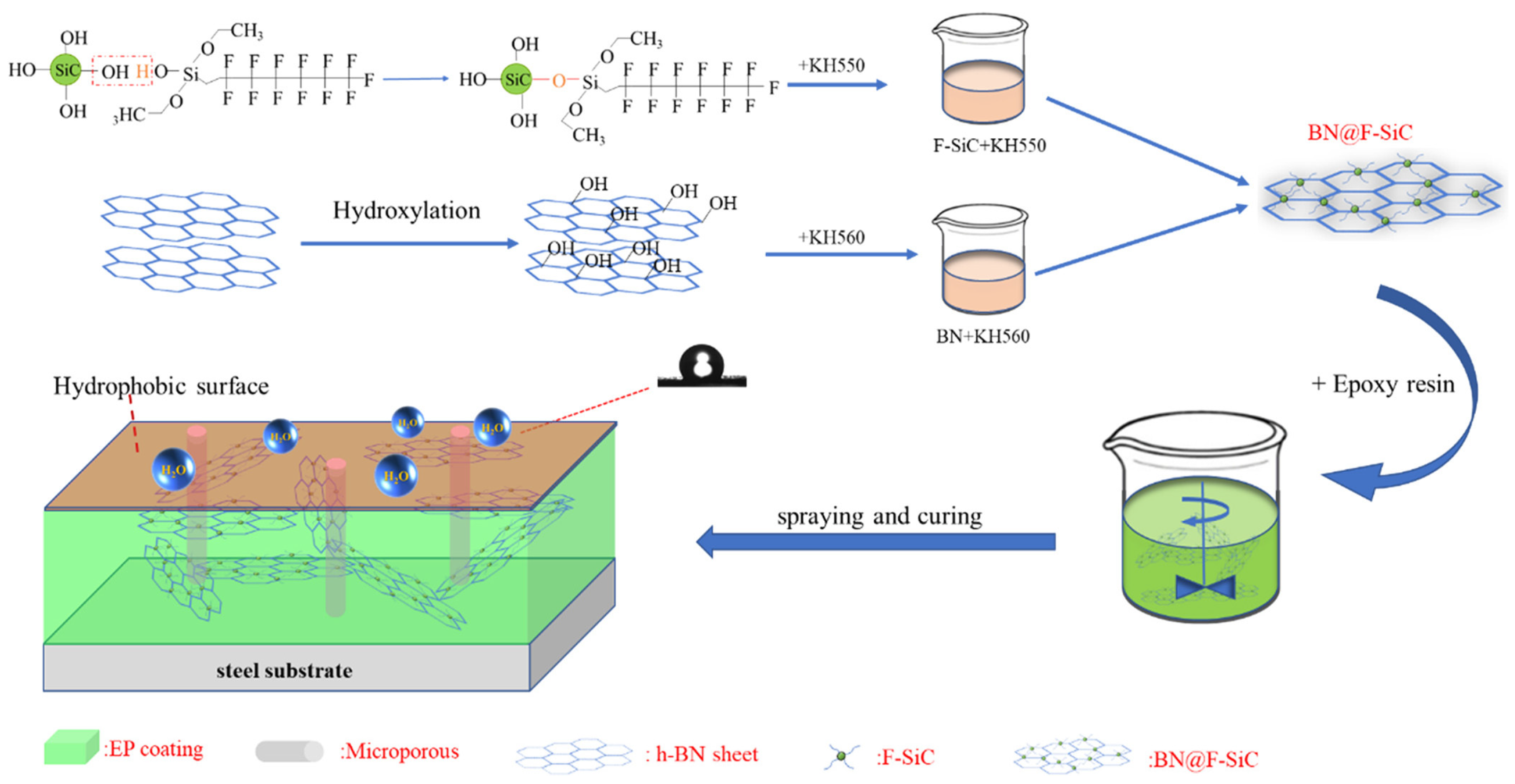
Zhang et al. [71] grafted KH550 and trifluorotrimethyltriethoxysilane (FAS) onto KH560 to prepare a BN@F-SiC composite material, dispersed the material in epoxy resin, and tested the performance of the prepared coating. The research results show that when the ratio of BN@F-SiC is 2:1, the material dispersion performance is the best, and the water contact angle of the coating reaches 118°; compared with the F-SiC coating, the friction coefficient is reduced by 89.68%. EIS analysis shows that when the BN@F-SiC(2:1)/EP coating is immersed for 400 h and the frequency is 10−2 Hz (|Z|0.01 Hz), the electrochemical impedance of the coating reaches 1010 Ω·cm2, which has excellent anticorrosion and friction resistance performance. The preparation and mechanisms of BN@F-SiC are shown in Figure 16.
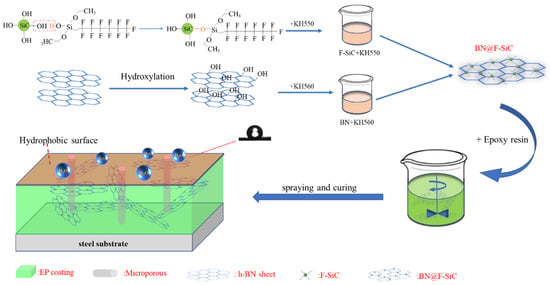
Figure 16.
Schematic diagram and mechanisms of preparation of the BN@F-SiC composite and BN@F-SiC(2:1)/EP coating [71]. Nano-SiC was grafted by γ-aminopropyl-triethoxysilane and 1H,1H,2H,2H-trifluoro-noctyltrie-thoxysilane, BN was grafted by glycidoxy-propyltrimethox-ysilane. The BN@F-SiC filler was prepared by depositing modified Nano-SiC on BN and then added into epoxy resin to prepare the anticorrosion coatings (With permission from Ref. [71], 2023, ACS).
2.3.5. Self-Repair Coating
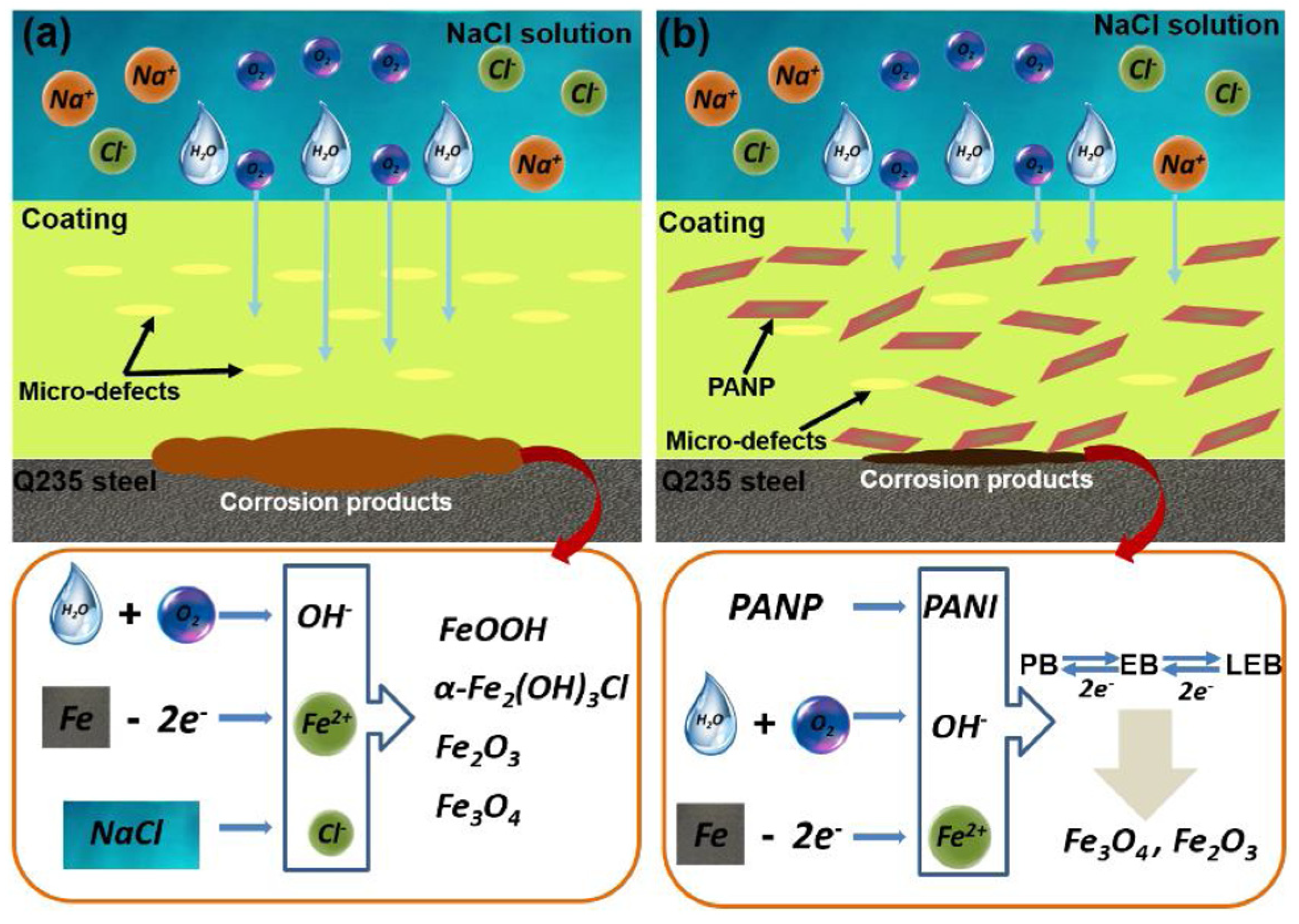
Liu et al. [72] synthesized a polyaniline-based plate (PANP) via chemical oxidation and introduced it into an epoxy resin system to study the anticorrosion performance and self-healing ability of the coating. The research results show that PANPs with good dispersion performance can have a strong shielding performance, and the electrical activity of PANPs can promote the self-repairing phenomenon of the coating. The EP coating with 1.0 wt.% PANP introduced has good anticorrosion performance. After 40 d of soaking, the water absorption rate is 2.23%. The O2 shielding effect is 7.92 × 10−15 cm3 cm cm−2s−1Pa−1. After immersion in a 3.5 wt.% NaCl solution for 40 d, the electrochemical impedance of the coating can still reach 1.94 × 109 Ω·cm2, and because of the presence of the polyaniline molecules, it is shown that a passivation layer composed of Fe2O3 and Fe3O4 is formed on the substrate, which makes the coating exhibit a self-repairing ability. The anticorrosive mechanism of the coating is shown in Figure 17.
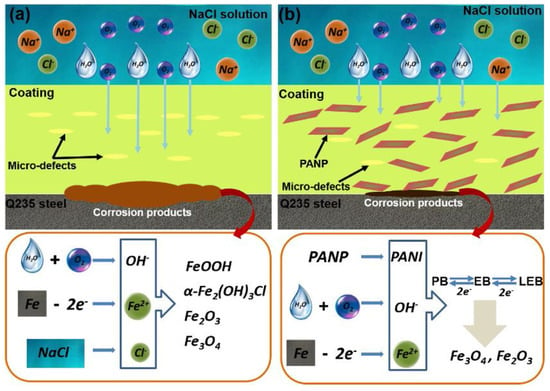
Figure 17.
Anticorrosion mechanism diagram of the EP (a) and PANP/EP (b) coatings [72]. For the pure EP coating, corrosive substances are likely to reach the surface of metal substrate because of the poor barrier effect. However, for the as-prepared PANP-doped coating, the protective effect originates from the combination of the barrier and corrosion inhibition effects. Furthermore, the redox behavior of the PANI can induce the formation of passive layers that play a key role in the corrosion protection of mild steel (With permission from Ref. [72], License number: 5645950089916, 2023, Elsevier).
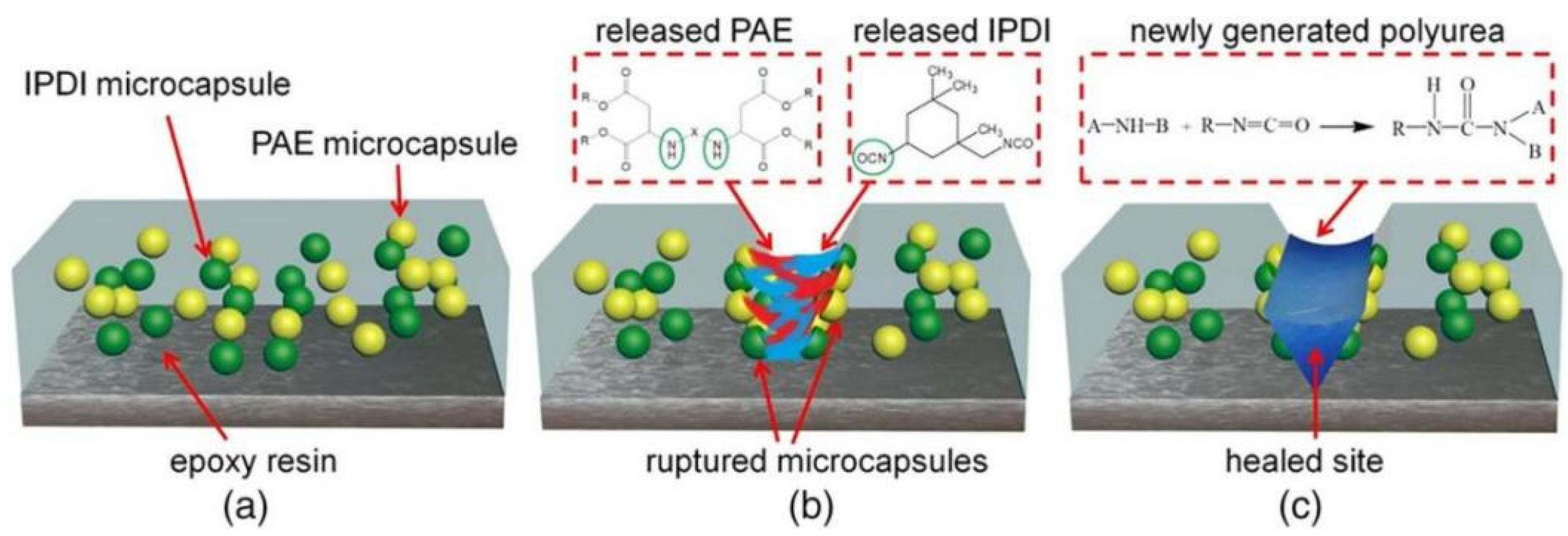
Guo et al. [73] microencapsulated polyaspartate (PAE) and diisocyanate (IPDI) through an in situ synthesis method. The diameter of the microcapsules was between 2.66–11.25 μm, and the prepared PAE-IPDI double microcapsules were introduced into the epoxy coating to study the self-repairing and anticorrosive capabilities of the microcapsules in the coating. The self-healing mechanism of the double microcapsules is shown in Figure 18. The research results show that when microdamage occurs inside the coating, the IPDI can react with the PAE to form a self-healing PU layer to prevent the further penetration of corrosive media and effectively protect the substrate. With increasing microcapsule load, the repair and anticorrosion efficiency also increases. When 15 wt.% is added, the repair rate can reach approximately 93%.
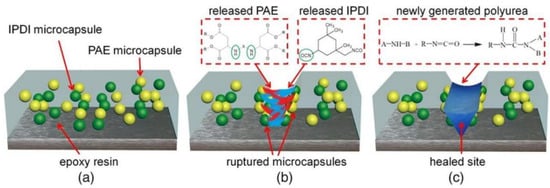
Figure 18.
Schematic diagram of the self-healing mechanism of the double microcapsules [73]. (a) First, the IPDI microcapsules and PAE microcapsules were evenly embedded into the epoxy resin matrix. (b) Upon the external force worked on epoxy coating, the formation and extension of a microcrack would result in microcapsules broken mechanically, and the IPDI and PAE released and contacted with each other on account of the capillary effect. (c) Finally, the scratched area was filled by the newly formed PU layer via the nucleophilic addition reaction of isocyanate-amine at room temperature. In this manner, the dual-component microcapsules autonomously healed their destruction and thus protected the substrate from further corroding (With permission from Ref. [73], License number: 5672360866998, 2023, Wiley).
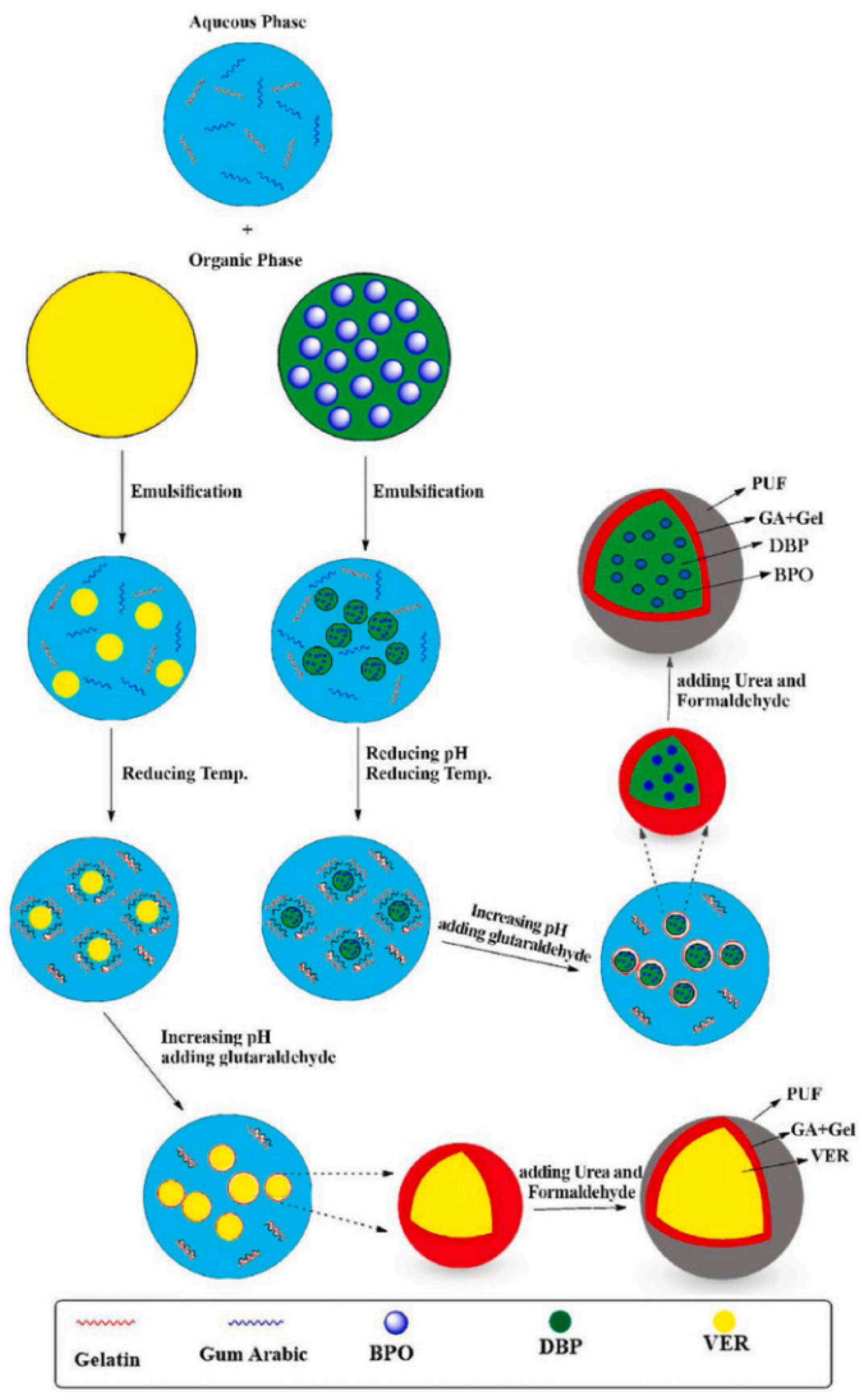
Adibzadeh et al. [20] prepared a microcapsule material filled with vinyl ester resin (VER) and benzoyl peroxide (BPO). The synthesis method of the microcapsule is shown in Figure 19, and the microcapsule is added to the epoxy resin system. The corrosion resistance and self-repair performance of the coating are studied through salt spray tests and EIS. The results of the study showed that as the number of microcapsules introduced gradually increased, the self-healing ability of the coating gradually increased. When the number of microcapsules introduced was 20 wt.%, the self-healing ability of the coating was best. After the coating was immersed in NaCl electrolyte for 336 h, the coating resistance decreased from 7.3 × 109 Ω to 1.6 × 109 Ω; the coating with a content of 20 wt.% was applied to the surface of carbon steel, exposed to 50% in the salt spray test, and corrosion occurred.
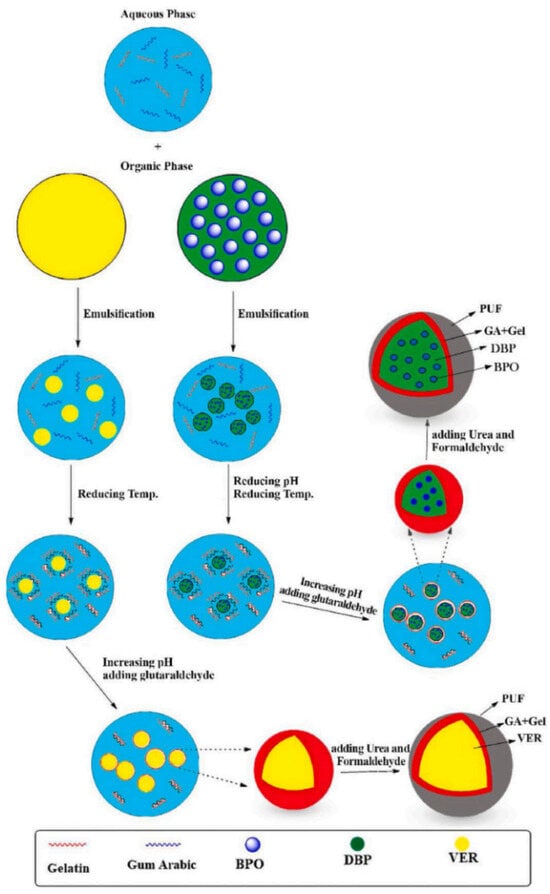
Figure 19.
Schematic diagram of the synthesis of the microcapsules containing VER or BPO [20]. The VER or BPO/DPB were added to the GA/Gel solution to form solid-in-oil-in-water. And then, the pH of the dispersion was adjusted by adding a NaOH solution. Then glutaraldehyde solution was added to the mixture to form a microcapsule (With permission from Ref. [20], License number: 5672361333700, 2023, Elsevier).
3. Summary and Outlook
As the most effective way to prevent the corrosion of metal materials, anticorrosion coatings provide a safe and reliable solution for the protection of metal materials. With the gradual increase in the amount of metal used, the use environment is gradually becoming more complicated, especially in marine facilities, high-humidity and cold environments, and chemical production. The anticorrosion performance requirements of other fields are gradually increasing. However, as research on the anticorrosion mechanism and functional new materials continues, more new anticorrosion coating products will be applied. To comprehensively improve the performance of anticorrosion coatings and manufacture new functional anticorrosion coatings, work can be carried out via the following aspects:
- (1)
- Research and develop new anticorrosive resin products. As the basis of coating products, resin performance often determines the basic performance of the coating. The preparation of functionally modified anticorrosion resin products can provide a basic guarantee for the development and production of anticorrosion coating products.
- (2)
- Develop functional anticorrosion additives. The research and development of functional anticorrosion additives gives anticorrosion coating products excellent anticorrosion performance. Research on new materials and the functional modification of existing materials has high research significance.
- (3)
- In-depth study and expansion of the corrosion mechanism. The mechanism of anticorrosion coatings mainly focuses on preventing media penetration, extending the diffusion path, electrochemical protection, metal surface passivation, and self-repair. The in-depth study of the anticorrosion mechanism, discussion of new methods, and expansion of anticorrosion methods from a theoretical level should be conducted, and direction for the research of anticorrosion coatings should be provided.
- (4)
- Take water-based anticorrosive coatings as the research focus. As the country pays increasing attention to environmental issues, solvent-based coatings have been unable to meet the requirements of an environmentally friendly society. The development of high-efficiency anticorrosive water-based and solvent-free coating products and the promotion of the “oil-to-water” transformation of the coating industry have become the main directions of the future development of the coating industry.
Author Contributions
Conceptualization, X.Z. (Xingjun Zhang), Z.M. and X.W.; investigation, X.Z. (Xingjun Zhang), Y.F., X.Z. (Xingyu Zhang) and G.L.; writing—original draft preparation, X.Z. (Xingjun Zhang); writing—review and editing, Z.M. and X.W. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge receiving financial support from the National Natural Science Foundation of China (Grant No. 52075524), the Gansu Province Science and Technology Plan (22JR5RA094), and the Natural Science Foundation of Shandong Province (ZR2023QE329).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support this study can be available upon request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cui, C.; Lim, A.T.O.; Huang, J. A cautionary note on graphene anti-corrosion coatings. Nat. Nanotechnol. 2017, 12, 834–835. [Google Scholar] [CrossRef]
- Stankiewicz, A.; Szczygieł, I.; Szczygieł, B. Self-healing coatings in anti-corrosion applications. J. Mater. Sci. 2013, 48, 8041–8051. [Google Scholar] [CrossRef]
- Yan, M.; Gelling, V.J.; Hinderliter, B.R.; Battocchi, D.; Tallman, D.E.; Bierwagen, G.P. SVET method for characterizing anti-corrosion performance of metal-rich coatings. Corros. Sci. 2010, 52, 2636–2642. [Google Scholar] [CrossRef]
- Kyhl, L.; Nielsen, S.F.; Čabo, A.G.; Cassidy, A.; Miwa, J.A.; Hornekær, L. Graphene as an anti-corrosion coating layer. Faraday Discuss. 2015, 180, 495–509. [Google Scholar] [CrossRef]
- Dastpak, A.; Yliniemi, K.; Monteiro, M.C.D.O.; Höhn, S.; Virtanen, S.; Lundström, M.; Wilson, B.P. From Waste to Valuable Resource: Lignin as a Sustainable Anti-Corrosion Coating. Coatings 2018, 8, 454. [Google Scholar] [CrossRef]
- Qian, Y.; Li, Y.; Jungwirth, S.; Seely, N.; Fang, Y.; Shi, X. Review: The Application of Anti-Corrosion Coating for Preserving the Value of Equipment Asset in Chloride-Laden Environments: A Review. Int. J. Electrochem. Sci. 2015, 10, 10756–10780. [Google Scholar] [CrossRef]
- Popoola AP, I.; Olorunniwo, O.E.; Ige, O.O. Corrosion resistance through the application of anti-corrosion coatings. Dev. Corros. Prot. 2014, 13, 241–270. [Google Scholar]
- Ding, R.; Chen, S.; Lv, J.; Zhang, W.; Zhao, X.-D.; Liu, J.; Wang, X.; Gui, T.-J.; Li, B.-J.; Tang, Y.-Z.; et al. Study on graphene modified organic anti-corrosion coatings: A comprehensive review. J. Alloys Compd. 2019, 806, 611–635. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Duan, C.Y.; Liu, H.Y.; Chen, Y.F.; Wang, Y. Graphene coating for anti-corrosion and the investigation of failure mechanism. J. Phys. D Appl. Phys. 2017, 50, 114001. [Google Scholar] [CrossRef]
- Lu, J.L.; Liu, N.J.; Wang, X.H.; Li, J.; Jing, X.B.; Wang, F.S. Mechanism and life study on polyaniline anti-corrosion coating. Synth. Met. 2003, 135, 237–238. [Google Scholar] [CrossRef]
- Ou, B.; Wang, Y.; Lu, Y. A review on fundamentals and strategy of epoxy-resin-based anticorrosive coating materials. Polym. Technol. Mater. 2020, 60, 601–625. [Google Scholar] [CrossRef]
- Han, B.; Wang, H.; Yuan, S.; Li, Y.; Zhang, X.; Lin, D.; Chen, L.; Zhu, Y. Durable and anti-corrosion superhydrophobic coating with bistratal structure prepared by ambient curing. Prog. Org. Coat. 2020, 149, 105922. [Google Scholar] [CrossRef]
- Pan, L.; Xue, P.; Wang, M.; Wang, F.; Guo, H.; Yuan, X.; Zhong, L.; Yu, J. Novel superhydrophobic carbon fiber/epoxy composites with anti-icing properties. J. Mater. Res. 2021, 36, 1695–1704. [Google Scholar] [CrossRef]
- Yu, Z.; Di, H.; Ma, Y.; He, Y.; Liang, L.; Lv, L.; Ran, X.; Pan, Y.; Luo, Z. Preparation of graphene oxide modified by titanium dioxide to enhance the anti-corrosion performance of epoxy coatings. Surf. Coat. Technol. 2015, 276, 471–478. [Google Scholar] [CrossRef]
- Böhm, S. Graphene against corrosion. Nat. Nanotechnol. 2014, 9, 741–742. [Google Scholar] [CrossRef]
- Bopp, C.; Santhanam, K. Corrosion Protection of Monel Alloy Coated with Graphene Quantum Dots Starts with a Surge. Chemengineering 2019, 3, 80. [Google Scholar] [CrossRef]
- Lei, J.; Hu, Y.; Liu, Z.; Cheng, G.J.; Zhao, K. Defects Mediated Corrosion in Graphene Coating Layer. ACS Appl. Mater. Interfaces 2017, 9, 11902–11908. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, M.; Wu, L. Synthesis of UV-Responsive Dual-Functional Microspheres for Highly Efficient Self-Healing Coatings. Chem. Eng. J. 2021, 422, 130034. [Google Scholar] [CrossRef]
- Zhang, D.; Yuan, T.; Wei, G.; Wang, H.; Gao, L.; Lin, T. Preparation of self-healing hydrophobic coating on AA6061 alloy surface and its anti-corrosion property. J. Alloys Compd. 2019, 774, 495–501. [Google Scholar] [CrossRef]
- Adibzadeh, E.; Mirabedini, S.M.; Behzadnasab, M.; Farnood, R.R. A novel two-component self-healing coating comprising vinyl ester resin-filled microcapsules with prolonged anticorrosion performance. Prog. Org. Coat. 2021, 154, 106220. [Google Scholar] [CrossRef]
- Sababi, M.; Pan, J.; Augustsson, P.-E.; Sundell, P.-E.; Claesson, P.M. Influence of polyaniline and ceria nanoparticle additives on corrosion protection of a UV-cure coating on carbon steel. Corros. Sci. 2014, 84, 189–197. [Google Scholar] [CrossRef]
- Solano, R.; Patiño-Ruiz, D.; Herrera, A. Preparation of modified paints with nano-structured additives and its potential applications. Nanomater. Nanotechnol. 2020, 10, 1847980420909188. [Google Scholar] [CrossRef]
- Gao, X.Z.; Liu, H.J.; Cheng, F.; Chen, Y. Thermoresponsive polyaniline nanoparticles: Preparation, characterization, and their potential application in waterborne anticorrosion coatings. Chem. Eng. J. 2016, 283, 682–691. [Google Scholar] [CrossRef]
- Essien, E.A.; Kavaz, D.; Ituen, E.B.; Umoren, S.A. Synthesis, characterization and anticorrosion property of olive leaves extract-titanium nanoparticles composite. J. Adhes. Sci. Technol. 2018, 32, 1773–1794. [Google Scholar] [CrossRef]
- Seyedmehdi, S.A.; Zhang, H.; Zhu, J. Superhydrophobic RTV silicone rubber insulator coatings. Appl. Surf. Sci. 2012, 258, 2972–2976. [Google Scholar] [CrossRef]
- El-Sayed, A.; Khalil, I.A.; Kogure, K.; Futaki, S.; Harashima, H. Octaarginine- and Octalysine-modified Nanoparticles Have Different Modes of Endosomal Escape. J. Biol. Chem. 2008, 283, 23450–23461. [Google Scholar] [CrossRef]
- Dhoke, S.K.; Khanna, A. Electrochemical behavior of nano-iron oxide modified alkyd based waterborne coatings. Mater. Chem. Phys. 2009, 117, 550–556. [Google Scholar] [CrossRef]
- Yuan, H.; Qi, F.; Zhao, N.; Wan, P.; Zhang, B.; Xiong, H.; Liao, B.; Ouyang, X. Graphene Oxide Decorated with Titanium Nanoparticles to Reinforce the Anti-Corrosion Performance of Epoxy Coating. Coatings 2020, 10, 129. [Google Scholar] [CrossRef]
- Yang, W.; Feng, W.; Liao, Z.; Yang, Y.; Miao, G.; Yu, B.; Pei, X. Protection of mild steel with molecular engineered epoxy nanocomposite coatings containing corrosion inhibitor functionalized nanoparticles. Surf. Coat. Technol. 2021, 406, 126639. [Google Scholar] [CrossRef]
- Huang, H.; Sheng, X.; Tian, Y.; Zhang, L.; Chen, Y.; Zhang, X. Two-Dimensional Nanomaterials for Anticorrosive Polymeric Coatings: A Review. Ind. Eng. Chem. Res. 2020, 59, 15424–15446. [Google Scholar] [CrossRef]
- Dai, Y.; Wei, W.; Zhang, J.; Anastaiia, A.; Chen, M. Effect of Si-Based Compound Nanoparticles on Anticorrosive Properties of Epoxy Resin. J. Nanosci. Nanotechnol. 2020, 20, 4961–4970. [Google Scholar] [CrossRef]
- Xu, Y.; Gao, D.; Dong, Q.; Li, M.; Liu, A.; Wang, X.; Wang, S.; Liu, Q. Anticorrosive behavior of epoxy coating modified with hydrophobic nano-silica on phosphatized carbon steel. Prog. Org. Coat. 2021, 151, 106051. [Google Scholar] [CrossRef]
- Shafaamri, A.; Cheng, C.H.; Ma, I.A.W.; Baig, S.B.; Kasi, R.; Subramaniam, R.; Balakrishnan, V. Effects of TiO2 Nanoparticles on the Overall Performance and Corrosion Protection Ability of Neat Epoxy and PDMS Modified Epoxy Coating Systems. Front. Mater. 2020, 6, 336. [Google Scholar] [CrossRef]
- Mo, C.; Zheng, Y.; Wang, F.; Mo, Q. A Simple Process for Fabricating Organic/TiO2 Super-Hydrophobic and Anti-Corrosion Coating. Int. J. Electrochem. Sci. 2015, 10, 7380–7391. [Google Scholar] [CrossRef]
- Zhong, B.; Shen, L.; Zhang, X.; Li, C.; Bao, N. Reduced graphene oxide/silica nanocomposite-reinforced anticorrosive fluorocarbon coating. J. Appl. Polym. Sci. 2021, 138, 49689. [Google Scholar] [CrossRef]
- Jiang, W.; Jin, X.; Li, H.; Zhang, S.; Zhou, T.; Xie, H. Modification of nano-hybrid silicon acrylic resin with anticorrosion and hydrophobic properties. Polym. Test. 2020, 82, 106287. [Google Scholar] [CrossRef]
- Osipchik, V.S.; Kostromina, N.V.; Kravchenko, T.P.; Mezhuev, Y.O. Development of Corrosion-Resistant Materials Using ED-20 Epoxy Resin Modified with Viniflex. Polym. Sci. Ser. D 2021, 14, 205–207. [Google Scholar] [CrossRef]
- Ding, J.; Zhao, H.; Yu, H. Epidermis microstructure inspired mica-based coatings for smart corrosion protection. Prog. Org. Coat. 2021, 152, 106126. [Google Scholar] [CrossRef]
- Pan, D.; Zhang, X.; Yang, G.; Shang, Y.; Su, F.; Hu, Q.; Patil, R.R.; Liu, H.; Liu, C.; Guo, Z. Thermally Conductive Anticorrosive Epoxy Nanocomposites with Tannic Acid-Modified Boron Nitride Nanosheets. Ind. Eng. Chem. Res. 2020, 59, 20371–20381. [Google Scholar] [CrossRef]
- Ouyang, D.-D.; Hu, L.-B.; Wang, G.; Dai, B.; Yu, F.; Zhang, L.-L. A review of biomass-derived graphene and graphene-like carbons for electrochemical energy storage and conversion. New Carbon Mater. 2021, 36, 350–372. [Google Scholar] [CrossRef]
- Chang, C.-H.; Huang, T.-C.; Peng, C.-W.; Yeh, T.-C.; Lu, H.-I.; Hung, W.-I.; Weng, C.-J.; Yang, T.-I.; Yeh, J.-M. Novel anticorrosion coatings prepared from polyaniline/graphene composites. Carbon 2012, 50, 5044–5051. [Google Scholar] [CrossRef]
- Cui, M.; Ren, S.; Zhao, H.; Xue, Q.; Wang, L. Polydopamine coated graphene oxide for anticorrosive reinforcement of water-borne epoxy coating. Chem. Eng. J. 2018, 335, 255–266. [Google Scholar] [CrossRef]
- Xiaogen, F.A.; Si, W.U.; Huixia, L.I.; Yuzheng, X.I.; Shuxian, S.H. Research progress of dispersion modification and anticorrosion mechanism of graphene and its derivatives in coatings. Acta Mater. Compos. Sin. 2021, 38, 2383–2395. [Google Scholar]
- Cui, G.; Bi, Z.; Zhang, R.; Liu, J.; Yu, X.; Li, Z. A comprehensive review on graphene-based anti-corrosive coatings. Chem. Eng. J. 2019, 373, 104–121. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Z.; Zhang, L.; Ma, J.; Sun, D.; Zhang, D.; Liu, J.; Bai, H.; Wang, B. Preparation of polyvinylpyrrolidone/graphene oxide/epoxy resin composite coatings and the study on their anticorrosion performance. J. Appl. Polym. Sci. 2021, 138, 50596. [Google Scholar] [CrossRef]
- Zhu, X.; Zhao, H.; Wang, L.; Xue, Q. Bioinspired ultrathin graphene nanosheets sandwiched between epoxy layers for high performance of anticorrosion coatings. Chem. Eng. J. 2021, 410, 128301. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, Q.; Zhang, C.; Chi, M.; Zhang, X. Preparation and study of graphite nanoplatelets/waterborne acrylate composite anticorrosive coating. J. Mater. Sci. Mater. Electron. 2021, 32, 6228–6238. [Google Scholar] [CrossRef]
- Li, H.; Xue, C.; Gao, L.; Wang, X.; Wei, H.; Nan, H.; Wang, G.; Lin, H. “Labyrinthine structure” anticorrosive water-based composite coatings. Prog. Org. Coat. 2021, 150, 105974. [Google Scholar] [CrossRef]
- Shen, L.; Zhao, W.; Miao, L. Designed a novel EP + GO/ZRC + GO coating with bilayered structure for enhancing corrosion resistance of steel substrate. J. Hazard. Mater. 2021, 403, 123670. [Google Scholar] [CrossRef]
- Shi, H.; Liu, W.; Xie, Y.; Yang, M.; Liu, C.; Zhang, F.; Wang, S.; Liang, L.; Pi, K. Synthesis of carboxymethyl chitosan-functionalized graphene nanomaterial for anticorrosive reinforcement of waterborne epoxy coating. Carbohydr. Polym. 2021, 252, 117249. [Google Scholar] [CrossRef]
- Vu, C.M.; Bach, Q.V. Oxidized multiwall carbon nanotubes filled epoxy-based coating: Fabrication, anticorrosive, and mechanical characteristics. Polym. Bull. 2021, 78, 2329–2339. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, A. Study on the synthesis of PANI/CNT nanocomposite and its anticorrosion mechanism in waterborne coatings. Prog. Org. Coat. 2021, 159, 106447. [Google Scholar] [CrossRef]
- Nayak, S.R.; Mohana, K.N.S.; Hegde, M.B.; Rajitha, K.; Madhusudhana, A.M.; Naik, S.R. Functionalized multi-walled carbon nanotube/polyindole incorporated epoxy: An effective anti-corrosion coating material for mild steel. J. Alloys Compd. 2021, 856, 158057. [Google Scholar] [CrossRef]
- Cui, X.; Zhu, G.; Pan, Y.; Shao, Q.; Zhao, C.; Dong, M.; Zhang, Y.; Guo, Z. Polydimethylsiloxane-titania nanocomposite coating: Fabrication and corrosion resistance. Polymer 2018, 138, 203–210. [Google Scholar] [CrossRef]
- Leyland, A.; Matthews, A. On the significance of the H/E ratio in wear control: A nanocomposite coating approach to optimised tribological behaviour. Wear 2000, 246, 1–11. [Google Scholar] [CrossRef]
- Dastjerdi, R.; Montazer, M.; Shahsavan, S. A novel technique for producing durable multifunctional textiles using nanocomposite coating. Colloids Surf. B Biointerfaces 2010, 81, 32–41. [Google Scholar] [CrossRef]
- Dong, M.; Li, Q.; Liu, H.; Liu, C.; Wujcik, E.K.; Shao, Q.; Ding, T.; Mai, X.; Shen, C.; Guo, Z. Thermoplastic polyurethane-carbon black nanocomposite coating: Fabrication and solid particle erosion resistance. Polymer 2018, 158, 381–390. [Google Scholar] [CrossRef]
- Ma, I.A.W.; Sh, A.; Ramesh, K.; Vengadaesvaran, B.; Ramesh, S.; Arof, A.K. Anticorrosion properties of epoxy-nanochitosan nanocomposite coating. Prog. Org. Coat. 2017, 113, 74–81. [Google Scholar]
- Amiri, S.; Rahimi, A. Hybrid nanocomposite coating by sol–gel method: A review. Iran. Polym. J. 2016, 25, 559–577. [Google Scholar] [CrossRef]
- Lakshmi, R.; Bharathidasan, T.; Bera, P.; Basu, B.J. Fabrication of superhydrophobic and oleophobic sol–gel nanocomposite coating. Surf. Coat. Technol. 2012, 206, 3888–3894. [Google Scholar] [CrossRef]
- Luo, X.; Mather, P.T. Shape Memory Assisted Self-Healing Coating. ACS Macro Lett. 2013, 2, 152–156. [Google Scholar] [CrossRef]
- Zhang, F.; Ju, P.; Pan, M.; Zhang, D.; Huang, Y.; Li, G.; Li, X. Self-healing mechanisms in smart protective coatings: A review. Corros. Sci. 2018, 144, 74–88. [Google Scholar] [CrossRef]
- Das, P.; Sharma, N.; Puzari, A.; Kakati, D.K.; Devi, N. Synthesis and characterization of neem (Azadirachta indica) seed oil-based alkyd resins for efficient anticorrosive coating application. Polym. Bull. 2021, 78, 457–479. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; He, Y.; Chen, C.; Zhang, C.; Xie, P.; Zhong, F.; Li, H.; Chen, J.; Li, Z. APTES Modification of Molybdenum Disulfide to Improve the Corrosion Resistance of Waterborne Epoxy Coating. Coatings 2021, 11, 178. [Google Scholar] [CrossRef]
- Sumi, V.; Arunima, S.; Deepa, M.; Sha, M.A.; Riyas, A.; Meera, M.; Saji, V.S.; Shibli, S. PANI-Fe2O3 composite for enhancement of active life of alkyd resin coating for corrosion protection of steel. Mater. Chem. Phys. 2020, 247, 122881. [Google Scholar] [CrossRef]
- Mohammadi, I.; Shahrabi, T.; Mahdavian, M.; Izadi, M. Chemical modification of LDH conversion coating with diethyldithiocarbamate as a novel anti-corrosive film for AA2024-T3. J. Ind. Eng. Chem. 2021, 95, 134–147. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, S.; Yao, H.; Di, Y.; Shu, M.; Li, J.; Zhang, B.; Cao, G.; Guan, S. Positive effect of the addition of polyaniline on the anticorrosive property of polyethersulfone two-layer composite coating. J. Appl. Polym. Sci. 2021, 138, 50758. [Google Scholar] [CrossRef]
- Yin, S.; Yang, H.; Dong, Y.; Qu, C.; Liu, J.; Guo, T.; Duan, K. Environmentally favorable magnesium phosphate anti-corrosive coating on carbon steel and protective mechanisms. Sci. Rep. 2021, 11, 197. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, L.; Qiu, S.; Zhao, H. PEDOT-hydroxypropyl-β-cyclodextrin Inclusion Complex as Additive for Epoxy Coating with Enhanced Anticorrosion Performance. Int. J. Electrochem. Sci. 2021, 16, 210443. [Google Scholar] [CrossRef]
- Sili, H.; Yuntao, L.; Chunxia, Z.; Jiaojiao, W.; Hui, L.; Dong, X. Advanced anticorrosion coatings prepared from polybenzoxazine/siloxane-containing epoxy resin. Polym. Eng. Sci. 2020, 60, 1812–1821. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, H.; Qi, F.; Zhao, N.; Zhang, B.; Ouyang, X. Functionalized Modified BN@ F-SiC Particle-Incorporating Epoxy: An Effective Hydrophobic Antiwear and Anticorrosion Coating Material. Ind. Eng. Chem. Res. 2021, 60, 8430–8441. [Google Scholar] [CrossRef]
- Liu, T.; Wei, J.; Ma, L.; Liu, S.; Zhang, D.; Zhao, H. Effect of polyaniline-based plate on the anticorrosion performance of epoxy coating. Prog. Org. Coat. 2021, 151, 106109. [Google Scholar] [CrossRef]
- Guo, M.; He, Y.; Wang, J.; Zhang, X.; Li, W. Microencapsulation of oil soluble polyaspartic acid ester and isophorone diisocyanate and their application in self-healing anticorrosive epoxy resin. J. Appl. Polym. Sci. 2020, 137, 48478. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).