Novel Photocatalyst Ag/ZnO/BC Nanofilms Degradation of Low Concentration Ammonia Nitrogen Wastewater
Abstract
:1. Introduction
2. Experiment
2.1. Reagents
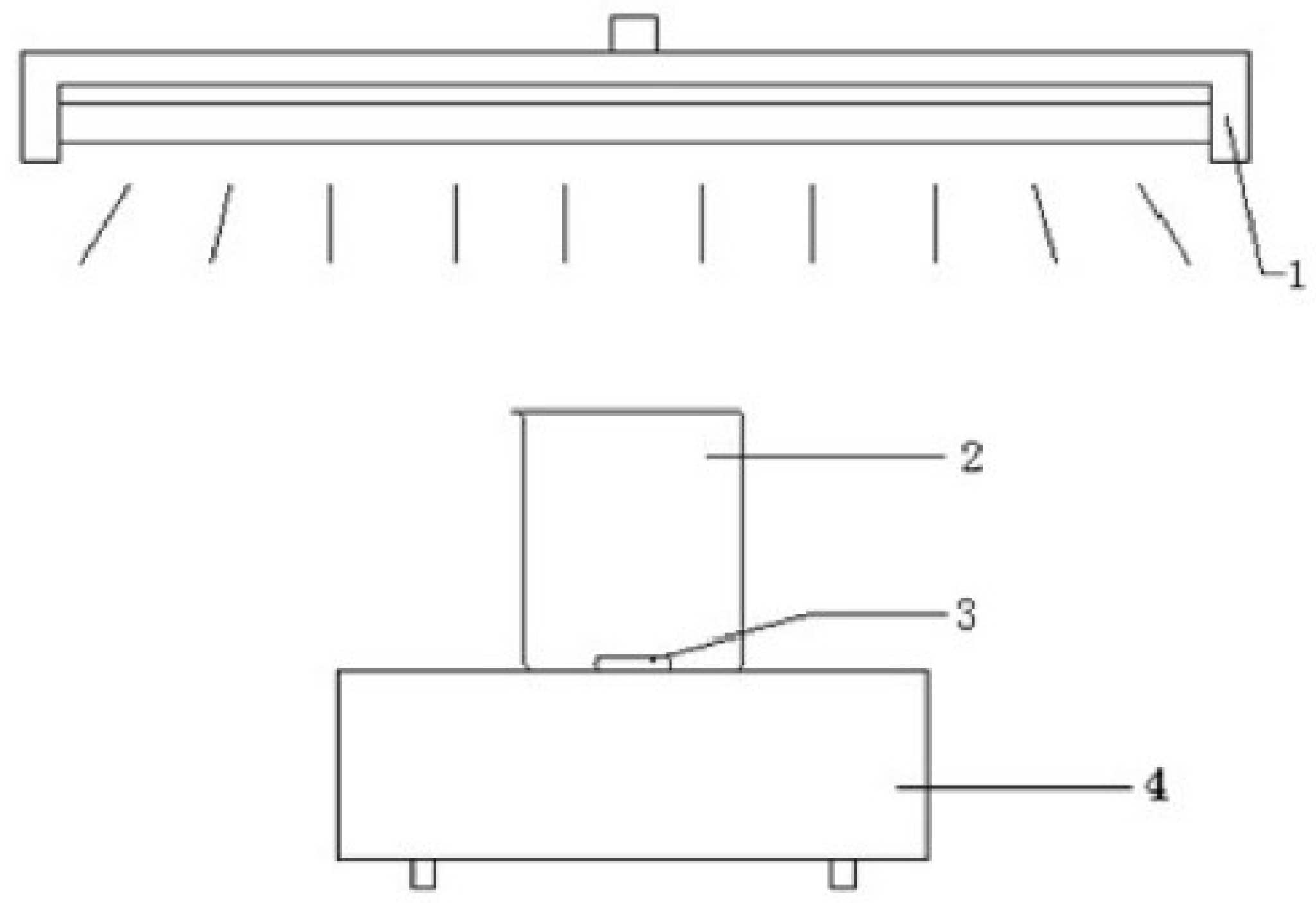
2.2. Experimental Setup
2.3. Preparation of ZnO and Ag/ZnO materials
2.4. Preparation of Ag/ZnO/BC Nanofilm composites
2.5. Sample Characterisation
2.6. Photocatalytic Activity Test
3. Results and Discussion
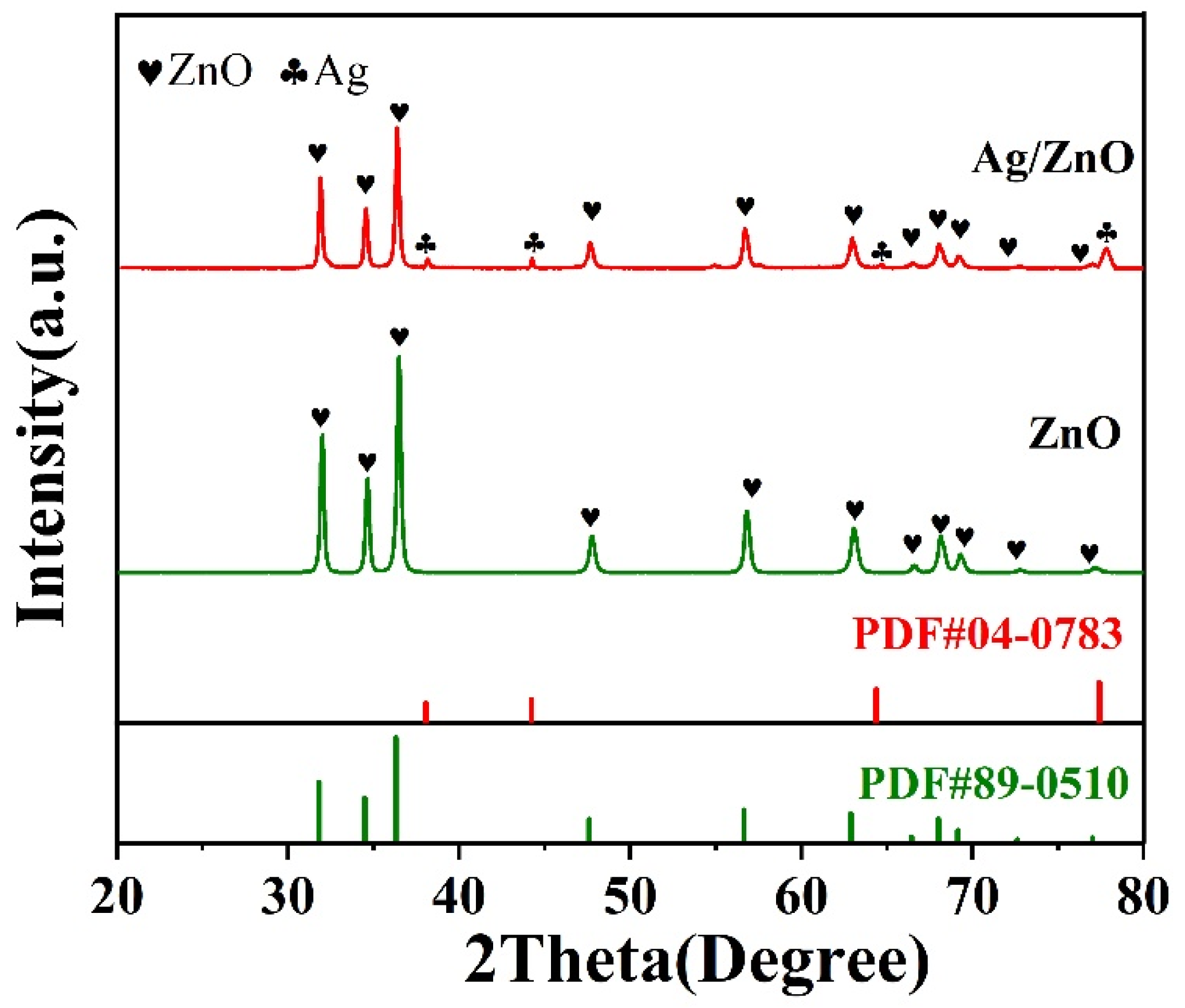
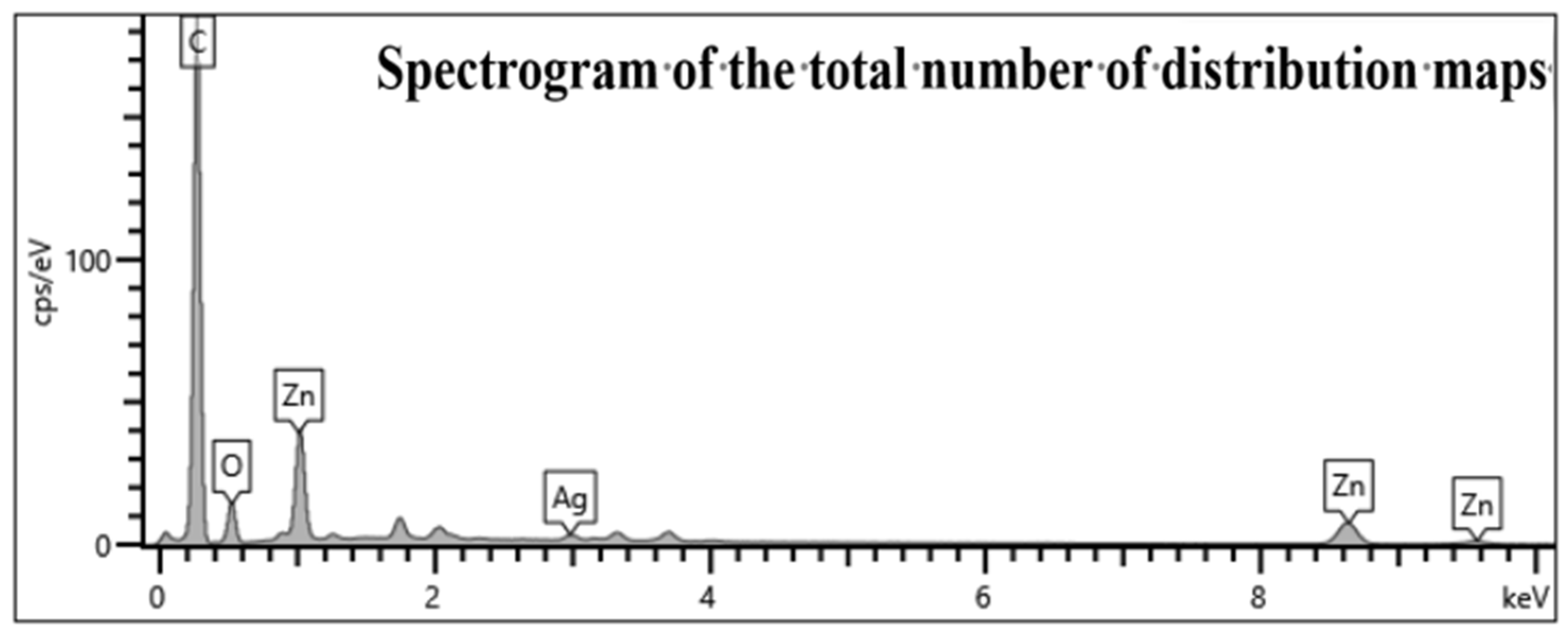
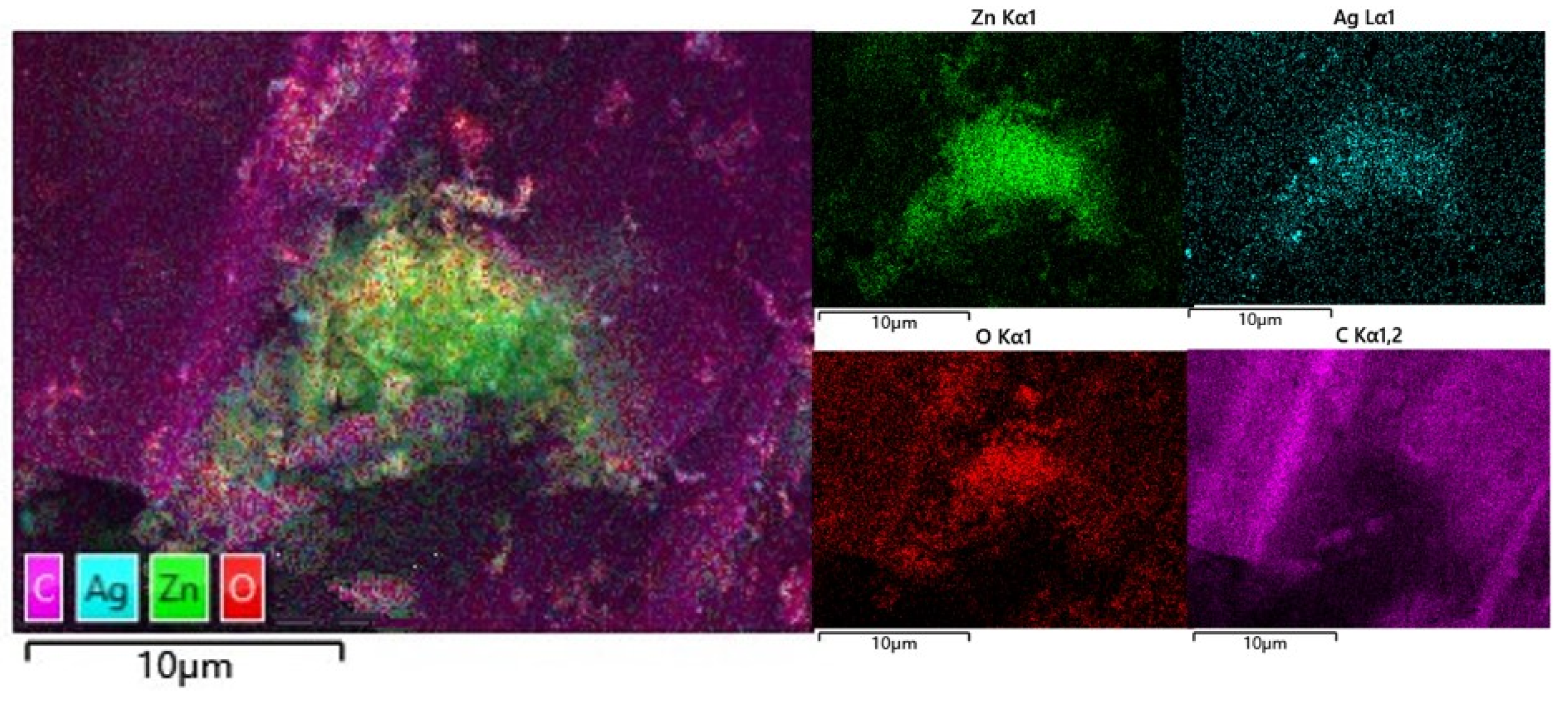
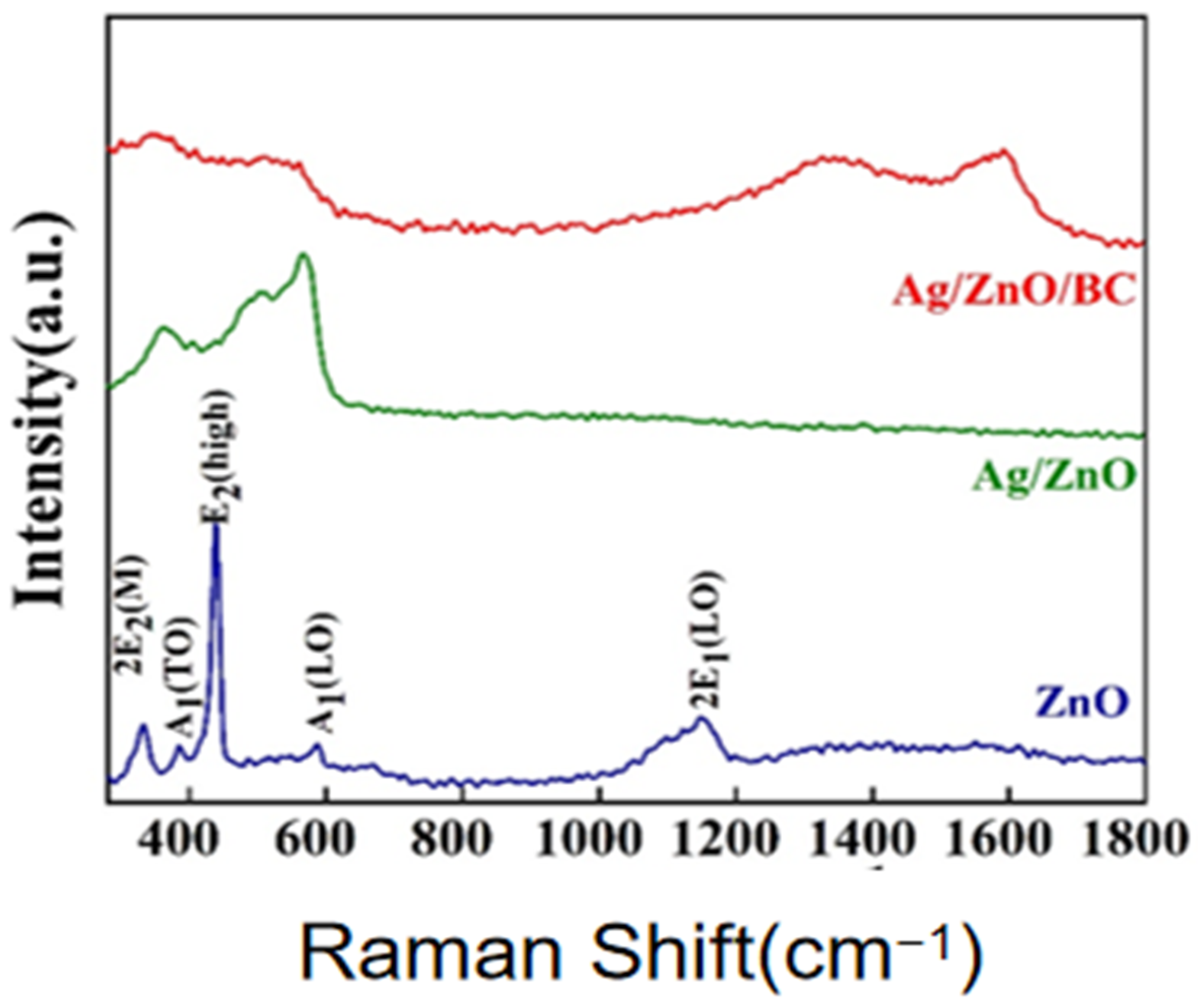
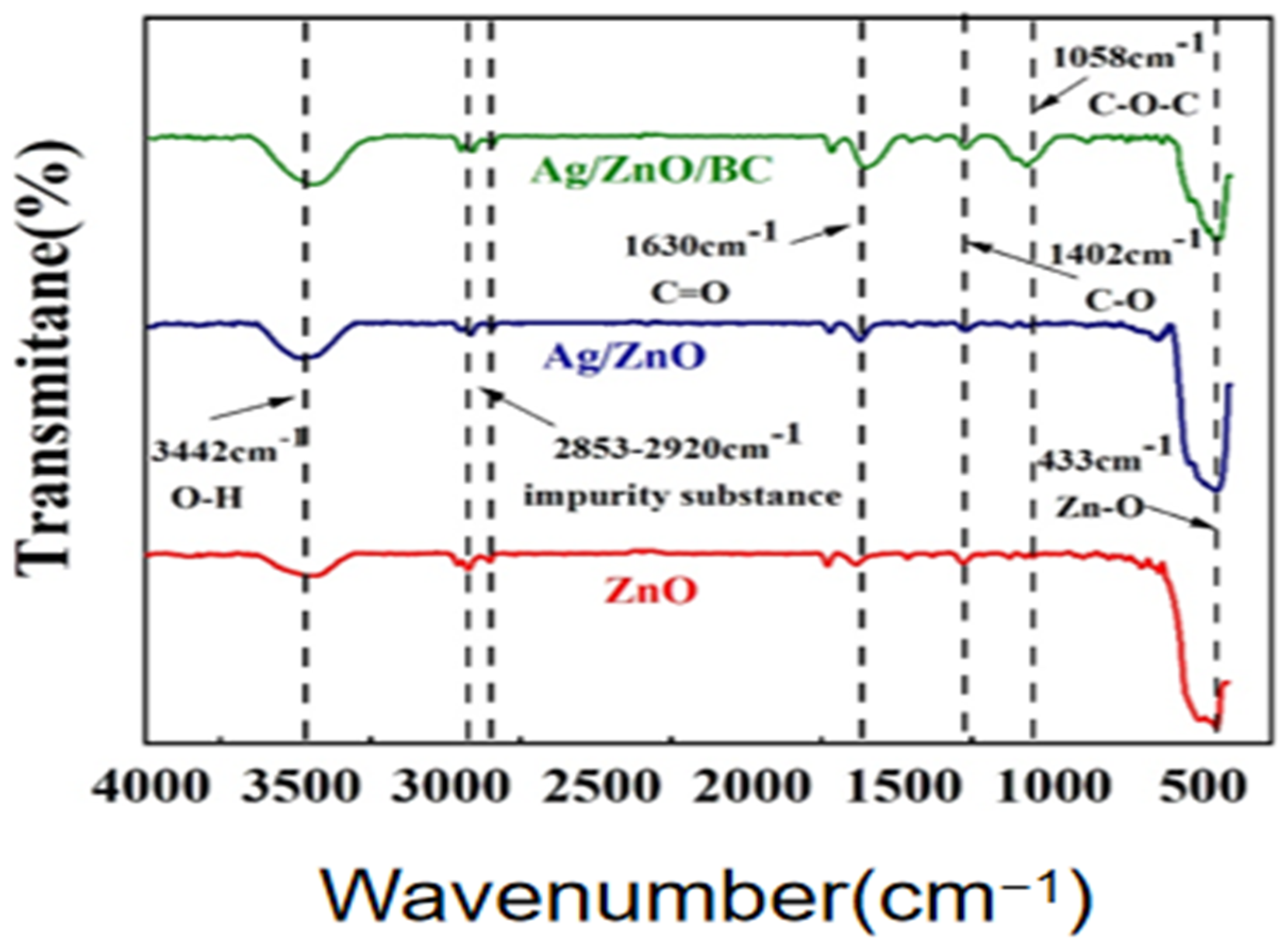
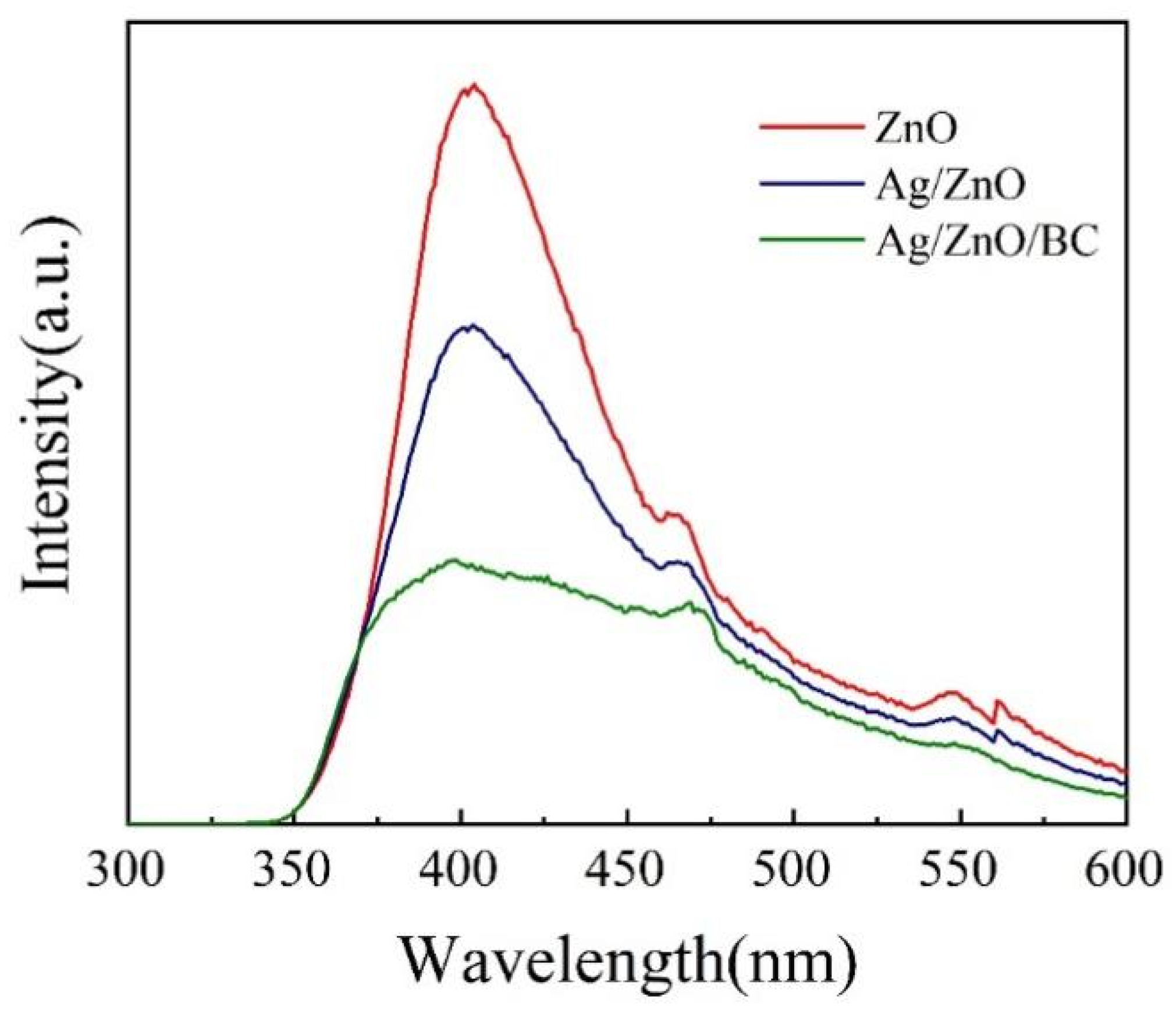
3.1. Sample Characterisation Analysis
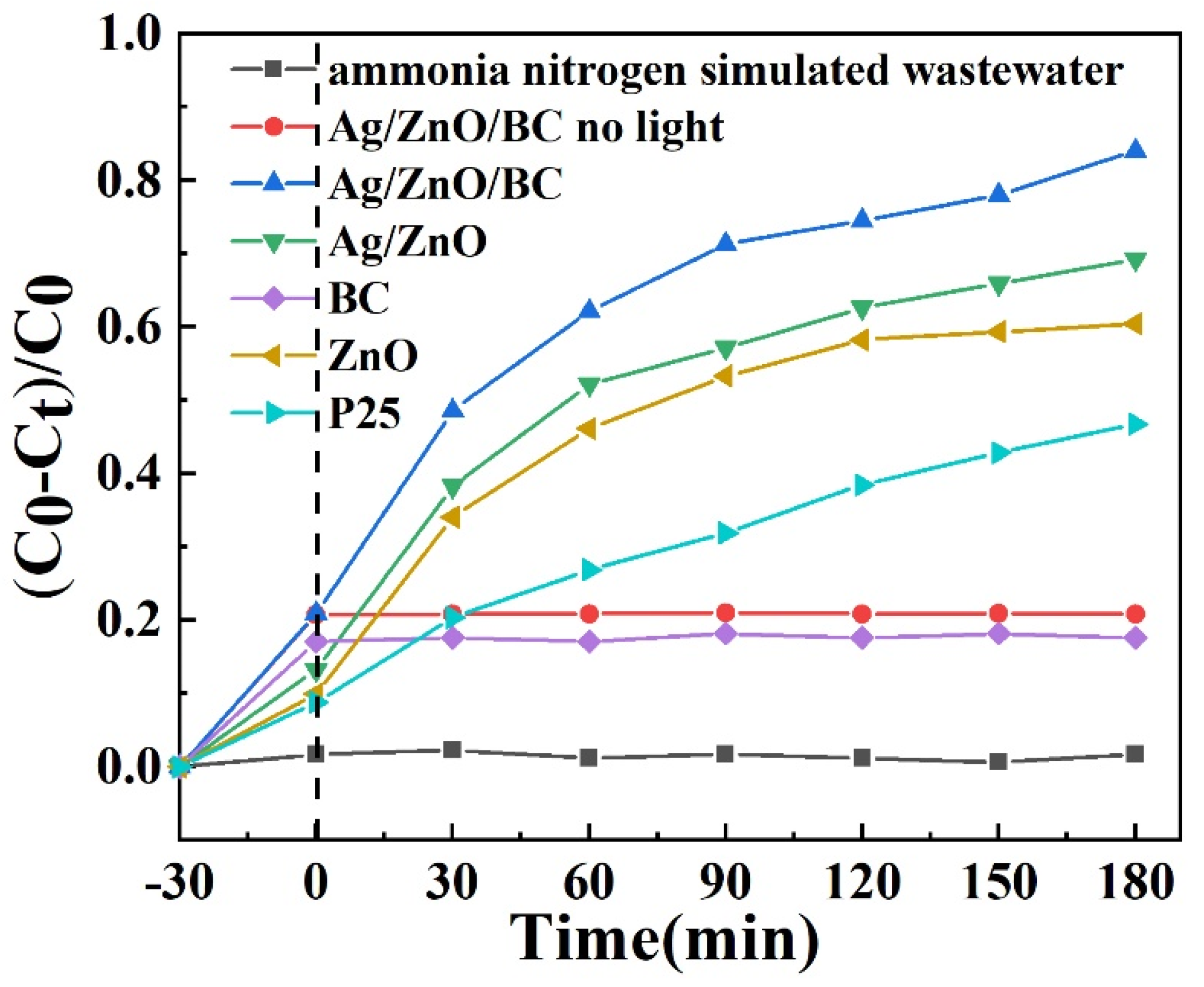
3.2. Photocatalytic Performance Tests
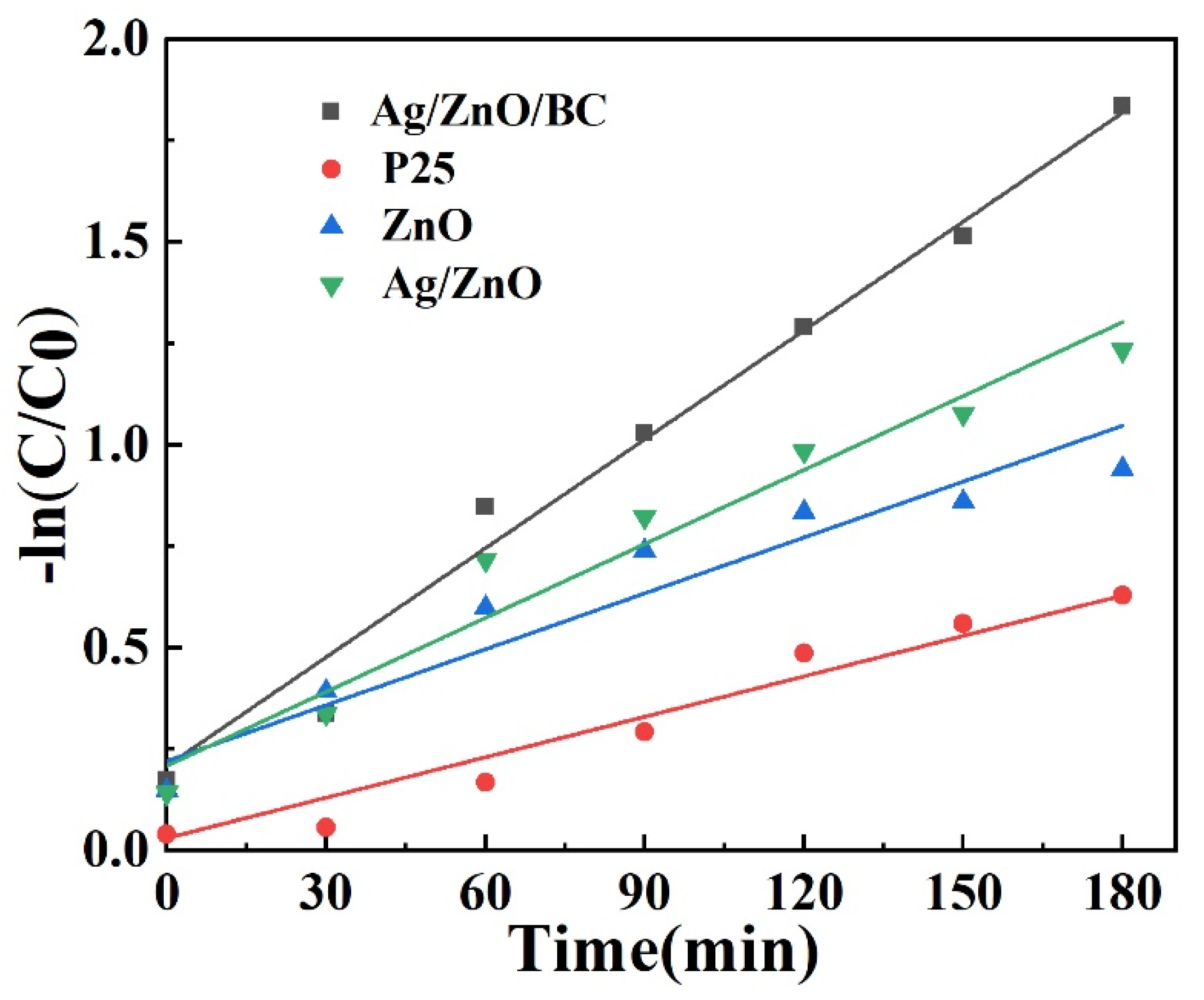
3.3. Kinetic Analysis
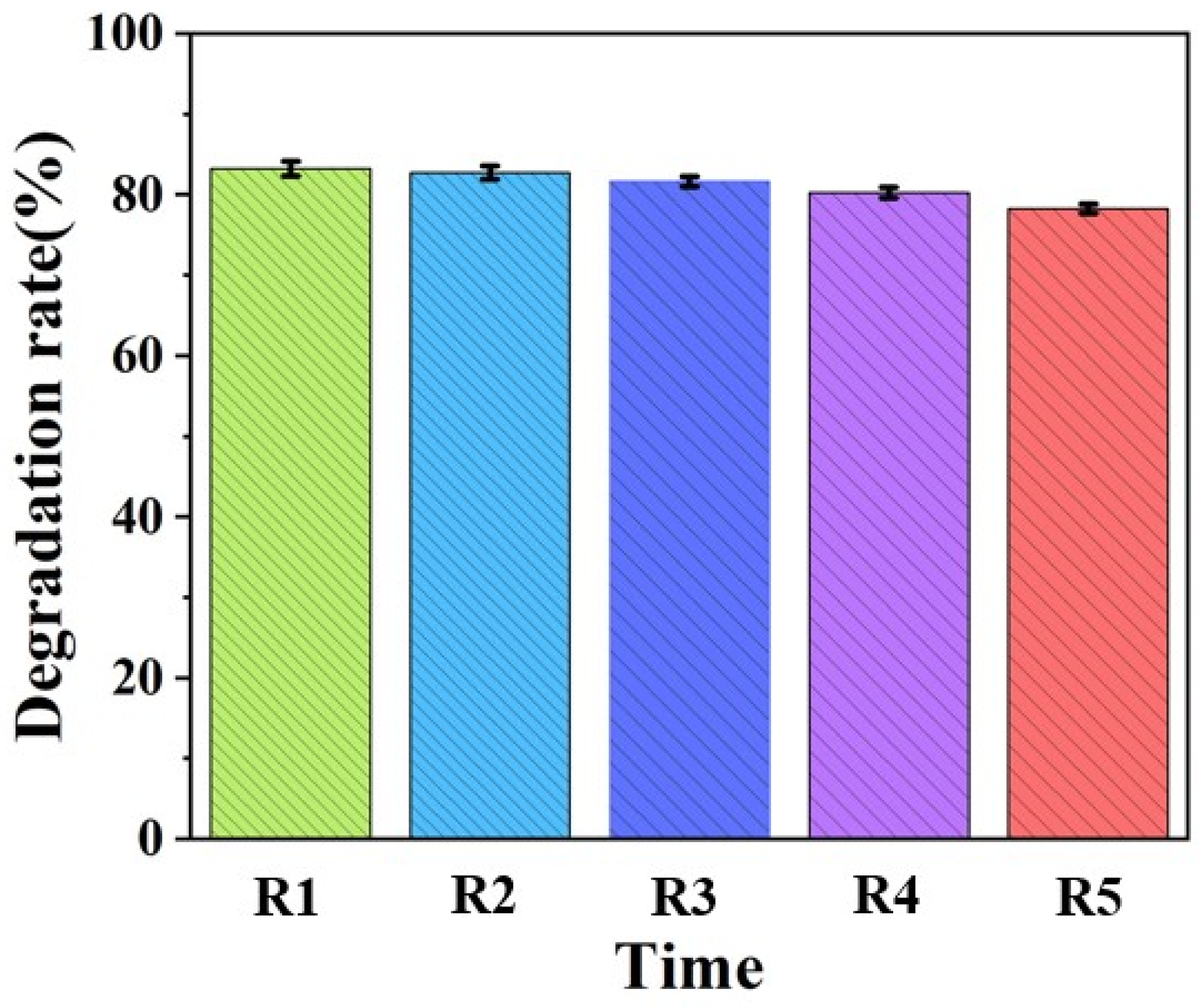
3.4. Photocatalytic Stability Analysis
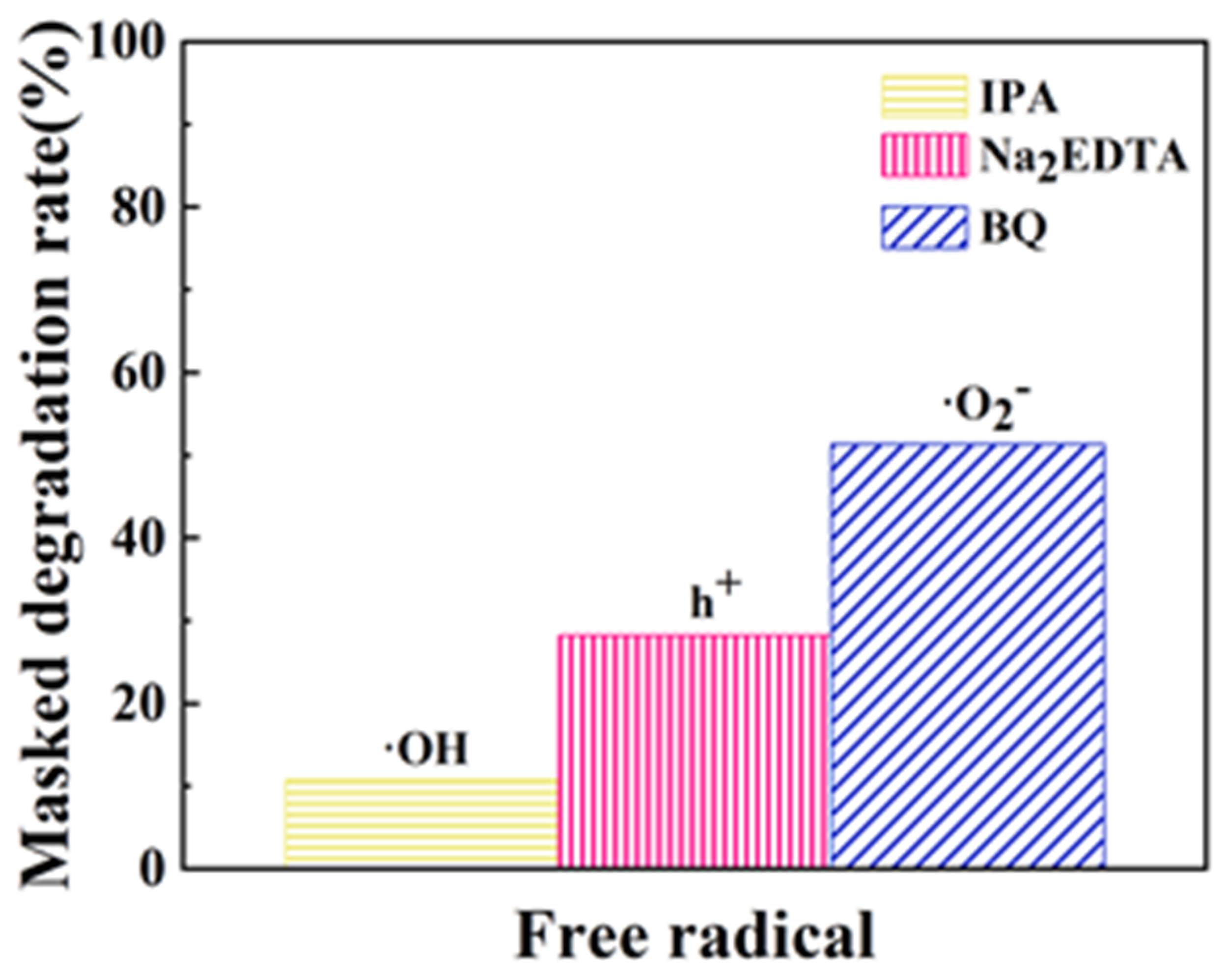
3.5. Free Radical Masking Experiments
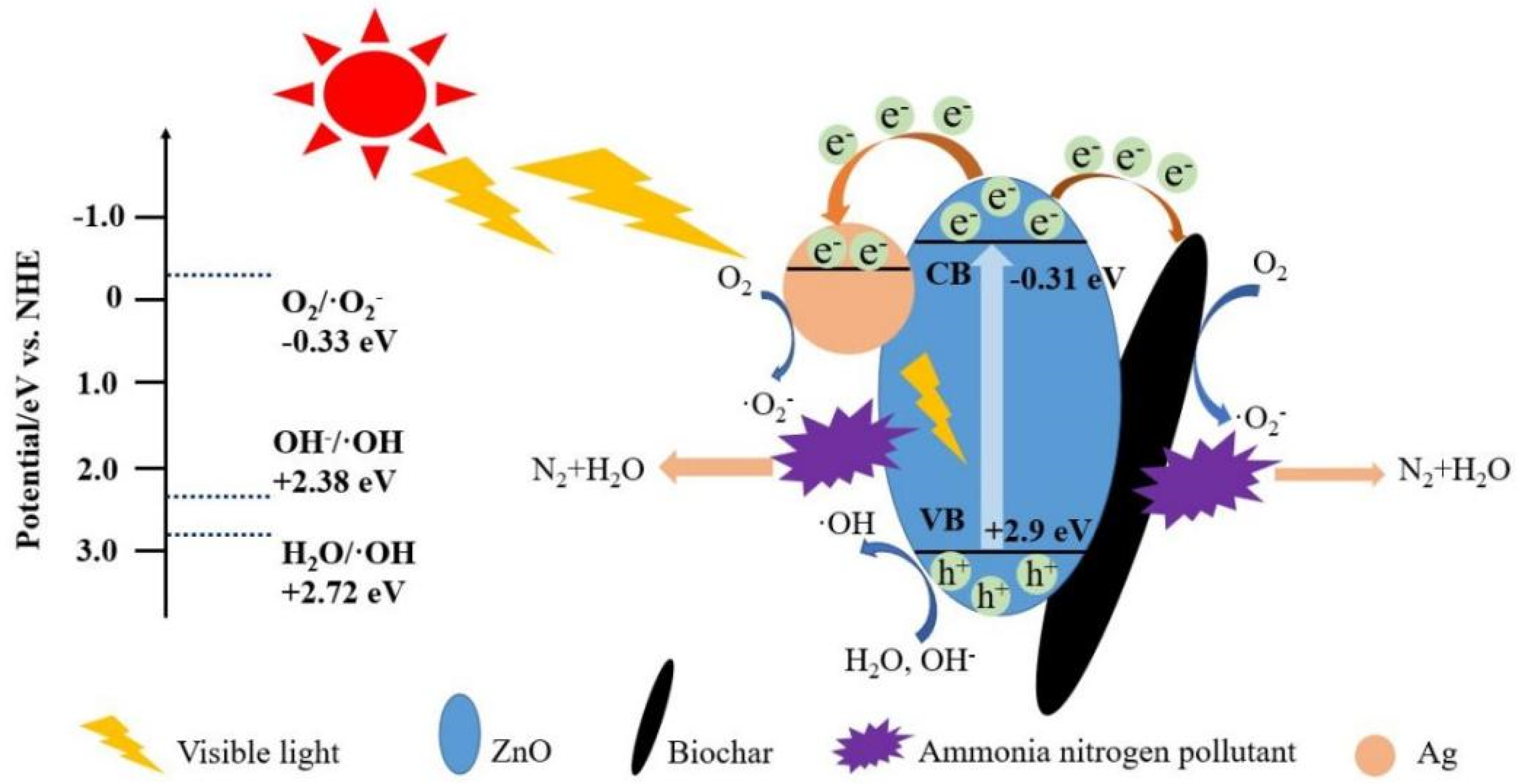
3.6. Analysis of the Photocatalytic Mechanism
4. Conclusions
- (1)
- Characterization results substantiate that Ag/ZnO nanocomposites are effectively anchored onto the biochar nanofilms. The introduction of biochar increased the specific surface area of Ag/ZnO/BC nanomembrane composite, thereby improving the adsorption performance, accelerating the adsorption of ammonia nitrogen on its surface, increasing the contact between active substances and pollutant molecules, and improving the catalytic activity. Under the condition of photoactivation, the degradation rate of ammonia nitrogen pollutants reached 84.03% within 180 min after the reaction Consequently, the utilization of biochar as a substrate for catalyst loading not only augments photocatalytic performance, but also facilitates catalyst recovery.
- (2)
- The photocatalytic stability experiment showed that the catalyst had a very high stability and still had a very high catalytic efficiency after four cycles, and the catalytic efficiency could still reach 78.3%.
- (3)
- Results from these masking experiments unequivocally indicate that superoxide anions (·O2) serve as the most influential reactive species in the photocatalytic mechanisms of the Ag/ZnO/BC nanofilm composite. This is followed in significance by photogenerated holes (h+), while hydroxyl radicals (·OH) exerted the least impact on the composite’s photocatalytic activity.
- (4)
- This methodology thereby opens up a novel avenue for the synthesis of loaded composite photocatalysts and expands their potential applications, particularly in the removal of ammonia and nitrogenous pollutants from wastewater.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quartaroli, L.; Silva, C.M.; Silva, L.C.F.; Lima, H.S.; de Paula, S.O.; Dias, R.S.; da Silva, C.C. Effect of the gradual increase of salt on stability and microbial diversity of granular sludge and ammonia removal. J. Environ. Manag. 2019, 248, 109273. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Chen, Y.; Cui, X. Study on the treatment of ammonia-nitrogen wastewater by oxidization methods. Adv. Mater. Res. 2015, 1092–1093, 938–942. [Google Scholar] [CrossRef]
- Yuan, R.; Qin, Y.; He, C.; Wang, Z.; Bai, L.; Zhao, H.; He, X. Fe-Mn-Cu-Ce/Al2O3 as an efficient catalyst for catalytic ozonation of bio-treated coking wastewater: Characteristics, efficiency, and mechanism. Arab. J. Chem. 2023, 16, 104415. [Google Scholar] [CrossRef]
- Vaiano, V.; Jaramillo-Paez, C.A.; Matarangolo, M. UV and visible-light driven photocatalytic removal of caffeine using ZnO modified with different noble metals (Pt, Ag and Au). Mater. Res. Bull. 2019, 112, 251–260. [Google Scholar] [CrossRef]
- Yu, F.; Tian, F.; Zou, H. ZnO/biochar nanocomposites via solvent free ball milling for enhanced adsorption and photocatalytic degradation of methylene blue. J. Hazard. Mater. 2021, 415, 125511. [Google Scholar] [CrossRef] [PubMed]
- HJ 535-2009; Water Quality-Determination of Ammonia Nitrogen—Nessler’s Reagent Spectrophotometry. Ministry of Environmental Protection: Beijing, China, 2009.
- Mou, H.; Song, C.; Zhou, Y.; Zhang, B.; Wang, D. Design and synthesis of porous Ag/ZnO nanosheets assemblies as super photocatalysts for enhanced visible-light degradation of 4-nitrophenol and hydrogen evolution. Appl. Catal. B Environ. 2018, 221, 565–573. [Google Scholar] [CrossRef]
- Shi, L.; Liang, L.; Ma, J.; Meng, Y.; Zhong, S.; Wang, F.; Sun, J. Highly efficient visible light-driven Ag/AgBr/ZnO composite photocatalyst for degrading Rhodamine B. Ceram. Int. 2014, 40, 3495–3502. [Google Scholar] [CrossRef]
- Jiang, X.; Li, Z.; Lin, Q.; Dong, K.; Zhang, Y.; Sun, Z. Structure, optical properties and photocatalysis performance of Cu2O microspheres prepared by hydrothermal method. J. Mater. Sci. Mater. Electron. 2016, 27, 8856–8861. [Google Scholar] [CrossRef]
- Liang, Y.; Jiao, C.; Pan, L.; Zhao, T.; Liang, J.; Xiong, J.; Zhou, Q. Degradation of chlorine dioxide bleaching wastewater and response of bacterial community in the intimately coupled system of visible-light photocatalysis and biodegradation. Environ. Res. 2021, 195, 110840. [Google Scholar] [CrossRef]
- Bera, M.; Lee, D.S.; Cho, E.J. Advances in N-centered intermediates by energy transfer photocatalysis. Trends Chem. 2021, 3, 877–891. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, X.; Mi, R.; Liu, M.; Chen, Y.; Chen, C.; Ruan, S. On the high response towards TEA of gas sensors based on Ag-loaded 3D porous ZnO microspheres. Sens. Actuators B Chem. 2018, 270, 492–499. [Google Scholar] [CrossRef]
- Meshram, S.P.; Adhyapak, P.V.; Amalnerkar, D.P.; Mulla, I.S. Cu doped ZnO microballs as effective sunlight driven photocatalyst. Ceram. Int. 2016, 42, 7482–7489. [Google Scholar] [CrossRef]
- Ozdemir, E.; Orkan Ucar, I.; Akay, R.G.; Aytac, A. The effect of Ag, ZnO, and CuO nanoparticles on the properties of the compatibilized polyethylene/thermoplastic starch blend films. J. Vinyl Addit. Technol. 2021, 27, 543–554. [Google Scholar] [CrossRef]
- Kasim, N.; Hassan, Z.; Lim, W.F.; Quah, H.J. A comparison study of ZnO, InZnO, GaZnO and InGaZnO physical properties and optical bandgap. Int. J. Nanotechnol. 2022, 19, 241–251. [Google Scholar] [CrossRef]
- Amirouche, B.; Abderrahmane, Y. Effect of ZnO, SiO2 and Al2O3 Doped on Morphological, Optical, Structural and Mechanical Properties of Polylactic Acid. Key Eng. Mater. 2022, 6323, 105–113. [Google Scholar]
- Jung, K.-W.; Lee, S.Y.; Lee, Y.J. Hydrothermal synthesis of hierarchically structured birnessite-type MnO2/biochar composites for the adsorptive removal of Cu(II) from aqueous media. Bioresour. Technol. 2018, 260, 204–212. [Google Scholar] [CrossRef]
- Soltani, S.; Khanian, N.; Rashid, U.; Choong, T.S.Y. Core-shell ZnO-TiO2 hollow spheres synthesized by in-situ hydrothermal method for ester production application. Renew. Energy 2020, 151, 1076–1081. [Google Scholar] [CrossRef]
- Khan, H.; Habib, M.; Khan, A.; Boffito, D.C. A modified sol-gel synthesis to yield a stable Fe3+/ZnO photocatalyst: Degradation of water pollutants and mechanistic insights under UV and visible light. Engineering 2020, 8, 104282. [Google Scholar] [CrossRef]
- Ambedkar, A.K.; Singh, M.; Kumar, V.; Kumar, V.; Singh, B.P.; Kumar, A.; Gautam, Y.K. Structural, optical and thermoelectric properties of Al-doped ZnO thin films prepared by spray pyrolysis. Surf. Interfaces 2020, 19, 100504. [Google Scholar] [CrossRef]
- Jung, S.H.; Oh, E.; Lee, K.H.; Yang, Y.; Park, C.G.; Park, W.; Jeong, S.H. Sonochemical preparation of shape selective ZnO nanostructures. Cryst. Growth Des. 2008, 8, 265–269. [Google Scholar] [CrossRef]
- Mahana, A.; Guliy, O.I.; Momin, S.C.; Lalmuanzeli, R.; Mehta, S.K. Sunlight-driven photocatalytic degradation of methylene blue using ZnO nanowires prepared through ultrasonication-assisted biological process using aqueous extract of Anabaena doliolum. Cryst. Growth Des. 2020, 108, 110205. [Google Scholar] [CrossRef]
- Nava, O.J.; Luque, P.A.; Gómez-Gutiérrez, C.M.; Vilchis-Nestor, A.R.; Castro-Beltrán, A.; Mota-González, M.L.; Olivas, A. Influence of Camellia sinensis extract on Zinc Oxide nanoparticle green synthesis. Opt. Mater. 2017, 1134, 121–125. [Google Scholar] [CrossRef]
- Zhou, Q.; Yin, H.; Wang, A.; Si, Y. Preparation of hollow B-SiO_2@TiO_2 composites and their photocatalytic performances for degradation of ammonia-nitrogen and green algae in aqueous solution. Chin. J. Chem. Eng. 2019, 27, 2535–2543. [Google Scholar] [CrossRef]
- Manzoor, M.F.; EjazAhmed; Ahmad, M. Enhanced photocatalytic activity of hydrogen evolution through Cu incorporated ZnO nano composites. Mater. Sci. Semicond. Process. 2020, 120, 105278. [Google Scholar] [CrossRef]
- Gharaghani, M.A.; Malakootian, M. Photocatalytic deg-radation of the antibiotic ciprofloxacin by ZnO nanoparti-cles immobilized on a glass plate. Desalnation Water Treat. 2017, 89, 304–314. [Google Scholar] [CrossRef]
- Gong, H.; Hu, J.Q.; Wang, J.H.; Ong, C.H.; Zhu, F.R. Nano-crystalline Cu-doped ZnO thin film gas sensor for CO. Sens. Actuators B Chem. 2006, 115, 247–251. [Google Scholar] [CrossRef]
- Emamifar, A.; Kadivar, M.; Shahedi, M.; Soleimanian-Zad, S. Evaluation of nanocomposite packaging containing Ag and ZnO on shelf life of fresh orange juice. Innov. Food Sci. Emerg. Technol. 2010, 11, 742–748. [Google Scholar] [CrossRef]
- Shafei, A.E.; Abou-Okeil, A. ZnO/carboxymethyl chitosan bionano-composite to impart antibacterial and UV protection for cotton fabric. Carbohydr. Polym. 2011, 83, 920–925. [Google Scholar] [CrossRef]
- Wu, C.L. Facile one-step synthesis of N-doped ZnO micropolyhedrons for efficient photocatalytic degradation of formaldehyde under visible-light irradiation. Appl. Surf. Sci. 2014, 319, 237–243. [Google Scholar] [CrossRef]
- Jalili-Jahani, N.; Rabbani, F.; Fatehi, A. Rapid one-pot synthesis of Ag-decorated ZnO nanoflowers for photocatalytic degradation of tetracycline and product analysis by LC/APCI-MS and direct probe ESI-MS. Adv. Powder Technol. 2021, 32, 3075–3089. [Google Scholar] [CrossRef]





| Samples Sample | Specific Surface Area Specific Surface Area/(m2·g)−1 | Kong Rong Pore Volume (cm3/g) | Average Pore Size Average Aperture (nm) |
|---|---|---|---|
| ZnO | 41.38 | 0.113 | 10.93 |
| Ag/ZnO | 27.37 | 0.07 | 9.75 |
| Ag/ZnO/BC nanofilms | 62.14 | 0.13 | 8.37 |
| Samples | k (min)−1 | R2 |
|---|---|---|
| Ag/ZnO/BC nanofilms | 0.0095 | 0.986 |
| P25 | 0.0033 | 0.945 |
| ZnO | 0.0046 | 0.930 |
| Ag/ZnO | 0.0061 | 0.966 |
| Material/Method Name | Processing Object | Concentration of Ammonia Nitrogen in Primary Solution/(mg·L−1) | Removal Rate/(mg·g−1) |
|---|---|---|---|
| TiO2 (Hydrothermal process) | Simulated ammonia nitrogen wastewater | 30.00 mg/L | 79.00% |
| ZnO (Hydrothermal process) | Simulated ammonia nitrogen wastewater | 50.00 mg/L | 64.80% |
| BiOI/BiOBr/1 wt%MoS2 | Simulated ammonia nitrogen wastewater | 50.00 mg/L | 80.52% |
| ZnO-PMMA | Simulated ammonia nitrogen wastewater | 50.00 mg/L | 66.00% |
| ZnFe2O4/NG | Simulated ammonia nitrogen wastewater | 100.00 mg/L | 62.84% |
| TiO2-CuO/HSC | Simulated ammonia nitrogen wastewater | 100.00 mg/L | 60.7% |
| Fe3O4/ZnO—BC | Simulated ammonia nitrogen wastewater | 50.00 mg/L | 80.50% |
| Ag/ZnO/BC | Simulated ammonia nitrogen wastewate | 50.00 mg/L | 84.03% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Li, S.; Jiang, L.; Xu, J.; Li, J.; Xia, Z.; Tan, C.; Zuo, J.; Wang, Y. Novel Photocatalyst Ag/ZnO/BC Nanofilms Degradation of Low Concentration Ammonia Nitrogen Wastewater. Coatings 2023, 13, 2043. https://doi.org/10.3390/coatings13122043
Li J, Li S, Jiang L, Xu J, Li J, Xia Z, Tan C, Zuo J, Wang Y. Novel Photocatalyst Ag/ZnO/BC Nanofilms Degradation of Low Concentration Ammonia Nitrogen Wastewater. Coatings. 2023; 13(12):2043. https://doi.org/10.3390/coatings13122043
Chicago/Turabian StyleLi, Junsheng, Sihang Li, Liming Jiang, Jialun Xu, Jiahui Li, Zhi Xia, Chong Tan, Jinlong Zuo, and Yuyang Wang. 2023. "Novel Photocatalyst Ag/ZnO/BC Nanofilms Degradation of Low Concentration Ammonia Nitrogen Wastewater" Coatings 13, no. 12: 2043. https://doi.org/10.3390/coatings13122043





