Facilitating Synthesis of FeP/C@CoP Composites as High-Performance Anode Materials for Sodium-Ion Batteries
Abstract
:1. Introduction
2. Experimental Section
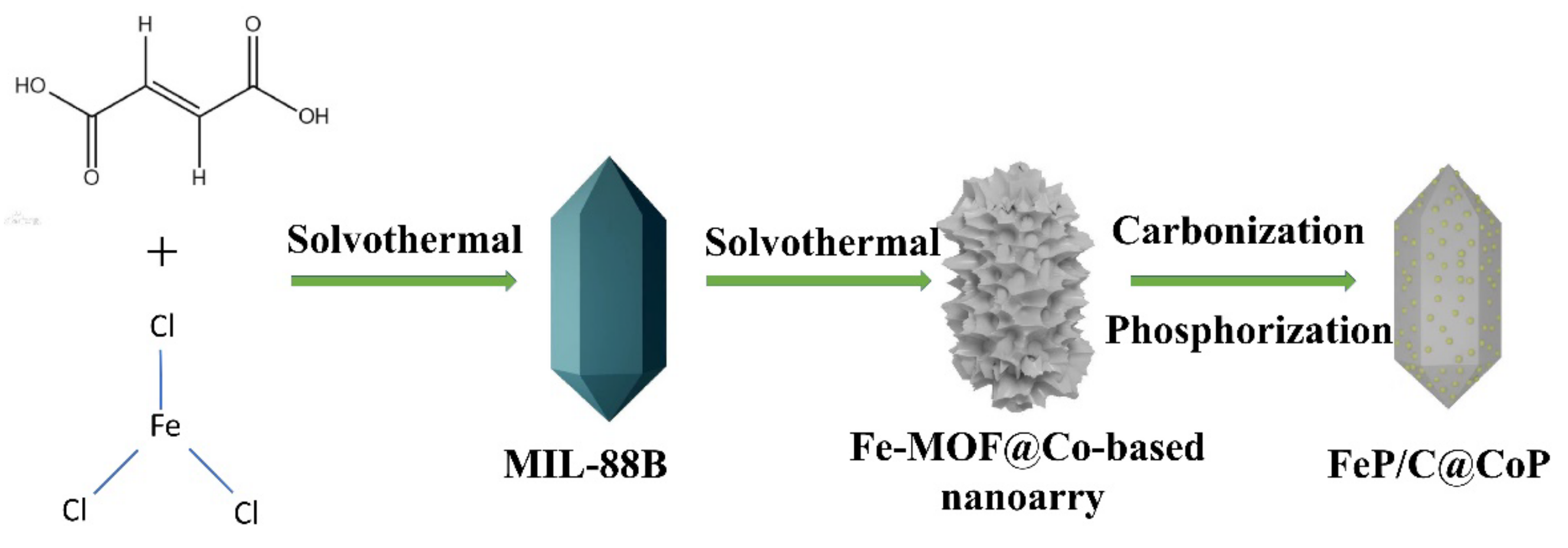
2.1. Materials Preparation
2.1.1. Synthesis of Fe-MOF (MIL-88)
2.1.2. Synthesis of Co3Fe7/C
2.1.3. Synthesis of FeP/C@CoP
2.1.4. Synthesis of CoP/C
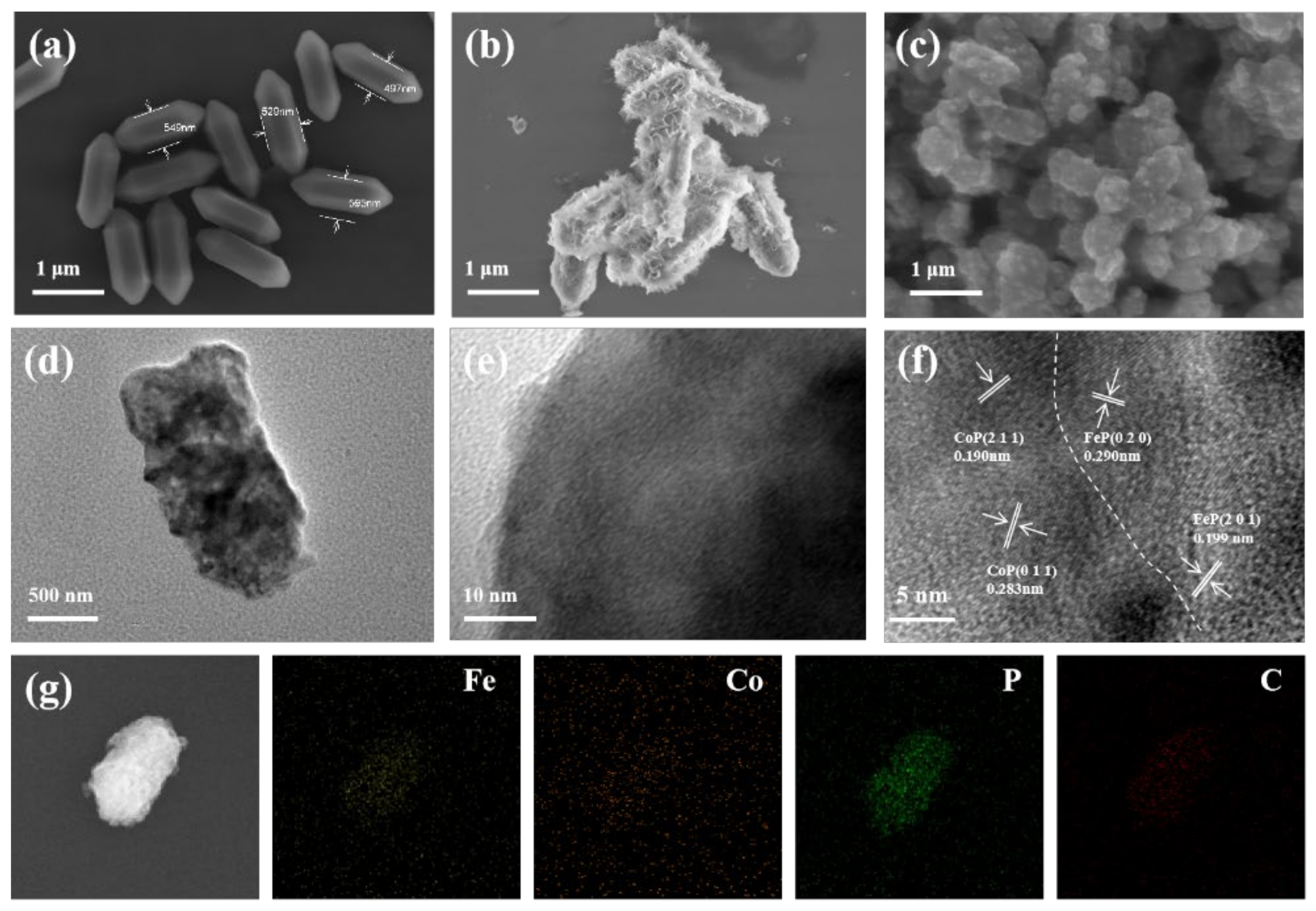
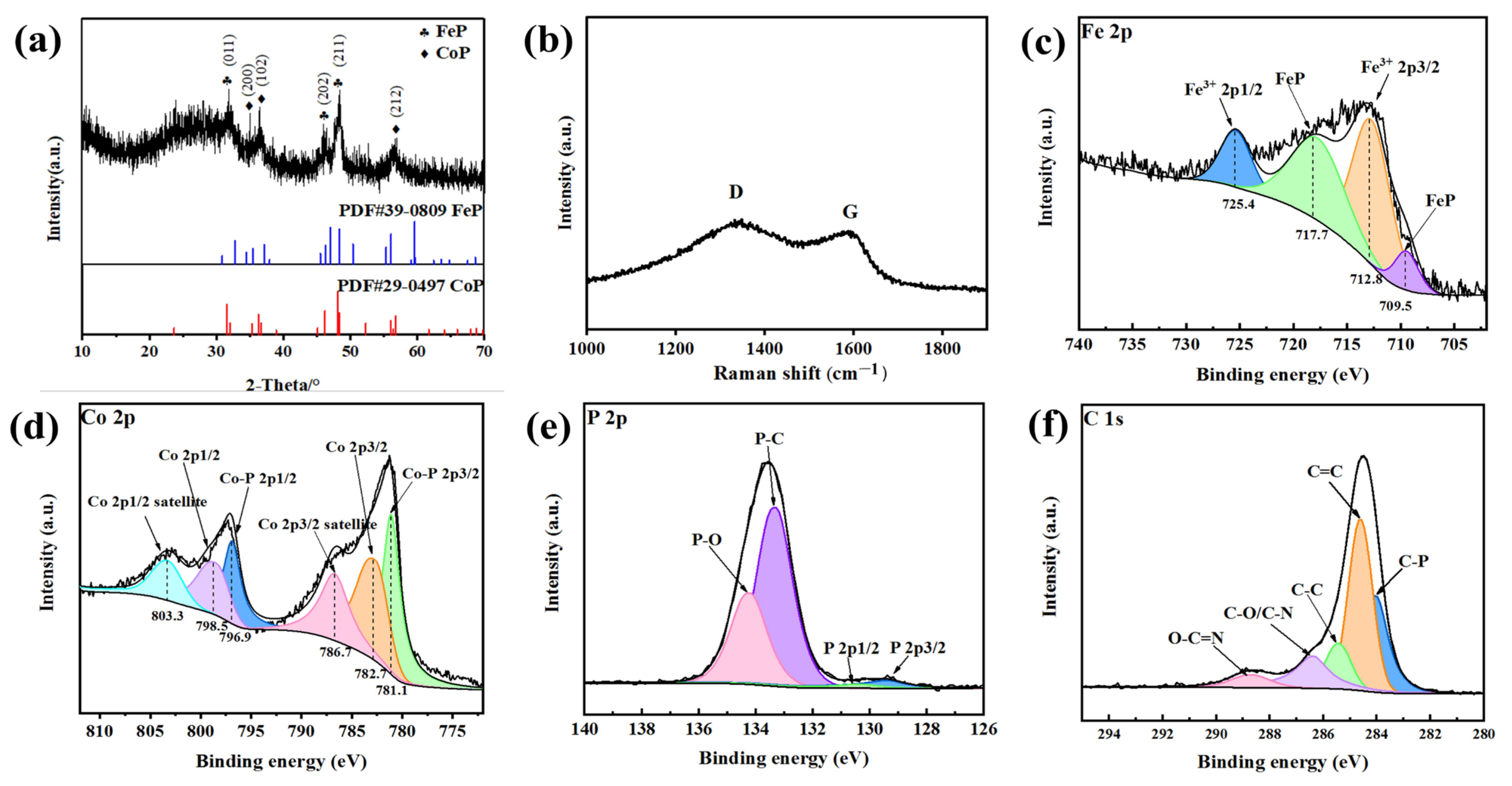
2.2. Materials Characterization
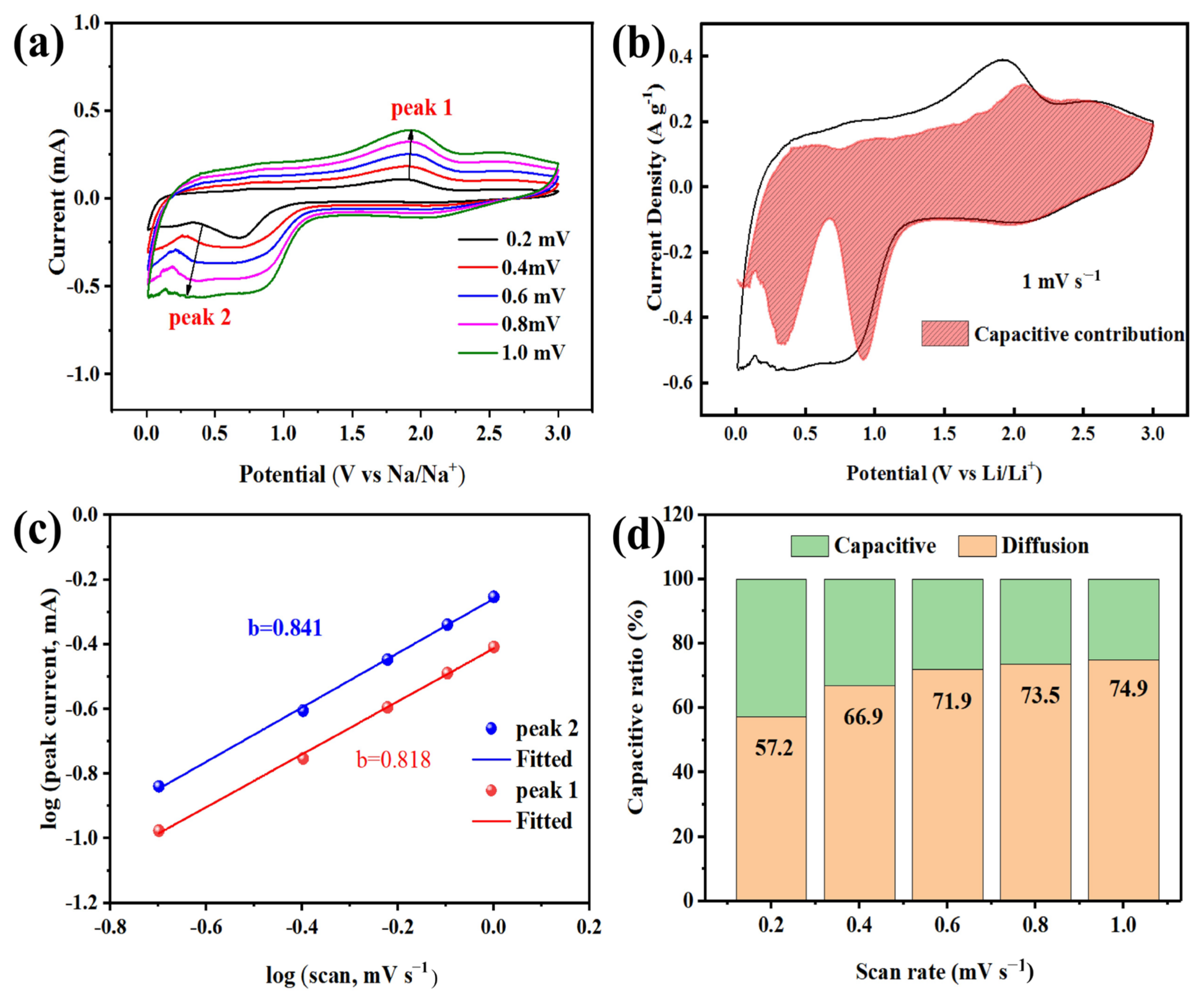
2.3. Electrochemical Measurements
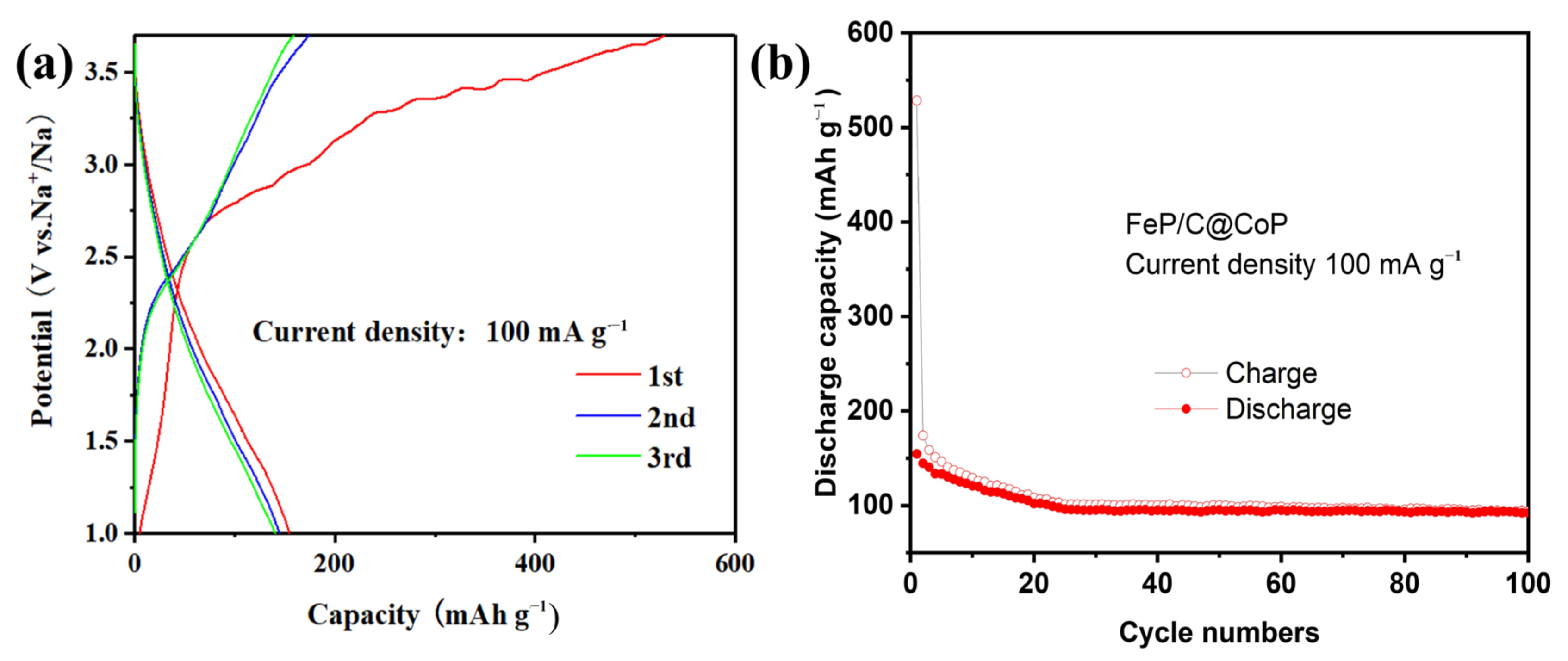
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, J.E.; Chen, J.H.; Peng, Y.H.; Zheng, Y.Q.; Zeb, A.; Lin, X.M. Metal-organic framework-derived transition metal sulfides and their composites for alkali-ion batteries: A review. Coord. Chem. Rev. 2022, 472, 214781. [Google Scholar] [CrossRef]
- Zeng, L.C.; Huang, L.C.; Zhu, J.H.; Li, P.P.; Chu, P.K.; Wang, J.H.; Yu, X.F. Phosphorus-Based Materials for High-Performance Alkaline Metal Ion Batteries: Progress and Prospect. Small 2022, 18, 2201808. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Lan, X.X.; Hu, R.Z.; Yao, Y.; Yu, Y.; Zhu, M. Tin-Based Anode Materials for Stable Sodium Storage: Progress and Perspective. Adv. Mater. 2022, 34, 2106895. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fu, W.; Lee, D.C.; Bell, C.; Drexler, M.; Ma, Z.F.; Magasinski, A.; Yushin, G.; Alamgir, F.M. Porous FeP/C composite nanofibers as high-performance anodes for Li-ion/Na-ion batteries. Mater. Today Energy 2020, 16, 100410. [Google Scholar] [CrossRef]
- Xu, E.; Zhang, Y.; Wang, H.; Zhu, Z.; Quan, J.; Chang, Y.; Li, P.; Yu, D.; Jiang, Y. Ultrafast kinetics net electrode assembled via MoSe2/MXene heterojunction for high-performance sodium-ion batteries. Chem. Eng. J. 2020, 385, 123839. [Google Scholar] [CrossRef]
- Wang, Y.; Lim, Y.V.; Huang, S.Z.; Ding, M.; Kong, D.Z.; Pei, Y.Y.; Xu, T.T.; Shi, Y.M.; Li, X.J.; Yang, H.Y. Enhanced sodium storage kinetics by volume regulation and surface engineering rationally designed hierarchical porous FeP@C/rGO. Nanoscale 2020, 12, 4341–4351. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Yang, H.; Zhou, J.; Luo, Z.; Fang, G.; Liu, S.; Pan, A.; Liang, S. Nitrogen doped hollow MoS2/C nanospheres as anode for long-life sodium-ion batteries. Chem. Eng. J. 2017, 327, 522–529. [Google Scholar] [CrossRef]
- Zhu, C.; Qu, X.; Zhang, M.; Wang, J.; Li, Q.; Geng, Y.; Ma, Y.; Su, Z. Planar NiC3 as a reversible anode material with high storage capacity for lithium-ion and sodium-ion batteries. J. Mater. Chem. A 2019, 7, 13356–13363. [Google Scholar] [CrossRef]
- Wu, C.H.; Song, H.; Tang, C.; Du, A.J.; Yu, C.Z.; Huang, Z.D.; Wu, M.H.; Zhang, H.J. Ultralarge interlayer distance and C,N-codoping enable superior sodium storage capabilities of MoS nanoonions. Chem. Eng. J. 2019, 378, 122249. [Google Scholar] [CrossRef]
- Zhao, D.; Zhao, R.; Dong, S.; Miao, X.; Zhang, Z.; Wang, C.; Yin, L. Alkali-induced 3D crinkled porous Ti3C2 MXene architectures coupled with NiCoP bimetallic phosphide nanoparticles as anodes for high-performance sodium-ion batteries. Energy Environ. Sci. 2019, 12, 2422–2432. [Google Scholar] [CrossRef]
- Shi, S.; Li, Z.; Sun, Y.; Wang, B.; Liu, Q.; Hou, Y.; Huang, S.; Huang, J.; Zhao, Y. A covalent heterostructure of monodisperse Ni2P immobilized on N, P-co-doped carbon nanosheets for high performance sodium/lithium storage. Nano Energy 2018, 48, 510–517. [Google Scholar] [CrossRef]
- Tan, Q.W.; Zhao, W.; Han, K.; Li, P.; Wang, W.; He, D.L.; Liu, Z.W.; Yu, Q.Y.; Qin, M.L.; Qu, X.H. The multi-yolk/shell structure of FeP@foam-like graphenic scaffolds: Strong P-C bonds and electrolyte- and binder-optimization boost potassium storage. J. Mater. Chem. A 2019, 7, 15673–15682. [Google Scholar] [CrossRef]
- Zhao, X.J.; Wang, J.H.; Wang, B.Y.; Wang, Y.; Fan, J.L.; Ma, H.Z. Preparing a graphene/carbon nanotube coated hollow nickel phosphides microsphere anode with high stability for high-performance lithium/sodium battery. J. Electroanal. Chem. 2023, 939, 117479. [Google Scholar] [CrossRef]
- Zhang, K.F.; Zhu, Z.Q.; Lin, J.H.; Zhang, R.Z.; Zhao, C.J. One-step simultaneously heteroatom doping and phosphating to construct 3D FeP/C nanocomposite for lithium storage. Appl. Surf. Sci. 2020, 500, 144055. [Google Scholar] [CrossRef]
- Shi, S.S.; Sun, C.L.; Yin, X.P.; Shen, L.Y.; Shi, Q.H.; Zhao, K.N.; Zhao, Y.F.; Zhang, J.J. FeP Quantum Dots Confined in Carbon-Nanotube-Grafted P-Doped Carbon Octahedra for High-Rate Sodium Storage and Full-Cell Applications. Adv. Funct. Mater. 2020, 30, 1909283. [Google Scholar] [CrossRef]
- Guo, K.K.; Xi, B.J.; Wei, R.C.; Li, H.B.; Feng, J.K.; Xiong, S.L. Hierarchical Microcables Constructed by CoP@C⊂Carbon Framework Intertwined with Carbon Nanotubes for Efficient Lithium Storage. Adv. Energy Mater. 2020, 10, 1902913. [Google Scholar] [CrossRef]
- Chen, J.S.; Wei, L.; Mahmood, A.; Pei, Z.X.; Zhou, Z.; Chen, X.C.; Chen, Y. Prussian blue, its analogues and their derived materials for electrochemical energy storage and conversion. Energy Storage Mater. 2020, 25, 585–612. [Google Scholar] [CrossRef]
- Wang, S.; Cao, F.; Li, Y.; Zhang, Z.; Zhou, D.; Yang, Y.; Tang, Z. MoS2-Coupled Carbon Nanosheets Encapsulated on Sodium Titanate Nanowires as Super-Durable Anode Material for Sodium-Ion Batteries. Adv. Sci. 2019, 6, 1900028. [Google Scholar] [CrossRef]
- Li, X.D.; Zhang, W.D.; Cai, J.X.; Yan, H.J.; Cui, M.Q.; Wu, G.X.; Li, M.C. Hierarchical nanosheets constructed by integration of bimetallic sulfides into N-Doped carbon: Enhanced diffusion kinetics and cycling stability for sodium storage. Nano Energy 2019, 62, 239–249. [Google Scholar] [CrossRef]
- Lin, J.; Yao, L.; Zhang, C.; Ding, H.; Wu, Y.; Li, S.; Han, J.; Yue, G.; Peng, D. Construction of Sb2S3@SnS@C Tubular Heterostructures as High-Performance Anode Materials for Sodium-Ion Batteries. ACS Sustain. Chem. Eng. 2021, 9, 11280–11289. [Google Scholar] [CrossRef]
- Li, Y.; Qian, J.; Zhang, M.H.; Wang, S.; Wang, Z.H.; Li, M.S.; Bai, Y.; An, Q.Y.; Xu, H.J.; Wu, F.; et al. Co-Construction of Sulfur Vacancies and Heterojunctions in Tungsten Disulfide to Induce Fast Electronic/Ionic Diffusion Kinetics for Sodium-Ion Batteries. Adv. Mater. 2020, 32, 2005802. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.R.; Hou, Y.; Jiang, K.; Zhao, L.; Olsen, T.; Fan, Y.C.; Jiang, J.L.; Xu, Z.X.; Ma, Z.F.; Legut, D.; et al. In situ cross-linking construction of 3D mesoporous bimetallic phosphide-in-carbon superstructure with atomic interface toward enhanced sodium ion storage performance. Chem. Eng. J. 2021, 413, 127449. [Google Scholar] [CrossRef]
- Xu, X.; Cao, R.; Jeong, S.; Cho, J. Spindle-like Mesoporous α-Fe2O3 Anode Material Prepared from MOF Template for High-Rate Lithium Batteries. Nano Lett. 2012, 12, 4988–4991. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.; Su, J.; Li, M.; Jiang, P.; Yang, Y.; Chen, Q. A MOF-derived self-template strategy toward cobalt phosphide electrodes with ultralong cycle life and high capacity. J. Mater. Chem. A 2017, 5, 10321–10327. [Google Scholar] [CrossRef]
- Zhou, D.; Fan, L.Z. Co2P nanoparticles encapsulated in 3D porous N-doped carbon nanosheet networks as an anode for high-performance sodium-ion batteries. J. Mater. Chem. A 2018, 6, 2139–2147. [Google Scholar] [CrossRef]
- Li, D.; Zhou, J.; Chen, X.; Song, H. Achieving Ultrafast and Stable Na-Ion Storage in FeSe2 Nanorods/Graphene Anodes by Controlling the Surface Oxide. ACS Appl. Mater. Interfaces 2018, 10, 22841–22850. [Google Scholar] [CrossRef]
- Li, Z.Q.; Zhang, L.Y.; Ge, X.L.; Li, C.X.; Dong, S.H.; Wang, C.X.; Yin, L.W. Core-shell structured CoP/FeP porous microcubes interconnected by reduced graphene oxide as high performance anodes for sodium ion batteries. Nano Energy 2017, 32, 494–502. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Hou, H.S.; Shao, L.D.; Zhang, Y.; Zou, G.Q.; Chen, J.; Ji, X.B. Large-Area Carbon Nanosheets Doped with Phosphorus: A High-Performance Anode Material for Sodium-Ion Batteries. Adv. Sci. 2017, 4, 1600243. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, W.; Liu, Y.; He, J.; Yang, S.; Liu, M.; Wang, Q.; Guo, Z. Constructing CoO/Co3S4 Heterostructures Embedded in N-doped Carbon Frameworks for High-Performance Sodium-Ion Batteries. Adv. Funct. Mater. 2019, 29, 1901925. [Google Scholar] [CrossRef]
- Fang, Y.J.; Zhang, J.X.; Xiao, L.F.; Ai, X.P.; Cao, Y.L.; Yang, H.X. Phosphate Framework Electrode Materials for Sodium Ion Batteries. Adv. Sci. 2017, 4, 1600392. [Google Scholar] [CrossRef]
- Latorre-Sánchez, M.; Primo, A.; García, H. P-Doped Graphene Obtained by Pyrolysis of Modified Alginate as a Photocatalyst for Hydrogen Generation from Water–Methanol Mixtures. Angew. Chem. Int. Ed. 2013, 52, 11813–11816. [Google Scholar] [CrossRef] [PubMed]
- Grosvenor, A.P.; Wik, S.D.; Cavell, R.G.; Mar, A. Examination of the Bonding in Binary Transition-Metal Monophosphides MP (M = Cr, Mn, Fe, Co) by X-Ray Photoelectron Spectroscopy. Inorg. Chem. 2005, 44, 8988–8998. [Google Scholar] [CrossRef]
- Huang, Y.R.; Li, M.G.; Yang, W.W.; Yu, Y.S.; Hao, S.E. 3D ordered mesoporous cobalt ferrite phosphides for overall water splitting. Sci. China Mater. 2020, 63, 240–248. [Google Scholar] [CrossRef]
- Li, X.Y.; Qian, X.; Xu, Y.L.; Wu, H.; Dan, Y.Y.; Chen, L.Z.; Yu, Q. Fe-Co-P/C with strong coupling interaction for enhanced sodium ion batteries and oxygen evolution reactions. Electrochim. Acta 2019, 321, 134646. [Google Scholar] [CrossRef]
- Chen, J.H.; Liu, J.W.; Xie, J.Q.; Ye, H.Q.; Fu, X.Z.; Sun, R.; Wong, C.P. Co-Fe-P nanotubes electrocatalysts derived from metal-organic frameworks for efficient hydrogen evolution reaction under wide pH range. Nano Energy 2019, 56, 225–233. [Google Scholar] [CrossRef]
- Du, Y.M.; Qu, H.Q.; Liu, Y.R.; Han, Y.; Wang, L.; Dong, B. Bimetallic CoFeP hollow microspheres as highly efficient bifunctional electrocatalysts for overall water splitting in alkaline media. Appl. Surf. Sci. 2019, 465, 816–823. [Google Scholar] [CrossRef]
- Li, W.J.; Chou, S.L.; Wang, J.Z.; Liu, H.K.; Dou, S.X. A new, cheap, and productive FeP anode material for sodium-ion batteries. Chem. Commun. 2015, 51, 4720. [Google Scholar] [CrossRef]
- Madej, M.; Matoga, D.; Skaźnik, K.; Porada, R.; Baś, B.; Kochana, J. A voltammetric sensor based on mixed proton-electron conducting composite including metal-organic framework JUK-2 for determination of citalopram. Microchim. Acta 2021, 188, 184. [Google Scholar] [CrossRef]
- Hu, X.; Jia, J.C.; Wang, G.X.; Chen, J.X.; Zhan, H.B.; Wen, Z.H. Reliable and General Route to Inverse Opal Structured Nanohybrids of Carbon-Confined Transition Metal Sulfides Quantum Dots for High-Performance Sodium Storage. Adv. Energy Mater. 2018, 8, 1801452. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Zheng, Y.H.; Li, X.; Adams, F.; Luo, W.; Huang, Y.H.; Hu, L.B. Electrode Materials of Sodium-Ion Batteries toward Practical Application. ACS Energy Lett. 2018, 3, 1604–1612. [Google Scholar] [CrossRef]
- Li, X.; Li, K.; Zhu, S.; Fan, K.; Lyu, L.; Yao, H.; Li, Y.; Hu, J.; Huang, H.; Mai, Y.W.; et al. Fiber-in-Tube Design of Co9S8-Carbon/Co9S8: Enabling Efficient Sodium Storage. Angew. Chem. Int. Ed. 2019, 58, 6239–6243. [Google Scholar] [CrossRef]
- Shen, Q.; Jiang, P.; He, H.; Chen, C.; Liu, Y.; Zhang, M. Encapsulation of MoSe2 in carbon fibers as anodes for potassium ion batteries and nonaqueous battery–supercapacitor hybrid devices. Nanoscale 2019, 11, 13511–13520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Park, M.; Zhou, L.; Lee, G.H.; Shin, J.; Hu, Z.; Chou, S.L.; Chen, J.; Kang, Y.M. Cobalt-Doped FeS2 Nanospheres with Complete Solid Solubility as a High-Performance Anode Material for Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2016, 55, 12822–12826. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Chen, K.; Wang, G.; Liu, X.J.; Wang, H. Rational Design of Three-Dimensional Graphene Encapsulated with Hollow FeP@Carbon Nanocomposite as Outstanding Anode Material for Lithium Ion and Sodium Ion Batteries. ACS Nano 2017, 11, 11602–11616. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, C.D.; Liang, J.W.; Zuo, J.; Yang, Q. Synthesis of nanorod-FeP@C composites with hysteretic lithiation in lithium-ion batteries. Dalton Trans. 2015, 44, 10297–10303. [Google Scholar] [CrossRef] [PubMed]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef]
- Laghlimi, C.; Ziat, Y.; Moutcine, A.; Hammi, M.; Zarhri, Z.; Ifguis, O.; Chtaini, A. A new sensor based on graphite carbon paste modified by an organic molecule for efficient determination of heavy metals in drinking water. Chem. Data Collect. 2021, 31, 100595. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, T.; Hong, Z.; Ding, H.; Li, J.; Xia, Y.; Zhou, Z.; Yue, G. Facilitating Synthesis of FeP/C@CoP Composites as High-Performance Anode Materials for Sodium-Ion Batteries. Coatings 2023, 13, 2056. https://doi.org/10.3390/coatings13122056
Mao T, Hong Z, Ding H, Li J, Xia Y, Zhou Z, Yue G. Facilitating Synthesis of FeP/C@CoP Composites as High-Performance Anode Materials for Sodium-Ion Batteries. Coatings. 2023; 13(12):2056. https://doi.org/10.3390/coatings13122056
Chicago/Turabian StyleMao, Tianle, Zheyu Hong, Haoran Ding, Jintang Li, Yongji Xia, Zhidong Zhou, and Guanghui Yue. 2023. "Facilitating Synthesis of FeP/C@CoP Composites as High-Performance Anode Materials for Sodium-Ion Batteries" Coatings 13, no. 12: 2056. https://doi.org/10.3390/coatings13122056




