Preparation and Characterization of Biomimetic SiO2-TiO2-PDMS Composite Hydrophobic Coating with Self-Cleaning Properties for Wall Protection Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Substrates
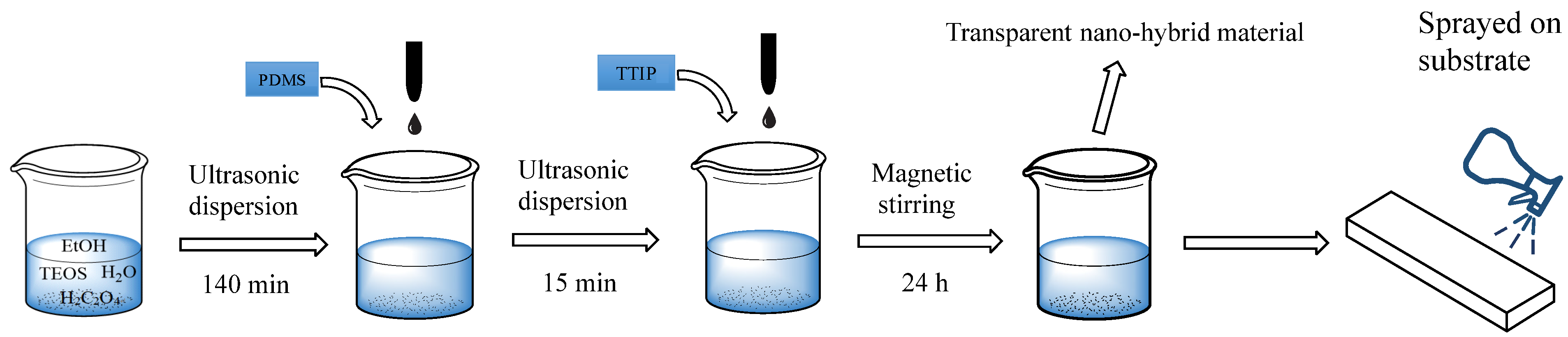
2.3. Synthesis and Deposition of the Surface Coating
2.4. Characterization
2.5. Water Absorption Test
2.6. Self-Cleaning Test
2.7. Mechanical Stability Test
3. Results and Discussion
3.1. Characterization of the SiO2-TiO2-PDMS Sol and Coating






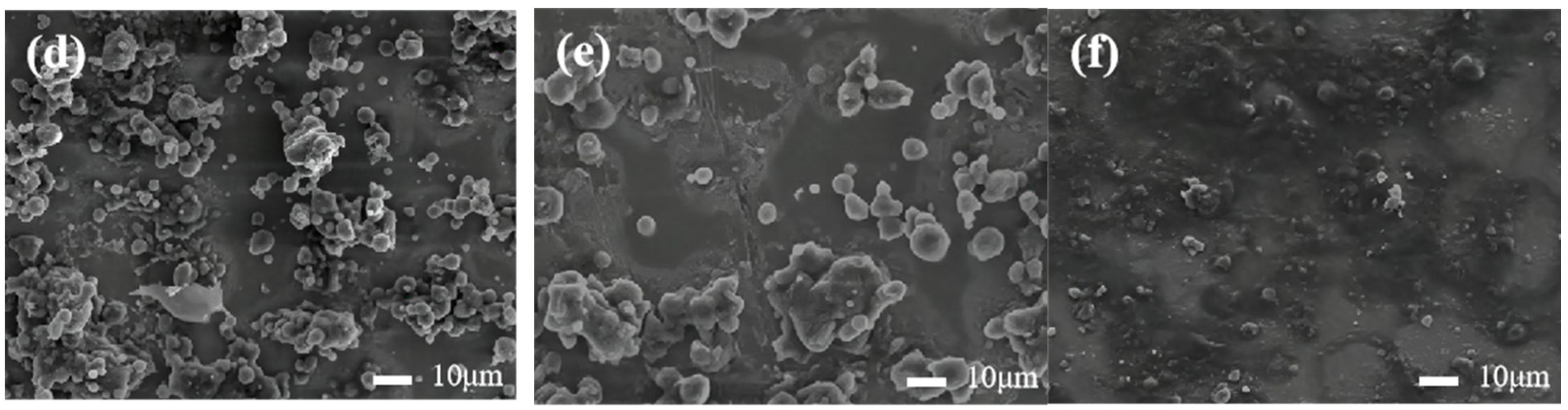
3.2. Microstructure of the Coating
3.3. Water-Repellent Performance
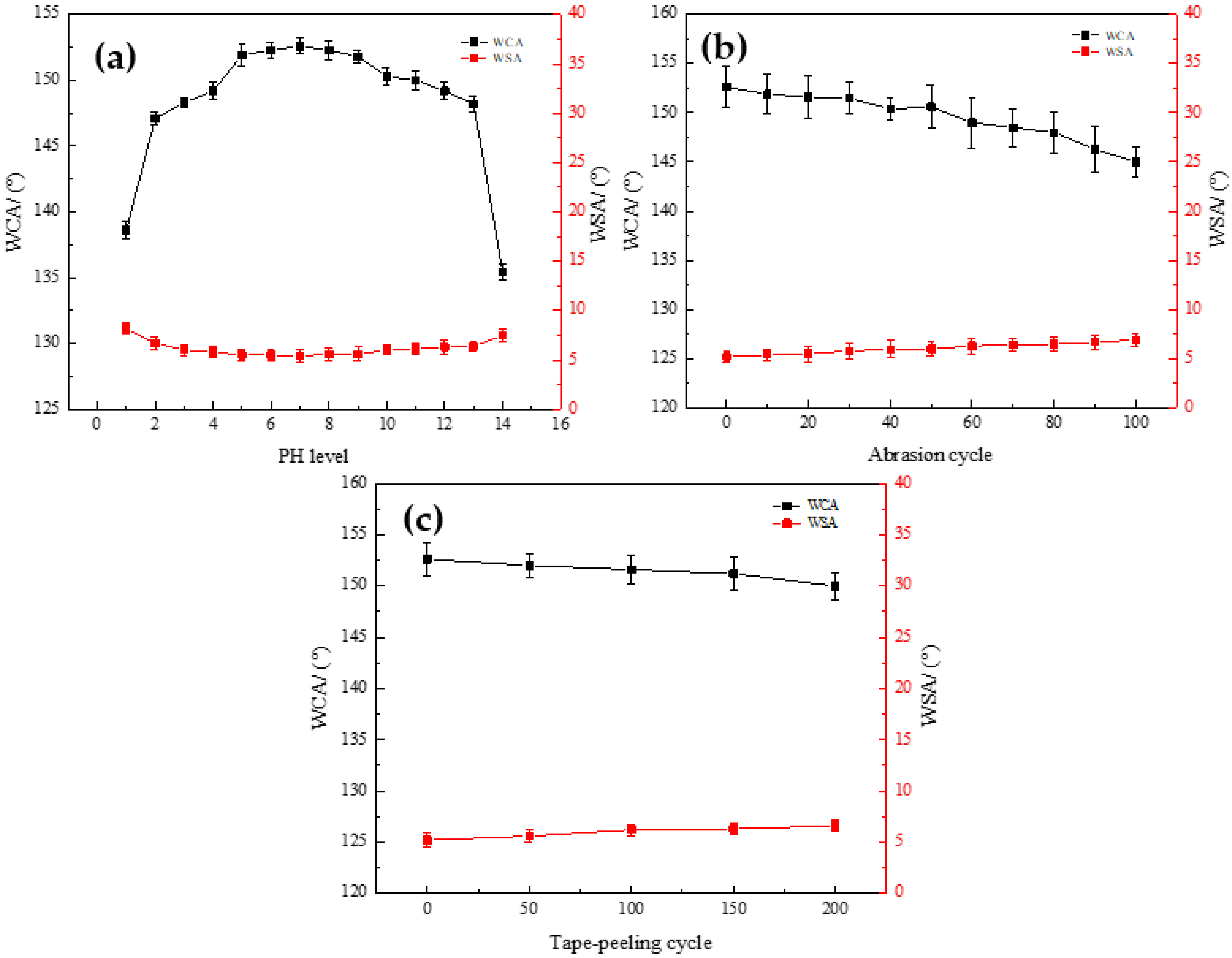
3.3.1. Contact Angle
3.3.2. Water Absorption
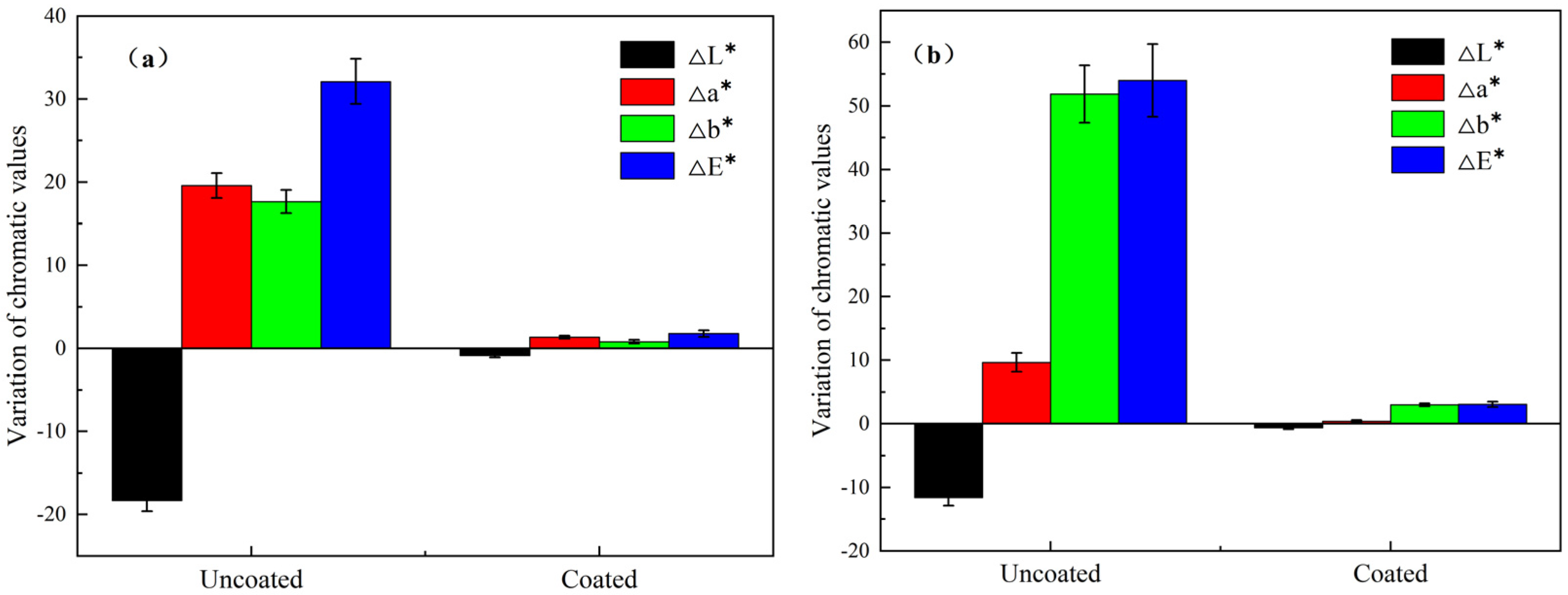
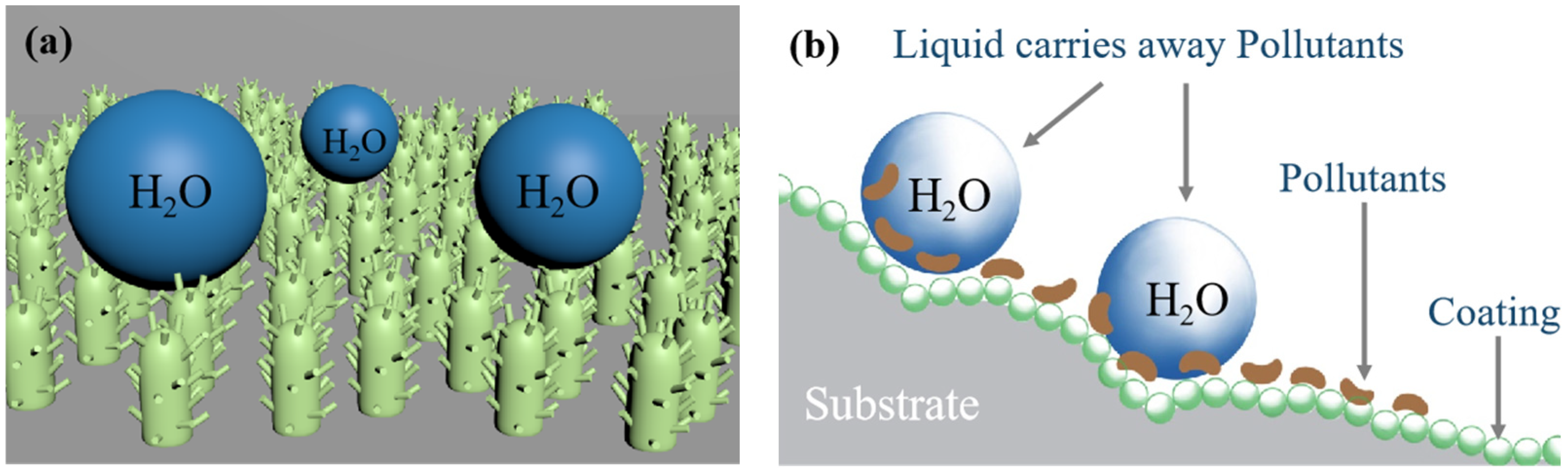
3.4. Self-Cleaning Ability of the Coating
3.5. Mechanical Stability of the Coating
4. Conclusions
- (1)
- A novel hybrid material for building wall protection was prepared by the sol-gel process with TEOS and TTIP as precursors and PDMS as low surface energy substances under the catalytic action of oxalic acid. When the molar ratio of PDMS to TEOS was 1:5, the static contact angle of the coating surface was 152.6°, and the superhydrophobic state was achieved.
- (2)
- The coating exhibited a hydrophobic protective effect on the building wall. Without coating, the building wall reached water absorption saturation at 24 h with 5.28% water absorption. After coating, the building wall reached water absorption saturation after 4 days, and the water absorption rate was 3.3%, which was 37.5% lower than before.
- (3)
- The results of FTIR showed that the coating formed a micro-nano secondary structure similar to the surface of lotus leaf after condensation and co-polymerization. Short term immersion measurements revealed that the coating exhibited excellent chemical durability at different pH concentrations.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gradeci, K.; Labonnote, N.; Time, B.; Köhler, J. A Probabilistic-Based Methodology for Predicting Mould Growth in Façade Constructions. Build. Environ. 2018, 128, 33–45. [Google Scholar] [CrossRef]
- Chen, G.; Wang, H.; Chen, Y.; Liu, X.; Guo, X. Contrastive Study on Indoor Temperature-humidity Threshold Value for Mould Germination Risk on the Hygroscopic Wall. J. Univ. S. China (Sci. Technol.) 2019, 33, 1–7. [Google Scholar] [CrossRef]
- Kurbanova, A.; Myrzakhmetova, N.; Akimbayeva, N.; Kishibayev, K.; Nurbekova, M.; Kanagat, Y.; Tursynova, A.; Zhunussova, T.; Seralin, A.; Kudaibergenova, R.; et al. Superhydrophobic SiO2/Trimethylchlorosilane Coating for Self-Cleaning Application of Construction Materials. Coatings 2022, 12, 1422. [Google Scholar] [CrossRef]
- Salimifard, P.; Rim, D.; Gomes, C.; Kremer, P.; Freihaut, J.D. Resuspension of Biological Particles from Indoor Surfaces: Effects of Humidity and Air Swirl. Sci. Total Environ. 2017, 583, 241–247. [Google Scholar] [CrossRef]
- Dalawai, S.P.; Saad Aly, M.A.; Latthe, S.S.; Xing, R.; Sutar, R.S.; Nagappan, S.; Ha, C.S.; Kumar Sadasivuni, K.; Liu, S. Recent Advances in Durability of Superhydrophobic Self-Cleaning Technology: A Critical Review. Prog. Org. Coat. 2020, 138, 105381. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, H.; Shao, Y.; Zhang, H.; Zhu, J. Recent Progresses of Superhydrophobic Coatings in Different Application Fields: An Overview. Coatings 2021, 11, 116. [Google Scholar] [CrossRef]
- Xiang, T.; Lv, Z.; Wei, F.; Liu, J.; Dong, W.; Li, C.; Zhao, Y.; Chen, D. Superhydrophobic Civil Engineering Materials: A Review from Recent Developments. Coatings 2019, 9, 753. [Google Scholar] [CrossRef] [Green Version]
- Neinhuis, C.; Barthlott, W. Characterization and Distribution of Water-Repellent, Self-Cleaning Plant Surfaces. Ann. Bot. 1997, 79, 667–677. [Google Scholar] [CrossRef] [Green Version]
- Qian, B.; Shen, Z. Fabrication of Superhydrophobic Surfaces by Dislocation-Selective Chemical Etching on Aluminum, Copper, and Zinc Substrates. Langmuir 2005, 21, 9007–9009. [Google Scholar] [CrossRef]
- Yu, J.; Qin, L.; Hao, Y.; Kuang, S.; Bai, X.; Chong, Y.M.; Zhang, W.; Wang, E. Vertically Aligned Boron Nitride Nanosheets: Chemical Vapor Synthesis, Ultraviolet Light Emission, and Superhydrophobicity. ACS Nano 2010, 4, 414–422. [Google Scholar] [CrossRef]
- Chaudhary, A.; Barshilia, H.C. Nanometric Multiscale Rough CuO/Cu(OH)2 Superhydrophobic Surfaces Prepared by a Facile One-Step Solution-Immersion Process: Transition to Superhydrophilicity with Oxygen Plasma Treatment. J. Phys. Chem. C 2011, 115, 18213–18220. [Google Scholar] [CrossRef]
- Rao, A.V.; Latthe, S.S.; Mahadik, S.A.; Kappenstein, C. Mechanically Stable and Corrosion Resistant Superhydrophobic Sol–Gel Coatings on Copper Substrate. Appl. Surf. Sci. 2011, 257, 5772–5776. [Google Scholar] [CrossRef]
- Dong, C.; Gu, Y.; Zhong, M.; Li, L.; Sezer, K.; Ma, M.; Liu, W. Fabrication of Superhydrophobic Cu Surfaces with Tunable Regular Micro and Random Nano-Scale Structures by Hybrid Laser Texture and Chemical Etching. J. Mater. Proc. Technol. 2011, 211, 1234–1240. [Google Scholar] [CrossRef]
- Guo, X.J.; Xue, C.H.; Sathasivam, S.; Page, K.; He, G.; Guo, J.; Promdet, P.; Heale, F.L.; Carmalt, C.J.; Parkin, I.P. Fabrication of Robust Superhydrophobic Surfaces via Aerosol-Assisted CVD and Thermo-Triggered Healing of Superhydrophobicity by Recovery of Roughness Structures. J. Mater. Chem. A 2019, 7, 17604–17612. [Google Scholar] [CrossRef]
- Li, S.; Page, K.; Sathasivam, S.; Heale, F.; He, G.; Lu, Y.; Lai, Y.; Chen, G.; Carmalt, C.J.; Parkin, I.P. Efficiently Texturing Hierarchical Superhydrophobic Fluoride-Free Translucent Films by AACVD with Excellent Durability and Self-Cleaning Ability. J. Mater. Chem. A 2018, 6, 17633–17641. [Google Scholar] [CrossRef] [Green Version]
- Wang, F. Study on Bionic Preparation of Micro-Nanostructures and Properties of Plant Leaves onto Bamboo Surfaces through Morph-Genetic Method. Ph.D. Thesis, Zhejiang A&F University, Zhejiang, China, 2018. [Google Scholar]
- Wang, F.; Li, S. The super-hydrophobic structure of lotus leaf generated on the bamboo surface based on soft lithography. Sci. Technol. Rev. 2016, 34, 101–104. [Google Scholar] [CrossRef]
- Wang, F.; Wu, H.; Li, S.; Zhang, W. The Bionic Rose-like Super-hydrophobic Structure on Bamboo Surface. J. Bamboo Res. 2017, 36, 49–52. [Google Scholar] [CrossRef]
- He, X.; Fu, S.; Dai, Y.; Jin, C.; Wang, F. Research on Surface Characteristics of Bamboo with Bionic Super-hydrophobic Chinese Rose/TiO2. World Bamboo Ratt. 2018, 15, 11–15. [Google Scholar] [CrossRef]
- Wen, N.; Miao, X.; Yang, X.; Long, M.; Deng, W.; Zhou, Q.; Deng, W. An Alternative Fabrication of Underoil Superhydrophobic or Underwater Superoleophobic Stainless Steel Meshes for Oil-Water Separation: Originating from One-Step Vapor Deposition of Polydimethylsiloxane. Sep. Purif. Technol. 2018, 204, 116–126. [Google Scholar] [CrossRef]
- Xie, A.; Cui, J.; Chen, Y.; Lang, J.; Li, C.; Yan, Y.; Dai, J. One-Step Facile Fabrication of Sustainable Cellulose Membrane with Superhydrophobicity via a Sol-Gel Strategy for Efficient Oil/Water Separation. Surf. Coat. Technol. 2019, 361, 19–26. [Google Scholar] [CrossRef]
- Arun Kumar, K.V.; John, J.; Sooraj, T.R.; Raj, S.A.; Unnikrishnan, N.V.; Selvaraj, N.B. Surface Plasmon Response of Silver Nanoparticles Doped Silica Synthesised via Sol-Gel Route. Appl. Surf. Sci. 2019, 472, 40–45. [Google Scholar] [CrossRef]
- Calia, A.; Lettieri, M.; Masieri, M. Durability Assessment of Nanostructured TiO2 Coatings Applied on Limestones to Enhance Building Surface with Self-Cleaning Ability. Build. Environ. 2016, 110, 1–10. [Google Scholar] [CrossRef]
- Pedna, A.; Pinho, L.; Frediani, P.; Mosquera, M.J. Obtaining SiO2–Fluorinated PLA Bionanocomposites with Application as Reversible and Highly-Hydrophobic Coatings of Buildings. Prog. Org. Coat. 2016, 90, 91–100. [Google Scholar] [CrossRef]
- Peng, M.; Wang, L.; Guo, L.; Guo, J.; Zheng, L.; Yang, F.; Ma, Z.; Zhao, X. A Durable Nano-SiO2-TiO2/Dodecyltrimethoxysilane Superhydrophobic Coating for Stone Protection. Coatings 2022, 12, 1397. [Google Scholar] [CrossRef]
- Adamopoulos, F.G.; Vouvoudi, E.C.; Pavlidou, E.; Achilias, D.S.; Karapanagiotis, I. TEOS-Based Superhydrophobic Coating for the Protection of Stone-Built Cultural Heritage. Coatings 2021, 11, 135. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, H.; Niu, H.; Zhao, Y.; Xu, Z.; Lin, T. A Waterborne Coating System for Preparing Robust, Self-Healing, Superamphiphobic Surfaces. Adv. Funct. Mater. 2017, 27, 1604261. [Google Scholar] [CrossRef]
- Wei, X.; Chen, F.; Wang, H.; Zhou, H.; Ji, Z.; Lin, T. Efficient Removal of Aerosol Oil-Mists Using Superoleophobic Filters. J. Mater. Chem. A 2018, 6, 871–877. [Google Scholar] [CrossRef]
- Zhong, M. Study on the Preparation and Application of Superhydrophobic Silica Coatings. Master’s Thesis, Fuzhou University, Fuzhou, China, 2018. [Google Scholar]
- Yang, C.; Wang, F.; Li, W.; Ou, J.; Li, C.; Amirfazli, A. Anti-Icing Properties of Superhydrophobic ZnO/PDMS Composite Coating. Appl. Phys. A Mater. Sci. Process. 2016, 122, 1. [Google Scholar] [CrossRef]
- Deng, Z.Y.; Wang, W.; Mao, L.H.; Wang, C.F.; Chen, S. Versatile Superhydrophobic and Photocatalytic Films Generated from TiO2–SiO2@PDMS and Their Applications on Fabrics. J. Mater. Chem. A 2014, 2, 4178–4184. [Google Scholar] [CrossRef]
- Song, Y.; Lei, X.; Liu, J.; Guan, X.; Yuan, H. Preparation and Properties of Superhydrophobic Surface with Titanium Dioxide and Attapulgite. J. Chin. Ceram. Soc. 2021, 49, 2242–2250. [Google Scholar] [CrossRef]
- Zárraga, R.; Cervantes, J.; Salazar-Hernandez, C.; Wheeler, G. Effect of the Addition of Hydroxyl-Terminated Polydimethylsiloxane to TEOS-Based Stone Consolidants. J. Cult. Herit. 2010, 11, 138–144. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, J.; Yuan, C.; Lin, J.; Yin, Z. A single TiO2-coated side-glowing optical fiber for photocatalytic wastewater treatment. Chin. Sci. Bull. 2005, 50, 1978–1984. [Google Scholar] [CrossRef]
- Peng, S.; Meng, W.; Guo, J.; Wang, B.; Wang, Z.; Xu, N.; Li, X.; Wang, J.; Xu, J. Photocatalytically Stable Superhydrophobic and Translucent Coatings Generated from PDMS-Grafted-SiO2/TiO2@PDMS with Multiple Applications. Langmuir 2019, 35, 2760–2771. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Xu, L.; Meng, Y.; Shen, Y.; Wang, L.; Pan, H. Preparation of superhydrophobic, photocatalytic and UV-blocking textiles based on SiO2/TiO2 composite aerogels. J. Text. Res. 2019, 40, 90–96. [Google Scholar] [CrossRef]
- Liu, X.; Gu, Y.; Mi, T.; Wang, X.; Zhang, X. Dip-Coating Approach to Fabricate Durable PDMS/STA/SiO2 Superhydrophobic Polyester Fabrics. Coatings 2021, 11, 326. [Google Scholar] [CrossRef]
- Test Method of Cement Mortar Strength (ISO Method). Available online: https://wenku.baidu.com/view/bf9f196074232f60ddccda38376baf1ffd4fe379.html?_wkts_=1674023709816 (accessed on 3 November 2022).
- Aslanidou, D.; Karapanagiotis, I.; Panayiotou, C. Tuning the Wetting Properties of Siloxane-Nanoparticle Coatings to Induce Superhydrophobicity and Superoleophobicity for Stone Protection. Mater. Des. 2016, 108, 736–744. [Google Scholar] [CrossRef]
- Chatzigrigoriou, A.; Karapanagiotis, I.; Poulios, I. Superhydrophobic Coatings Based on Siloxane Resin and Calcium Hydroxide Nanoparticles for Marble Protection. Coatings 2020, 10, 334. [Google Scholar] [CrossRef] [Green Version]
- Yin, Z.; Chen, X.; Zhou, T.; Xue, M.; Li, M.; Liu, K.; Zhou, D.; Ou, J.; Xie, Y.; Ren, Z.; et al. Mussel-Inspired Fabrication of Superior Superhydrophobic Cellulose-Based Composite Membrane for Efficient Oil Emulsions Separation, Excellent Anti-Microbial Property and Simultaneous Photocatalytic Dye Degradation. Sep. Purif. Technol. 2022, 286, 120504. [Google Scholar] [CrossRef]
- Standard Test Method for Determining the Water Absorption of Hardened Concrete Treated with a Water Repellent Coating. Available online: https://www.astm.org/d6489-99r20.html (accessed on 3 November 2022).
- La Russa, M.F.; Ruffolo, S.A.; Rovella, N.; Belfiore, C.M.; Palermo, A.M.; Guzzi, M.T.; Crisci, G.M. Multifunctional TiO2 Coatings for Cultural Heritage. Prog. Org. Coat. 2012, 74, 186–191. [Google Scholar] [CrossRef]
- García, O.; Malaga, K. Definition of the Procedure to Determine the Suitability and Durability of an Anti-Graffiti Product for Application on Cultural Heritage Porous Materials. J. Cult. Herit. 2012, 13, 77–82. [Google Scholar] [CrossRef]
- Elzaabalawy, A.; Meguid, S.A. Development of Novel Superhydrophobic Coatings Using Siloxane-Modified Epoxy Nanocomposites. Chem. Eng. J. 2020, 398, 125403. [Google Scholar] [CrossRef]
- Zhang, X.; Si, Y.; Mo, J.; Guo, Z. Robust Micro-Nanoscale Flowerlike ZnO/Epoxy Resin Superhydrophobic Coating with Rapid Healing Ability. Chem. Eng. J. 2017, 313, 1152–1159. [Google Scholar] [CrossRef]
- Yin, Z.; Yuan, F.; Li, M.; Xue, M.; Zhou, D.; Chen, Y.; Liu, X.; Luo, Y.; Hong, Z.; Xie, C.; et al. Self-Cleaning, Underwater Writable, Heat-Insulated and Photocatalytic Cellulose Membrane for High-Efficient Oil/Water Separation and Removal of Hazardous Organic Pollutants. Prog. Org. Coat 2021, 157, 106311. [Google Scholar] [CrossRef]
- Yin, Z.; Yuan, F.; Xue, M.; Xue, Y.; Xie, Y.; Ou, J.; Luo, Y.; Hong, Z.; Xie, C. A Multifunctional and Environmentally Safe Superhydrophobic Membrane with Superior Oil/Water Separation, Photocatalytic Degradation and Anti-Biofouling Performance. J. Colloid Interface Sci. 2022, 611, 93–104. [Google Scholar] [CrossRef]
- Yap, S.W.; Johari, N.; Mazlan, S.A.; Hassan, N.A. Mechanochemical Durability and Self-Cleaning Performance of Zinc Oxide-Epoxy Superhydrophobic Coating Prepared via a Facile One-Step Approach. Ceram. Int. 2021, 47, 15825–15833. [Google Scholar] [CrossRef]
- Vazhnova, T.; Lukyanov, D.B. Fourier Self-Deconvolution of the IR Spectra as a Tool for Investigation of Distinct Functional Groups in Porous Materials: Brønsted Acid Sites in Zeolites. Anal. Chem. 2013, 85, 11291–11296. [Google Scholar] [CrossRef]
- Kauppinen, J.K.; Moffatt, D.J.; Mantsch, H.H.; Cameron, D.G. Fourier Self-Deconvolution: A Method for Resolving Intrinsically Overlapped Bands. Appl. Spectrosc. 1981, 35, 271–276. [Google Scholar] [CrossRef]
- Jia, S.; Liu, M.; Qing, Y.; Wang, S.; Wu, Y. Preparation of Superhydrophobic Surface on Wood Substrate by One-step Sol-gel. China Wood Ind. 2016, 30, 17–20. [Google Scholar] [CrossRef]
- Song, X.; Cui, W.; Gao, G. Water Resistance of Catalyzed and Hybridized TEOS Waterproof Agent. Bull. Chin. Ce Ramic Soc. 2016, 35, 358–362. [Google Scholar] [CrossRef]
- Kapridaki, C.; Maravelaki-Kalaitzaki, P. TiO2–SiO2–PDMS Nano-Composite Hydrophobic Coating with Self-Cleaning Properties for Marble Protection. Prog. Org. Coat. 2013, 76, 400–410. [Google Scholar] [CrossRef]
- Zhou, S. PreParation of PDMS/SiO2 Hybrid Material and Its Application in the Protection of Ancient Ivory. Master’s Thesis, Chengdu University of Technology, Chengdu, China, 2008. [Google Scholar]
- Wang, H. Study on the PreParation and Properties of PDMS/SiO2 Hybrid Membrafl. Master’s Thesis, Beijing Jiaotong University, Beijing, China, 2011. [Google Scholar]
- Ye, X.; Cai, D.; Hou, J.; Ruan, X. Superhydrophobic and self-cleaning coating for building wall protection. Acta Mater. Compos. Sin. 2018, 35, 3271–3279. [Google Scholar] [CrossRef]
- Yin, Z.; Xue, M.; Luo, Y.; Hong, Z.; Xie, C.; Ren, Z.; Wang, H. Excellent Static and Dynamic Anti-Icing Properties of Hierarchical Structured ZnO Superhydrophobic Surface on Cu Substrates. Chem. Phys. Lett. 2020, 755, 137806. [Google Scholar] [CrossRef]
- Yin, Z.; Yuan, F.; Zhou, D.; Xue, M.; Luo, Y.; Hong, Z.; Xie, C. Ultra Dynamic Water Repellency and Anti-Icing Performance of Superhydrophobic ZnO Surface on the Printed Circuit Board (PCB). Chem. Phys. Lett. 2021, 771, 138558. [Google Scholar] [CrossRef]

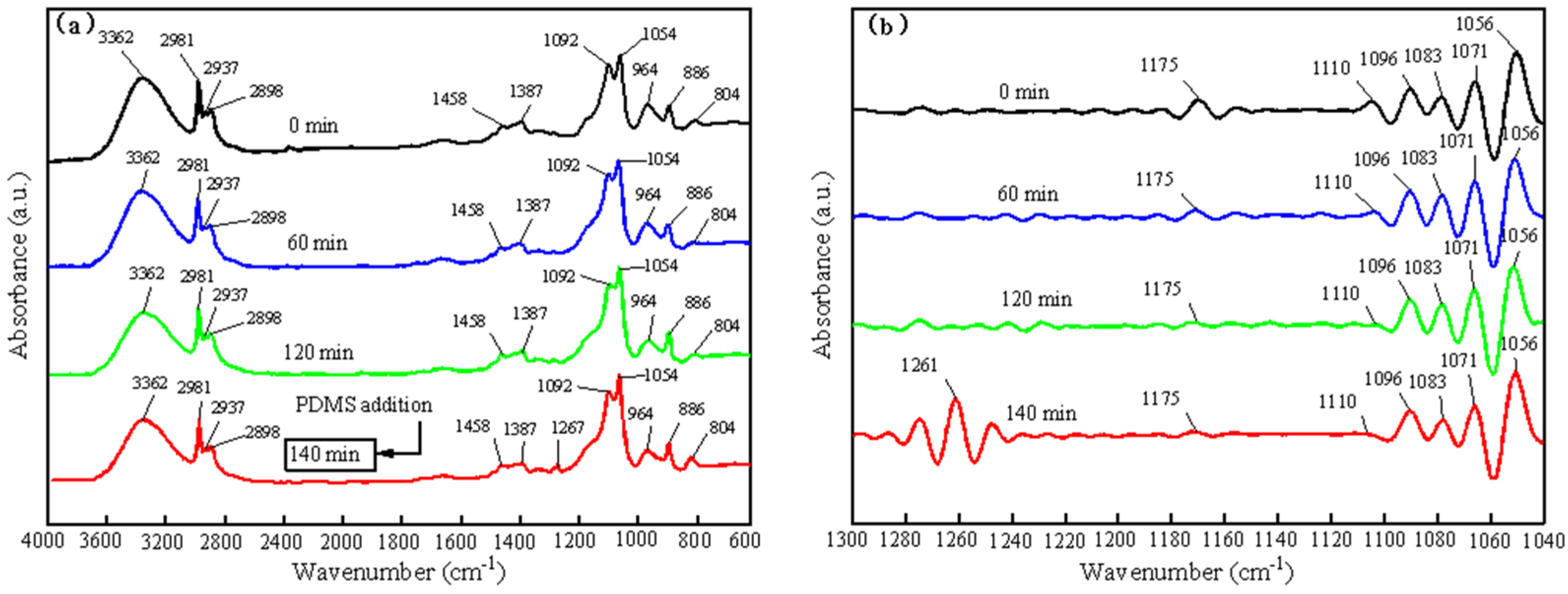
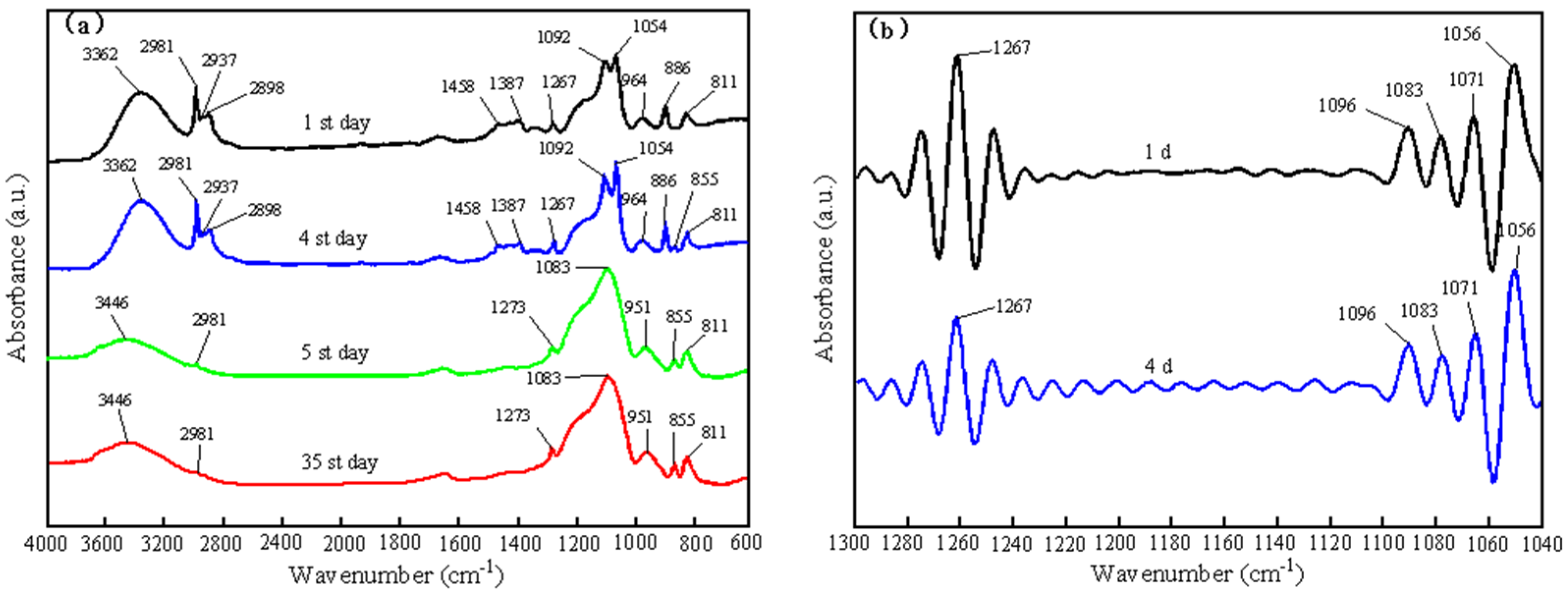






| Wavenumber (cm−1) | Characteristic Peaks | References |
|---|---|---|
| 2981, 2933 | Antisymmetric stretching of C-H bond in EtOH and TEOS | [48,49] |
| 2968 | Antisymmetric stretching of C-H bond in PDMS | [51] |
| 2911 | Symmetric stretching of C-H bonds in PDMS | [51] |
| 2898 | Symmetric stretching of C-H bond in EtOH and TEOS | [48,49] |
| 1460 | Antisymmetric bending of the C-H bond in EtOH | [49] |
| 1451 | Antisymmetric bending of the C-H bond in TEOS | [49] |
| 1396 | Symmetric bending of C-H bond in EtOH and TEOS | [48,49] |
| 1267 | Symmetric bending of C-H bond in PDMS | [51] |
| 1175 | Rocking of C-H bond in TEOS | [49] |
| 1089 | Rocking of C-H bond in EtOH | [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, X.; Liu, J.; Liu, Y.; Lei, Z.; Han, Y.; Zheng, Z.; Yin, J. Preparation and Characterization of Biomimetic SiO2-TiO2-PDMS Composite Hydrophobic Coating with Self-Cleaning Properties for Wall Protection Applications. Coatings 2023, 13, 224. https://doi.org/10.3390/coatings13020224
Xia X, Liu J, Liu Y, Lei Z, Han Y, Zheng Z, Yin J. Preparation and Characterization of Biomimetic SiO2-TiO2-PDMS Composite Hydrophobic Coating with Self-Cleaning Properties for Wall Protection Applications. Coatings. 2023; 13(2):224. https://doi.org/10.3390/coatings13020224
Chicago/Turabian StyleXia, Xiaojing, Jue Liu, Yang Liu, Zijie Lei, Yutong Han, Zeping Zheng, and Jian Yin. 2023. "Preparation and Characterization of Biomimetic SiO2-TiO2-PDMS Composite Hydrophobic Coating with Self-Cleaning Properties for Wall Protection Applications" Coatings 13, no. 2: 224. https://doi.org/10.3390/coatings13020224
APA StyleXia, X., Liu, J., Liu, Y., Lei, Z., Han, Y., Zheng, Z., & Yin, J. (2023). Preparation and Characterization of Biomimetic SiO2-TiO2-PDMS Composite Hydrophobic Coating with Self-Cleaning Properties for Wall Protection Applications. Coatings, 13(2), 224. https://doi.org/10.3390/coatings13020224





