Influence of Process Parameters on the Electrodeposition of Vanadium in NaCl-KCl-NaF-V2O3 Molten Salt
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
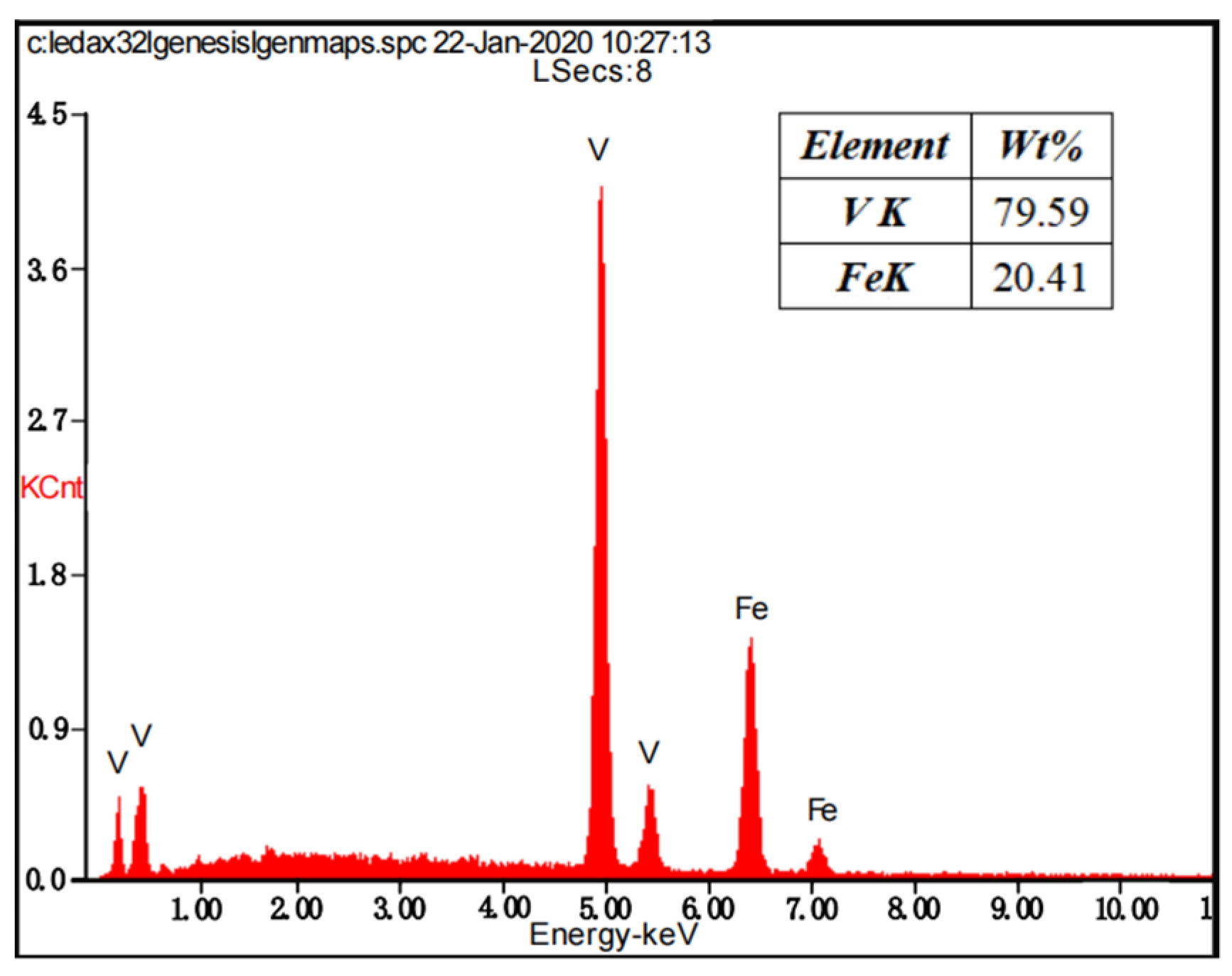
3.1. The Reduction Mechanism of V2O3
3.2. Influence of NaF Content
3.2.1. Influence of NaF Content on the Thickness of the Deposition Layer
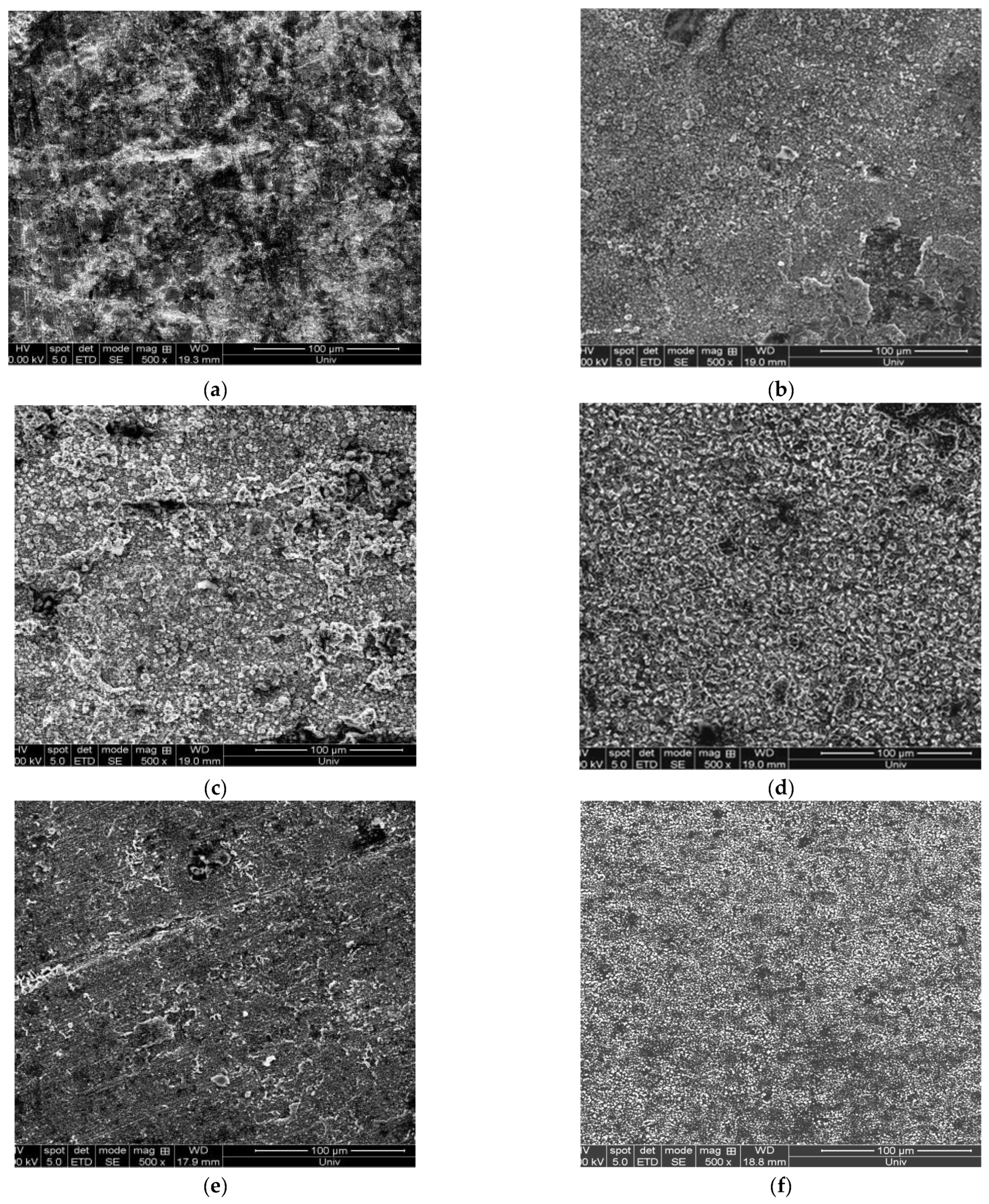
3.2.2. Influence of NaF Content on the Surface Morphology of the Deposition Layer
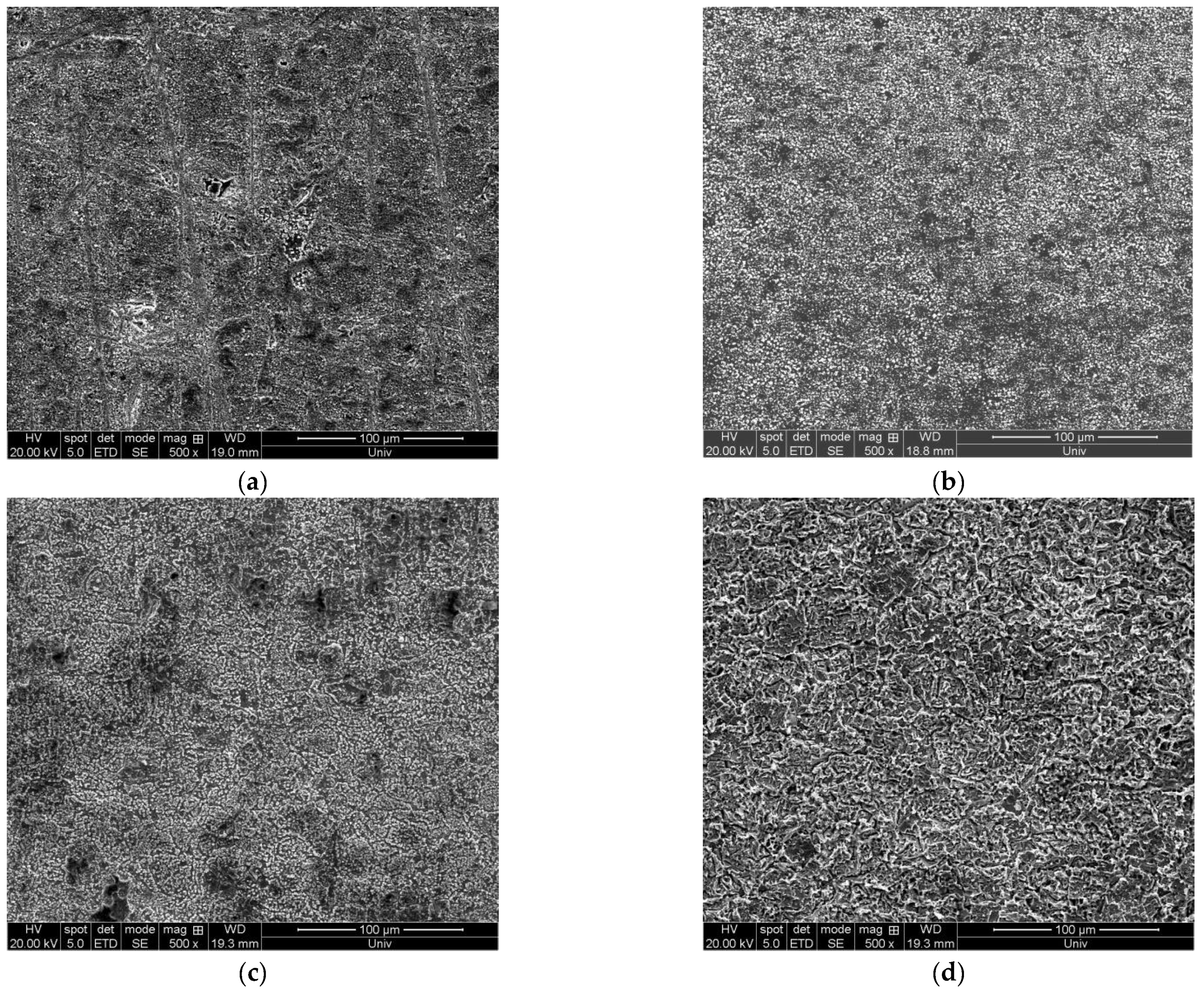
3.3. Influence of Current Waveform
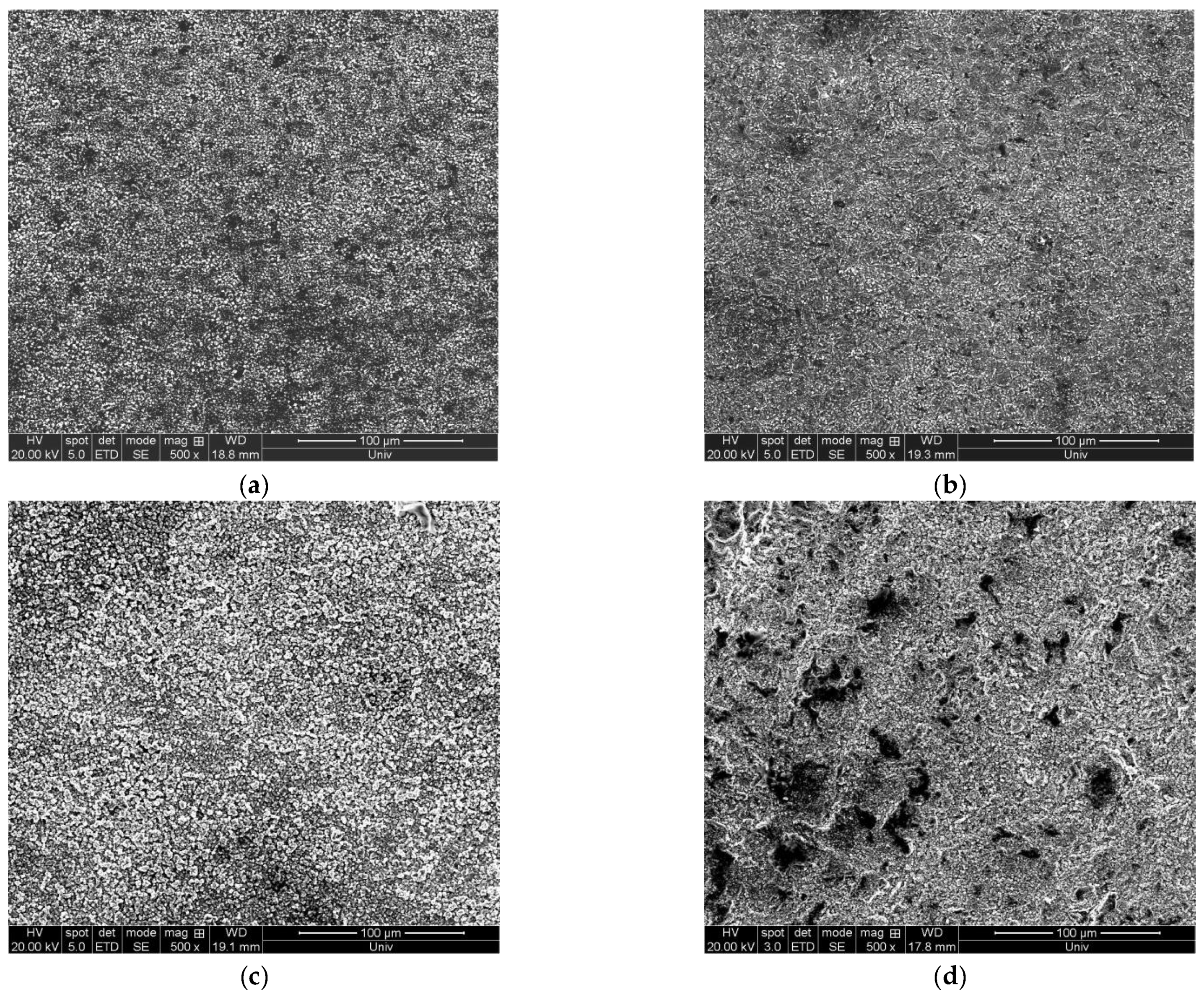
3.4. Influence of Current Density
3.4.1. Influence of Current Density on the Thickness of the Deposition Layer
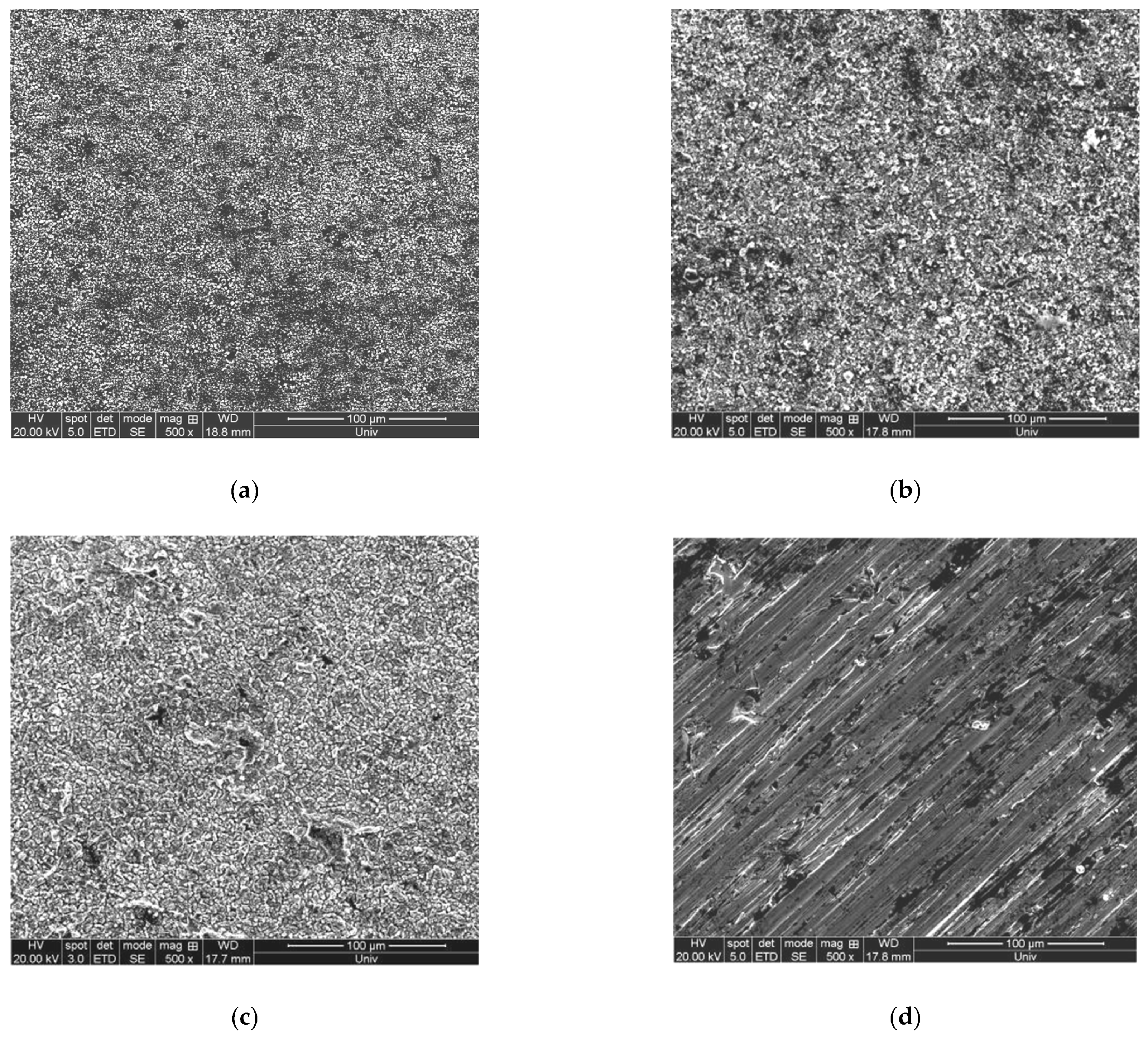
3.4.2. Influence of Current Density on the Surface Morphology of the Deposition Layer
3.5. Influence of Electrodeposition Temperature
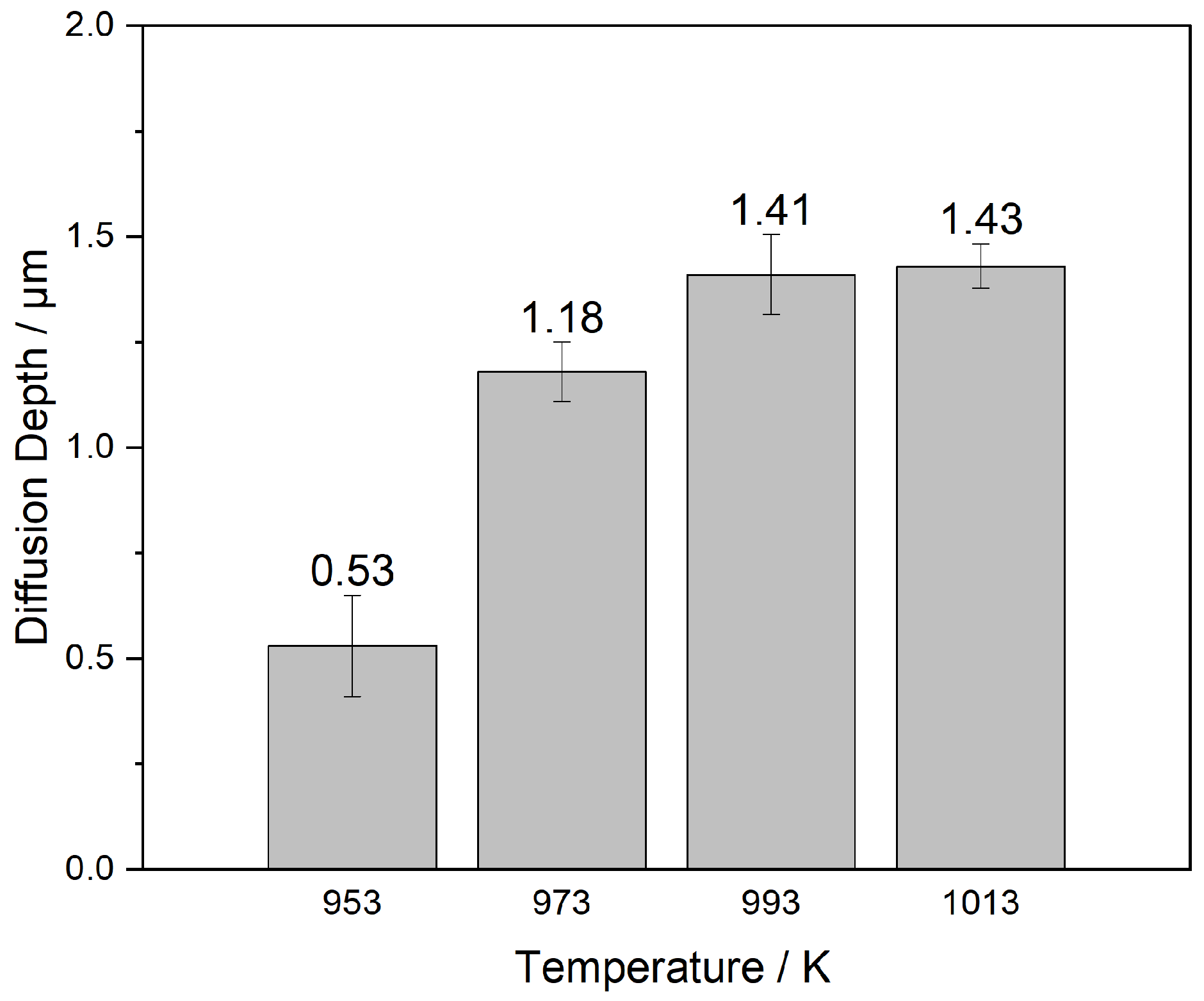
3.5.1. Influence of Electrodeposition Temperature on the Thickness of Vanadium Deposition layer
3.5.2. Influence of Electrodeposition Temperature on the Surface Morphology of Vanadium Deposition Layer
3.6. Influence of Electrodeposition Time
3.6.1. Influence of Electrodeposition Time on the Thickness of the Deposition Layer
3.6.2. The Influence of Electrodeposition Time on the Surface Morphology of the Deposition Layer
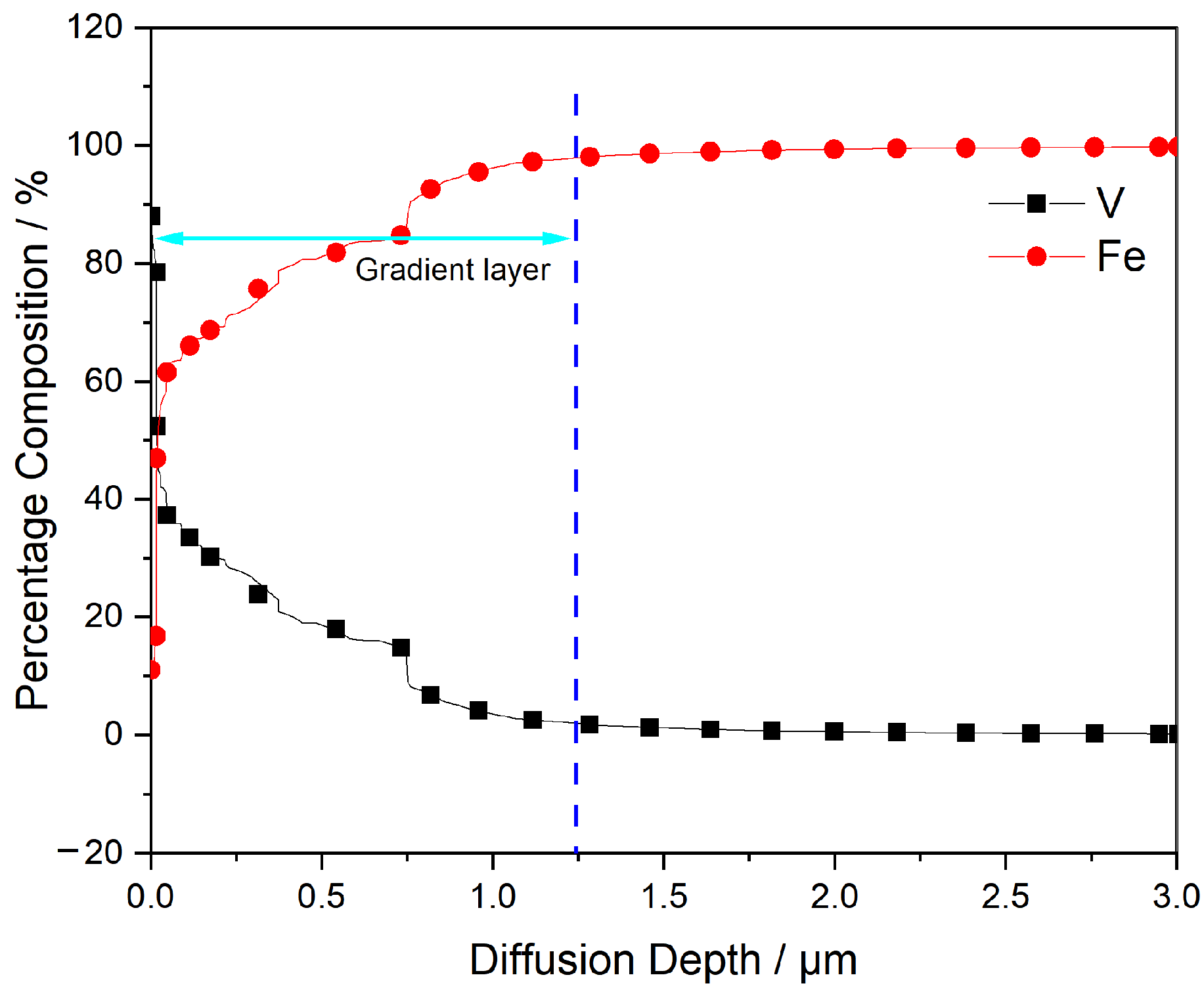
3.7. Cross-Section Analysis of Vanadium Deposits under Optimum Preparation Conditions
4. Conclusions
- (1)
- When the system ratio is XNaCl = 0.4, XKCl = 0.4, and XNaF = 0.2, the quality of the obtained vanadium deposition layer is the best;
- (2)
- The electrodeposition method adopts the bidirectional pulse method, and the surface quality of the obtained vanadium deposition layer is better than that of DC electrodeposition. The process parameters are: pulse period T = 1000 ms; positive and negative current ratio i p/i n = 6:1; positive and negative time ratio t p/t n = 3:1;
- (3)
- The current density of electrodeposition has a great influence on the thickness of the vanadium deposition layer; when the current density is 200 mA·cm−2, a better vanadium deposition layer is obtained;
- (4)
- The electrodeposition temperature is an important factor affecting the thickness of the vanadium deposition layer; when the electrodeposition temperature is 973 K, it can not only ensure good fluidity of molten salt but also maintain the stability of the complex anion in the molten salt and reduce the volatilization of molten salt;
- (5)
- With the prolongation of electrodeposition time, the thickness of the vanadium deposition layer increases continuously, but too long a deposition time will also make the surface of the deposition layer rough and uneven. The quality of the vanadium deposition layer obtained by electrodeposition for 30 min is better.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, S.Q. Production status and development trend of vanadium product in world in recent years. Iron Steel Vanadium Titan 2014, 35, 55–62. [Google Scholar]
- Xin, X.Y.; Li, S.J. Progress in the research of vanadium metal preparation. Sichuan Nonferrous Met. 2009, 1, 11–14. [Google Scholar]
- Zuo, H.; Chen, C.; Wang, H. Research on the indusrtial production processes of high purity metal vanadium. Henan Sci. Technol. 2018, 2, 151–152. [Google Scholar]
- Joly, M.P. In Proceedings of the Second United Nations International Conference on Peaceful Uses of Atomic Energy. Geneva, Switzerland, 1–13 September 1956; p. 309. [Google Scholar]
- Zhang, Y.Y.; Yu, L.; Yu, H.; Fu, T.; Wang, J.; Shen, F.Q.; Cui, K.K. Microstructure evolution and growth mechanism of Si-MoSi2 composite coatings on TZM (Mo-0.5Ti-0.1Zr-0.02 C). Alloys Compd. 2022, 894, 62403. [Google Scholar] [CrossRef]
- Xian, X.B.; Ye, L.S.; Leng, B.Y. Study on Preparation and Properties of Pure Vanadium. Rare Met. Mater. Eng. 2010, 39, 928–931. [Google Scholar]
- Block, F.E. Metallothermic Reduction of Vanadium Chlorides. Report of Investigations 5722; Bureau of Mines, US Department of Interior: Washington, DC, USA, 1961; p. 17.
- Carlson, O.N.; Burkholder, H.R.; Martsching, G.A.; Schmidt, F.A. Preparation of High Purity Vanadium, AIME Extraction Metallurgy of Refractory Metals Meeting; Ames Lab.: Chicago, CA, USA, 1981; p. 1. [Google Scholar]
- Rostoker, W.; Kolodney, M. The Metallurgy of Vanadium. J. Electrochem. Soc. 1958, 105, 197–206. [Google Scholar] [CrossRef]
- Wang, Z.F. Preparation of Vanadium Alloy by Electrochamical Reduction of Solid in Molten Salt; Northeastern University: Shenyang, China, 2013. [Google Scholar]
- Wang, J.; Zhang, Y.Y.; Cui, K.K.; Fu, T.; Gao, J.J.; Hussain, S.; AlGarni, T.S. Pyrometallurgical recovery of zinc and valuable metals from electric arc furnace dust-A review. J. Clean. Prod. 2021, 298, 126788. [Google Scholar] [CrossRef]
- Yang, S.Z. Vanadium Metallurgy, 1st ed.; Metallurgical Industry Press: Beijing, China, 2010; pp. 96–97. [Google Scholar]
- Tripathy, P.K.; Suri, A.K. A New Process for the Preparation of Vanadium Metal. High Temp. Mater. Process. 2002, 21, 127–137. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.Y.; Luo, D.M.; Chen, H.S.; Sui, Z.T. Preparation of High Density VC by Vacuum Carbon Reduction Method. Rare Met. 2003, 1, 132–134. [Google Scholar]
- Li, M.J.; Zhang, S.L.; Ding, X.M.; Luo, W.; Xiong, M. Preparation of Tungsten-doped VO2 Thin Film with Low Phase Transition Temperature by Hydrogen Reduction Method. Iron Steel Vanadium Titan. 2010, 2, 6–9. [Google Scholar]
- Wu, Z.W.; Yang, H.T.; Wu, L.R.; Hu, C.Q.; Gao, W.G.; Huang, L.Y.; Li, L. Research progress in preparation of metal powders by pressurized hydrogen reduction. Int. J. Hydrogen Energy 2021, 46, 35102–35120. [Google Scholar] [CrossRef]
- Abolpour, B.; Afsahi, M.M.; Azizkarimi, M. Hydrogen reduction of magnetite concentrate particles. Miner. Process. Extr. Metall. Rev. 2018, 130, 1–14. [Google Scholar] [CrossRef]
- Wu, C.L.; Wang, B.H.; Chen, X.; Wang, H.L.; Wang, N. Preparation of Vanadium Powder by Calcium Thermic Reduction of Calcium Vanadate in Molten Salts. Nonferrous Met. (Extr. Metall.) 2019, 6, 52–54+75. [Google Scholar]
- Cheng, W.; Huang, M.S.; Wang, Z.J.; Yang, L.H. Preparation of High-purity Lanthanum by Calciothermic Reduction. Min. Metall. Eng. 2013, 3, 104–106+109. [Google Scholar]
- Cai, Z.F.; Zhang, Z.M.; Guo, Z.C. Direct electrochemical reduction of solid vanadium oxide to metal vanadium at low temperature in molten CaCl2-NaCl. Int. J. Miner. Metall. Mater. 2012, 19, 499–505. [Google Scholar] [CrossRef]
- Wang, S.L.; Li, S.C.; Wan, L.F. Electro-deoxidation of V2O3 in molten CaCl2-NaCl-CaO. Int. J. Miner. Metall. Mater. 2012, 19, 212–216. [Google Scholar] [CrossRef]
- Liu, Y.X.; Yan, H.W.; Liu, Z.W.; Qin, B.; Yang, P.C. Direct electrolytic preparation of titanium in NaF-K2TiF6-TiO2 system. J. Kunming Univ. Sci. Technol. (Nat. Sci.) 2021, 46, 9–15+64. [Google Scholar]
- Zhang, M.J. Principles and Applications of Molten Salt Electrochemistry, 1st ed.; Chemical Industry Press: Beijing, China, 2006. [Google Scholar]
- Aguilera, L.; Leyet, Y.; Romaguera-Barcelay, Y.; Thaines, E.; Terezo, A.; Souza, G.L.; Freitas, R.G.; Passos, R.R.; Pocrifka, L.A. Influence of electrodeposition temperature in the electrochemical properties of Ni(OH)2: An experimental and theoretical study. Thin Solid Films.Influence of electrodeposition temperature in the electrochemical properties of Ni(OH)2: An experimental and theoretical study. Thin Solid Film. 2019, 670, 24–33. [Google Scholar]








| Sample | Molten Salt Mole Ratio | Current Waveform | Electrodeposition Temperature (K) | Current Density (mA·cm−2) | Electrodeposition Time (min) |
|---|---|---|---|---|---|
| 1 | XNaCl:XKC:XNaF = 0.4:0.4:0.2 | Bidirectional pulse | 973 | 200 | 10 |
| 2 | XNaCl:XKC:XNaF = 0.35:0.35:0.3 | Bidirectional pulse | 973 | 200 | 10 |
| 3 | XNaCl:XKC:XNaF = 0.3:0.3:0.4 | Bidirectional pulse | 973 | 200 | 10 |
| 4 | XNaCl:XKC:XNaF = 0.4:0.4:0.2 | Bidirectional pulse | 973 | 90 | 10 |
| 5 | XNaCl:XKC:XNaF = 0.4:0.4:0.2 | DC | 973 | 120 | 10 |
| 6 | XNaCl:XKC:XNaF = 0.4:0.4:0.2 | Bidirectional pulse | 973 | 120 | 10 |
| 7 | XNaCl:XKC:XNaF = 0.4:0.4:0.2 | DC | 973 | 150 | 10 |
| 8 | XNaCl:XKC:XNaF = 0.4:0.4:0.2 | Bidirectional pulse | 973 | 150 | 10 |
| 9 | XNaCl:XKC:XNaF = 0.4:0.4:0.2 | DC | 973 | 200 | 10 |
| 10 | XNaCl:XKC:XNaF = 0.4:0.4:0.2 | Bidirectional pulse | 973 | 250 | 10 |
| 11 | XNaCl:XKC:XNaF = 0.4:0.4:0.2 | Bidirectional pulse | 953 | 200 | 10 |
| 12 | XNaCl:XKC:XNaF = 0.4:0.4:0.2 | Bidirectional pulse | 993 | 200 | 10 |
| 13 | XNaCl:XKC:XNaF = 0.4:0.4:0.2 | Bidirectional pulse | 1013 | 200 | 10 |
| 14 | XNaCl:XKC:XNaF = 0.4:0.4:0.2 | Bidirectional pulse | 973 | 200 | 20 |
| 15 | XNaCl:XKC:XNaF = 0.4:0.4:0.2 | Bidirectional pulse | 973 | 200 | 30 |
| 16 | XNaCl:XKC:XNaF = 0.4:0.4:0.2 | Bidirectional pulse | 973 | 200 | 50 |
| Order Number | Current Waveform | Current Density/mA·cm−2 | Surface Quality |
|---|---|---|---|
| (a) | DC electrodeposition | 120 | The surface has deposits attached and a peeling phenomenon |
| (b) | Bidirectional pulse electrodeposition | 120 | The surface is relatively flat, but there is a local peeling phenomenon |
| (c) | DC electrodeposition | 150 | The surface is rough, and the deposits are unevenly distributed. |
| (d) | Bidirectional pulse electrodeposition | 150 | The surface is relatively flat, and the deposited particles are larger. |
| (e) | DC electrodeposition | 200 | The surface is relatively flat, and the deposition layer is thin and not dense. |
| (f) | Bidirectional pulse electrodeposition | 200 | The surface is flat, and the deposit’s thickness is uniform and tight. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Li, C.; Li, Y. Influence of Process Parameters on the Electrodeposition of Vanadium in NaCl-KCl-NaF-V2O3 Molten Salt. Coatings 2023, 13, 234. https://doi.org/10.3390/coatings13020234
Tian Y, Li C, Li Y. Influence of Process Parameters on the Electrodeposition of Vanadium in NaCl-KCl-NaF-V2O3 Molten Salt. Coatings. 2023; 13(2):234. https://doi.org/10.3390/coatings13020234
Chicago/Turabian StyleTian, Ying, Changqing Li, and Yungang Li. 2023. "Influence of Process Parameters on the Electrodeposition of Vanadium in NaCl-KCl-NaF-V2O3 Molten Salt" Coatings 13, no. 2: 234. https://doi.org/10.3390/coatings13020234





