Nanomaterials and Equipment for Chemical–Mechanical Polishing of Single-Crystal Sapphire Wafers
Abstract
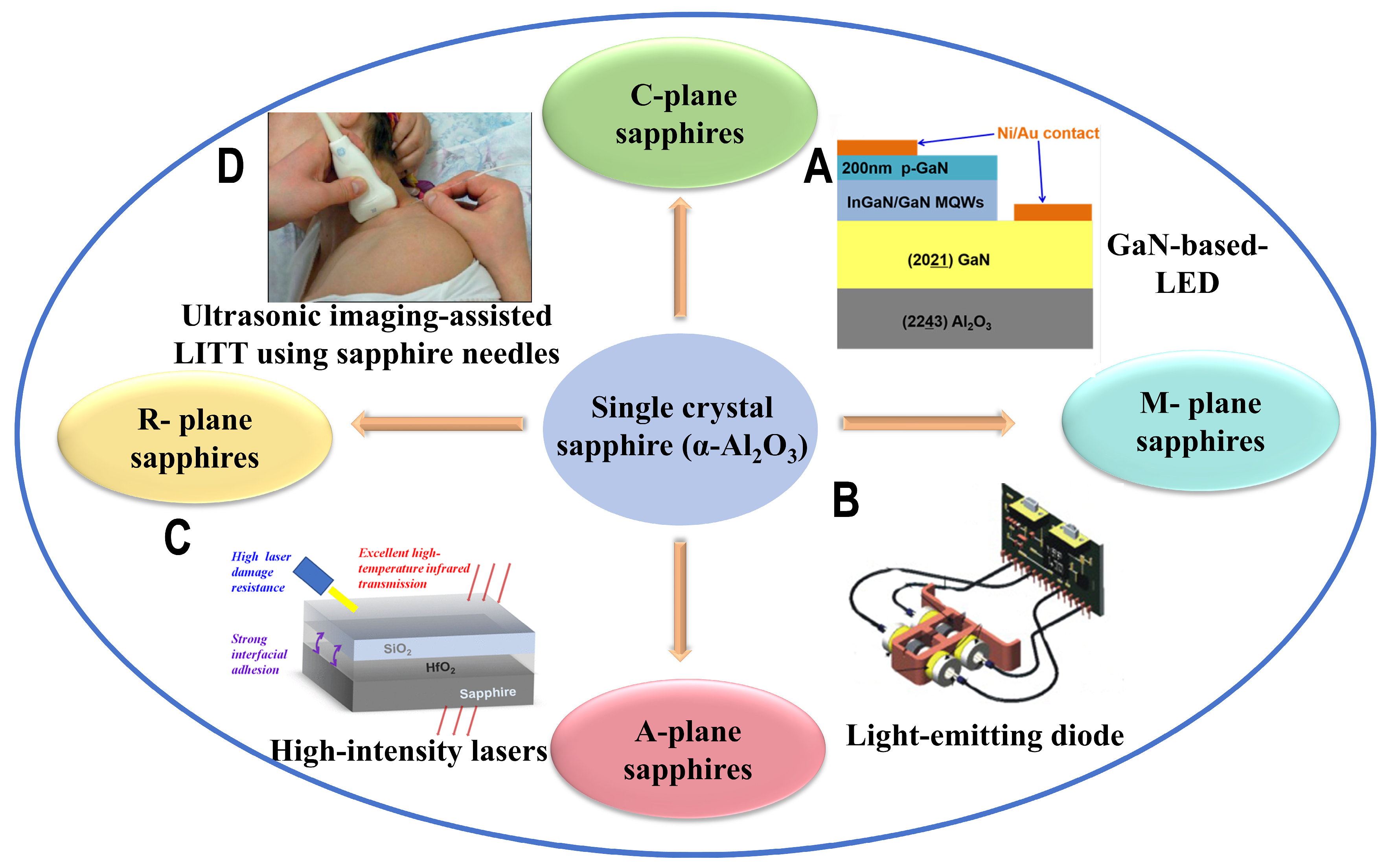
:1. Introduction
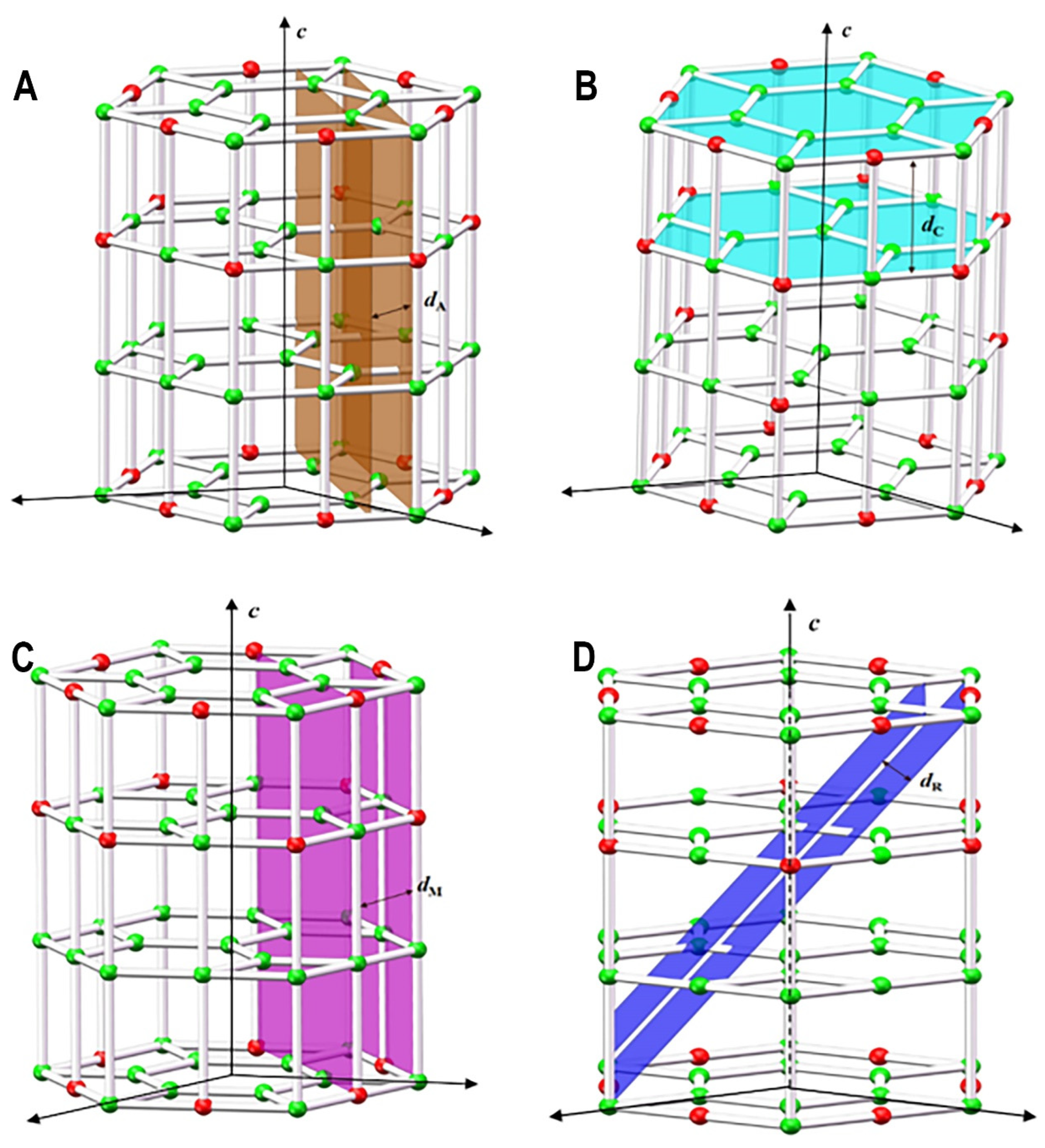
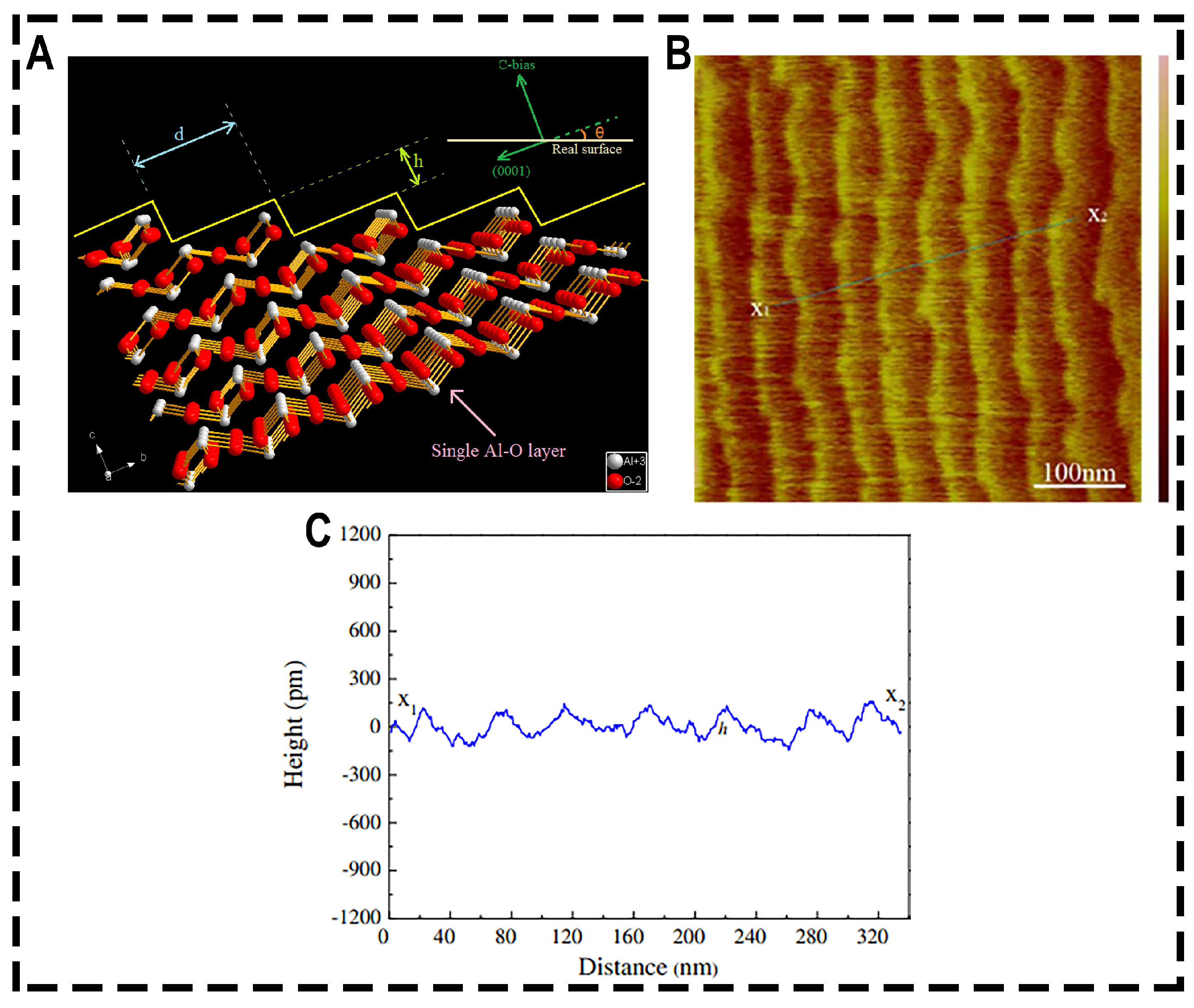
2. The Mechanism of Sapphire Polishing
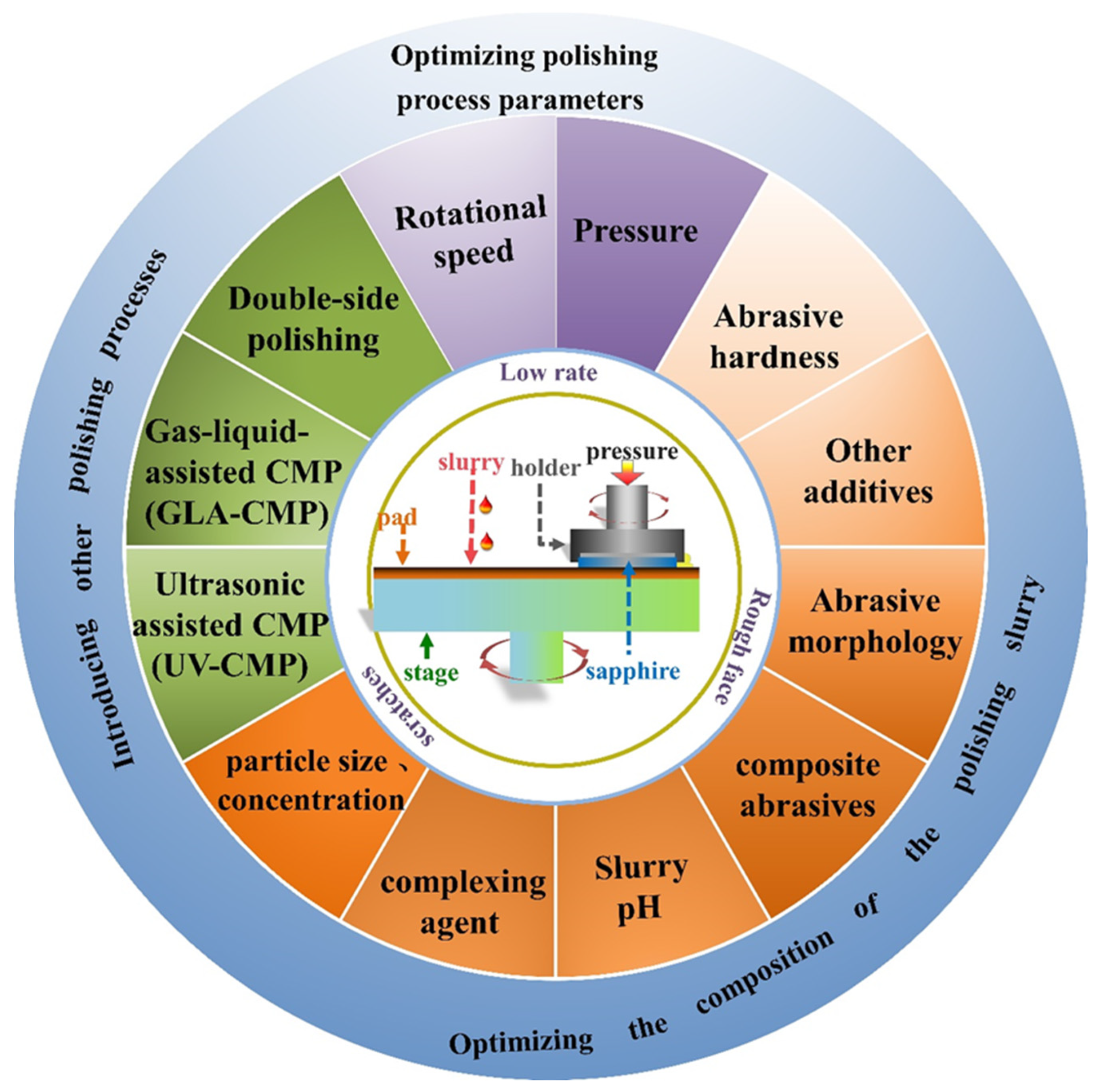
3. The Methods to Boost the Sapphire CMP Efficiency Productivity
3.1. Optimizing Polishing-Process Parameters
3.2. Optimizing the Composition of the Polishing Slurry
3.2.1. Changing the Hardness of the Abrasive
3.2.2. Changing the Particle Size and Concentration of the Abrasive
3.2.3. Changing the Morphology of Abrasive
3.2.4. Constructing Composite Abrasives
3.2.5. Changing the pH of the Slurry
3.2.6. Changing the Complexing Agent
3.2.7. Other Additives
3.3. Introducing Other Polishing Processes
4. Summary and Outlook
- The change of polishing parameters will influence the polishing efficiency of sapphire CMP, but the mechanism by how polishing parameters affect the effect of sapphire CMP is still controversial.
- The deep mechanism of the influence of abrasive hardness, and particle size and concentration on sapphire CMP has not been fully explored, and the interaction mechanism between the abrasive and sapphire is still controversial.
- The mechanism of interaction between other components such as surfactants, oxidants, and chelating agents in the polishing fluid and sapphire needs to be further explored. Only in this way, we can clearly understand the role of each component in the sapphire CMP, and better configure the polishing slurry.
- New and simple polishing supplementary means should be introduced into the CMP process of sapphire to improve the polishing efficiency and polishing quality of sapphire.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gong, Z. Layer-scale and chip-scale transfer techniques for functional devices and systems: A review. Nanomaterials 2021, 11, 842. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Xiang, S.; Han, G.; Zhang, J.; Ma, X.; Zhu, Z.; Guo, X.; Zhang, Y.; Han, Y.; Song, Z.; et al. Recent progress of integrated circuits and optoelectronic chips. Sci. China Inf. Sci. 2021, 64, 201401. [Google Scholar] [CrossRef]
- Juska, V.B.; Maxwell, G.; Estrela, P.; Pemble, M.E.; O’Riordan, A. Silicon microfabrication technologies for biology integrated advance devices and interfaces. Biosens. Bioelectron. 2023, 237, 115503. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, M.; Butt, M.A.; Piramidowicz, R. Breakthrough in silicon photonics technology in telecommunications, biosensing, and gas sensing. Micromachines 2023, 14, 1637. [Google Scholar] [CrossRef]
- Fujii, T.; Hiraki, T.; Aihara, T.; Nishi, H.; Takeda, K.; Sato, T.; Kakitsuka, T.; Tsuchizawa, T.; Matsuo, S. Development of an epitaxial growth technique using III-V on a Si platform for heterogeneous integration of membrane photonic devices on Si. Appl. Sci. 2021, 11, 1801. [Google Scholar] [CrossRef]
- Li, K.H.; Fu, W.Y.; Choi, H.W. Chip-scale GaN integration. Prog. Quantum Electron. 2020, 70, 100247. [Google Scholar] [CrossRef]
- Iyengar, S.A.; Bhattacharyya, S.; Roy, S.; Glavin, N.R.; Roy, A.K.; Ajayan, P.M. A researcher’s perspective on unconventional lab-to-fab for 2D semiconductor devices. ACS Nano 2023, 17, 12955–12970. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, Y.; Wang, J.; Miao, P.; Liu, H.; Cheng, Y.; Gou, Y.; Wang, S.; Deng, G.; Zhou, S. High-power and low-threshold 1200 nm InGaAs/GaAs quantum-wells VECSEL grown by MOCVD. J. Lumin. 2023, 263, 120022. [Google Scholar] [CrossRef]
- Gao, Z.; Ju, X.; Zhang, H.; Liu, X.; Chen, H.; Li, W.; Zhang, H.; Liang, L.; Cao, H. InP quantum dots tailored oxide thin film phototransistorfor bioinspired visual adaptation. Adv. Funct. Mater. 2023, 2305959. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, Y.; Yin, Y. Reliability of wide band gap power electronic semiconductor and packaging: A review. Energies 2022, 15, 6670. [Google Scholar] [CrossRef]
- Li, G.; Wang, W.; Yang, W.; Lin, Y.; Wang, H.; Lin, Z.; Zhou, S. GaN-based light-emitting diodes on various substrates: A critical review. Rep. Prog. Phys. 2016, 79, 056501. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Park, J.H.; Kim, J.K.; Schubert, E.F. White light-emitting diodes: History, progress, and future. Laser Photonics Rev. 2017, 11, 1600147. [Google Scholar] [CrossRef]
- Lin, J.; Jiang, F.; Wen, Q.; Wu, Y.; Lu, J.; Tian, Z.; Wang, N. Deformation anisotropy of nano-scratching on C-plane of sapphire: A molecular dynamics study and experiment. Appl. Surf. Sci. 2021, 546, 149091. [Google Scholar] [CrossRef]
- Ham, D.; Oh, S.; Kang, H.C. Competing phases in epitaxial SnO2 thin films deposited on sapphire (0001) substrates using radio-frequency powder sputtering. Ceram. Int. 2022, 48, 28396–28403. [Google Scholar] [CrossRef]
- Luo, Q.; Lu, J.; Xu, X.; Jiang, F. Removal mechanism of sapphire substrates (0001, 1120 and 1010) in mechanical planarization machining. Ceram. Int. 2017, 43, 16178–16184. [Google Scholar] [CrossRef]
- Song, J.; Choi, J.; Xiong, K.; Xie, Y.; Cha, J.J.; Han, J. Semipolar (2021) GaN and InGaN light-emitting diodes grown on sapphire. ACS Appl. Mater. Interfaces 2017, 9, 14088–14092. [Google Scholar] [CrossRef] [PubMed]
- Armitage, R.; Hirayama, H. M-plane GaN grown on m-sapphire by metalorganic vapor phase epitaxy. Appl. Phys. Lett. 2008, 92, 092121. [Google Scholar] [CrossRef]
- Rajabi, K.; Wang, J.; Jin, J.; Xing, Y.; Wang, L.; Han, Y.; Sun, C.; Hao, Z.; Luo, Y.; Qian, K.; et al. Improving modulation bandwidth of c-plane GaN-based light-emitting diodes by an ultra-thin quantum wells design. Opt. Express 2018, 26, 24985–24991. [Google Scholar] [CrossRef]
- Zhao, D.Q.; Xu, F.; Wang, G.G.; Zhang, S.Y.; Qin, G.S.; Wang, B.L.; Han, J.C. SiO2/HfO2 laser film with enhanced protection and antireflection for sapphire infrared windows at high temperatures. ACS Appl. Electron. Mater. 2021, 3, 4611–4617. [Google Scholar] [CrossRef]
- Wang, W.; Yang, W.; Wang, H.; Zhu, Y.; Yang, M.; Gao, J.; Li, G. A comparative study on the properties of c-plane and a-plane GaN epitaxial films grown on sapphire substrates by pulsed laser deposition. Vacuum 2016, 128, 158–165. [Google Scholar] [CrossRef]
- Xu, M.; Xue, Z.; Wang, J.; Zhao, Y.; Duan, Y.; Zhu, G.; Yu, L.; Xu, J.; Wang, J.; Shi, Y.; et al. Heteroepitaxial writing of silicon-on-sapphire nanowires. Nano Lett. 2016, 16, 7317–7324. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Buric, M.; Ohodnicki, P.R.; Nakano, J.; Liu, B.; Chorpening, B.T. Review and perspective: Sapphire optical fiber cladding development for harsh environment sensing. Appl. Phys. Rev. 2018, 5, 011102. [Google Scholar] [CrossRef]
- Gong, S.; Zhu, X.; Sun, Y.; Tang, B.; Su, Z. Experimental research on surface characteristics and subsurface damage behavior of monocrystal sapphire induced by helical micro abrasive tools. Ceram. Int. 2022, 48, 21459–21472. [Google Scholar] [CrossRef]
- Yu, Y.; Zhu, W.; Ma, Y.; Song, X.; Zou, H.; Zu, G.; Han, Y.; Ran, X. Novel design of sapphire/spinel transparent ceramic joints with double glass interlayers by coating and bonding. Ceram. Int. 2024, 50, 1591–1600. [Google Scholar]
- De Vargas-Sansalvador, I.M.P.; Fay, C.; Phelan, T.; Fernández-Ramos, M.D.; Capitán-Vallvey, L.F.; Diamond, D.; Benito-Lopez, F. A new light emitting diode-light emitting diode portable carbon dioxide gas sensor based on an interchangeable membrane system for industrial applications. Anal. Chim. Acta 2011, 699, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Katyba, G.M.; Zaytsev, K.I.; Dolganova, I.N.; Shikunova, I.A.; Chernomyrdin, N.V.; Yurchenko, S.O.; Komandin, G.A.; Reshetov, I.V.; Nesvizhevsky, V.V.; Kurlov, V.N. Sapphire shaped crystals for waveguiding, sensing and exposure applications. Prog. Cryst. Growth Charact. Mater. 2018, 64, 133–151. [Google Scholar] [CrossRef]
- Fu, J.H.; Min, J.; Chang, C.K.; Tseng, C.C.; Wang, Q.; Sugisaki, H.; Li, C.; Chang, Y.M.; Alnami, I.; Syong, W.R.; et al. Oriented lateral growth of two-dimensional materials on c-plane sapphire. Nat. Nanotechnol. 2023, 18, 1289–1294. [Google Scholar] [CrossRef] [PubMed]
- Panasyuk, G.P.; Azarova, L.A.; Belan, V.N.; Semenov, E.A.; Danchevskaya, M.N.; Voroshilov, I.L.; Kozerozhets, I.V.; Pershikov, S.A. Preparation of fine-grained corundum powders with given properties: Crystal size and habit control. Theor. Found. Chem. Eng. 2018, 52, 879–886. [Google Scholar] [CrossRef]
- Zhu, Z.; Gao, Y.; Shi, Z.; Zhang, X. Study on surface characteristics of as-sawn sapphire crystal wafer considering diamond saw wire wear. Wear 2023, 530–531, 205037. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Chang, C.Y.; Hsiao, Y.K.; Chen, C.C.A.; Tu, C.C.; Kuo, H.C. Recent advances in silicon carbide chemical mechanical polishing technologies. Micromachines 2022, 13, 1752. [Google Scholar] [CrossRef]
- Ng, C.K.; Chen, C.; Yang, Y.; Zhang, F.; Ju, B.F.; Chen, Y.L. Femtosecond laser micro-machining of three-dimensional surface profiles on flat single crystal sapphire. Opt. Laser Technol. 2024, 170, 110205. [Google Scholar] [CrossRef]
- Chen, J.; Lu, X.; Li, Z.; Wen, Q.; Lu, J.; Jiang, F. Anisotropy of material removal during laser-induced plasma assisted ablation of sapphire. Ceram. Int. 2022, 48, 13880–13889. [Google Scholar] [CrossRef]
- Dang, G.T.; Yasuoka, T.; Kawaharamura, T. Sub-μm features patterned with laser interference lithography for the epitaxial lateral overgrowth of α-Ga2O3 via mist chemical vapor deposition. Appl. Phys. Lett. 2021, 119, 041902. [Google Scholar] [CrossRef]
- Uhlmann, E.; List, M.; Patraschkov, M.; Tracht, G. A new process design for manufacturing sapphire wafers. Precis. Eng. 2018, 53, 146–150. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, J.; Xu, X.; Chen, C.C.A.; Lin, Y. Study on high efficient sapphire wafer processing by coupling SG-mechanical polishing and GLA-CMP. Int. J. Mach. Tools Manuf. 2018, 130–131, 12–19. [Google Scholar] [CrossRef]
- Zhu, H.; Tessaroto, L.A.; Sabia, R.; Greenhut, V.A.; Smith, M.; Niesz, D.E. Chemical mechanical polishing (CMP) anisotropy in sapphire. Appl. Surf. Sci. 2004, 236, 120–130. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.L.; Tan, B.M.; Li, W.W.; Zhou, J.W. Fine polishing of sapphire substrates. Microelectronics 2006, 36, 46–48. [Google Scholar]
- Herzog, A.H.; Walsh, R.J. Process for Polishing Semi-Conductor Materials. U.S. Patent 3170273 A, 23 February 1965. [Google Scholar]
- Krishnan, M.; Nalaskowski, J.W.; Cook, L.M. Chemical mechanical planarization: Slurry chemistry, materials and mechanisms. Chem. Rev. 2010, 110, 178–204. [Google Scholar] [CrossRef]
- Sharma, S.; Favela, C.A.; Yu, B.; Galstyan, E.; Selvamanickam, V. Conversion efficiency improvement of ELO GaAs solar cell, deposited on water soluble sacrificial buffer. Surf. Coat. Technol. 2023, 456, 129282. [Google Scholar] [CrossRef]
- Lai, S.M.; Chen, Y.Y.; Liu, C.P.; Hsieh, C.K.; Lin, J.Y. Degradation of inhibitor in alkaline cleaning solution for post-Cu CMP cleaning. Surf. Coat. Technol. 2018, 350, 1080–1084. [Google Scholar] [CrossRef]
- Zhou, Y.; Pan, G.; Shi, X.; Gong, H.; Luo, G.; Gu, Z. Chemical mechanical planarization (CMP) of on-axis Si-face SiC wafer using catalyst nanoparticles in slurry. Surf. Coat. Technol. 2014, 251, 48–55. [Google Scholar] [CrossRef]
- Wei, K.H.; Wang, Y.S.; Liu, C.P.; Chen, K.W.; Wang, Y.L.; Cheng, Y.L. The influence of abrasive particle size in copper chemical mechanical planarization. Surf. Coat. Technol. 2013, 231, 543–545. [Google Scholar] [CrossRef]
- Lin, Z.C.; Chen, C.C. Distribution of polishing times for a wafer with different patterned polishing pads during CMP and CCMP. Surf. Coat. Technol. 2010, 204, 3101–3107. [Google Scholar] [CrossRef]
- Bun-Athuek, N.; Takazaki, H.; Yoshimoto, Y.; Khajornrungruang, P.; Yasunaga, T.; Suzuki, K. Effects of mixed ultrafine colloidal silica particles on chemical mechanical polishing of sapphire. Jpn. J. Appl. Phys. 2018, 57, 07MD03. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Z.; Wu, B.; Hu, W.; Meng, F.; Li, Y. A review: Green chemical mechanical polishing for metals and brittle wafers. J. Phys. D Appl. Phys. 2021, 54, 373001. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, B.; Li, Z.C. An overview of recent advances in chemical mechanical polishing (CMP) of sapphire substrates. ECS Trans. 2013, 52, 495–500. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, X.; Yuan, J.; Guo, L.; Hong, T.; Hang, W.; Ma, Y. Study on the influence of sapphire crystal orientation on its chemical mechanical polishing. Appl. Sci. 2020, 10, 8065. [Google Scholar] [CrossRef]
- Zhou, Y.; Pan, G.S.; Shi, X.L.; Gong, H.; Zou, C.L.; Tang, J.N. Atomic step morphology research of LED sapphire substrate polishing surface and its periodicity. Opt. Precis. Eng. 2017, 25, 100–106. (In Chinese) [Google Scholar] [CrossRef]
- Zhou, Y.; Pan, G.; Shi, X.; Gong, H.; Xu, L.; Zou, C. AFM and XPS studies on material removal mechanism of sapphire wafer during chemical mechanical polishing (CMP). J. Mater. Sci. Mater. Electron. 2015, 26, 9921–9928. [Google Scholar] [CrossRef]
- Shi, X.; Pan, G.; Zhou, Y.; Xu, L.; Zou, C.; Gong, H. A study of chemical products formed on sapphire (0001) during chemical-mechanical polishing. Surf. Coat. Technol. 2015, 270, 206–220. [Google Scholar] [CrossRef]
- Shi, X.; Xu, L.; Zhou, Y.; Zou, C.; Wang, R.; Pan, G. An in situ study of chemical-mechanical polishing behaviours on sapphire (0001) via simulating the chemical product-removal process by AFM-tapping mode in both liquid and air environments. Nanoscale 2018, 10, 19692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, W.; Song, Z.; Hu, X. Two-step chemical mechanical polishing of sapphire substrate. J. Electrochem. Soc. 2010, 157, H688–H691. [Google Scholar] [CrossRef]
- Vovk, E.A.; Budnikov, A.T.; Dobrotvorskaya, M.V.; Krivonogov, S.I.; Dan’ko, A.Y. Mechanism of the interaction between Al2O3 and SiO2 during the chemical-mechanical polishing of sapphire with silicon dioxide. J. Surf. Investig. X-ray Synchrotron Neutron Tech. 2012, 6, 115–121. [Google Scholar] [CrossRef]
- Yu, H.; Tang, X.; Kong, X.; Li, X.; Li, Y.; Xi, M.; Chang, T.; Meng, D.; Yang, S.; Guo, W.; et al. Electrostatic self-assembled composite abrasives for chemical mechanical polishing of A-plane sapphire. ECS J. Solid State Sci. Technol. 2021, 10, 114002. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, W.; Zhang, L.; Liu, W.; Song, Z. Effect of mechanical process parameters on friction behavior and material removal during sapphire chemical mechanical polishing. Microelectron. Eng. 2011, 88, 3020–3023. [Google Scholar] [CrossRef]
- Lee, H.; Jeong, H. A wafer-scale material removal rate profile model for copper chemical mechanical planarization. Int. J. Mach. Tools Manuf. 2011, 51, 395–403. [Google Scholar] [CrossRef]
- Oh, S.; Seok, J. An integrated material removal model for silicon dioxide layers in chemical mechanical polishing processes. Wear 2009, 266, 839–849. [Google Scholar] [CrossRef]
- Zhao, G.; Wei, Z.; Wang, W.; Feng, D.; Xu, A.; Liu, W.; Song, Z. Review on modeling and application of chemical mechanical polishing. Nanotechnol. Rev. 2020, 9, 182–189. [Google Scholar] [CrossRef]
- Xu, W.; Cheng, Y.; Zhong, M. Effects of process parameters on chemical-mechanical interactions during sapphire polishing. Microelectron. Eng. 2019, 216, 111029. [Google Scholar] [CrossRef]
- Li, Z.; Deng, Z.; Hu, Y. Effects of polishing parameters on surface quality in sapphire double-sided CMP. Ceram. Int. 2020, 46, 13356–13364. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J.; Hu, W.; Zhang, L.; Xie, W.; Liao, L. Chemical mechanical polishing for sapphire wafers using a developed slurry. J. Manuf. Process. 2021, 62, 762–771. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, D.; Chen, T. Chemico-mechanical polishing technique of monocrystal sapphire substrate wafer. Surf. Technol. 2017, 46, 253–261. (In Chinese) [Google Scholar]
- Wang, H.B.; Yu, Q.C.; Liu, W.L.; Song, Z.T.; Zhang, K.L. Process conditions affected chemical mechanical polishing (CMP) on sapphire. J. Funct. Mater. Devices 2010, 16, 206–210. (In Chinese) [Google Scholar]
- Chen, G.; Chen, F.; Ni, Z.; Bai, Y. Study on chemical mechanical polishing of A-plane sapphire wafer. Electroplat. Finish. 2023, 42, 75–82. (In Chinese) [Google Scholar]
- Lin, Z.C.; Huang, W.S.; Tsai, J.S. A study of material removal amount of sapphire wafer in application of chemical mechanical polishing with different polishing pads. J. Mech. Sci. Technol. 2012, 26, 2353–2364. [Google Scholar] [CrossRef]
- Zhu, H.; Niesz, D.E.; Greenhut, V.A. The effect of abrasive hardness on the chemical-assisted polishing of (0001) plane sapphire. J. Mater. Res. 2005, 20, 504–520. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Z.; Lu, S. Study of several silica properties influence on sapphire CMP. J. Electr. Eng. Technol. 2018, 13, 886–891. [Google Scholar]
- Hu, X.; Song, Z.; Pan, Z.; Liu, W.; Wu, L. Planarization machining of sapphire wafers with boron carbide and colloidal silica as abrasives. Appl. Surf. Sci. 2009, 255, 8230–8234. [Google Scholar] [CrossRef]
- Xiong, W.; Chu, X.; Dong, Y.; Lei, B.; Ye, M.; Sun, W. Effect of different abrasives on sapphire chemical-mechanical polishing. J. Synth. Cryst. 2013, 42, 1064–1106. (In Chinese) [Google Scholar]
- Zhong, M.; Yuan, R.; Li, X.; Chen, J.; Xu, W. Effects of abrasive particles and pads’ characteristics on ultrasonic assisted chemical mechanical polishing for sapphire. China Surf. Eng. 2018, 31, 125–132. (In Chinese) [Google Scholar]
- Gao, S.; Kang, R.K.; Jin, Z.J.; Dong, Z.G. Research on the polishing performance of CMP slurry for the sapphire crystal. Adv. Mater. Res. 2011, 325, 457–463. [Google Scholar] [CrossRef]
- Zhang, Z.; Jin, Z.; Guo, J. The effect of the interface reaction mode on chemical mechanical polishing. CIRP J. Manuf. Sci. Technol. 2020, 31, 539–547. [Google Scholar] [CrossRef]
- Kozerozhets, I.; Semenov, E.; Kozlova, L.; Ioni, Y.; Avdeeva, V.; Ivakin, Y. Mechanism to form nanosized oxides when burning aqueous carbohydrate salt solutions. Mater. Chem. Phys. 2023, 309, 128387. [Google Scholar] [CrossRef]
- Dong, Y.; Lei, H.; Liu, W.; Chen, Y. Preparation of ellipsoidal rod-shaped silica nanocomposite abrasives by Chromium ion/PEG200 induced method for sapphire substrates chemical mechanical polishing. J. Alloys Compd. 2019, 777, 1294–1303. [Google Scholar] [CrossRef]
- Seyedi, S.S.; Shabgard, M.R.; Mousavi, S.B.; Heris, S.Z. The impact of SiC, Al2O3, and B2O3 abrasive particles and temperature on wear characteristics of 18Ni (300) maraging steel in abrasive flow machining (AFM). Int. J. Hydrog. Energy 2021, 46, 33991–34001. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, W.; Zhang, S.; Yan, W. Study on chemical mechanical polishing performances of sapphire wafer (0001) using silica-based slurry. ECS J. Solid State Sci. Technol. 2017, 6, P723–P727. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Xiang, X.; Bian, N. Effects of abrasive on removal rate of sapphire substrate. Semicond. Technol. 2010, 35, 1053–1064. (In Chinese) [Google Scholar]
- Lin, Z.C.; Wang, R.Y. Abrasive removal depth for polishing a sapphire wafer by a cross-patterned polishing pad with different abrasive particle sizes. Int. J. Adv. Manuf. Technol. 2014, 74, 25–36. [Google Scholar] [CrossRef]
- Niu, X.H.; Huang, Y.H.; Zhou, J.W.; Han, L.Y.; Yuan, G.H. Influence of nano abrasive on chemical mechanical ultra-precision machining of sapphire substrate surfaces. Key Eng. Mater. 2014, 609–610, 130–134. [Google Scholar] [CrossRef]
- Bun-Athuek, N.; Yoshimoto, Y.; Sakai, K.; Khajornrungruang, P.; Suzuki, K. Study on effect of the surface variation of colloidal silica abrasive during chemical mechanical polishing of sapphire. Jpn. J. Appl. Phys. 2017, 56, 07KB01. [Google Scholar] [CrossRef]
- Park, C.; Kim, H.; Lee, S.; Jeong, H. The influence of abrasive size on high-pressure chemical mechanical polishing of sapphire wafer. Int. J. Precis. Eng. Manuf. Green Technol. 2015, 2, 157–162. [Google Scholar] [CrossRef]
- Yan, W.X.; Zhang, Z.F.; Guo, X.H.; Liu, W.L.; Song, Z.T. Effect of abrasive concentration on chemical mechanical polishing of sapphire. Chin. Phys. Lett. 2015, 32, 088301. [Google Scholar] [CrossRef]
- Chen, G.; Xu, Y.; Ni, Z.; Bai, Y.; Fan, Q.; Chen, Z. Effects of surfactants on the chemical mechanical polishing performance of a-plane sapphire substrates. ECS J. Solid State Sci. Technol. 2023, 12, 094003. [Google Scholar] [CrossRef]
- Dong, Y.; Lei, H.; Chen, Y.; Liu, W.; Xu, L.; Wang, T.; Dai, S. Preparation of irregular silica nanoparticles by the polymer templating for chemical mechanical polishing of sapphire substrates. J. Electron. Mater. 2019, 48, 4598–4606. [Google Scholar] [CrossRef]
- Dong, Y.; Lei, H.; Liu, W. Preparation of irregular silica nano-abrasives for the chemical mechanical polishing behaviour on sapphire substrates. Micro Nano Lett. 2019, 14, 1328–1333. [Google Scholar] [CrossRef]
- Xu, L.; Lei, H.; Wang, T.; Dong, Y.; Dai, S. Preparation of flower-shaped silica abrasives by double system template method and its effect on polishing performance of sapphire wafers. Ceram. Int. 2019, 45, 8471–8476. [Google Scholar] [CrossRef]
- Dong, Y.; Lei, H.; Liu, W.; Wang, T.; Xu, L. Preparation of non-spherical silica composite abrasives by lanthanum ion-induced effect and its chemical-mechanical polishing properties on sapphire substrates. J. Mater. Sci. 2018, 53, 10732–10742. [Google Scholar] [CrossRef]
- Zhang, B.; Lei, H.; Chen, Y. Preparation of Ag2O modified silica abrasives and their chemical mechanical polishing performances on sapphire. Friction 2017, 5, 429–436. [Google Scholar] [CrossRef]
- Lei, H.; Liu, T.; Xu, L. Synthesis of Sm-doped colloidal SiO2 composite abrasives and their chemical mechanical polishing performances on sapphire substrates. Mater. Chem. Phys. 2019, 237, 121819. [Google Scholar] [CrossRef]
- Lei, H.; Tong, K.; Wang, Z. Preparation of Ce-doped colloidal SiO2 composite abrasives and their chemical mechanical polishing behavior on sapphire substrates. Mater. Chem. Phys. 2016, 172, 26–31. [Google Scholar] [CrossRef]
- Lei, H.; Tong, K.; Zhang, B.; Chen, Y. Preparation of monodisperse Ti-doped colloidal SiO2 composite abrasives and their chemical mechanical polishing performances on sapphire substrates. ECS J. Solid State Sci. Technol. 2016, 5, 674–679. [Google Scholar] [CrossRef]
- Lei, H.; Tong, K. Preparation of La-doped colloidal SiO2 composite abrasives and their chemical mechanical polishing behavior on sapphire substrates. Precis. Eng. 2016, 44, 124–130. [Google Scholar] [CrossRef]
- Ma, P.; Lei, H.; Chen, R. Preparation of cobalt-doped colloidal silica abrasives and their chemical mechanical polishing performances on sapphire. Micro Nano Lett. 2015, 10, 657–661. [Google Scholar] [CrossRef]
- Lei, H.; Gu, Q.; Chen, R.; Wang, Z. Preparation of Fe-doped colloidal SiO2 abrasives and their chemical mechanical polishing behavior on sapphire substrates. Appl. Opt. 2015, 54, 7188–7194. [Google Scholar] [CrossRef]
- Lei, H.; Gu, Q. Preparation of Cu-doped colloidal SiO2 abrasives and their chemical mechanical polishing behavior on sapphire substrates. J. Mater. Sci. Mater. Electron. 2015, 26, 10194–10200. [Google Scholar] [CrossRef]
- Ma, P.; Lei, H.; Chen, Y.; Chen, R. Preparation of Ni-Doped colloidal silica abrasives and their chemical mechanical polishing performances on sapphire. ECS J. Solid State Sci. Technol. 2016, 5, 132–136. [Google Scholar] [CrossRef]
- Lei, H.; Huang, L.; Gu, Q. Synthesis of Zn-doped colloidal SiO2 abrasives and their applications in sapphire chemical mechanical polishing slurry. J. Mater. Sci. Mater. Electron. 2017, 28, 1229–1237. [Google Scholar] [CrossRef]
- Zhou, C.; Xu, X.; Dai, L.; Gong, H.; Lin, S. Chemical-mechanical polishing performance of core-shell structured polystyrene@ceria/nanodiamond ternary abrasives on sapphire wafer. Ceram. Int. 2021, 47, 31691–31701. [Google Scholar] [CrossRef]
- Wang, T.; Lei, H.; Dong, Y.; Xu, L.; Dai, S. Highly efficient removal of sapphire by composite nanoabrasive with novel inorganic polyelectrolyte as a binder. J. Alloys Compd. 2019, 782, 709–715. [Google Scholar] [CrossRef]
- Wang, T.; Lei, H. Novel polyelectrolyte-Al2O3/SiO2 composite nanoabrasives for improved chemical mechanical polishing (CMP) of sapphire. J. Mater. Res. 2019, 34, 1073–1082. [Google Scholar] [CrossRef]
- Yin, D.; Niu, X.; Zhang, K.; Wang, J.; Cui, Y. Preparation of MgO doped colloidal SiO2 abrasive and their chemical mechanical polishing performance on c-, r- and a-plane sapphire substrate. Ceram. Int. 2018, 44, 14631–14637. [Google Scholar] [CrossRef]
- Dai, S.; Lei, H.; Fu, J. Preparation of SiC/SiO2 hard core–soft shell abrasive and its CMP behavior on sapphire substrate. J. Electron. Mater. 2020, 49, 1301–1307. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, J.; Xu, X. Study on planarization machining of sapphire wafer with soft-hard mixed abrasive through mechanical chemical polishing. Appl. Surf. Sci. 2016, 389, 713–720. [Google Scholar] [CrossRef]
- Wang, X.; Lei, H. Preparation of γ-alumina/silica core–shell abrasives and their chemical mechanical polishing performances on sapphire substrates. Micro Nano Lett. 2018, 13, 1315–1320. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.Q.; Suo, S.X.; Yu, S.M. Preparation of CeO2/ZrO2 colloidal SiO2 composite abrasive and its polishing behavior on sapphire. Bull. Chin. Ceram. Soc. 2018, 37, 3021–3027. (In Chinese) [Google Scholar]
- Zhou, C.; Xu, X.Y.; Lin, S.T.; Yao, Y.F. Preparation of DND@CeO2 core-shell abrasives and their chemical mechanical polishing performances on sapphire wafers. Min. Metall. Eng. 2021, 41, 115–120. (In Chinese) [Google Scholar]
- Bai, L.S.; Xiong, W.; Chu, X.F.; Dong, Y.P.; Zhang, W.B. Preparation of nano SiO2/CeO2 composite particles and their applications to CMP on sapphire substrates. Opt. Precis. Eng. 2014, 22, 1189–1295. (In Chinese) [Google Scholar]
- Wang, H.; Yang, J.; Lu, S.; Zhang, Z.; Jiang, X.; Wang, F.; Fan, M.; Peng, Y. Effect of particle hybrid on sapphire polishing performance. Lubr. Eng. 2018, 43, 43–54. (In Chinese) [Google Scholar]
- Park, C.; Kim, H.; Cho, H.; Lee, T.; Kim, D.; Lee, S.; Jeong, H. Efect of relative surface charge of colloidal silica and sapphire on removal rate in chemical mechanical polishing. Int. J. Precis. Eng. Manuf. Green Technol. 2019, 6, 339–347. [Google Scholar] [CrossRef]
- Liu, T.; Lei, H. Nd3+-doped colloidal SiO2 composite abrasives: Synthesis and the effects on chemical mechanical polishing (CMP) performances of sapphire wafers. Appl. Surf. Sci. 2017, 413, 16–26. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Q.; Liu, W.; Song, Z. Effect of particle size distribution, pH, and Na+ concentration on the chemical mechanical polishing of sapphire and 4H-SiC (0001). ECS J. Solid State Sci. Technol. 2022, 11, 044004. [Google Scholar] [CrossRef]
- Yin, D.; Niu, X.; Yang, L.; Zhang, K.; Wang, J.; Cui, Y.; Wang, R. Effect of Sr(OH)2 as a pH regulator on different plane sapphire substrate chemical mechanical polishing. ECS J. Solid State Sci. Technol. 2019, 8, 63–69. [Google Scholar] [CrossRef]
- Wang, J.; Niu, X.; Zhao, X.; Liu, Y. Effect of a pH regulator on sapphire substrate CMP. ECS J. Solid State Sci. Technol. 2017, 6, 832–838. [Google Scholar] [CrossRef]
- Deng, H.; Zhong, M.; Xu, W. Investigation of green alkaline pH regulators on sapphire UV-CMP. Tribol. Int. 2023, 178, 108047. [Google Scholar] [CrossRef]
- Yan, W.; Zhang, Z.; Guo, X.; Liu, W.; Song, Z. The effect of pH on sapphire chemical mechanical polishing. ECS J. Solid State Sci. Technol. 2015, 4, 108–111. [Google Scholar] [CrossRef]
- Xie, W.; Zhang, Z.; Liao, L.; Liu, J.; Su, H.; Wang, S.; Guo, D. Green chemical mechanical polishing of sapphire wafers using a novel slurry. Nanoscale 2020, 12, 22518. [Google Scholar] [CrossRef]
- Qu, M.; Niu, X.; Hou, Z.; Yan, H.; Luo, F. Effect of hydroxy carboxylates as complexing agent on improving chemical mechanical polishing performance of M-plane sapphire and action mechanism analysis. Ceram. Int. 2023, 49, 9622–9631. [Google Scholar] [CrossRef]
- Zhang, W.; Lei, H.; Liu, W.; Zhang, Z. Effect of the carboxyl group number of the complexing agent on polishing performance of alumina slurry in sapphire CMP. Ceram. Int. 2023, 49, 13687–13697. [Google Scholar] [CrossRef]
- Qu, M.; Niu, X.; Hou, Z.; Yan, H.; Luo, F.; Zhang, Y.; Zhu, Y. Effect of chitosan oligosaccharide as a complexing agent on chemical mechanical polishing performance of c-, a-, and r-plane sapphire substrate. ECS J. Solid State Sci. Technol. 2022, 11, 104005. [Google Scholar] [CrossRef]
- Zhao, X.; Niu, X.; Wang, J.; Yin, D.; Yao, C. Role of a new type chelating agent in chemical mechanical polishing of r-plane sapphire substrate. ECS J. Solid State Sci. Technol. 2017, 6, 618–625. [Google Scholar] [CrossRef]
- Xiong, W.; Bai, L.; Chu, X.; Dong, Y.; Chen, J.; Bi, L.; Ye, M. Effect of chelating agent on chemical mechanical polishing quality of sapphire. Mech. Sci. Technol. Aerosp. Eng. 2014, 33, 1027–1030. (In Chinese) [Google Scholar]
- Niu, X.; Liu, X.; Wang, S.; Tan, B. High precision finishing technique of sapphire substrate surface for photoconducting device. Mater. Sci. Forum 2011, 663–665, 80–83. [Google Scholar] [CrossRef]
- Bai, Y.W.; Chen, G.M.; Teng, K.; Ni, Z.F. Effect of cationic surfactant on chemical mechanical polishing efficiency of a-plane sapphire. J. Synth. Cryst. 2018, 47, 470–475. (In Chinese) [Google Scholar]
- Chen, G.; Du, C.; Ni, Z.; Bai, Y.; Liu, Y.; Zhao, Y. Effect of amphoteric surfactant on the chemical mechanical polishing of A-plane sapphire wafer. Modern Manuf. Eng. 2020, 483, 83–87. (In Chinese) [Google Scholar]
- Zhao, X.; Niu, X.; Yin, D.; Wang, J.; Zhang, K. Research on r-plane sapphire substrate CMP removal rate based on a new-type alkaline slurry. ECS J. Solid State Sci. Technol. 2018, 7, P135–P141. [Google Scholar] [CrossRef]
- Deng, H.; Zhong, M.; Xu, W. Effects and mechanisms of different types of surfactants on sapphire ultrasonic polishing. Tribol. Int. 2023, 187, 108734. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, W.; Song, Z. Particle size and surfactant effects on chemical mechanical polishing of glass using silica-based slurry. Appl. Opt. 2010, 49, 5480–5485. [Google Scholar] [CrossRef]
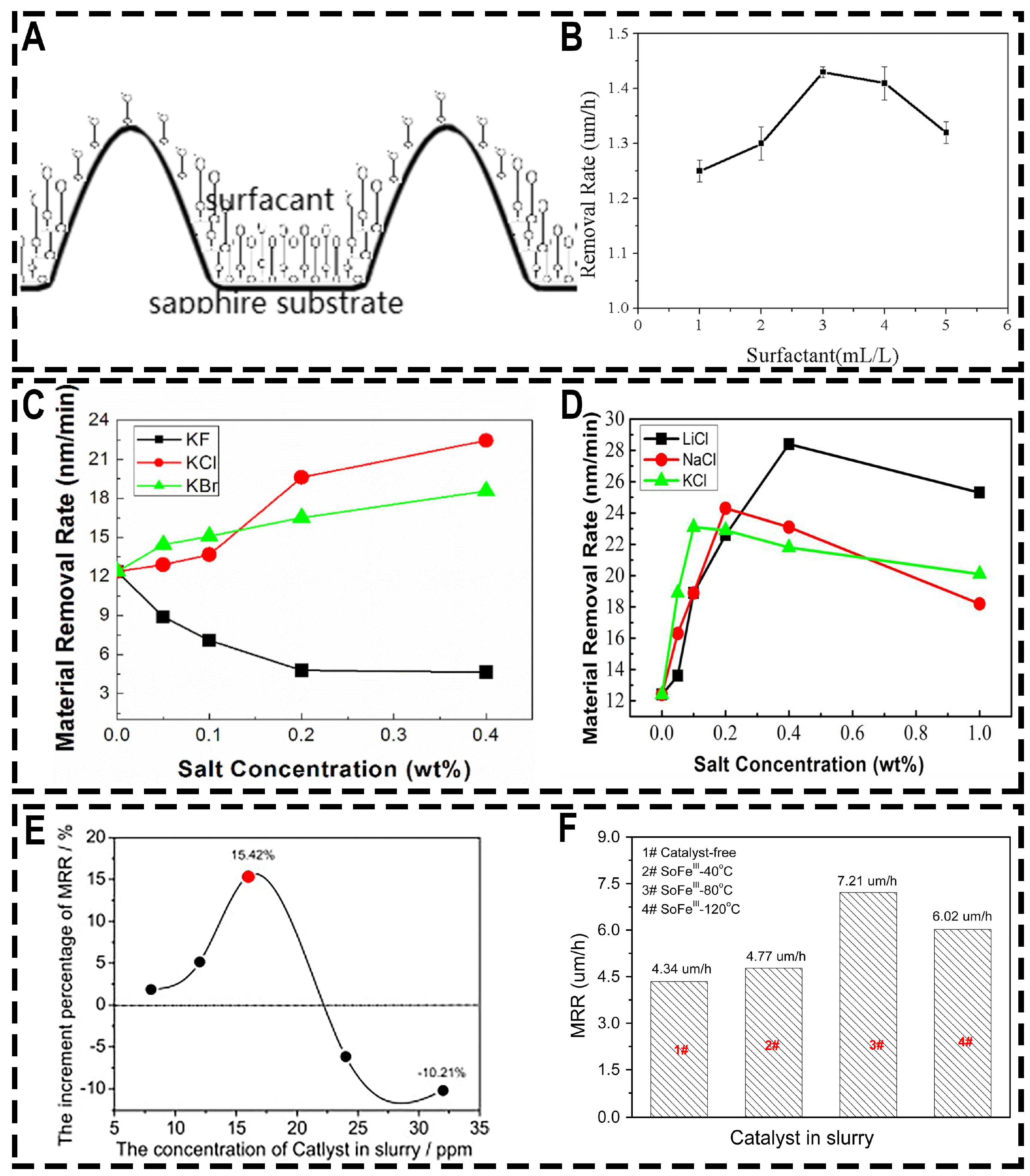
- Cui, Y.; Niu, X.; Zhou, J.; Wang, Z.; Wang, R.; Zhang, J. Effect of chloride ions on the chemical mechanical planarization efficiency of sapphire substrate. ECS J. Solid State Sci. Technol. 2019, 8, 488–495. [Google Scholar] [CrossRef]
- Lu, Y.; Niu, X.; Yang, C.; Huo, Z.; Cui, Y.; Zhou, J.; Wang, Z. Effect of potassium persulfate as an additive on chemical mechanical polishing performance on c-, a- and r-plane sapphire. ECS J. Solid State Sci. Technol. 2020, 9, 064006. [Google Scholar] [CrossRef]
- Xu, L.; Zou, C.; Shi, X.; Pan, G.; Luo, G.; Zhou, Y. Fe-Nx/C assisted chemical-mechanical polishing for improving the removal rate of sapphire. Appl. Surf. Sci. 2015, 343, 115–120. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, X.; Kang, C.; Wang, R.; Zou, C.; Zhou, Y.; Pan, G. Preparation of a novel catalyst (SoFeIII) and its catalytic performance towards the removal rate of sapphire substrate during CMP process. Tribol. Int. 2018, 120, 99–104. [Google Scholar] [CrossRef]
- Xu, W.; Lu, X.; Pan, G.; Lei, Y.; Luo, J. Effects of the ultrasonic flexural vibration on the interaction between the abrasive particles; pad and sapphire substrate during chemical mechanical polishing (CMP). Appl. Surf. Sci. 2011, 257, 2905–2911. [Google Scholar] [CrossRef]
- Xu, W.; Lu, X.; Pan, G.; Lei, Y.; Luo, J. Ultrasonic flexural vibration assisted chemical mechanical polishing for sapphire substrate. Appl. Surf. Sci. 2010, 256, 3936–3940. [Google Scholar] [CrossRef]
- Zhou, M.; Cheng, Y.; Zhong, M.; Xu, W. Macro and micro-nano machining mechanism for ultrasonic vibration assisted chemical mechanical polishing of sapphire. Appl. Surf. Sci. 2023, 640, 158343. [Google Scholar] [CrossRef]
- Deng, H.; Zhong, M.; Xu, W. Effects of different dispersants on chemical reaction and material removal in ultrasonic assisted chemical mechanical polishing of sapphire. ECS J. Solid State Sci. Technol. 2022, 11, 033007. [Google Scholar] [CrossRef]
- Zhou, M.; Zhong, M.; Xu, W. Novel model of material removal rate on ultrasonic-assisted chemical mechanical polishing for sapphire. Friction 2023, 11, 2073–2090. [Google Scholar] [CrossRef]
- Li, Z.; Deng, Z.; Ge, J.; Zhuo, R.; Wan, L. Material removal rate prediction for sapphire double-sided CMP based on RSM-SVM. ECS J. Solid State Sci. Technol. 2022, 11, 084002. [Google Scholar] [CrossRef]
- Li, Z.; Deng, Z.; Ge, J.; Liu, T.; Wan, L. Experimental and theoretical analysis of single-sided and double-sided chemical mechanical polishing of sapphire wafers. Int. J. Adv. Manuf. Technol. 2022, 119, 5095–5106. [Google Scholar] [CrossRef]
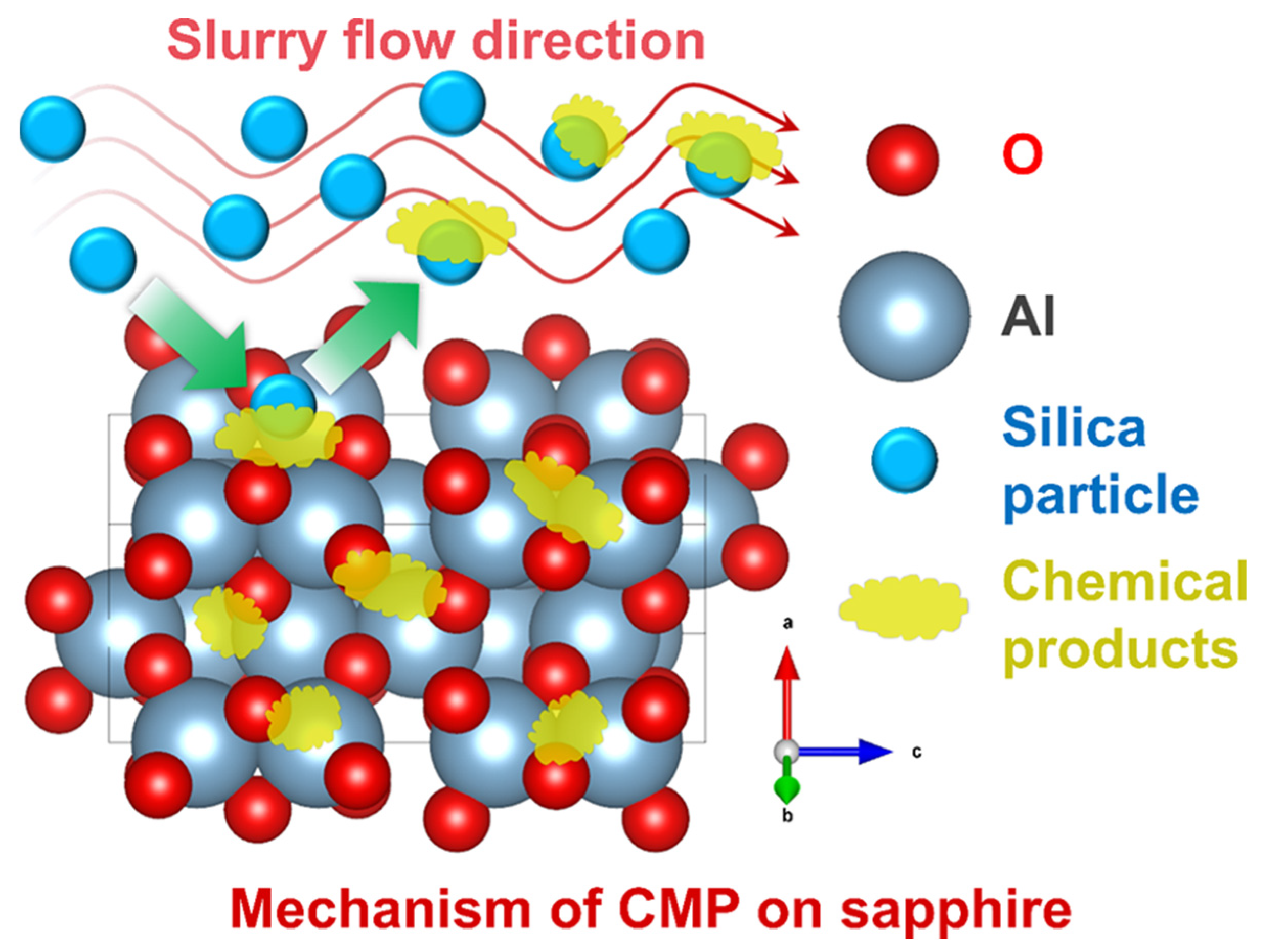
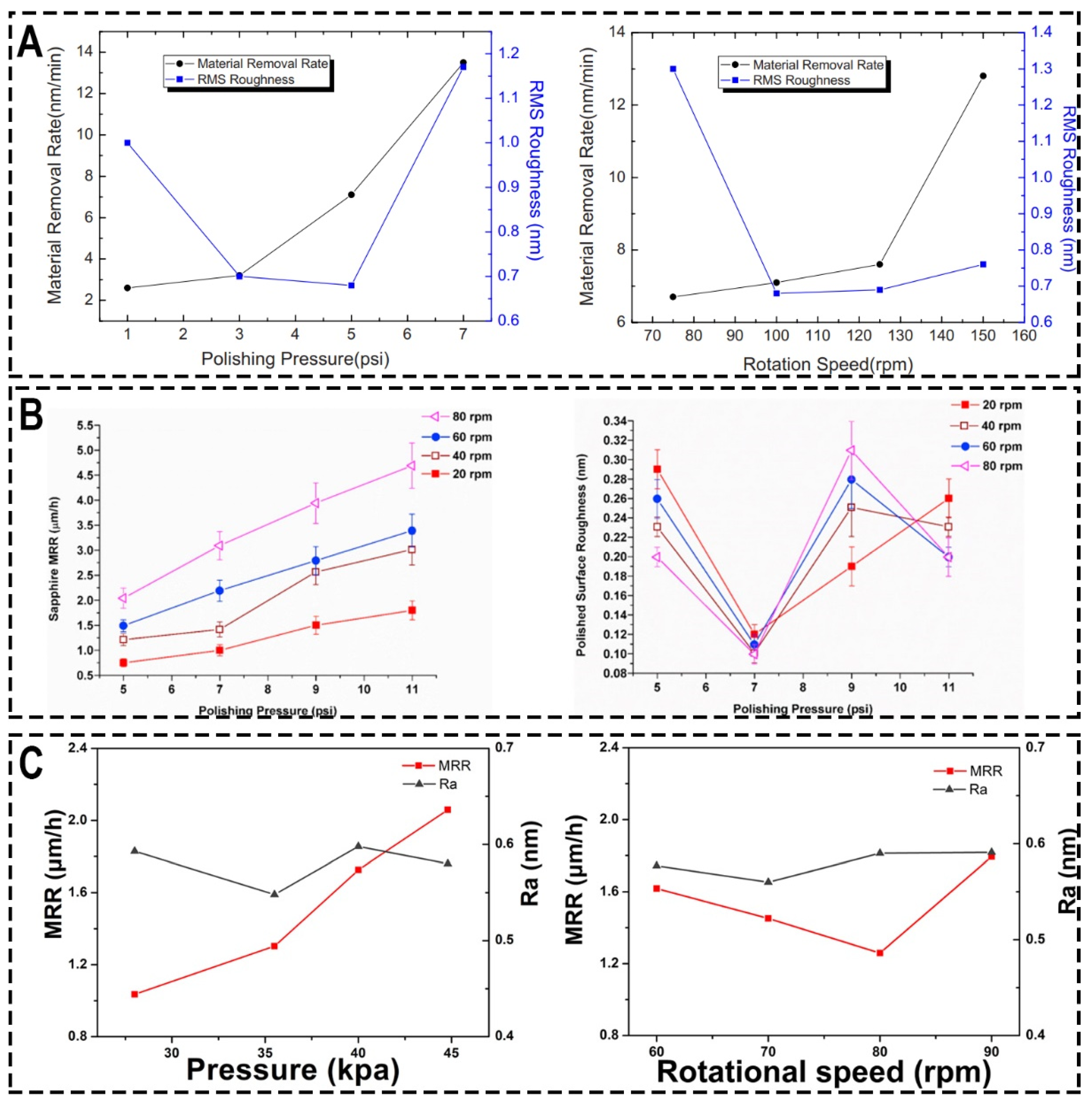
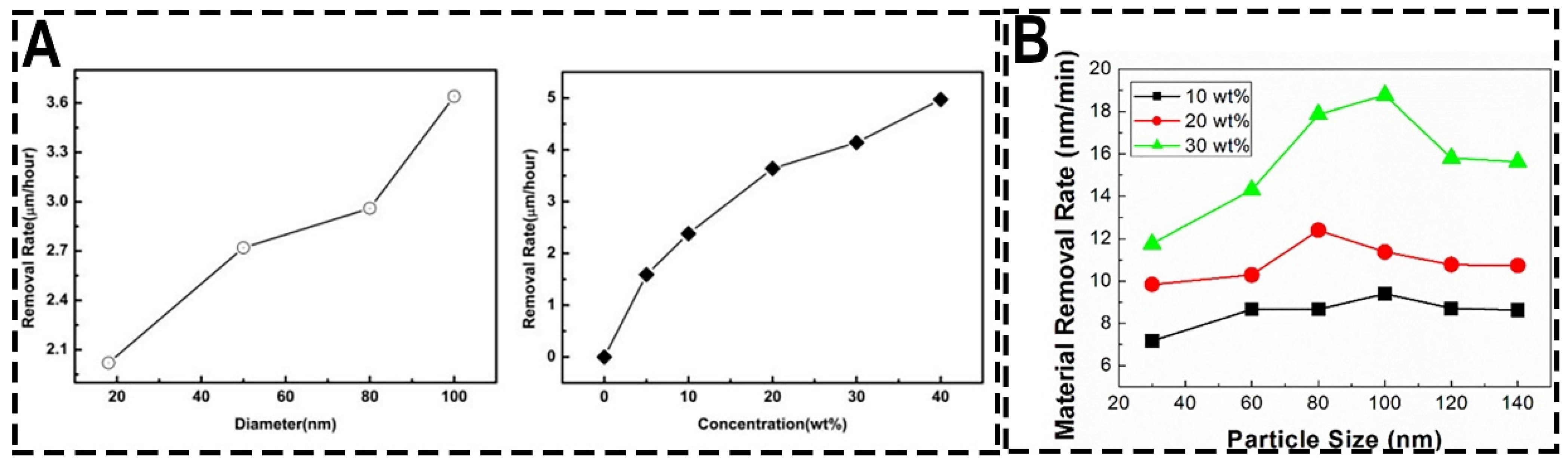
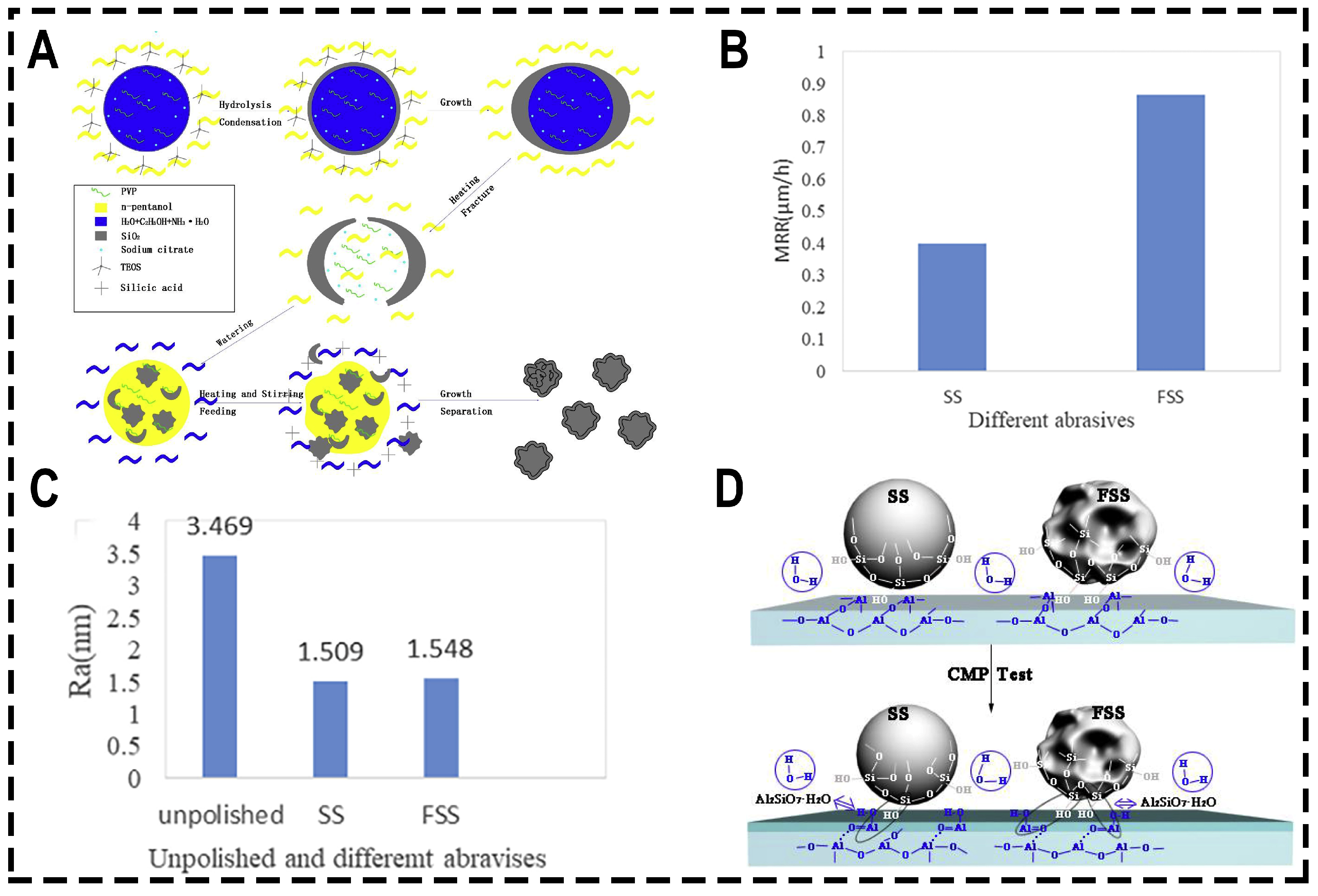
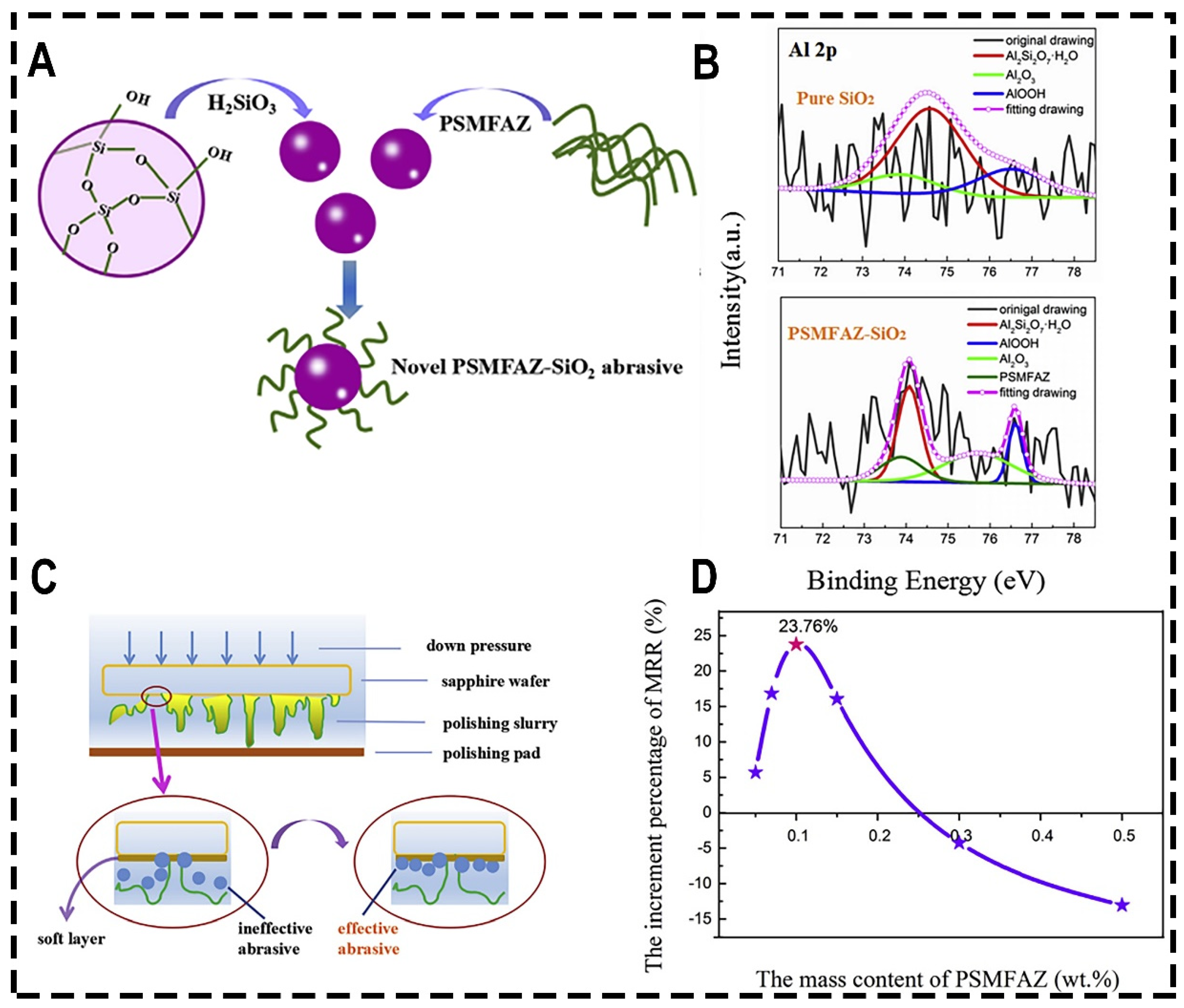



| Pressure (psi) | Rotational Speed (rpm) | MRR (μm/h) | Ra (nm) | Ref. |
|---|---|---|---|---|
| 1, 3, 5, 7 | 100 | MRR increases with increasing pressure | With the change of pressure, it decreases first, reaches the minimum at 5 psi, and then increases | [53] |
| 5 | 75, 100, 125, 150 | MRR increases with increasing rotational speed | With the change of rotational speed, it decreases first, reaches the minimum at 100 rpm, and then increases | [53] |
| 5 | 75, 100, 125, 150 | MRR increases with increasing rotational speed | / | [56] |
| 5, 7, 9, 11 | 60 | MRR increases with increasing pressure | With the change of pressure, it decreases first, reaches the minimum at 7 psi, and then increases | [60] |
| 3.2, 4.17, 5.13, 6.09 | 10, 20, 30, 40 | MRR increases with increasing pressure and rotational speed | With the change of pressure and rotational speed, it decreases first, reaches the minimum, and then increases | [61] |
| 4.06, 5.15, 5.8, 6.5 | / | MRR increases with increasing pressure | With the change of pressure, it decreases first, reaches the minimum, and then increases | [62] |
| / | 60, 70, 80, 90 | With the change of rotational speed, it decreases first, reaches the minimum, and then increases | With the change of rotational speed, it decreases first, reaches the minimum, and then increases | [62] |
| 2.47, 3.39, 5.04, 6.54, 8.27, 10.01 | 40 | MRR increases with increasing pressure | / | [63] |
| 6.54 | 20, 30, 40, 50, 60 | MRR increases with increasing rotational speed | / | [63] |
| 3, 5, 7, 10 | / | With the change of pressure, it increase first, reaches the minimum, and then increases | With the change of pressure, it decreases first, reaches the minimum, and then increases | [64] |
| 1.4, 2.1, 2.8, 3.49, 4.2 | / | MRR increases with increasing pressure | With the change of pressure, it decreases first, reaches the minimum, and then increases | [65] |
| Abrasive | Size (μm) | Mohs Hardness | MRR of Sapphire (mg/h) | Ra (nm) | Scratch (nm) |
|---|---|---|---|---|---|
| Monodiamond | 0–0.25 | 10 | 0.22 ± 0.04 | 2.3 | 12 |
| Polydiamond | 0–0.25 | 10 | 0.13 ± 0.02 | 0.5 | 2 |
| α-Al2O3 | 0.16 | 9 | 0.47 ± 0.08 | 0.4 | 0.8 |
| α-Al2O3 | 0.2 | 9 | 0.43 ± 0.08 | 0.3 | 0.8 |
| γ-Al2O3 | 3 | 8 | 0.2 ± 0.04 | 1.0 | 1 |
| Silica | 0.2 | 7 | 0.11 ± 0.03 | 0.4 | 1.0 |
| ZrO2 | 0.4 | 6.5 | 0.13 ± 0.01 | 0.4 | 1.0 |
| CeO2 | 0.64 | 6 | 0.00 ± 0.01 | 16 | 30 |
| Abrasive | Size (nm) | Concentration (wt%) | MRR (μm/h) | Conclusion | Ref. |
|---|---|---|---|---|---|
| colloid silica | 20, 50, 80, 100 | 20 | 2.02–3.64 | With the increase of size and concentration of abrasive, the sapphire MRRs increase. | [68] |
| colloid silica | 100 | 0–40 | 0–4.97 | [68] | |
| colloid silica | 30, 60, 80, 100, 120, 140 | 10–30 | / | [77] | |
| colloid silica | 20–60 | 0–70 | / | [78] | |
| colloid silica | 20, 30, 40, 50, 60 | / | / | [79] | |
| mixed sol of SiO2-Al2O3 | 20–30 | SiO2 is 33, Al2O3 is 0–2.5 | 6–11.25 | [80] | |
| colloid silica | 20, 55 | / | 0.42, 1.42 | [81] | |
| colloid silica | 40, 72, 82.5 | / | 0.36, 0.56, 0.39 at 400 g/cm3 pressure | [82] | |
| colloid silica | 40 | 6, 20, 40 wt% | 0.36, 0.86, 1.23 | [82] | |
| silica particle | / | 5–40 wt% | 0.3–1.38 | [83] | |
| colloidal silica | 80 | 2–12 wt% | 1.25–2.027 | [84] |
| Composite Abrasives | The Changing Trend of MRR | The Changing Trend of Ra | Conclusion | Ref. |
|---|---|---|---|---|
| Ag-doped colloidal silica | MRR increases with the increase of Ag+ doping amount | With increase of Ag+ doping amount, Ra decreases first, reaches the minimum, and then increases | In the polishing process, the doping of metal ions can accelerate the solid-state reaction between the abrasive and the sapphire, thereby increasing the MRR and reducing the Ra of sapphire | [89] |
| Sm-doped colloidal silica | MRR increases with the increase of Sm3+ doping amount | With increase of Sm3+ doping amount, Ra decreases first, reaches the minimum, and then increases | [90] | |
| CeO2-doped colloidal SiO2 | MRR increases with the increase of CeO2 doping amount | Ra decreases with increase of CeO2 doping amount | [91] | |
| Ti-doped colloidal SiO2 | MRR increases with the increase of Ti doping amount | Ra decreases with increase of Ti doping amount | [92] | |
| Zn-doped colloidal SiO2 | With increase of Zn(OH)2 doping amount, MRR increases first, reaches the maximum, and then decreases | With increase of Zn(OH)2 doping amount, Ra decreases first, reaches the minimum, and then increases | [98] | |
| PS@CeO2/DND | MRR for PS@CeO2, PS@DND and PS@CeO2/DND reached 1.2, 1.6 and 1.7 μm/h, respectively | Ra for PS@CeO2, PS@DND and PS@CeO2/DND reached 1.25, 0.63 and 0.52 nm, respectively | Under the combined action of super hard diamond particles, chemically active abrasive CeO2 and elastic PS balls, PS@CeO2/DND has good CMP performance | [99] |
| PSMFAZ-SiO2 | With increase of PSMFAZ doping amount, MRR increases first, reaches the maximum, and then decreases | With increase of PSMFAZ doping amount, Ra decreases first, reaches the minimum, and then increases | The addition of PSMFAZ reduces the electrostatic repulsion between particles and increases the contact between sapphire and abrasive, thus obtaining a higher MRR | [100] |
| γ-Al2O3/SiO2 | With increase of SiO2 doping amount, MRR increases first, reaches the maximum, and then decreases | With increase of SiO2 doping amount, Ra decreases first, reaches the minimum, and then increases | After the introduction of silica, the hydration of gamma-alumina is prevented, and the removal amount is increased | [105] |
| pH Conditioning Agent | The Changing Trend of MRR | The Changing Trend of Ra | Conclusion | Ref. |
|---|---|---|---|---|
| KOH, HNO3 | As the pH increases, from acidic to basic, the MRR gradually increases until it is saturated | Ra has barely changed | When pH changes, the MRR of sapphire is mainly affected by the change of Zeta potential of abrasive and hydration layer formed in sapphire. Due to electrostatic attraction, more abrasives are involved in the polishing process under acidic conditions, which increases the MRR under acidic conditions. Under alkaline conditions, the surface of sapphire is more likely to form a hydration layer, so the MRR of sapphire under alkaline conditions is higher | [110] |
| KOH | Under basic conditions, MRR increased with the increase of pH value | Ra decreases with increase of pH value | The higher the pH value, the thicker the hydration layer formed on the sapphire surface, and the faster the sapphire removal rate | [112] |
| Sr(OH)2 | With increase of pH value, MRR increases first, reaches the maximum, and then decreases | / | Sr(OH)2 can not only condition the pH of the polishing liquid, but also hydrate with the sapphire, promoting the chemical reaction between the slurry and the sapphire substrate | [113] |
| FA/O and KOH | When the volume ratio of FA/O to KOH is 1:20, the MRR is the highest | When the volume ratio of FA/O to KOH is 1:20, the Ra is the lowest | When the mixed base of the organic base and KOH is used as a pH regulator, the strong base can maintain the alkaline environment, enhance the stability of the slurry, the organic base can precipitate OH− precipitate, and the reaction product can be removed in time, resulting in the reduction of MRR | [114] |
| Complexing Agent | The Changing Trend of MRR | Conclusion | Ref. |
|---|---|---|---|
| Sorbitol | / | The Al(OH)4− ions formed on the sapphire surface chelate with the chelating agent to form chelates. Chelates are soluble and easily removed from the sapphire surface by abrasives, so the MRR of a sapphire can be increased | [117] |
| PT, Cit, Gluc | With the addition of PT, Cit, and Gluc, MRR increases first and then decreases | [118] | |
| NaAc, Na2C2O4, NTA, EDTA-2Na, and DTPA | MRR first increased and then decreased with the increase of carboxylic acid in the complex agent | [119] | |
| Chitosan oligosaccharide | With the increase of chitosan oligosaccharide doping amount, MRR increases first, reaches the maximum, and then decreases | [120] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Fu, J.; He, Z.; Luo, Y.; Wu, S. Nanomaterials and Equipment for Chemical–Mechanical Polishing of Single-Crystal Sapphire Wafers. Coatings 2023, 13, 2081. https://doi.org/10.3390/coatings13122081
Li S, Fu J, He Z, Luo Y, Wu S. Nanomaterials and Equipment for Chemical–Mechanical Polishing of Single-Crystal Sapphire Wafers. Coatings. 2023; 13(12):2081. https://doi.org/10.3390/coatings13122081
Chicago/Turabian StyleLi, Shaoping, Jieni Fu, Zhaobo He, Yue Luo, and Shuilin Wu. 2023. "Nanomaterials and Equipment for Chemical–Mechanical Polishing of Single-Crystal Sapphire Wafers" Coatings 13, no. 12: 2081. https://doi.org/10.3390/coatings13122081
APA StyleLi, S., Fu, J., He, Z., Luo, Y., & Wu, S. (2023). Nanomaterials and Equipment for Chemical–Mechanical Polishing of Single-Crystal Sapphire Wafers. Coatings, 13(12), 2081. https://doi.org/10.3390/coatings13122081






