Nanomaterials for Potential Detection and Remediation: A Review of Their Analytical and Environmental Applications
Abstract
:1. Introduction
2. Nanomaterials for the Detection of Heavy Metals
2.1. Noble Metal NPs for the Colorimetric Detection of Heavy Metals
2.2. Nanocomposites for the Detection of Heavy Metals
3. Nanomaterials for the Detection of Agrochemicals: Pesticides, Herbicides, and Insecticides
3.1. Nanomaterials for the Detection of Organophosphorous Pesticides
3.2. Nanomaterials for the Detection of Atrazine
3.3. Nanomaterials for the Detection of Carbamates and Dithiocarbamates
3.4. Nanomaterials for the Detection of Glyphosate
3.5. Nanomaterials for the Detection of Triazine Pymetrozine
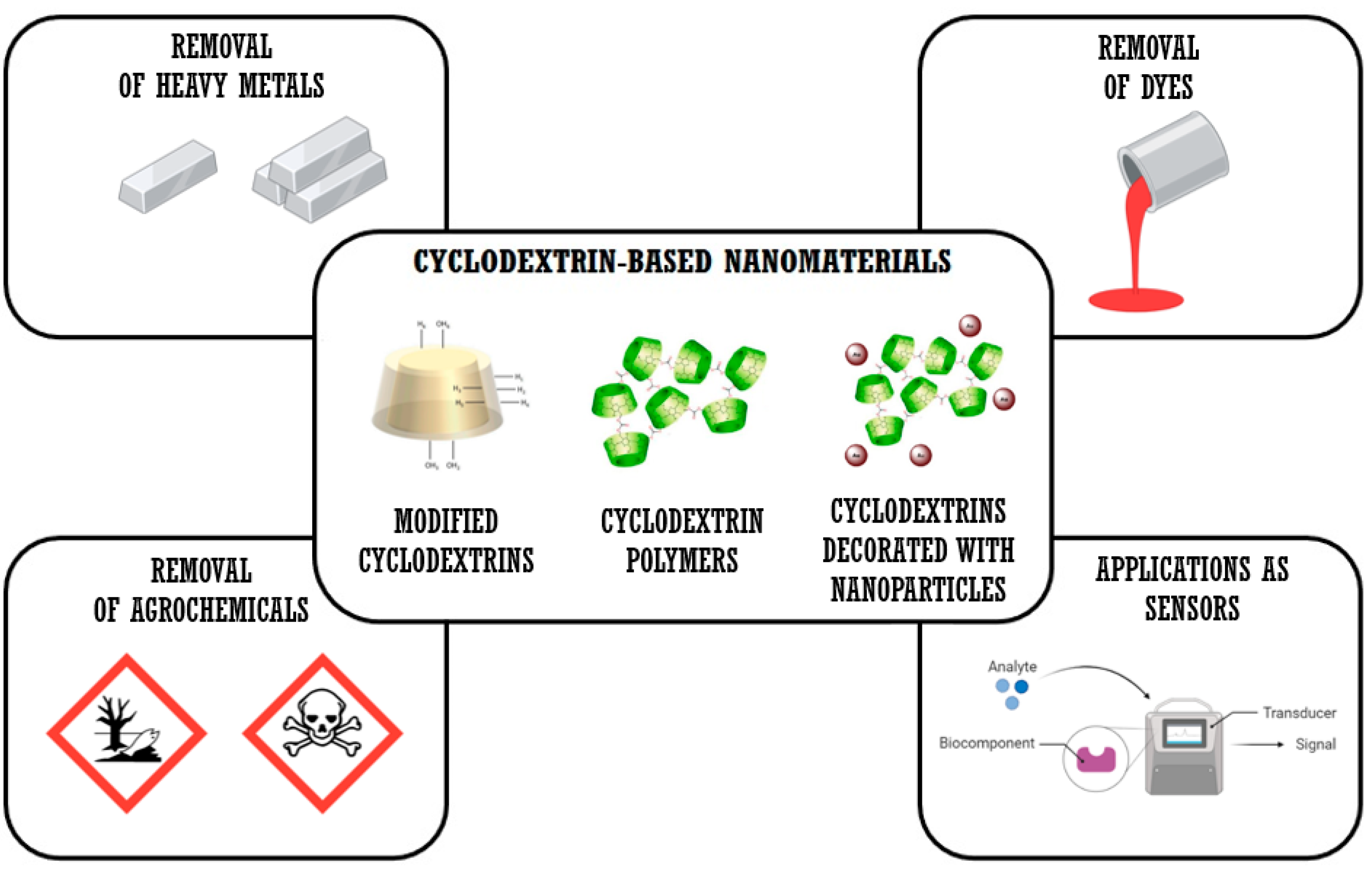
4. Cyclodextrin-Based Materials and Cyclodextrin Polymers for the Remediation and Detection of Pollutants
4.1. Cyclodextrin Monomers and Cyclodextrin-Based Polymers
4.2. Potential Applications of CD Monomers and CD Polymers in Environmental Remediation
4.3. CDs Monomers and CD-Based Polymers in the Remediation of Heavy Metals
4.4. CDs Monomers and CD-Based Polymers in the Remediation of Dyes
4.5. CDs Monomers and CD-Based Polymers in the Remediation of Pesticides and Agrochemicals
4.6. CDs Monomers and CD-Based Polymers as Electrochemical Sensors
5. Nanomaterials for the Remediation of Pharmaceutical Pollutants
6. Nanomaterials for the Photodegradation of Pollutants
7. Innovative Nanomaterials for Environmental Applications
8. Nanomaterials: Challenges, and Future Prospects
9. Conclusions and Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NPs | Nanoparticles |
| AgNPs | Silver Nanoparticles |
| AuNPs | Gold Nanoparticles |
| CuNPs | Copper Nanoparticles |
| PdNPs | Palladium Nanoparticles |
| PtNPs | Platinum Nanoparticles |
| MagNPs | Magnetite Nanoparticles |
| SPR | Surface Plasmon Resonance |
| 11-MUA | Mercaptoundecanoic acid |
| BSA | Bovine Serum Albumin |
| 3-MPS | 3-mercaptopropyl trimethoxy silane |
| APD | 2-aminopyrimidine-4,6-diol |
| AMP | Adenosine Monophosphate |
| TMB | Tetramethylbenzidine |
| 4-MPY | 4-mercaptopyridine |
| PAC | Porous Activated Carbon |
| GCE | Glassy Carbon Electrode |
| rGO | Reduced Graphene Oxide |
| ZnONRs | Zinc Oxide Nanorods |
| CNTs | Carbon Nanotubes |
| CNCs | Cellulose Nanocrystals |
| MOF | Metallic Organic Framework |
| AChE | Acetylcholinesterase |
| PYM | Triazine Pymetrozine |
| SDS | Sodium Dodecyl Sulfate |
| VNSWCNTs | Nitrogen-Doped Single-Walled Carbon Nanotubes |
| MW-CNTs | Multi-Walled Carbon Nanotubes |
| LOD | Limit of Detection |
| LOQ | Limit of Quantification |
| DTCs | Dithiocarbamates |
| MS | Mass Spectrometry |
| HPLC | High Performance Liquid Chromatography |
| GC-MS | Gas Chromatography Mass Spectroscopy |
| LC-MS | Liquid Chromatography Mass Spectroscopy |
| EIS | Electrochemical Impedance Spectroscopy |
| CV | Cyclic Voltammetry |
| DPV | Differential Pulse Voltammetry |
| DLS | Dynamic Light Scattering |
| SEM | Scanning Electron Microscopy |
| TEM | Transmission Electron Microscopy |
| FT-IR | Fourier Transform Infrared Spectroscopy |
| XRPD | X-ray Powder Diffraction |
| EPI | Epichlorohydrin |
| CDs | Cyclodextrins |
| NSs | Nanosponges |
| TPE | Tetrakis (4-hydroxyphenyl) ethene |
| PMDA | Pyromellitic Dianhydride |
References
- Saravanan, A.; Kumar, P.S.; Jeevanantham, S.; Anubha, M.; Jayashree, S. Degradation of toxic agrochemicals and pharmaceutical pollutants: Effective and alternative approaches toward photocatalysis. Environ. Pollut. 2022, 298, 118844. [Google Scholar] [CrossRef]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Jin, M.; Yuan, H.; Liu, B.; Peng, J.; Xu, L.; Yang, D. Review of the distribution and detection methods of heavy metals in the environment. Anal. Methods 2020, 12, 5747–5766. [Google Scholar] [CrossRef]
- Wagner, M.; Lin, K.Y.A.; Da Oh, W.; Lisak, G. Metal-organic frameworks for pesticidal persistent organic pollutants detection and adsorption—A mini review. J. Hazard. Mater. 2021, 413, 125325. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.; Zeng, Z.; Zeng, G.; Xiao, R.; Wang, Y.; Hu, Y.; Tang, L.; Feng, C. Sensors for the environmental pollutant detection: Are we already there? Coord. Chem. Rev. 2020, 431, 213681. [Google Scholar] [CrossRef]
- Wen, N.; Zhang, L.; Jiang, D.; Wu, Z.; Li, B.; Sun, C.; Guo, Z. Emerging flexible sensors based on nanomaterials: Recent status and applications. J. Mater. Chem. A 2020, 8, 25499–25527. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Beitollahi, H.; Kumar, P.S.; Tajik, S.; Jahani, P.M.; Karimi, F.; Karaman, C.; Vasseghian, Y.; Baghayeri, M.; Rouhi, J.; et al. Recent advances in carbon nanomaterials-based electrochemical sensors for food azo dyes detection. Food Chem. Toxicol. 2022, 164, 112961. [Google Scholar] [CrossRef]
- Saleh, T.A. Nanomaterials: Classification, properties, and environmental toxicities. Environ. Technol. Innov. 2020, 20, 101067. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W.; Kammakakam, I. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Ethaib, S.; Al-Qutaifia, S.; Al-Ansari, N.; Zubaidi, S.L. Function of Nanomaterials in Removing Heavy Metals for Water and Wastewater Remediation: A Review. Environments 2022, 9, 123. [Google Scholar] [CrossRef]
- Emenike, E.C.; Iwuozor, K.O.; Anidiobi, S.U. Heavy Metal Pollution in Aquaculture: Sources, Impacts and Mitigation Techniques. Biol. Trace Element Res. 2021, 200, 4476–4492. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Bhagat, N.R.; Giri, A. Nanotechnology for Detection and Removal of Heavy Metals From Contaminated Water; Wiley: Hoboken, NJ, USA, 2021. [Google Scholar]
- Shrivastava, P.; Jain, V.K.; Nagpal, S. Nanoparticle intervention for heavy metal detection: A review. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100667. [Google Scholar] [CrossRef]
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V.H. Heavy metal water pollution: A fresh look about hazards, novel and conventional remediation methods. Environ. Technol. Innov. 2021, 22, 101504. [Google Scholar] [CrossRef]
- Rajendran, S.; Priya, T.; Khoo, K.S.; Hoang, T.K.; Ng, H.-S.; Munawaroh, H.S.H.; Karaman, C.; Orooji, Y.; Show, P.L. A critical review on various remediation approaches for heavy metal contaminants removal from contaminated soils. Chemosphere 2021, 287, 132369. [Google Scholar] [CrossRef]
- Gopinath, K.P.; Madhav, N.V.; Krishnan, A.; Malolan, R.; Rangarajan, G. Present applications of titanium dioxide for the photocatalytic removal of pollutants from water: A review. J. Environ. Manag. 2020, 270, 110906. [Google Scholar] [CrossRef]
- Hosny, M.; Eltaweil, A.S.; Mostafa, M.; El-Badry, Y.A.; Hussein, E.E.; Omer, A.M.; Fawzy, M. Facile Synthesis of Gold Nanoparticles for Anticancer, Antioxidant Applications, and Photocatalytic Degradation of Toxic Organic Pollutants. ACS Omega 2022, 7, 3121–3133. [Google Scholar] [CrossRef]
- Prosposito, P.; Burratti, L.; Venditti, I. Silver nanoparticles as colorimetric sensors for water pollutants. Chemosensors 2020, 8, 26. [Google Scholar] [CrossRef]
- Sultana, K.A.; Islam, M.T.; Silva, J.A.; Turley, R.S.; Hernandez-Viezcas, J.A.; Gardea-Torresdey, J.L.; Noveron, J.C. Sustainable synthesis of zinc oxide nanoparticles for photocatalytic degradation of organic pollutant and generation of hydroxyl radical. J. Mol. Liq. 2020, 307, 112931. [Google Scholar] [CrossRef]
- Alberti, G.; Zanoni, C.; Magnaghi, L.R.; Biesuz, R. Gold and silver nanoparticle-based colorimetric sensors: New trends and applications. Chemosensors 2021, 9, 305. [Google Scholar] [CrossRef]
- Vilela, D.; González, M.C.; Escarpa, A. Sensing colorimetric approaches based on gold and silver nanoparticles aggregation: Chemical creativity behind the assay. A review. Anal. Chim. Acta 2012, 751, 24–43. [Google Scholar] [CrossRef]
- Maghsoudi, A.S.; Hassani, S.; Mirnia, K.; Abdollahi, M. Recent advances in nanotechnology-based biosensors development for detection of arsenic, lead, mercury, and cadmium. Int. J. Nanomed. 2021, 16, 803–832. [Google Scholar] [CrossRef]
- Dong, Y.; Ding, L.; Jin, X.; Zhu, N. Silver nanoparticles capped with chalcon carboxylic acid as a probe for colorimetric determination of cadmium(II). Microchim. Acta 2017, 184, 3357–3362. [Google Scholar] [CrossRef]
- Battocchio, C.; Meneghini, C.; Fratoddi, I.; Venditti, I.; Russo, M.V.; Aquilanti, G.; Maurizio, C.; Bondino, F.; Matassa, R.; Rossi, M.; et al. Silver nanoparticles stabilized with thiols: A close look at the local chemistry and chemical structure. J. Phys. Chem. C 2012, 116, 19571–19578. [Google Scholar] [CrossRef]
- Rossi, A.; Zannotti, M.; Cuccioloni, M.; Minicucci, M.; Petetta, L.; Angeletti, M.; Giovannetti, R. Silver nanoparticle-based sensor for the selective detection of nickel ions. Nanomaterials 2021, 11, 1733. [Google Scholar] [CrossRef]
- Mochi, F.; Burratti, L.; Fratoddi, I.; Venditti, I.; Battocchio, C.; Carlini, L.; Iucci, G.; Casalboni, M.; De Matteis, F.; Casciardi, S.; et al. Plasmonic sensor based on interaction between silver nanoparticles and Ni2+ or Co2+ in water. Nanomaterials 2018, 8, 488. [Google Scholar] [CrossRef]
- Schiesaro, I.; Burratti, L.; Meneghini, C.; Fratoddi, I.; Prosposito, P.; Lim, J.; Scheu, C.; Venditti, I.; Iucci, G.; Battocchio, C. Hydrophilic Silver Nanoparticles for Hg(II) Detection in Water: Direct Evidence for Mercury-Silver Interaction. J. Phys. Chem. C 2020, 124, 25975–25983. [Google Scholar] [CrossRef]
- Prasad, K.S.; Shruthi, G.; Shivamallu, C. Functionalized silver nano-sensor for colorimetric detection of Hg2+ ions: Facile synthesis and docking studies. Sensors 2018, 18, 2698. [Google Scholar] [CrossRef]
- Sharma, P.; Mourya, M.; Choudhary, D.; Goswami, M.; Kundu, I.; Dobhal, M.P.; Tripathi, C.S.P.; Guin, D. Thiol terminated chitosan capped silver nanoparticles for sensitive and selective detection of mercury (II) ions in water. Sens. Actuators B Chem. 2018, 268, 310–318. [Google Scholar] [CrossRef]
- Garg, N.; Bera, S.; Ballal, A. SPR responsive xylenol orange functionalized gold nanoparticles- optical sensor for estimation of Al3+ in water. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 228, 117701. [Google Scholar] [CrossRef]
- Sadani, K.; Nag, P.; Mukherji, S. LSPR based optical fiber sensor with chitosan capped gold nanoparticles on BSA for trace detection of Hg (II) in water, soil and food samples. Biosens. Bioelectron. 2019, 134, 90–96. [Google Scholar] [CrossRef]
- Yuan, H.; Ji, W.; Chu, S.; Liu, Q.; Qian, S.; Guang, J.; Wang, J.; Han, X.; Masson, J.-F.; Peng, W. Mercaptopyridine-Functionalized Gold Nanoparticles for Fiber-Optic Surface Plasmon Resonance Hg2+ Sensing. ACS Sens. 2019, 4, 704–710. [Google Scholar] [CrossRef]
- Zhu, Y.; Fan, W.; Zhou, T.; Li, X. Removal of chelated heavy metals from aqueous solution: A review of current methods and mechanisms. Sci. Total Environ. 2019, 678, 253–266. [Google Scholar] [CrossRef]
- Feng, J.; Jin, W.; Huang, P.; Wu, F. Highly selective colorimetric detection of Ni2+ using silver nanoparticles cofunctionalized with adenosine monophosphate and sodium dodecyl sulfonate. J. Nanoparticle Res. 2017, 19, 306. [Google Scholar] [CrossRef]
- Prosposito, P.; Burratti, L.; Bellingeri, A.; Protano, G.; Faleri, C.; Corsi, I.; Battocchio, C.; Iucci, G.; Tortora, L.; Secchi, V.; et al. Bifunctionalized silver nanoparticles as Hg2+ plasmonic sensor in water: Synthesis, characterizations, and ecosafety. Nanomaterials 2019, 9, 1353. [Google Scholar] [CrossRef]
- Prakashan, V.; George, G.; Sanu, M.; Sajna, M.; Saritha, A.; Sudarsanakumar, C.; Biju, P.; Joseph, C.; Unnikrishnan, N. Investigations on SPR induced Cu@Ag core shell doped SiO2-TiO2-ZrO2 fiber optic sensor for mercury detection. Appl. Surf. Sci. 2019, 507, 144957. [Google Scholar] [CrossRef]
- Hutter, E.; Fendler, J.H. Exploitation of localized surface plasmon resonance. Adv. Mater. 2004, 16, 1685–1706. [Google Scholar] [CrossRef]
- Liu, R.; Zuo, L.; Huang, X.; Liu, S.; Yang, G.; Li, S.; Lv, C. Colorimetric determination of lead(II) or mercury(II) based on target induced switching of the enzyme-like activity of metallothionein-stabilized copper nanoclusters. Microchim. Acta 2019, 186, 250. [Google Scholar] [CrossRef]
- Laghari, G.N.; Nafady, A.; Al-Saeedi, S.I.; Sirajuddin; Sherazi, S.T.H.; Nisar, J.; Shah, M.R.; Abro, M.I.; Arain, M.; Bhargava, S.K. Ranolazine-functionalized copper nanoparticles as a colorimetric sensor for trace level detection of as3+. Nanomaterials 2019, 9, 83. [Google Scholar] [CrossRef]
- Yoon, S.J.; Nam, Y.S.; Lee, H.J.; Lee, Y.; Lee, K.B. Colorimetric probe for Ni2+ based on shape transformation of triangular silver nanoprisms upon H2O2 etching. Sens. Actuators B Chem. 2019, 300, 127045. [Google Scholar] [CrossRef]
- Gan, Y.; Liang, T.; Hu, Q.; Zhong, L.; Wang, X.; Wan, H.; Wang, P. In-situ detection of cadmium with aptamer functionalized gold nanoparticles based on smartphone-based colorimetric system. Talanta 2020, 208, 120231. [Google Scholar] [CrossRef]
- Zhang, T.; Jin, H.; Fang, Y.; Guan, J.; Ma, S.; Pan, Y.; Zhu, H.; Liu, X.; Du, M. Detection of trace Cd2+, Pb2+ and Cu2+ ions via porous activated carbon supported palladium nanoparticles modified electrodes using SWASV. Mater. Chem. Phys. 2019, 225, 433–442. [Google Scholar] [CrossRef]
- Yukird, J.; Kongsittikul, P.; Qin, J.; Chailapakul, O.; Rodthongkum, N. ZnO@graphene nanocomposite modified electrode for sensitive and simultaneous detection of Cd (II) and Pb (II). Synth. Met. 2018, 245, 251–259. [Google Scholar] [CrossRef]
- Hanif, F.; Tahir, A.; Akhtar, M.; Waseem, M.; Haider, S.; Aboud, M.F.A.; Shakir, I.; Imran, M.; Warsi, M.F. Ultra-selective detection of Cd2+ and Pb2+ using glycine functionalized reduced graphene oxide/polyaniline nanocomposite electrode. Synth. Met. 2019, 257, 116185. [Google Scholar] [CrossRef]
- Hamid, H.A.; Lockman, Z.; Nor, N.M.; Zakaria, N.D.; Razak, K.A. Sensitive detection of Pb ions by square wave anodic stripping voltammetry by using iron oxide nanoparticles decorated zinc oxide nanorods modified electrode. Mater. Chem. Phys. 2021, 273, 125148. [Google Scholar] [CrossRef]
- Giao, N.Q.; Dang, V.H.; Yen, P.T.H.; Phong, P.H.; Ha, V.T.T.; Duy, P.K.; Chung, H. Au nanodendrite incorporated graphite pencil lead as a sensitive and simple electrochemical sensor for simultaneous detection of Pb(II), Cu(II) and Hg(II). J. Appl. Electrochem. 2019, 49, 839–846. [Google Scholar] [CrossRef]
- Sebastian, M.; Aravind, A.; Mathew, B. Green silver-nanoparticle-based dual sensor for toxic Hg(II) ions. Nanotechnology 2018, 29, 355502. [Google Scholar] [CrossRef]
- Sánchez-Calvo, A.; Fernández-Abedul, M.T.; Blanco-López, M.C.; Costa-García, A. Paper-based electrochemical transducer modified with nanomaterials for mercury determination in environmental waters. Sens. Actuators B Chem. 2019, 290, 87–92. [Google Scholar] [CrossRef]
- Bertolacci, L.; Valentini, P.; Pompa, P.P. A nanocomposite hydrogel with catalytic properties for trace-element detection in real-world samples. Sci. Rep. 2020, 10, 18340. [Google Scholar] [CrossRef]
- Kumar, A.; Arya, K.; Mehra, S.; Kumar, A.; Mehta, S.K.; Kataria, R. Detection and sorption of heavy metal ions in aqueous media using Zn-based luminescent metal-organic framework. Sep. Purif. Technol. 2023, 333, 125875. [Google Scholar] [CrossRef]
- Arabbani, F.K.; Vasu, D.; Sakthinathan, S.; Chiu, T.W.; Liu, M.C. A High Efficient Electrocatalytic Activity of Metal-organic Frameworks ZnO/Ag/ZIF-8 Nanocomposite for Electrochemical Detection of Toxic Heavy Metal Ions. Electroanalysis 2022, 35, e202200284. [Google Scholar] [CrossRef]
- Gao, J.; He, D.; Zhang, J.; Sun, B.; Wang, G.; Suo, H.; Zhang, L.; Zhao, C. In-situ growth of porous rod-like tungsten oxide for electrochemical determination of cupric ion. Anal. Chim. Acta 2023, 1276, 341645. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Sivanesan, S.; Panneerselvam, P. Turn-On fluorescence sensor based detection of heavy metal ion using carbon dots@graphitic-carbon nitride nanocomposite probe. J. Photochem. Photobiol. A Chem. 2019, 389, 112204. [Google Scholar] [CrossRef]
- Bano, S.; Raj, S.I.; Khalilullah, A.; Jaiswal, A.; Uddin, I. Selective and sensitive cation exchange reactions in the aqueous starch capped ZnS nanoparticles with tunable composition, band gap and color for the detection and estimation of Pb2+, Cu2+ and Hg2+. J. Photochem. Photobiol. A Chem. 2020, 405, 112925. [Google Scholar] [CrossRef]
- Zeng, H.; Hu, Z.; Peng, C.; Deng, L.; Liu, S. Effective adsorption and sensitive detection of cr(Vi) by chitosan/cellulose nanocrystals grafted with carbon dots composite hydrogel. Polymers 2021, 13, 3788. [Google Scholar] [CrossRef]
- Essiedu, J.A.; Adepoju, F.O.; Ivantsova, M.N. Benefits and limitations in using biopesticides: A review. AIP Conf. Proc. 2020, 2313, 080002. [Google Scholar] [CrossRef]
- Rajmohan, K.S.; Chandrasekaran, R.; Varjani, S. A Review on Occurrence of Pesticides in Environment and Current Technologies for Their Remediation and Management. Indian J. Microbiol. 2020, 60, 125–138. [Google Scholar] [CrossRef]
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2020, 283, 124657. [Google Scholar] [CrossRef]
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, N.; Mehra, R.; Kumar, H.; Singh, V.P. Progress and challenges in the detection of residual pesticides using nanotechnology based colorimetric techniques. Trends Environ. Anal. Chem. 2020, 26, e00086. [Google Scholar] [CrossRef]
- Sharma, P.; Pandey, V.; Sharma, M.M.M.; Patra, A.; Singh, B.; Mehta, S.; Husen, A. A Review on Biosensors and Nanosensors Application in Agroecosystems. Nanoscale Res. Lett. 2021, 16, 136. [Google Scholar] [CrossRef]
- Mirres, A.C.d.M.; Silva, B.E.P.d.M.d.; Tessaro, L.; Galvan, D.; de Andrade, J.C.; Aquino, A.; Joshi, N.; Conte-Junior, C.A. Recent Advances in Nanomaterial-Based Biosensors for Pesticide Detection in Foods. Biosensors 2022, 12, 572. [Google Scholar] [CrossRef]
- Hara, T.O.; Singh, B. Electrochemical Biosensors for Detection of Pesticides and Heavy Metal Toxicants in Water: Recent Trends and Progress. ACS Environ. Sci. Technol. Water 2021, 1, 462–478. [Google Scholar] [CrossRef]
- Kundu, M.; Krishnan, P.; Kotnala, R.K.; Sumana, G. Recent developments in biosensors to combat agricultural challenges and their future prospects. Trends Food Sci. Technol. 2019, 88, 157–178. [Google Scholar] [CrossRef]
- Hazarika, A.; Yadav, M.; Yadav, D.K.; Yadav, H.S. An overview of the role of nanoparticles in sustainable agriculture. Biocatal. Agric. Biotechnol. 2022, 43, 102399. [Google Scholar] [CrossRef]
- Singh, R.P.; Handa, R.; Manchanda, G. Nanoparticles in sustainable agriculture: An emerging opportunity. J. Control. Release 2020, 329, 1234–1248. [Google Scholar] [CrossRef]
- Dimcheva, N. Nanostructures of noble metals as functional materials in biosensors. Curr. Opin. Electrochem. 2020, 19, 35–41. [Google Scholar] [CrossRef]
- Kucherenko, I.S.; Soldatkin, O.O.; Kucherenko, D.Y.; Soldatkina, O.V.; Dzyadevych, S.V. Advances in nanomaterial application in enzyme-based electrochemical biosensors: A review. Nanoscale Adv. 2019, 1, 4560–4577. [Google Scholar] [CrossRef]
- Rajagopalan, V.; Venkataraman, S.; Rajendran, D.S.; Kumar, V.V.; Kumar, V.V.; Rangasamy, G. Acetylcholinesterase biosensors for electrochemical detection of neurotoxic pesticides and acetylcholine neurotransmitter: A literature review. Environ. Res. 2023, 227, 115724. [Google Scholar] [CrossRef]
- Fenoy, G.E.; Marmisollé, W.A.; Azzaroni, O.; Knoll, W. Acetylcholine biosensor based on the electrochemical functionalization of graphene field-effect transistors. Biosens. Bioelectron. 2019, 148, 111796. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Oladoye, P.O.; Olanrewaju, C.A.; Akinsola, G.O. Organophosphorus pesticides: Impacts, detection and removal strategies. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100655. [Google Scholar] [CrossRef]
- Thakkar, J.B.; Gupta, S.; Prabha, C.R. Acetylcholine esterase enzyme doped multiwalled carbon nanotubes for the detection of organophosphorus pesticide using cyclic voltammetry. Int. J. Biol. Macromol. 2019, 137, 895–903. [Google Scholar] [CrossRef]
- Yi, Y.; Zhou, X.; Liao, D.; Hou, J.; Liu, H.; Zhu, G. High Peroxidase-Mimicking Metal-Organic Frameworks Decorated with Platinum Nanozymes for the Colorimetric Detection of Acetylcholine Chloride and Organophosphorus Pesticides via Enzyme Cascade Reaction. Inorg. Chem. 2023, 62, 13929–13936. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Wang, G. Peroxidase-like activity of acetylcholine-based colorimetric detection of acetylcholinesterase activity and an organophosphorus inhibitor. J. Mater. Chem. B 2018, 7, 2613–2618. [Google Scholar] [CrossRef]
- El Alami, A.; Lagarde, F.; Huo, Q.; Zheng, T.; Baitoul, M.; Daniel, P. Acetylcholine and acetylcholinesterase inhibitors detection using gold nanoparticles coupled with dynamic light scattering. Sens. Int. 2020, 1, 100007. [Google Scholar] [CrossRef]
- Kong, D.; Jin, R.; Zhao, X.; Li, H.; Yan, X.; Liu, F.; Sun, P.; Gao, Y.; Liang, X.; Lin, Y.; et al. Protein-Inorganic Hybrid Nanoflower-Rooted Agarose Hydrogel Platform for Point-of-Care Detection of Acetylcholine. ACS Appl. Mater. Interfaces 2019, 11, 11857–11864. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wu, W.; Zhao, Q.; Wei, X.; Lu, Q. Efficient immobilization of acetylcholinesterase onto amino functionalized carbon nanotubes for the fabrication of high sensitive organophosphorus pesticides biosensors. Biosens. Bioelectron. 2015, 68, 288–294. [Google Scholar] [CrossRef]
- Du, D.; Wang, M.; Cai, J.; Qin, Y.; Zhang, A. One-step synthesis of multiwalled carbon nanotubes-gold nanocomposites for fabricating amperometric acetylcholinesterase biosensor. Sens. Actuators B Chem. 2010, 143, 524–529. [Google Scholar] [CrossRef]
- Li, H.; Li, F.; Wu, J.; Yang, Q.; Li, Q. Two-dimensional MnO2 nanozyme-mediated homogeneous electrochemical detection of organophosphate pesticides without the interference of H2O2 and color. Anal. Chem. 2021, 93, 4084–4091. [Google Scholar] [CrossRef]
- Wang, S.; Ye, Z.; Wang, X.; Xiao, L. Etching of Single-MnO2-Coated Gold Nanoparticles for the Colorimetric Detection of Organophosphorus Pesticides. ACS Appl. Nano Mater. 2019, 2, 6646–6654. [Google Scholar] [CrossRef]
- Yan, X.; Kong, D.; Jin, R.; Zhao, X.; Li, H.; Liu, F.; Lin, Y.; Lu, G. Fluorometric and colorimetric analysis of carbamate pesticide via enzyme-triggered decomposition of Gold nanoclusters-anchored MnO2 nanocomposite. Sens. Actuators B Chem. 2019, 290, 640–647. [Google Scholar] [CrossRef]
- Shu, R.; Wu, Y.; Li, Z.; Zhang, J.; Wan, Z.; Liu, Y.; Zheng, M. Facile synthesis of cobalt-zinc ferrite microspheres decorated nitrogen-doped multi-walled carbon nanotubes hybrid composites with excellent microwave absorption in the X-band. Compos. Sci. Technol. 2019, 184, 107839. [Google Scholar] [CrossRef]
- Kumar, R.; Khan, M.A.; Anupama, A.V.; Krupanidhi, S.B.; Sahoo, B. Infrared photodetectors based on multiwalled carbon nanotubes: Insights into the effect of nitrogen doping. Appl. Surf. Sci. 2020, 538, 148187. [Google Scholar] [CrossRef]
- Li, N.; Shu, R.; Zhang, J.; Wu, Y. Synthesis of ultralight three-dimensional nitrogen-doped reduced graphene oxide/multi-walled carbon nanotubes/zinc ferrite composite aerogel for highly efficient electromagnetic wave absorption. J. Colloid Interface Sci. 2021, 596, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Jiang, S.; Jiang, B.; Zheng, J. Organophosphorus pesticides detection using acetylcholinesterase biosensor based on gold nanoparticles constructed by electroless plating on vertical nitrogen-doped single-walled carbon nanotubes. Int. J. Environ. Anal. Chem. 2019, 99, 913–927. [Google Scholar] [CrossRef]
- Kumar, T.H.V.; Sundramoorthy, A.K. Electrochemical biosensor for methyl parathion based on single-walled carbon nanotube/glutaraldehyde crosslinked acetylcholinesterase-wrapped bovine serum albumin nanocomposites. Anal. Chim. Acta 2019, 1074, 131–141. [Google Scholar] [CrossRef]
- Zhai, R.; Chen, G.; Liu, G.; Huang, X.; Xu, X.; Li, L.; Zhang, Y.; Wang, J.; Jin, M.; Xu, D.; et al. Enzyme inhibition methods based on Au nanomaterials for rapid detection of organophosphorus pesticides in agricultural and environmental samples: A review. J. Adv. Res. 2021, 37, 61–74. [Google Scholar] [CrossRef]
- Piovesan, J.V.; Haddad, V.F.; Pereira, D.F.; Spinelli, A. Magnetite nanoparticles/chitosan-modified glassy carbon electrode for non-enzymatic detection of the endocrine disruptor parathion by cathodic square-wave voltammetry. J. Electroanal. Chem. 2018, 823, 617–623. [Google Scholar] [CrossRef]
- Ma, L.; He, Y.; Wang, Y.; Wang, Y.; Li, R.; Huang, Z.; Jiang, Y.; Gao, J. Nanocomposites of Pt nanoparticles anchored on UiO66-NH2 as carriers to construct acetylcholinesterase biosensors for organophosphorus pesticide detection. Electrochim. Acta 2019, 318, 525–533. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, T.; Rong, S.; Zeng, D.; Yu, H.; Zhang, Z.; Chang, D.; Pan, H. A sensitive amperometric AChE-biosensor for organophosphate pesticides detection based on conjugated polymer and Ag-rGO-NH2 nanocomposite. Bioelectrochemistry 2019, 127, 163–170. [Google Scholar] [CrossRef]
- Nasiri, M.; Ahmadzadeh, H.; Amiri, A. Organophosphorus pesticides extraction with polyvinyl alcohol coated magnetic graphene oxide particles and analysis by gas chromatography-mass spectrometry: Application to apple juice and environmental water. Talanta 2021, 227, 122078. [Google Scholar] [CrossRef]
- Lazarević-Pašti, T.; Anićijević, V.; Baljozović, M.; Anićijević, D.V.; Gutić, S.; Vasić, V.; Skorodumova, N.V.; Pašti, I.A. The impact of the structure of graphene-based materials on the removal of organophosphorus pesticides from water. Environ. Sci. Nano 2018, 5, 1482–1494. [Google Scholar] [CrossRef]
- Cui, H.F.; Wu, W.W.; Li, M.M.; Song, X.; Lv, Y.; Zhang, T.T. A highly stable acetylcholinesterase biosensor based on chitosan-TiO2-graphene nanocomposites for detection of organophosphate pesticides. Biosens. Bioelectron. 2018, 99, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Jiang, B.; Zheng, J. A novel acetylcholinesterase biosensor based on gold nanoparticles obtained by electroless plating on three-dimensional graphene for detecting organophosphorus pesticides in water and vegetable samples. Anal. Methods 2019, 11, 2428–2434. [Google Scholar] [CrossRef]
- Ruan, X.; Wang, Y.; Kwon, E.Y.; Wang, L.; Cheng, N.; Niu, X.; Ding, S.; Van Wie, B.J.; Lin, Y.; Du, D. Nanomaterial-enhanced 3D-printed sensor platform for simultaneous detection of atrazine and acetochlor. Biosens. Bioelectron. 2021, 184, 113238. [Google Scholar] [CrossRef] [PubMed]
- Supraja, P.; Tripathy, S.; Vanjari, S.R.K.; Singh, V.; Singh, S.G. Electrospun tin (IV) oxide nanofiber based electrochemical sensor for ultra-sensitive and selective detection of atrazine in water at trace levels. Biosens. Bioelectron. 2019, 141, 111441. [Google Scholar] [CrossRef]
- Ravindran, N.; Kumar, S.; Yashini, M.; Rajeshwari, S.; Mamathi, C.A.; Thirunavookarasu, S.N.; Sunil, C.K. Recent advances in Surface Plasmon Resonance (SPR) biosensors for food analysis: A review. Crit. Rev. Food Sci. Nutr. 2021, 63, 1055–1077. [Google Scholar] [CrossRef]
- Zahran, M.; Khalifa, Z.; Zahran, M.A.H.; Azzem, M.A. Dissolved Organic Matter-Capped Silver Nanoparticles for Electrochemical Aggregation Sensing of Atrazine in Aqueous Systems. ACS Appl. Nano Mater. 2020, 3, 3868–3875. [Google Scholar] [CrossRef]
- Fang, L.; Jia, M.; Zhao, H.; Kang, L.; Shi, L.; Zhou, L.; Kong, W. Molecularly imprinted polymer-based optical sensors for pesticides in foods: Recent advances and future trends. Trends Food Sci. Technol. 2021, 116, 387–404. [Google Scholar] [CrossRef]
- Yılmaz, E.; Özgür, E.; Bereli, N.; Türkmen, D.; Denizli, A. Plastic antibody based surface plasmon resonance nanosensors for selective atrazine detection. Mater. Sci. Eng. C 2017, 73, 603–610. [Google Scholar] [CrossRef]
- Oliveira, T.M.; Ribeiro, F.W.; Sousa, C.P.; Salazar-Banda, G.R.; de Lima-Neto, P.; Correia, A.N.; Morais, S. Current overview and perspectives on carbon-based (bio)sensors for carbamate pesticides electroanalysis. TrAC Trends Anal. Chem. 2019, 124, 115779. [Google Scholar] [CrossRef]
- Moreira, S.; Silva, R.; Carrageta, D.F.; Alves, M.G.; Seco-Rovira, V.; Oliveira, P.F.; Pereira, M.d.L. Carbamate Pesticides: Shedding Light on Their Impact on the Male Reproductive System. Int. J. Mol. Sci. 2022, 23, 8206. [Google Scholar] [CrossRef] [PubMed]
- Öter, Ç.; Zorer, Ö.S. Molecularly imprinted polymer synthesis and selective solid phase extraction applications for the detection of ziram, a dithiocarbamate fungicide. Chem. Eng. J. Adv. 2021, 7, 100118. [Google Scholar] [CrossRef]
- Fanjul-Bolado, P.; Fogel, R.; Limson, J.; Purcarea, C.; Vasilescu, A. Advances in the detection of dithiocarbamate fungicides: Opportunities for biosensors. Biosensors 2020, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Ghoto, S.A.; Khuhawar, M.Y.; Jahangir, T.M. Silver nanoparticles with sodium dodecyl sulfate as a colorimetric probe for the detection of dithiocarbamate pesticides in environmental samples. Anal. Sci. 2019, 35, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Zúñiga, K.; Rebollar, G.; Avelar, M.; Campos-Terán, J.; Torres, E. Nanomaterial-Based Sensors for the Detection of Glyphosate. Water 2022, 14, 2436. [Google Scholar] [CrossRef]
- Valle, A.L.; Mello, F.C.C.; Alves-Balvedi, R.P.; Rodrigues, L.P.; Goulart, L.R. Glyphosate detection: Methods, needs and challenges. Environ. Chem. Lett. 2018, 17, 291–317. [Google Scholar] [CrossRef]
- Deng, H.-H.; Peng, H.-P.; Huang, K.-Y.; He, S.-B.; Yuan, Q.-F.; Lin, Z.; Chen, R.-T.; Xia, X.-H.; Chen, W. Self-referenced ratiometric detection of sulfatase activity with dual-emissive urease-encapsulated gold nanoclusters. ACS Sens. 2019, 4, 344–352. [Google Scholar] [CrossRef]
- Saenchoopa, A.; Klangphukhiew, S.; Somsub, R.; Talodthaisong, C.; Patramanon, R.; Daduang, J.; Daduang, S.; Kulchat, S. A disposable electrochemical biosensor based on screen-printed carbon electrodes modified with silver nanowires/hpmc/chitosan/urease for the detection of mercury (II) in water. Biosensors 2021, 11, 351. [Google Scholar] [CrossRef]
- Vaghela, C.; Kulkarni, M.; Haram, S.; Aiyer, R.; Karve, M. A novel inhibition based biosensor using urease nanoconjugate entrapped biocomposite membrane for potentiometric glyphosate detection. Int. J. Biol. Macromol. 2018, 108, 32–40. [Google Scholar] [CrossRef]
- Thanh, C.T.; Binh, N.H.; Duoc, P.N.D.; Thu, V.T.; Van Trinh, P.; Anh, N.N.; Van Tu, N.; Tuyen, N.V.; Van Quynh, N.; Tu, V.C.; et al. Electrochemical Sensor Based on Reduced Graphene Oxide/Double-Walled Carbon Nanotubes/Octahedral Fe3O4/Chitosan Composite for Glyphosate Detection. Bull. Environ. Contam. Toxicol. 2021, 106, 1017–1023. [Google Scholar] [CrossRef]
- Byrne, M.P.; Tobin, J.T.; Forrestal, P.J.; Danaher, M.; Nkwonta, C.G.; Richards, K.; Cummins, E.; Hogan, S.A.; O’callaghan, T.F. Urease and nitrification inhibitors-As mitigation tools for greenhouse gas emissions in sustainable dairy systems: A review. Sustainability 2020, 12, 6018. [Google Scholar] [CrossRef]
- Német, N.; Miele, Y.; Shuszter, G.; Tóth, E.L.; Maróti, J.E.; Szabó, P.J.; Rossi, F.; Lagzi, I. Inhibition of the urea-urease reaction by the components of the zeolite imidazole frameworks-8 and the formation of urease-zinc-imidazole hybrid compound. React. Kinet. Catal. Lett. 2022, 135, 15–28. [Google Scholar] [CrossRef]
- Climent, M.J.; Sánchez-Martín, J.; Rodríguez-Cruz, S.; Pedreros, P.; Urrutia, R.; Herrero-Hernández, E. DETERMINATION OF PESTICIDES IN RIVER SURFACE WATERS OF CENTRAL CHILE USING SPE-GC-MS MULTI-RESIDUE METHOD. J. Chil. Chem. Soc. 2018, 63, 4023–4031. [Google Scholar] [CrossRef]
- Sun, M.; Han, S.; Feng, J.; Li, C.; Ji, X.; Feng, J.; Sun, H. Recent Advances of Triazine-Based Materials for Adsorbent Based Extraction Techniques. Top. Curr. Chem. 2021, 379, 24. [Google Scholar] [CrossRef]
- Kang, J.-Y.; Zhang, Y.-J.; Li, X.; Dong, C.; Liu, H.-Y.; Miao, L.-J.; Low, P.J.; Gao, Z.-X.; Hosmane, N.S.; Wu, A.-G. Rapid and sensitive colorimetric sensing of the insecticide pymetrozine using melamine-modified gold nanoparticles. Anal. Methods 2017, 10, 417–421. [Google Scholar] [CrossRef]
- Mangolim, C.S.; Moriwaki, C.; Nogueira, A.C.; Sato, F.; Baesso, M.L.; Neto, A.M.; Matioli, G. Curcumin-β-cyclodextrin inclusion complex: Stability, solubility, characterisation by FT-IR, FT-Raman, X-ray diffraction and photoacoustic spectroscopy, and food application. Food Chem. 2014, 153, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.; Barati, A.; Tonelli, A.E.; Hamedi, H. Chitosan-based hydrogels loading with thyme oil cyclodextrin inclusion compounds: From preparation to characterization. Eur. Polym. J. 2019, 122, 109303. [Google Scholar] [CrossRef]
- Wang, Z.; Zou, W.; Liu, L.; Wang, M.; Li, F.; Shen, W. Characterization and bacteriostatic effects of β-cyclodextrin/quercetin inclusion compound nanofilms prepared by electrospinning. Food Chem. 2020, 338, 127980. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Y.; Lu, J.; Zhou, Y. Novel cyclodextrin-based adsorbents for removing pollutants from wastewater: A critical review. Chemosphere 2020, 241, 125043. [Google Scholar] [CrossRef]
- Duan, Z.; Li, Y.; Zhang, M.; Bian, H.; Wang, Y.; Zhu, L.; Xia, D. Towards cleaner wastewater treatment for special removal of cationic organic dye pollutants: A case study on application of supramolecular inclusion technology with β-cyclodextrin derivatives. J. Clean. Prod. 2020, 256, 120308. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Fourmentin, M.; Fourmentin, S.; Torri, G.; Crini, G. Synthesis of silica materials containing cyclodextrin and their applications in wastewater treatment. Environ. Chem. Lett. 2018, 17, 683–696. [Google Scholar] [CrossRef]
- Li, L.; Liu, H.; Li, W.; Liu, K.; Tang, T.; Liu, J.; Jiang, W. One-step synthesis of an environment-friendly cyclodextrin-based nanosponge and its applications for the removal of dyestuff from aqueous solutions. Res. Chem. Intermed. 2019, 46, 1715–1734. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Nanosponges for Water Treatment: Progress and Challenges. Appl. Sci. 2022, 12, 4182. [Google Scholar] [CrossRef]
- Krabicová, I.; Appleton, S.L.; Tannous, M.; Hoti, G.; Caldera, F.; Pedrazzo, A.R.; Cecone, C.; Cavalli, R.; Trotta, F. History of cyclodextrin nanosponges. Polymers 2020, 12, 1122. [Google Scholar] [CrossRef]
- Zhen, X.V.; Swanson, E.G.; Nelson, J.T.; Zhang, Y.; Su, Q.; Koester, S.J.; Bühlmann, P. Noncovalent monolayer modification of graphene using pyrene and cyclodextrin receptors for chemical sensing. ACS Appl. Nano Mater. 2018, 1, 2718–2726. [Google Scholar] [CrossRef]
- Verma, M.; Lee, I.; Sharma, S.; Kumar, R.; Kumar, V.; Kim, H. Simultaneous Removal of Heavy Metals and Ciprofloxacin Micropollutants from Wastewater Using Ethylenediaminetetraacetic Acid-Functionalized β-Cyclodextrin-Chitosan Adsorbent. ACS Omega 2021, 6, 34624–34634. [Google Scholar] [CrossRef]
- Zawierucha, I.; Nowik-Zajac, A.; Girek, T.; Lagiewka, J.; Ciesielski, W.; Pawlowska, B.; Biczak, R. Arsenic(V) Removal from Water by Resin Impregnated with Cyclodextrin Ligand. Processes 2022, 10, 253. [Google Scholar] [CrossRef]
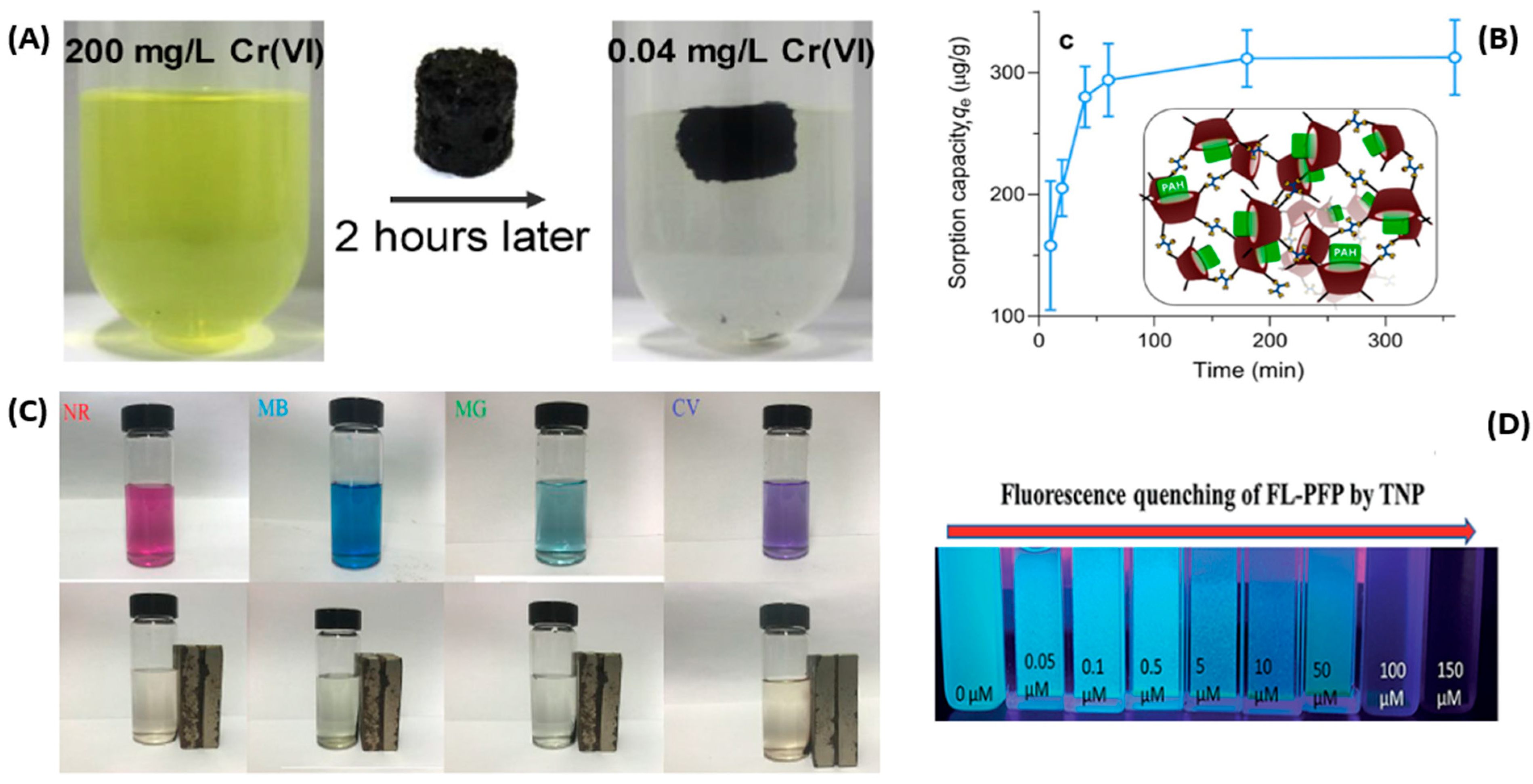
- Wang, Z.; Lin, F.; Huang, L.; Chang, Z.; Yang, B.; Liu, S.; Zheng, M.; Lu, Y.; Chen, J. Cyclodextrin functionalized 3D-graphene for the removal of Cr(VI) with the easy and rapid separation strategy. Environ. Pollut. 2019, 254, 112854. [Google Scholar] [CrossRef]
- Lin, S.; Zou, C.; Liang, H.; Peng, H.; Liao, Y. The effective removal of nickel ions from aqueous solution onto magnetic multi-walled carbon nanotubes modified by β-cyclodextrin. Colloids Surf. A Physicochem. Eng. Asp. 2021, 619, 126544. [Google Scholar] [CrossRef]
- Pedrazzo, A.R.; Smarra, A.; Caldera, F.; Musso, G.; Dhakar, N.K.; Cecone, C.; Hamedi, A.; Corsi, I.; Trotta, F. Eco-friendly ß-cyclodextrin and linecaps polymers for the removal of heavy metals. Polymers 2019, 11, 1658. [Google Scholar] [CrossRef] [PubMed]
- Badawi, M.A.; Negm, N.A.; Kana, M.T.H.A.; Hefni, H.H.; Moneem, M.M.A. Adsorption of aluminum and lead from wastewater by chitosan-tannic acid modified biopolymers: Isotherms, kinetics, thermodynamics and process mechanism. Int. J. Biol. Macromol. 2017, 99, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, H.; Li, L.; Liu, K.; Liu, J.; Tang, T.; Jiang, W. Green synthesis of citric acid-crosslinked β-cyclodextrin for highly efficient removal of uranium(VI) from aqueous solution. J. Radioanal. Nucl. Chem. 2019, 322, 2033–2042. [Google Scholar] [CrossRef]
- Cataldo, S.; Meo, P.L.; Conte, P.; Di Vincenzo, A.; Milea, D.; Pettignano, A. Evaluation of adsorption ability of cyclodextrin-calixarene nanosponges towards Pb2+ ion in aqueous solution. Carbohydr. Polym. 2021, 267, 118151. [Google Scholar] [CrossRef]
- Usman, M.; Ahmed, A.; Ji, Z.; Yu, B.; Shen, Y.; Cong, H. Environmentally friendly fabrication of new β-Cyclodextrin/ZrO2 nanocomposite for simultaneous removal of Pb(II) and BPA from water. Sci. Total Environ. 2021, 784, 147207. [Google Scholar] [CrossRef]
- Rodríguez-López, M.I.; Pellicer, J.A.; Gómez-Morte, T.; Auñón, D.; Gómez-López, V.M.; Yáñez-Gascón, M.J.; Gil-Izquierdo, Á.; Cerón-Carrasco, J.P.; Crini, G.; Núñez-Delicado, E.; et al. Removal of an Azo Dye from Wastewater through the Use of Two Technologies: Magnetic Cyclodextrin Polymers and Pulsed Light. Int. J. Mol. Sci. 2022, 23, 8406. [Google Scholar] [CrossRef]
- Martwong, E.; Chuetor, S.; Junthip, J. Adsorption of Cationic Contaminants by Cyclodextrin Nanosponges Cross-Linked with 1,2,3,4-Butanetetracarboxylic Acid and Poly(vinyl alcohol). Polymers 2022, 14, 342. [Google Scholar] [CrossRef]
- Semeraro, P.; Gabaldón, J.A.; Fini, P.; Núñez-Delicado, E.; Pellicer, J.A.; Rizzi, V.; Cosma, P. Removal of an Azo Textile Dye from Wastewater by Cyclodextrin-Epichlorohydrin Polymers. In Cyclodextrin—A Versatile Ingredient; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Taka, A.L.; Fosso-Kankeu, E.; Pillay, K.; Mbianda, X.Y. Metal nanoparticles decorated phosphorylated carbon nanotube/cyclodextrin nanosponge for trichloroethylene and Congo red dye adsorption from wastewater. J. Environ. Chem. Eng. 2019, 8, 103602. [Google Scholar] [CrossRef]
- Gao, Z.; Li, Y.; Ma, Y.; Ji, W.; Chen, T.; Ma, X.; Xu, H. Functionalized melamine sponge based on β-cyclodextrin-graphene oxide as solid-phase extraction material for rapidly pre-enrichment of malachite green in seafood. Microchem. J. 2019, 150, 104167. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Fronczyk, J.; Świątkowski, A. Adsorptive Removal of Rhodamine B Dye from Aqueous Solutions Using Mineral Materials as Low-Cost Adsorbents. Water Air Soil Pollut. 2023, 234, 531. [Google Scholar] [CrossRef]
- Sulaiman, N.S.; Zaini, M.A.A.; Arsad, A. Evaluation of dyes removal by beta-cyclodextrin adsorbent. Mater. Today Proc. 2020, 39, 907–910. [Google Scholar] [CrossRef]
- Xie, Z.-W.; Lin, J.-C.; Xu, M.-Y.; Wang, H.-Y.; Wu, Y.-X.; He, F.-A.; Jiang, H.-L. Novel Fe3O4Nanoparticle/β-Cyclodextrin-Based Polymer Composites for the Removal of Methylene Blue from Water. Ind. Eng. Chem. Res. 2020, 59, 12270–12281. [Google Scholar] [CrossRef]
- Salazar, S.; Guerra, D.; Yutronic, N.; Jara, P. Removal of aromatic chlorinated pesticides from aqueous solution using β-cyclodextrin polymers decorated with Fe3O4 nanoparticles. Polymers 2018, 10, 1038. [Google Scholar] [CrossRef] [PubMed]
- Salazar, S.; Yutronic, N.; Jara, P. Magnetic β-cyclodextrin nanosponges for potential application in the removal of the neonicotinoid dinotefuran from wastewater. Int. J. Mol. Sci. 2020, 21, 4079. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Ma, J.; Liu, K.; Jiang, Y.; Yang, G.; Liu, Y.; Lin, C.; Ye, X.; Shi, Y.; Liu, M.; et al. Rapid elimination of trace bisphenol pollutants with porous β-cyclodextrin modified cellulose nanofibrous membrane in water: Adsorption behavior and mechanism. J. Hazard. Mater. 2020, 403, 123666. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, A.; Topuz, F.; Yildiz, Z.I.; Uyar, T. Efficient Removal of Polycyclic Aromatic Hydrocarbons and Heavy Metals from Water by Electrospun Nanofibrous Polycyclodextrin Membranes. ACS Omega 2019, 4, 7850–7860. [Google Scholar] [CrossRef] [PubMed]
- Utzeri, G.; Murtinho, D.; Maria, T.M.; Pais, A.A.C.; Valente, A.J. Amine-β-cyclodextrin-based nanosponges. The role of cyclodextrin amphiphilicity in the imidacloprid uptake. Colloids Surf. A Physicochem. Eng. Aspects 2022, 635, 128044. [Google Scholar] [CrossRef]
- Wang, M.; Li, G.; Xia, C.; Jing, X.; Wang, R.; Liu, Q.; Cai, X. Facile preparation of cyclodextrin polymer materials with rigid spherical structure and flexible network for sorption of organic contaminants in water. Chem. Eng. J. 2021, 411, 128489. [Google Scholar] [CrossRef]
- Demasi, S.; Caser, M.; Caldera, F.; Dhakar, N.K.; Vidotto, F.; Trotta, F.; Scariot, V. Functionalized dextrin-based nanosponges as effective carriers for the herbicide ailanthone. Ind. Crops Prod. 2021, 164, 113346. [Google Scholar] [CrossRef]
- Martwong, E.; Sukhawipat, N.; Junthip, J. Adsorption of Cationic Pollutants from Water by Cotton Rope Coated with Cyclodextrin Polymers. Polymers 2022, 14, 2312. [Google Scholar] [CrossRef]
- Martwong, E.; Sukhawipat, N.; Junthip, J. Cotton Cord Coated with Cyclodextrin Polymers for Paraquat Removal from Water. Polymers 2022, 14, 2199. [Google Scholar] [CrossRef]
- Utzeri, G.; Verissimo, L.; Murtinho, D.; Pais, A.A.C.C.; Perrin, F.X.; Ziarelli, F.; Iordache, T.-V.; Sarbu, A.; Valente, A.J.M. Poly(β-cyclodextrin)-activated carbon gel composites for removal of pesticides from water. Molecules 2021, 26, 1426. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.H.; Moon, J.-Y.; Kim, S.-Y.; Lee, W.-C.; Park, S.-G.; Kim, D.-H.; Jung, H.S. A cyclodextrin-decorated plasmonic gold nanosatellite substrate for selective detection of bipyridylium pesticides. Analyst 2020, 146, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shi, Y.; Gao, Y. A simple but highly sensitive electropolymerization of L-citrulline and β-cyclodextrin based voltammetric sensor for metribuzin. Int. J. Environ. Anal. Chem. 2020, 102, 1784–1792. [Google Scholar] [CrossRef]
- Tu, X.; Gao, F.; Ma, X.; Zou, J.; Yu, Y.; Li, M.; Qu, F.; Huang, X.; Lu, L. Mxene/carbon nanohorn/β-cyclodextrin-Metal-organic frameworks as high-performance electrochemical sensing platform for sensitive detection of carbendazim pesticide. J. Hazard. Mater. 2020, 396, 122776. [Google Scholar] [CrossRef] [PubMed]
- Danquah, M.K.; Wang, S.; Wang, Q.; Wang, B.; Wilson, L.D. A porous β-cyclodextrin-based terpolymer fluorescence sensor for in situ trinitrophenol detection. RSC Adv. 2019, 9, 8073–8080. [Google Scholar] [CrossRef] [PubMed]
- Alrabiah, H.; Homoda, A.; Bakheit, A.; Mostafa, G.A. Cyclodextrin potentiometric sensors based on selective recognition sites for procainamide: Comparative and theoretical study. Open Chem. 2019, 17, 1222–1234. [Google Scholar] [CrossRef]
- Alrabiah, H.; Aljohar, H.I.; Bakheit, A.H.; Homoda, A.M.A.; Mostafa, G.A.H. Comparative study of β-cyclodextrin, γ-cyclodextrin and 4-tert-butylcalix[8]arene ionophores as electroactive materials for the construction of new sensors for trazodone based on host-guest recognition. Drug Des. Dev. Ther. 2019, 13, 2283–2293. [Google Scholar] [CrossRef]
- Hatami, E.; Ashraf, N.; Arbab-Zavar, M.H. Construction of β-Cyclodextrin-phosphomolybdate grafted polypyrrole composite: Application as a disposable electrochemical sensor for detection of propylparaben. Microchem. J. 2021, 168, 106451. [Google Scholar] [CrossRef]
- Bae, J.; Park, S.J.; Shin, D.-S.; Lee, J.; Park, S.; Kim, H.J.; Kwon, O.S. A Dual Functional Conductive Hydrogel Containing Titania@Polypyrrole-Cyclodextrin Hybrid Nanotubes for Capture and Degradation of Toxic Chemical. BioChip J. 2021, 15, 162–170. [Google Scholar] [CrossRef]
- Nikhil, S.; Karthika, A.; Suresh, P.; Suganthi, A.; Rajarajan, M. A selective and sensitive electrochemical determination of catechol based on reduced graphene oxide decorated β-cyclodextrin nanosheet modified glassy carbon electrode. Adv. Powder Technol. 2021, 32, 2148–2159. [Google Scholar] [CrossRef]
- Feng, G.; Huang, H.; Chen, Y. Effects of emerging pollutants on the occurrence and transfer of antibiotic resistance genes: A review. J. Hazard. Mater. 2021, 420, 126602. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Khan, S.U.; Ahmed, S.; Farooqi, I.H.; Yousefi, M.; Mohammadi, A.A.; Changani, F. Recent trends in disposal and treatment technologies of emerging-pollutants—A critical review. TrAC Trends Anal. Chem. 2019, 122, 115744. [Google Scholar] [CrossRef]
- Chaukura, N.; Gwenzi, W.; Tavengwa, N.; Manyuchi, M.M. Biosorbents for the removal of synthetic organics and emerging pollutants: Opportunities and challenges for developing countries. Environ. Dev. 2016, 19, 84–89. [Google Scholar] [CrossRef]
- Aguilar-Pérez, K.M.; Avilés-Castrillo, J.I.; Ruiz-Pulido, G. Nano-sorbent materials for pharmaceutical-based wastewater effluents—An overview. Case Stud. Chem. Environ. Eng. 2020, 2, 100028. [Google Scholar] [CrossRef]
- Sigonya, S.; Mokhothu, T.H.; Mokhena, T.C.; Makhanya, T.R. Mitigation of Non-Steroidal Anti-Inflammatory and Antiretroviral Drugs as Environmental Pollutants by Adsorption Using Nanomaterials as Viable Solution—A Critical Review. Appl. Sci. 2023, 13, 772. [Google Scholar] [CrossRef]
- Vicente-Martínez, Y.; Caravaca, M.; Soto-Meca, A.; Solana-González, R. Magnetic core-modified silver nanoparticles for ibuprofen removal: An emerging pollutant in waters. Sci. Rep. 2020, 10, 18288. [Google Scholar] [CrossRef]
- Chahm, T.; Rodrigues, C.A. Removal of ibuprofen from aqueous solutions using O-carboxymethyl-N-laurylchitosan/γ-Fe2O3. Environ. Nanotechnol. Monit. Manag. 2017, 7, 139–148. [Google Scholar] [CrossRef]
- Priyan, V.V.; Narayanasamy, S. Effective removal of pharmaceutical contaminants ibuprofen and sulfamethoxazole from water by Corn starch nanoparticles: An ecotoxicological assessment. Environ. Toxicol. Pharmacol. 2022, 94, 103930. [Google Scholar] [CrossRef]
- Yanyan, L.; Kurniawan, T.A.; Albadarin, A.B.; Walker, G. Enhanced removal of acetaminophen from synthetic wastewater using multi-walled carbon nanotubes (MWCNTs) chemically modified with NaOH, HNO3/H2SO4, ozone, and/or chitosan. J. Mol. Liq. 2018, 251, 369–377. [Google Scholar] [CrossRef]
- Tao, H.; Liang, X.; Zhang, Q.; Chang, C.T. Enhanced photoactivity of graphene/titanium dioxide nanotubes for removal of Acetaminophen. Appl. Surf. Sci. 2015, 324, 258–264. [Google Scholar] [CrossRef]
- Moradi, O.; Alizadeh, H.; Sedaghat, S. Removal of pharmaceuticals (diclofenac and amoxicillin) by maltodextrin/reduced graphene and maltodextrin/reduced graphene/copper oxide nanocomposites. Chemosphere 2022, 299, 134435. [Google Scholar] [CrossRef]
- Van Tran, T.; Nguyen, D.T.C.; Le, H.T.N.; Vo, D.V.N.; Nanda, S.; Nguyen, T.D. Optimization, equilibrium, adsorption behavior and role of surface functional groups on graphene oxide-based nanocomposite towards diclofenac drug. J. Environ. Sci. 2020, 93, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Van Tran, T.; Phan, T.-Q.T.; Nguyen, D.T.C.; Nguyen, T.T.; Nguyen, D.H.; Vo, D.-V.N.; Bach, L.G.; Nguyen, T.D. Recyclable Fe3O4@C nanocomposite as potential adsorbent for a wide range of organic dyes and simulated hospital effluents. Environ. Technol. Innov. 2020, 20, 101122. [Google Scholar] [CrossRef]
- ALOthman, Z.A.; Badjah, A.Y.; Alduhaish, O.M.; Rathinam, K.; Panglisch, S.; Ali, I. Synthesis, characterization, kinetics and modeling studies of new generation pollutant ketoprofen removal in water using copper nanoparticles. J. Mol. Liq. 2020, 323, 115075. [Google Scholar] [CrossRef]
- Hu, L.; Ding, Z.; Yan, F.; Li, K.; Feng, L.; Wang, H. Construction of Hexagonal Prism-like Defective BiOCL Hierarchitecture for Photocatalytic Degradation of Tetracycline Hydrochloride. Nanomaterials 2022, 12, 2700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, X.; Chen, Q.; He, Y.; Pan, M.; Huang, G.; Bi, J. Insights into Photocatalytic Degradation Pathways and Mechanism of Tetracycline by an Efficient Z-Scheme NiFe-LDH/CTF-1 Heterojunction. Nanomaterials 2022, 12, 4111. [Google Scholar] [CrossRef]
- Zang, S.; Cai, X.; Chen, M.; Teng, D.; Jing, F.; Leng, Z.; Zhou, Y.; Lin, F. Tunable Carrier Transfer of Polymeric Carbon Nitride with Charge-Conducting CoV2O6·2H2O for Photocatalytic O2 Evolution. Nanomaterials 2022, 12, 1931. [Google Scholar] [CrossRef]
- Schwarze, M.; Borchardt, S.; Frisch, M.L.; Collis, J.; Walter, C.; Menezes, P.W.; Strasser, P.; Driess, M.; Tasbihi, M. Degradation of Phenol via an Advanced Oxidation Process (AOP) with Immobilized Commercial Titanium Dioxide (TiO2) Photocatalysts. Nanomaterials 2023, 13, 1249. [Google Scholar] [CrossRef]
- Xu, H.; Hao, Z.; Feng, W.; Wang, T.; Li, Y. Mechanism of Photodegradation of Organic Pollutants in Seawater by TiO2-Based Photocatalysts and Improvement in Their Performance. ACS Omega 2021, 6, 30698–30707. [Google Scholar] [CrossRef]
- Zielińska-jurek, A.; Wei, Z.; Janczarek, M.; Wysocka, I.; Kowalska, E. Size-controlled synthesis of Pt particles on TiO2 surface: Physicochemical characteristic and photocatalytic activity. Catalysts 2019, 9, 940. [Google Scholar] [CrossRef]
- Verástegui-Domínguez, L.H.; Elizondo-Villarreal, N.; Martínez-Delgado, D.I.; Gracia-Pinilla, M.Á. Eco-Friendly Reduction of Graphene Oxide by Aqueous Extracts for Photocatalysis Applications. Nanomaterials 2022, 12, 3882. [Google Scholar] [CrossRef] [PubMed]
- Cifre-Herrando, M.; Roselló-Márquez, G.; García-García, D.M.; García-Antón, J. Degradation of Methylparaben Using Optimal WO3 Nanostructures: Influence of the Annealing Conditions and Complexing Agent. Nanomaterials 2022, 12, 4286. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Tian, X.; Yu, H.; Wang, Z.; Ren, C.; Zhou, L.; Lin, Y.-W.; Dou, L. WO3/Ag2CO3Mixed Photocatalyst with Enhanced Photocatalytic Activity for Organic Dye Degradation. ACS Omega 2021, 6, 26439–26453. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Pudukudy, M.; Harish, S.; Navaneethan, M.; Sohila, S.; Murugesan, K.; Ramesh, R. Facile construction of djembe-like ZnO and its composite with g-C3N4 as a visible-light-driven heterojunction photocatalyst for the degradation of organic dyes. Mater. Sci. Semicond. Process. 2019, 106, 104754. [Google Scholar] [CrossRef]
- Singh, J.; Kumari, P.; Basu, S. Degradation of toxic industrial dyes using SnO2/g-C3N4 nanocomposites: Role of mass ratio on photocatalytic activity. J. Photochem. Photobiol. A Chem. 2018, 371, 136–143. [Google Scholar] [CrossRef]
- Mohanta, D.; Ahmaruzzaman, M. Facile fabrication of novel Fe3O4-SnO2-gC3N4 ternary nanocomposites and their photocatalytic properties towards the degradation of carbofuran. Chemosphere 2021, 285, 131395. [Google Scholar] [CrossRef]
- Das, L.; Das, P.; Bhowal, A.; Bhattachariee, C. Synthesis of hybrid hydrogel nano-polymer composite using Graphene oxide, Chitosan and PVA and its application in waste water treatment. Environ. Technol. Innov. 2020, 18, 100664. [Google Scholar] [CrossRef]
- Salahuddin, B.; Aziz, S.; Gao, S.; Hossain, S.A.; Billah, M.; Zhu, Z.; Amiralian, N. Magnetic Hydrogel Composite for Wastewater Treatment. Polymers 2022, 14, 5074. [Google Scholar] [CrossRef]
- Khan, S.B.; Irfan, S.; Lam, S.S.; Sun, X.; Chen, S. 3D printed nanofiltration membrane technology for waste water distillation. J. Water Process. Eng. 2022, 49, 102958. [Google Scholar] [CrossRef]
- Wang, D.; Zhi, T.; Liu, L.; Yan, L.; Yan, W.; Tang, Y.; He, B.; Hu, L.; Jing, C.; Jiang, G. 3D printing of TiO2 nano particles containing macrostructures for As(III) removal in water. Sci. Total Environ. 2022, 815, 152754. [Google Scholar] [CrossRef] [PubMed]
- Figuerola, A.; Rodríguez, F.; Cabello, C.P.; Palomino, G.T. Carbon@ceramic 3D printed devices for bisphenol A and other organic contaminants extraction. Sep. Purif. Technol. 2022, 299, 121749. [Google Scholar] [CrossRef]
- Cui, J.; Li, F.; Wang, Y.; Zhang, Q.; Ma, W.; Huang, C. Electrospun nanofiber membranes for wastewater treatment applications. Sep. Purif. Technol. 2020, 250, 117116. [Google Scholar] [CrossRef]
- Pervez, N.; Talukder, E.; Mishu, M.R.; Buonerba, A.; Del Gaudio, P.; Stylios, G.K.; Hasan, S.W.; Zhao, Y.; Cai, Y.; Figoli, A.; et al. One-Step Fabrication of Novel Polyethersulfone-Based Composite Electrospun Nanofiber Membranes for Food Industry Wastewater Treatment. Membranes 2022, 12, 413. [Google Scholar] [CrossRef] [PubMed]
- Beiranvand, R.; Sarlak, N. Electrospun nanofiber mat of graphene/mesoporous silica composite for wastewater treatment. Mater. Chem. Phys. 2023, 309, 128311. [Google Scholar] [CrossRef]
- Ray, P.C.; Yu, H.; Fu, P.P. Toxicity and environmental risks of nanomaterials: Challenges and future needs. J. J. Environ. Sci. Health Part C 2009, 27, 1–35. [Google Scholar] [CrossRef]
- Zhang, N.; Xiong, G.; Liu, Z. Toxicity of metal-based nanoparticles: Challenges in the nano era. Front. Bioeng. Biotechnol. 2022, 10, 1001572. [Google Scholar] [CrossRef]
- Ganguly, P.; Breen, A.; Pillai, S.C. Toxicity of Nanomaterials: Exposure, Pathways, Assessment, and Recent Advances. ACS Biomater. Sci. Eng. 2018, 4, 2237–2275. [Google Scholar] [CrossRef]


| System | Heavy Metal | Altered State of the Metal NPs | Polluted Matrix | Reference |
|---|---|---|---|---|
| Chalcone carboxylic acid-capped AgNPs | Cd (II) | Carboxylic acid-Cd interaction induced AgNPs aggregation | Drinking water and lake water | [24] |
| 11 MUA—functionalized AgNPs | Ni (II) | Red shift in the SPR of AgNPs | Lab water | [26] |
| 3 MPS-AgNPs | Co (II) and Ni (II) | Aggregation of AgNPs induced by the formation of coordination compounds | Lab water | [27] |
| 3 MPS-AgNPs | Hg (II) | Formation of an Ag/Hg amalgam that causes changes in the SPR spectra | Lab water | [28] |
| APD-AgNPs | Hg (II) | Complex formation between APD-AgNPs and Hg2+, inducing change of the system color | Lab water | [29] |
| Chitosan-AgNPs | Hg (II) | Change in the structure of AgNPs | Drinking water | [30] |
| AMP-SDS-AgNPs | Ni (II) | Decrease in the plasmon absorbance band due to the interactions between Ni2+ and each capping agent | Tap water and lake water | [35] |
| L-cysteine-AgNPs | Hg (II) | Red shift of AgNPs SPR band | Salt water and drinking water | [36] |
| Silver nano prisms | Ni (II) | Changes in the triangular form of silver nano-prisms in a solution with O2 and H2O | Tap water and pond water | [41] |
| Xylenol orange-AuNPs | Al (III) | Aggregation and color change of AuNPs | Drinking water | [31] |
| Chitosan-BSA-AuNPs | Hg (II) | Hg (II) strong affinity towards chitosan and BSA promoted changes in the SPR of AuNPs | Water, soil, and food samples | [32] |
| 4-MPY-AuNPs | Hg (II) | Hg-pyridine complex formation and a coupling effect between Au film and AuNPs | Tap water | [33] |
| Aptamer-AuNPs | Cd (II) | Cd-Aptamer interaction promoted the aggregation of AuNPs | Drinking water | [42] |
| CuNPs-AgNPs-ternary matrix | Hg (II) | Variations in the intensity and shifts on the SPR bands | Drinking water | [37] |
| MT-CuNPs | Hg (II) and Pb (II) | Hg (II) and Pb (II) induced the aggregation of CuNPs | Tap and pond water | [39] |
| R-CuNPs | As (III) | The presence of As (III) promoted the release of R and the aggregation of CuNPs | Ground water | [40] |
| Nanocomposite | Heavy Metal | LOD (nM) | Polluted Matrix | Reference |
|---|---|---|---|---|
| Pd@PAC/GCE | Cd (II), Pb (II), Cu (II) | 13.33 (Cd2+), 6.60 (Pb2+), 11.92 (Cu2+) | Tap water | [43] |
| ZnO-G | Cd (II), Pb (II) | 0.05 (Cd2+), 0.03 (Pb2+) | Wastewater | [44] |
| rGO-glycine-polyaniline | Cd (II) and Pb (II) | 0.07 (Cd2+), 0.09 (Pb2+) | Tap water | [45] |
| Fe2O3 NPs/ZnONRs/ITO | Pb (II) | 10 | Sea water | [46] |
| GPL-Au nano-dendrites | Pb (II), Cu (II), Hg (II) | 57.8 (Pb2+), 18.8 (Cu2+), 60.1 (Hg2+) | Lake water | [47] |
| AgNPs-Agaricus Bispore-Platinum electrode | Hg (II) | 210 | Lake water | [48] |
| CNT-AuNPs-GO | Hg (II) | 30 | River water | [49] |
| PtNPs-TMB | Hg (II) | 80 | Lab water | [37] |
| Zn-based MOF | V (V) | 220 | Lab water | [51] |
| Ag-ZnO-ZIF-8 | Hg (II) | 40 | Tap water, river water, orange juice | [52] |
| SSM-WO3 | Cu (II) | 10 | Lab water | [53] |
| Carbon dots-GCN | Cr (VI), Cu (II), Pb (II) | 0.55 (Cr6+), 0.18 (Cu2+), 0.3 (Pb2+) | Tap water, pond water, river water | [54] |
| ZnS-starch | Pb (II), Cu (II), Hg (II) | 1 (Pb2+and Hg2+), 10 (Cu2+) | Tap water, pond water | [55] |
| Chitosan/CNCs/carbon dots | Cr (VI) | 20 | Lab water | [56] |
| System | Agrochemical | LOD (nM) | Polluted Matrix | Reference |
|---|---|---|---|---|
| AChE-CNT-GCE | Paraoxon | 0.08 | Vegetable samples | [78] |
| VNSWCNTs/AuNPs/AChE | Malathion | 0.3 | Cabbage samples | [79] |
| Au/VNSWCNTs | Methyl Parathion, Malathion, Chlorpyriphos | 0.03 (methyl parathion), 0.01 (malathion), 0.03 (chlorpyrifos) | River water | [81] |
| AChE/CS/Fe3O4 | Malathion | 0.3 | River water | [84] |
| Pt@UiO66-NH2 nanocomposite | Malathion | 4.9 × 10−6 | Garlic samples | [85] |
| Nafion/AuNPs/rGO/GCE | Malathion and Methyl Parathion | 0.08 (malathion), 0.07 (methyl parathion) | Cabbage sample, tap water, river water | [95] |
| HEMA-MA-aspartic acid-AuNPs | Atrazine | 3.3 | Lab water | [98] |
| BSA-stabilized AuNCs-MnO2 | Carbaryl | 0.63 | Lake water, soil | [99] |
| SDS capped AgNPs | Ziram, Zineb, and Maneb | 0.03–0.57 | Lab water | [101] |
| Urease-AuNPs agarose-guar gum | Glyphosate | 1.8 | River water | [113] |
| Melamine-modified AuNPs | Triazine Pymetrozine | 10–80 | Tap water, lake water | [76] |
| System | Heavy Metal | Maximum RE (%) or Adsorption Capacity (mg/g) | Optimal Conditions: pH, Metal Concentration, Contact Time | Reference |
|---|---|---|---|---|
| β-CD-chitosan-Fe3O4 | As (III) | 96% | pH 9, 0.1 mg/L, 20 min. | [129] |
| Permethylated β-CD | As (V) | 98% | pH 6, 0.1 mg/L, 30 min. | [130] |
| β-CD-graphene foam | Cr (VI) | 99.8% | pH 3, 50 mg/L, 240 min. | [131] |
| β-CD-CNT-Fe3O4 | Ni (II) | 103 | pH 6, 50 mg/L, 50 min. | [128] |
| β-CD-chitosan-EDTA | Pb (II), Cu (II), Ni (II) | 330.9 (Pb2+), 161 (Cu2+), 118.9 (Ni2+) | pH > 5, 25 mg/L, 300 min. | [132] |
| PMDA-β-CDNSs | Cu (II), Zn (II), Pb (II), Cd (II), and Fe (III) | Up to 94% | pH not reported; 500 ppm, 24 h. | [133] |
| Tannic acid β-CDNSs | Pb (II) | 81% | pH range 4–6, 200 mg/L, 3 min. | [134] |
| Citric acid β-CDNSs | U (VI) | 150 | pH 4; 60 mg/L, 60 min. | [134] |
| Calixarene-CDNSs | Pb (II) | Up to 85% | pH > 6, metal concentration not reported; 5–10 min. | [135] |
| Citric acid βCDNSs-ZrO2 | Pb (II) | 274.5 | pH 7, 200 mg/L, 120 min. | [136] |
| System | Dye | Maximum RE% or Adsorption Capacity (mg/g) | Optimal Conditions: pH, Contact Time | Reference |
|---|---|---|---|---|
| EPI NSs-Fe3O4 | Direct Red 83:1 | >90% | pH 5, 30 min. | [138] |
| 1,2,3,4-butane tetracarboxylic acid NSs | Malachite Green and Safranin | 98.3% for Malachite Green, 96% for Safranin | pH 8, 180 min. | [139] |
| α-CD-EPI, β-CD-EPI, γ-CD-EPI | Direct Blue 78 | 99% (β-CD-EPI); 97% (α-CD-EPI, γ-CD-EPI) | pH 6, 120 min. | [140] |
| NSs-CNT-TiO2-AgNPs | Congo Red | 146.7 | pH 8, 450 min. | [141] |
| β-CD-EPI-rGO | Malachite Green | 902.2 | pH 8, 90 min. | [142] |
| Halloysite-CD-NSs | Rhodamine B | 70% | pH > 4.5, 100 min. | [142] |
| β-CD-DPC NSs | Basic Red 46 and Rhodamine B | 101.3 (Basic Red); 52.3 (Rhodamine B) | pH 3–5, 120 min. for Basic Red; 180 min. for Rhodamine B | [124] |
| Citric Acid β-CD NSs | Methylene Blue and Congo Red | 5.1 for Methylene Blue; 12 for Congo Red | pH not reported; 1500 min. for Methylene Blue; 40 min. for Congo Red; | [143] |
| β-CD-Activated Charcoal-Alginate-Fe3O4 nanocomposite | Methylene Blue | 99.53% | pH 6, 90 min. | [144] |
| System | Organic Pollutants | Maximum Removal Efficiency | Optimal Conditions: pH, Contact Time | Reference |
|---|---|---|---|---|
| DPC NSs-Fe3O4 | 4-chlorophenoxyacetic acid and 2,3,4,6 tetra chlorophenol | 91% for 4-CPA, 78% for TCP | pH 9, 120 min. | [146] |
| DPC NSs-Fe3O4 | Dinotefuran | 90.3% | pH 7, 120 min. | [147] |
| β-CD-cellulose nanofiber | Bisphenol A, Bisphenol S, Bisphenol F | 88.1% | pH 7, 15 min. | [148] |
| Hydroxypropyl β-CD-1,2,3,4 butane tetracarboxylic acid NSs | PAHs | 92% (initial concentration of 400 μg/L of PAH) and 89% (initial concentration of 600 μg/L of PAH) | pH 7, 60 min. | [149] |
| Am6-NSs and Am12-NSs | Imidacloprid | 95% | pH 3.8, contact time not reported | [150] |
| β-CD-EPI NSs and γ-CD-EPI NSs functionalized with MnO2 nanorods | Atrazine, benalaxyl, bromacil, butachlor, fenamiphos, fipronil, flufiprole, and pretilachlor | Ranging between 43%–73% | pH 7, 120 min. | [151] |
| CDI NSs and PDA NSs | Ailanthone | 55.1% | pH not reported; 24 h. | [152] |
| Anionic β-CD-citric acid NSs-cotton cord | Paraquat, Methylene Blue, and Crystal Violet | 91% (Paraquat), 97% (Methylene Blue), 98% (Crystal Violet) | pH 6 for Paraquat; pH 4 for Methylene Blue and Crystal Violet, 360 min. | [153] |
| Anionic β-CD-1,2,3,4, butane tetracarboxylic acid NSs-cotton cord | Paraquat | 95.1% | pH 8, 360 min. | [154] |
| System | Organic Pollutants | LOD (nM) | Linear Range (nM) | Reference |
|---|---|---|---|---|
| Thiolated β-CD-gold nanosatellite | Paraquat, Diquat, Difenzoquat | 18.5 | 18.9–37.8 | [156] |
| L-Citrulline-CD-glassy carbon electrode | Metribuzin | 10 | 0.03–1 | [157] |
| β-CD-MOF-Mxrene-Carbon nanohorns | Carbendazim | 1 | 3–10 | [158] |
| Fluorescent β-CD-DL-TPE | Trinitrophenol and nitrobenzene | 5 | 10–150 | [159] |
| α-CD, β-CD, and γ-CD on PVC matrix | Procainamide | 240, 213, 238 for α-CD, β-CD, and γ-CD, respectively | 0.01–1.0 | [160] |
| β-CD and γ-CD on PVC matrix | Trazodone | 2.2 (β-CD) 0.15 (γ-CD) | 7–100 (β-CD) 0.5–100 (γ-CD) | [161] |
| β-CD incorporated into graphene/poly(dimethyl siloxane) composites | Propylparaben | 10 | 10–100 | [161] |
| Poly pyrrole nanotubes-β-CD-TiO2 | Methylparaben and Methylene Blue | 10 | 10–100 | [162] |
| rGO-β-CD-glassy carbon | Catechol | 1.3 | 100–800 | [163] |
| System | Pharmaceutical Pollutant | Maximum RE (%) | Optimal Conditions: pH, Contact Time | Reference |
|---|---|---|---|---|
| Fe3O4@AgNPs | Ibuprofen | 93% | pH 7, 45 min. | [170] |
| Chitosan-Fe2O3 | Ibuprofen | 98% | pH 7, 120 min. | [171] |
| Starch NPs | Ibuprofen and Sulfamethoxazole | 86% (Ibuprofen); 85% (Sulfamethoxazole) | pH 2–3, 300 min. | [172] |
| Ozone treated MW-CNTs | Acetaminophen | 95% | pH 4, 60 min. | [173] |
| Graphene-TiO2 | Acetaminophen | 97% | pH 9, 180 min. | [174] |
| Maltodextrin-GO-CuO | Diclofenac and Amoxicillin | 95% | pH 7, 10 min. | [175] |
| GO-CoFe2O4 | Diclofenac | 87% | pH 4, 120 min. | [176] |
| Fe3O4@C | Hospital effluent (antibiotics) | 85% | pH 6, 120 min. | [177] |
| CuNPs | Ketoprofen | 89% | pH 4.4, 50 min | [178] |
| System | Pollutant | Degradation (%) | Degradation Time (min) | Reference |
|---|---|---|---|---|
| TiO2 P90 on stainless steel | Phenol | 100 | 180 | [182] |
| Yb-TiO2-rGO | Phenol | 100 | 300 | [183] |
| PtNPs/TiO2 | Phenol | 90 | 30 | [184] |
| rGO-GOB | Methylene Blue | 90 | 120 | [185] |
| rGO-HAB | Methylene Blue | 60 | 120 | [186] |
| WO3/H2O2/citric acid | Methylparaben | 100 | 24 h. | [187] |
| WO3/Ag2CO3 | Rhodamine B | 99 | 8 | [188] |
| SnO2/C3N4 | Rhodamine B and Brilliant Red | 98 | 90 | [189] |
| Fe3O4/SnO2/C3N4 | Carbofuran | 89 | 70 | [190] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salazar Sandoval, S.; Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N.; Rojas-Romo, C.; González-Casanova, J.; Gómez, D.R.; Yutronic, N.; Urzúa, M.; et al. Nanomaterials for Potential Detection and Remediation: A Review of Their Analytical and Environmental Applications. Coatings 2023, 13, 2085. https://doi.org/10.3390/coatings13122085
Salazar Sandoval S, Bruna T, Maldonado-Bravo F, Jara P, Caro N, Rojas-Romo C, González-Casanova J, Gómez DR, Yutronic N, Urzúa M, et al. Nanomaterials for Potential Detection and Remediation: A Review of Their Analytical and Environmental Applications. Coatings. 2023; 13(12):2085. https://doi.org/10.3390/coatings13122085
Chicago/Turabian StyleSalazar Sandoval, Sebastián, Tamara Bruna, Francisca Maldonado-Bravo, Paul Jara, Nelson Caro, Carlos Rojas-Romo, Jorge González-Casanova, Diana Rojas Gómez, Nicolás Yutronic, Marcela Urzúa, and et al. 2023. "Nanomaterials for Potential Detection and Remediation: A Review of Their Analytical and Environmental Applications" Coatings 13, no. 12: 2085. https://doi.org/10.3390/coatings13122085
APA StyleSalazar Sandoval, S., Bruna, T., Maldonado-Bravo, F., Jara, P., Caro, N., Rojas-Romo, C., González-Casanova, J., Gómez, D. R., Yutronic, N., Urzúa, M., & Rodríguez-San Pedro, A. (2023). Nanomaterials for Potential Detection and Remediation: A Review of Their Analytical and Environmental Applications. Coatings, 13(12), 2085. https://doi.org/10.3390/coatings13122085






