Insight on Atmospheric Hydrothermal Aging for Polyester and Polyimide Film Used in Dry-Type Reactor
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Experimental Installation and Methods for Hydrothermal Aging
2.3. Characterization Methods
3. Results and Discussion
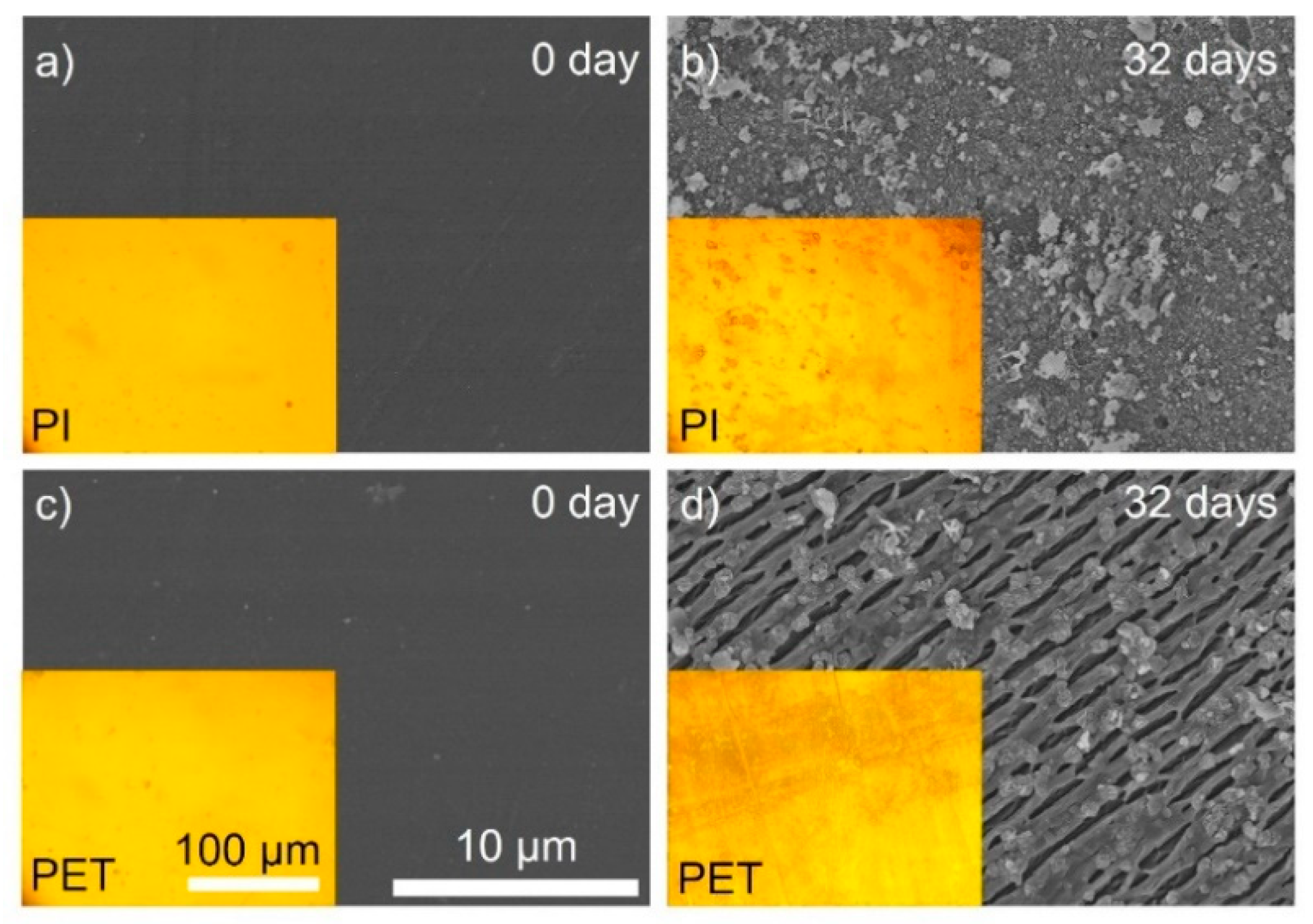
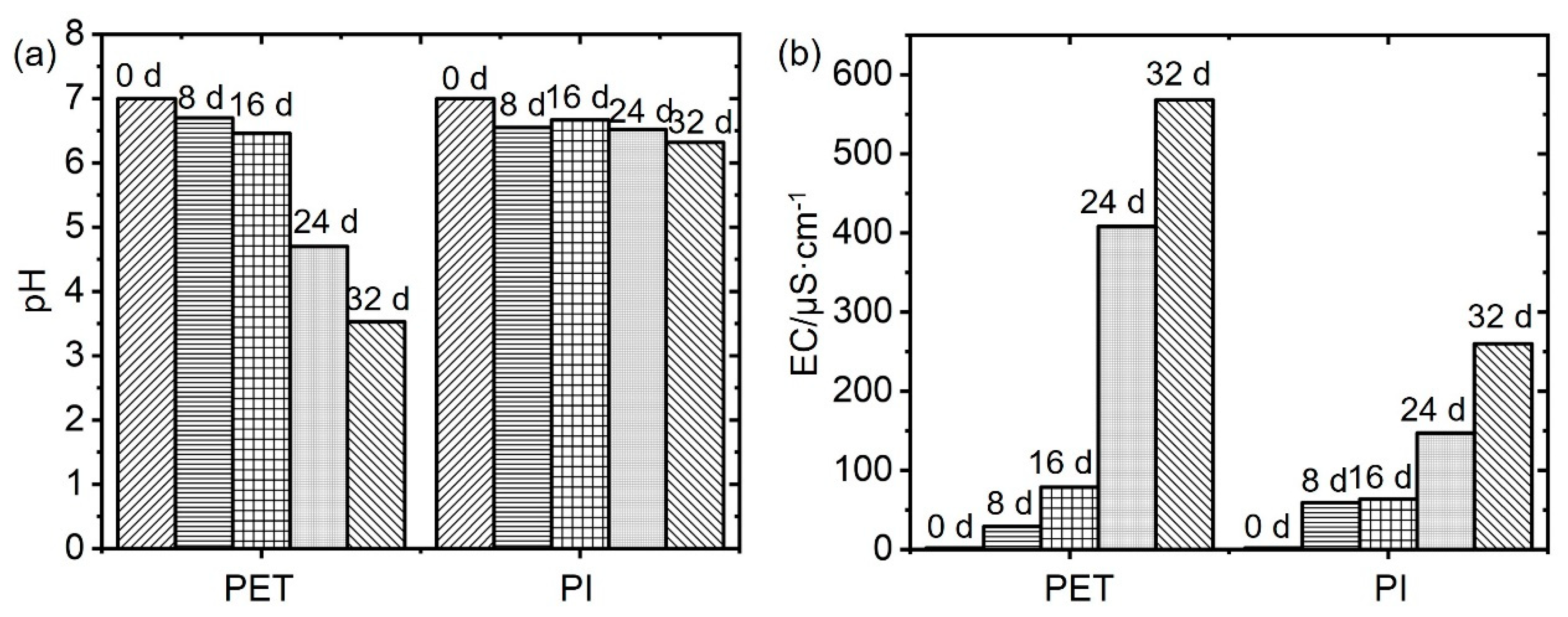
3.1. Characterization of PET and PI Films with Aging Time
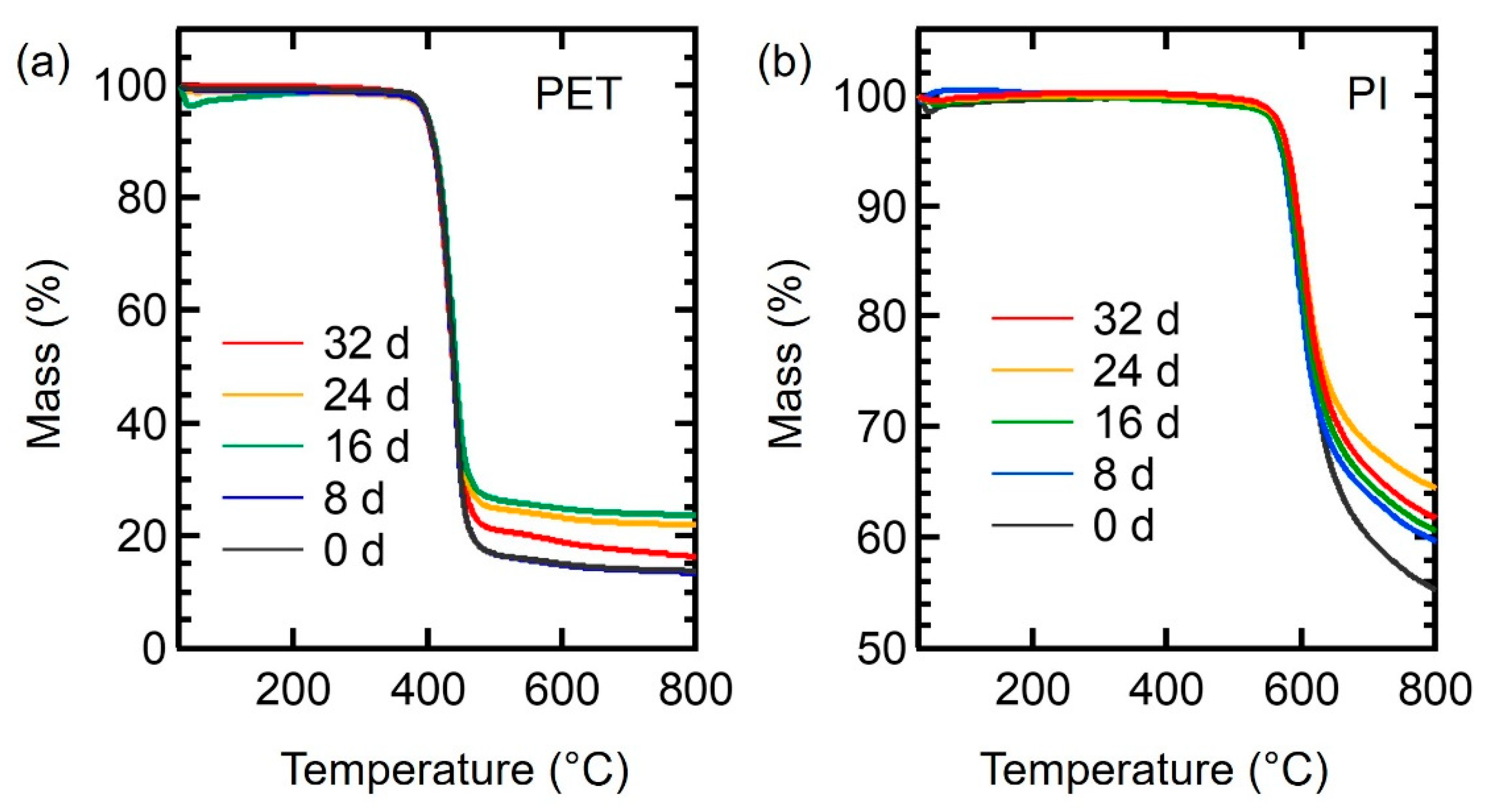
3.2. Mechanical Properties of Aged PET and PI Films
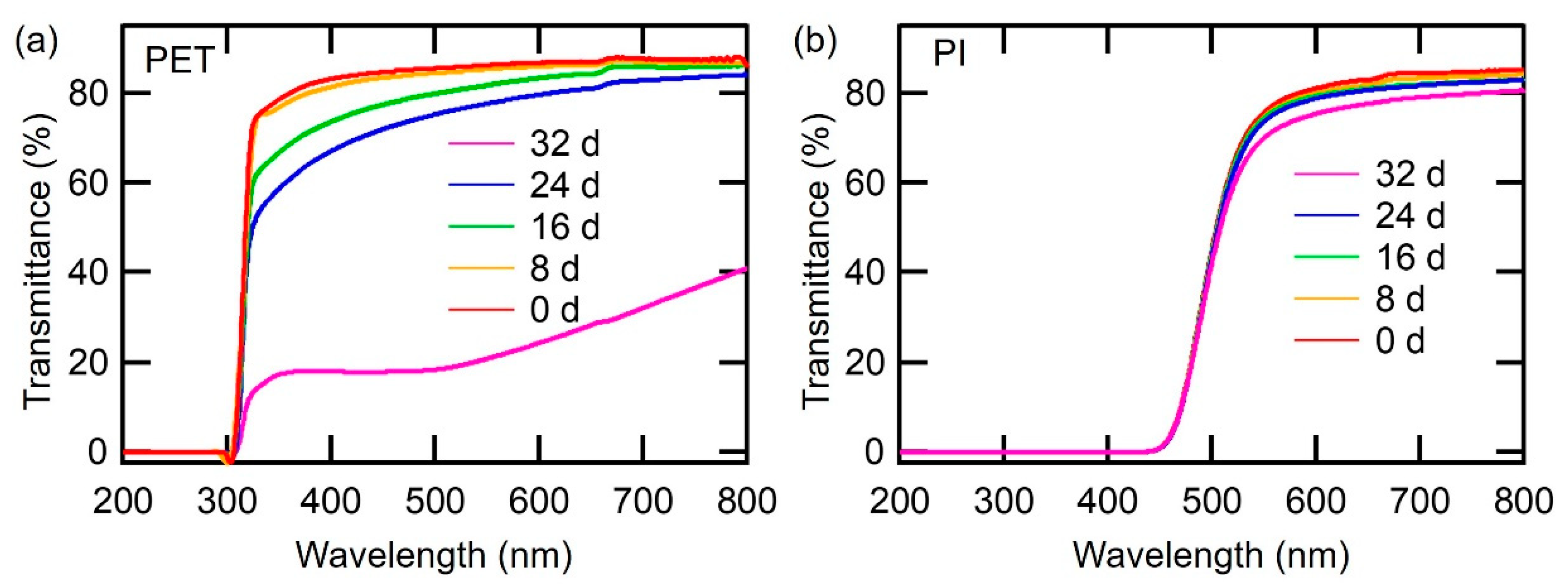
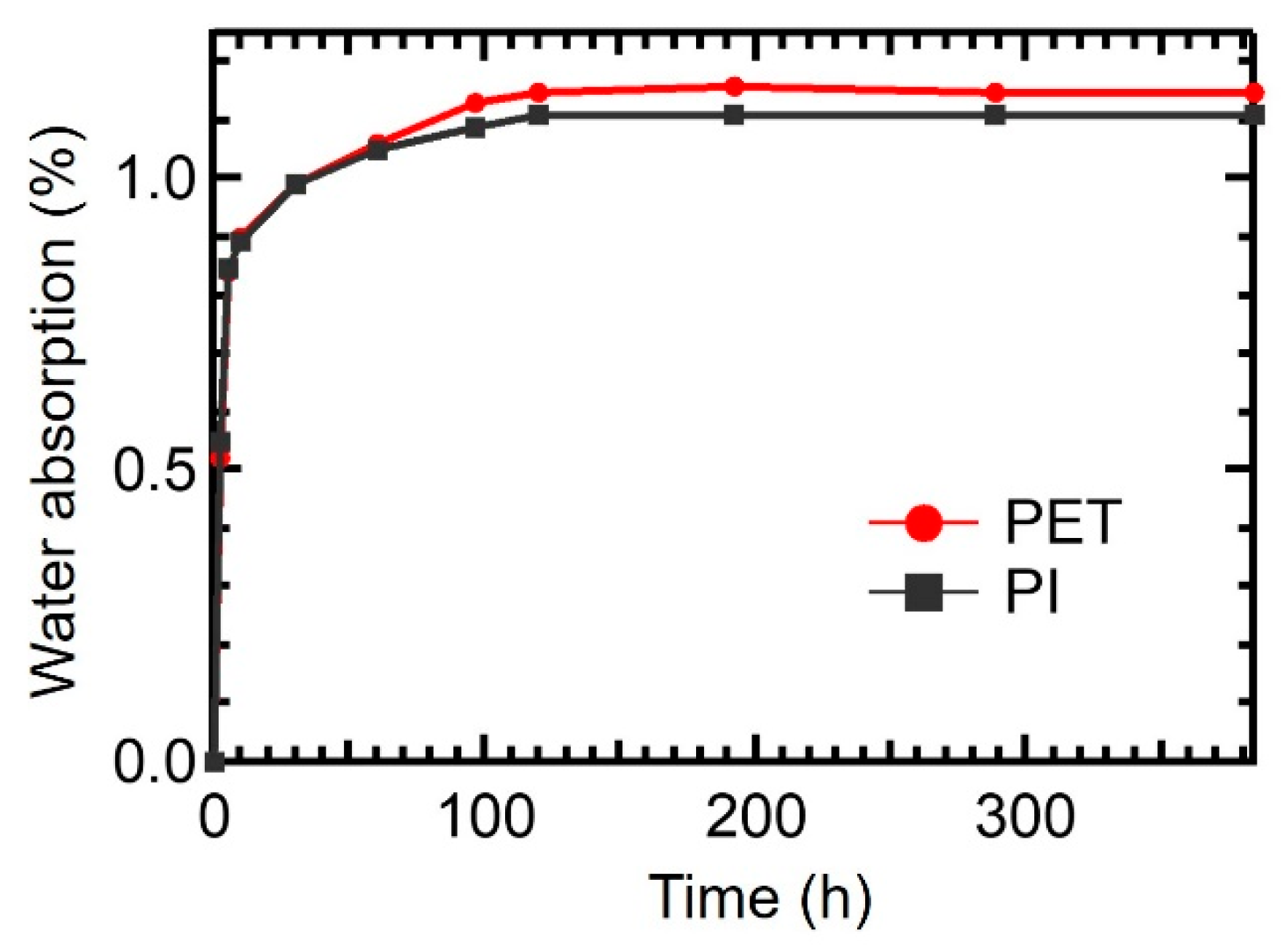
3.3. Hygroscopicity Analysis of PET and PI Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Enohnyaket, M.; Ekman, J. Analysis of Air-Core Reactors from DC to Very High Frequencies Using PEEC Models. IEEE Trans. Power Deliv. 2009, 24, 719–729. [Google Scholar] [CrossRef]
- Rodriguez, D.J.; Alonso Orcajo, G.; Cano, J.M.; Norniella, J.G.; Vicente, A. Thermal Analysis of Dry-Type Air-Core Coils for the Optimization of Passive Filtering Systems. Energies 2020, 13, 4540. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, X.; Yang, J. Online detection of inter-turn short circuit faults in dry-type air-core reactor. Int. J. Appl. Electromagn. Mech. 2012, 39, 443–449. [Google Scholar] [CrossRef]
- Lei, X.; Xu, J.; Feng, B.; Chen, S. Lightning faults of DC dry-type air-core reactor in HVDC substation. Energy Rep. 2022, 8, 1378–1385. [Google Scholar] [CrossRef]
- Nie, H.; Liu, X.; Wang, Y.; Yao, Y.; Gu, Z.; Zhang, C. Breaking Overvoltage of Dry-Type Air-Core Shunt Reactors and Its Cumulative Effect on the Interturn Insulation. IEEE Access. 2019, 7, 55707–55720. [Google Scholar] [CrossRef]
- Silva Almeida, M.L.; Peres, L.M.; Silva, K.M. On applying an Enhanced Generalized Alpha Plane to shunt reactor protection. Electr. Power Syst. Res. 2022, 212, 108387. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, Z.; Zhang, H.; Cheng, Y.; Ren, H. Analysis of Influence of Insulating Resin Paint Film on Enameled Wire Properties Based on Molecular Simulation. Coatings 2022, 12, 1352. [Google Scholar] [CrossRef]
- Ogbonna, V.E.; Popoola, A.P.I.; Popoola, O.M.; Adeosun, S.O. Recent progress on improving the mechanical, thermal and electrical conductivity properties of polyimide matrix composites from nanofillers perspective for technological applications. J. Polym. Eng. 2021, 41, 768–787. [Google Scholar] [CrossRef]
- Kausar, A. Progression from Polyimide to Polyimide Composite in Proton-Exchange Membrane Fuel Cell: A Review. Polym. Plast. Technol. Eng. 2017, 56, 1375–1390. [Google Scholar] [CrossRef]
- Zhang, M.; Niu, H.; Wu, D. Polyimide Fibers with High Strength and High Modulus: Preparation, Structures, Properties, and Applications. Macromol. Rapid Commun. 2018, 39, e1800141. [Google Scholar] [CrossRef]
- Lin, B.; Li, C.; Chen, F.; Liu, C. Continuous Blown Film Preparation of High Starch Content Composite Films with High Ultraviolet Aging Resistance and Excellent Mechanical Properties. Polymers 2021, 13, 3813. [Google Scholar] [CrossRef] [PubMed]
- Tapaswi, P.K.; Ha, C.-S. Recent Trends on Transparent Colorless Polyimides with Balanced Thermal and Optical Properties: Design and Synthesis. Macromol. Chem. Phys. 2019, 220, 1800313. [Google Scholar] [CrossRef]
- Dolez, P.I.; Tomer, N.S.; Malajati, Y. A quantitative method to compare the effect of thermal aging on the mechanical performance of fire protective fabrics. J. Appl. Polym. Sci. 2019, 136, 47045. [Google Scholar] [CrossRef]
- Panowicz, R.; Konarzewski, M.; Durejko, T.; Szala, M.; Lazinska, M.; Czerwinska, M.; Prasula, P. Properties of Polyethylene Terephthalate (PET) after Thermo-Oxidative Aging. Materials 2021, 14, 3833. [Google Scholar] [CrossRef]
- Oh, J.-H.; Park, C.H. Robust Fluorine-Free Superhydrophobic PET Fabric Using Alkaline Hydrolysis and Thermal Hydrophobic Aging Process. Macromol. Mater. Eng. 2018, 303, 1700673. [Google Scholar] [CrossRef]
- Zuo, P.; Fitoussi, J.; Shirinbayan, M.; Bakir, F.; Tcharkhtchi, A. Thermal aging effects on overall mechanical behavior of short glass fiber-reinforced polyphenylene sulfide composites. Polym. Eng. Sci. 2019, 59, 765–772. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Z.; Yuan, J.; Wang, H.; Wang, Z.; Yang, F.; Zhan, J.; Wang, L. A new recycling strategy for preparing flame retardants from polyphenylene sulfide waste textiles. Compos. Commun. 2021, 27, 100852. [Google Scholar] [CrossRef]
- Liu, Q.; Li, J.; Cong, C.; Cui, H.; Xu, L.; Zhang, Y.; Meng, X.; Zhou, Q. Thermal and thermo-oxidative degradation of tetrafluoroethylene-propylene elastomer above 300 degrees C. Polym. Degrad. Stab. 2020, 177, 109180. [Google Scholar] [CrossRef]
- Henri, V.; Dantras, E.; Lacabanne, C.; Dieudonne, A.; Koliatene, F. Thermal ageing of PTFE in the melted state: Influence of interdiffusion on the physicochemical structure. Polym. Degrad. Stab. 2020, 171, 109053. [Google Scholar] [CrossRef]
- Zhuo, T.; Xin, B.; Chen, Z.; Xu, Y.; Zhou, X.; Yu, J. Enhanced thermal insulation properties of PI nanofiber membranes achieved by doping with SiO2 nanoparticles. Eur. Polym. J. 2021, 153, 110489. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, Y.; Mo, Y.; Zhou, Z.; Sha, Y.; Lu, Z.; Cheng, Z. Dielectric property and charge evolution behavior in thermally aged polyimide films. Polym. Degrad. Stab. 2018, 156, 292–300. [Google Scholar] [CrossRef]
- Khazaka, R.; Locatelli, M.L.; Diaham, S.; Bidan, P. Effects of mechanical stresses, thickness and atmosphere on aging of polyimide thin films at high temperature. Polym. Degrad. Stab. 2013, 98, 361–367. [Google Scholar] [CrossRef]
- Qin, S.; Tu, Y.; Wang, S.; Cheng, Y.; Chen, B.; Wang, C.; Zhang, Y. Accelerated Aging of Fast Thermal Cycle Effects on the behavior of Space Charge in Polyimide. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 3182–3190. [Google Scholar] [CrossRef]
- Khazaka, R.; Locatelli, M.L.; Diaham, S.; Bidan, P. Endurance of Thin Insulation Polyimide Films for High-Temperature Power Module Applications. IEEE Trans. Compon. Packag. Manuf. Technol. 2013, 3, 811–817. [Google Scholar] [CrossRef]
- Han, T.; Cavallini, A. Dielectric properties and partial discharge endurance of thermally aged nano-structured polyimide. IEEE Electr. Insul. Mag. 2020, 36, 39–46. [Google Scholar] [CrossRef]
- Braun, C.A.; Nam, S.L.; de la Mata, A.P.; Harynuk, J.; Chung, H.J.; Dolez, P.I. Hydrothermal aging of polyimide film. J. Appl. Polym. Sci. 2022, 139, 1–11. [Google Scholar] [CrossRef]
- Zhang, Y.; Miyauchi, M.; Nutt, S. Moisture absorption and hydrothermal aging of phenylethynyl-terminated pyromellitic dianhydride-type asymmetric polyimide and composites. HIGH Perform. Polym. 2019, 31, 1020–1029. [Google Scholar] [CrossRef]
- Henri, V.; Dantras, E.; Lacabanne, C.; Koliatene, F.; Trebosc, C. Ageing of polyimide under thermal stress: Relaxational effects and charge transport. Polym. Degrad. Stab. 2021, 186, 109524. [Google Scholar] [CrossRef]
- Cooper, R.; Ferguson, D.; Engelhart, D.P.; Hoffmann, R. Effects of Radiation Damage on Polyimide Resistivity. J. Spacecr. Rockets 2017, 54, 343–348. [Google Scholar] [CrossRef]
- Kadiyala, A.K.; Sharma, M.; Bijwe, J. Exploration of thermoplastic polyimide as high temperature adhesive and understanding the interfacial chemistry using XPS, ToF-SIMS and Raman spectroscopy. Mater. Design 2016, 109, 622–633,1275. [Google Scholar] [CrossRef]
- Ahmed, E.M.; Aggor, F.S.; Awad, A.M.; El-Aref, A.T. An innovative method for preparation of nanometal hydroxide superabsorbent hydrogel. Carbohydr. Polym. 2013, 91, 693–698. [Google Scholar] [CrossRef] [PubMed]

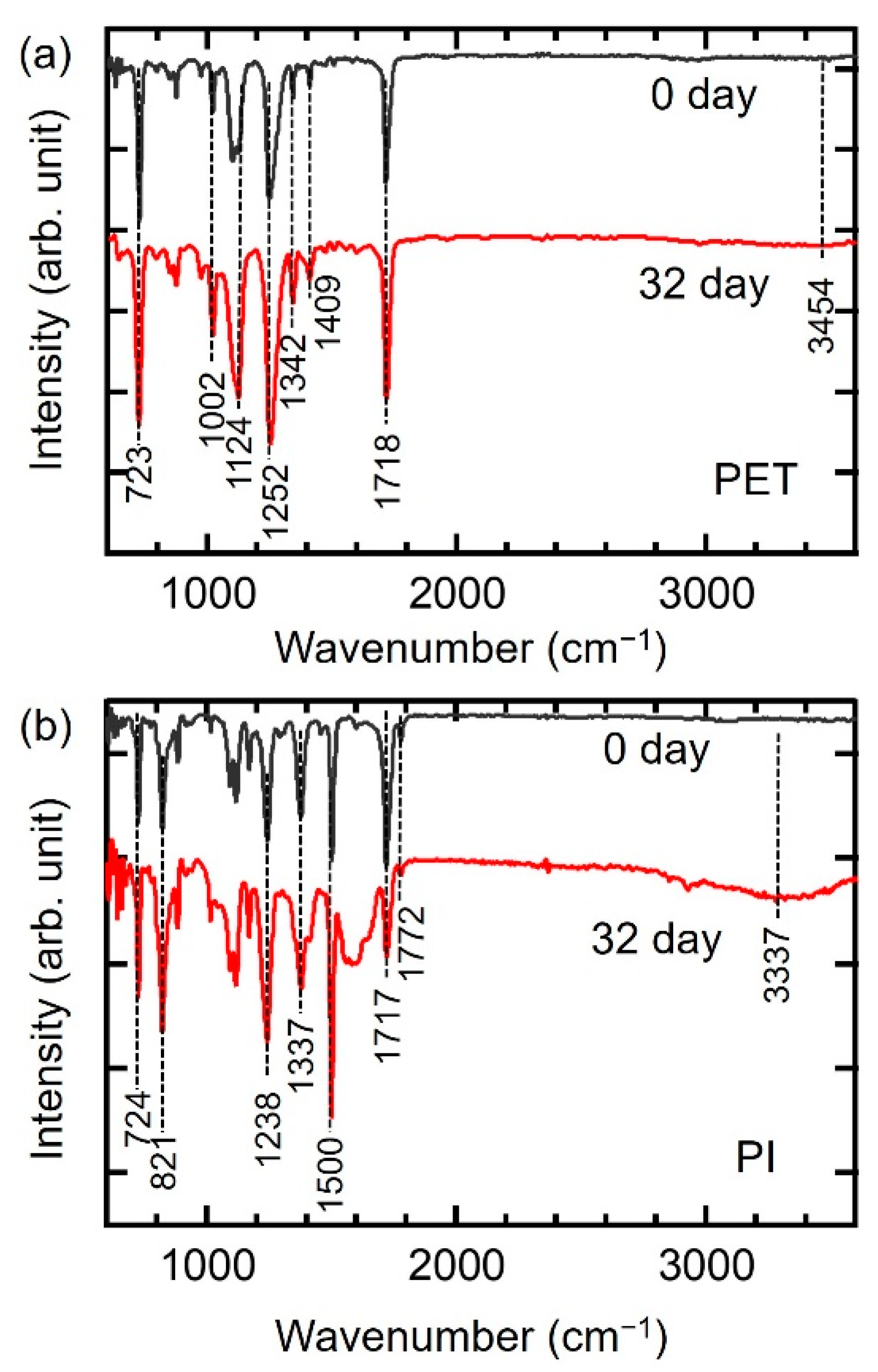

| Hydrothermal Aging Time (days) | PET | PI | ||
|---|---|---|---|---|
| T1718/T1409 | T1252/T1409 | T1772/T1500 | T1337/T1500 | |
| 0 | 4.78 | 5.37 | 0.164 | 0.697 |
| 32 | 3.48 | 4.40 | 0.048 | 0.485 |
| Aging Time (days) | PET | PI | ||||
|---|---|---|---|---|---|---|
| T5% (°C) | Tmax (°C) | ω (%) | T5% (°C) | Tmax (°C) | ω (%) | |
| 0 | 397.5 | 437.9 | 13.77 | 577.4 | 603.5 | 55.37 |
| 8 | 396.1 | 437.4 | 13.50 | 572.4 | 596.6 | 60.71 |
| 16 | 398.1 | 437.6 | 23.72 | 579.0 | 601.5 | 64.57 |
| 24 | 395.2 | 436.9 | 22.04 | 578.7 | 603.5 | 61.83 |
| 32 | 395.3 | 434.8 | 16.43 | 570.2 | 595.7 | 59.76 |
| Film | Pseudo-First-Order | Pseudo-Second-Order | Ritger–Peppas | |||
|---|---|---|---|---|---|---|
| k1 (h−1) | R2 | k2 (g∙mg–1∙h –1) | R2 | n | R2 | |
| PET | 0.0218 | 0.9517 | 0.165 | 0.9998 | 0.450 (<5 h) | 0.9873 |
| 0.099 | 0.9907 | |||||
| PI | 0.0217 | 0.9266 | 0.217 | 0.9999 | 0.452 (<5 h) | 0.9989 |
| 0.087 | 0.9977 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.; Guo, J.; Huang, X.; Jiang, S. Insight on Atmospheric Hydrothermal Aging for Polyester and Polyimide Film Used in Dry-Type Reactor. Coatings 2023, 13, 253. https://doi.org/10.3390/coatings13020253
Lin H, Guo J, Huang X, Jiang S. Insight on Atmospheric Hydrothermal Aging for Polyester and Polyimide Film Used in Dry-Type Reactor. Coatings. 2023; 13(2):253. https://doi.org/10.3390/coatings13020253
Chicago/Turabian StyleLin, Hao, Jiang Guo, Xiang Huang, and Shengbao Jiang. 2023. "Insight on Atmospheric Hydrothermal Aging for Polyester and Polyimide Film Used in Dry-Type Reactor" Coatings 13, no. 2: 253. https://doi.org/10.3390/coatings13020253
APA StyleLin, H., Guo, J., Huang, X., & Jiang, S. (2023). Insight on Atmospheric Hydrothermal Aging for Polyester and Polyimide Film Used in Dry-Type Reactor. Coatings, 13(2), 253. https://doi.org/10.3390/coatings13020253





