Study of Argon and Oxygen Mixtures in Low Temperature Plasma for Improving PLA Film Wettability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plasma Activation
2.3. Contact Angle Measurements and Calculation of Surface Free Energy
2.4. Chemical and Topographic Surface Analysis
2.5. Weight Loss and Change in Mechanical Properties
2.6. Statistical Analysis
3. Results and Discussion
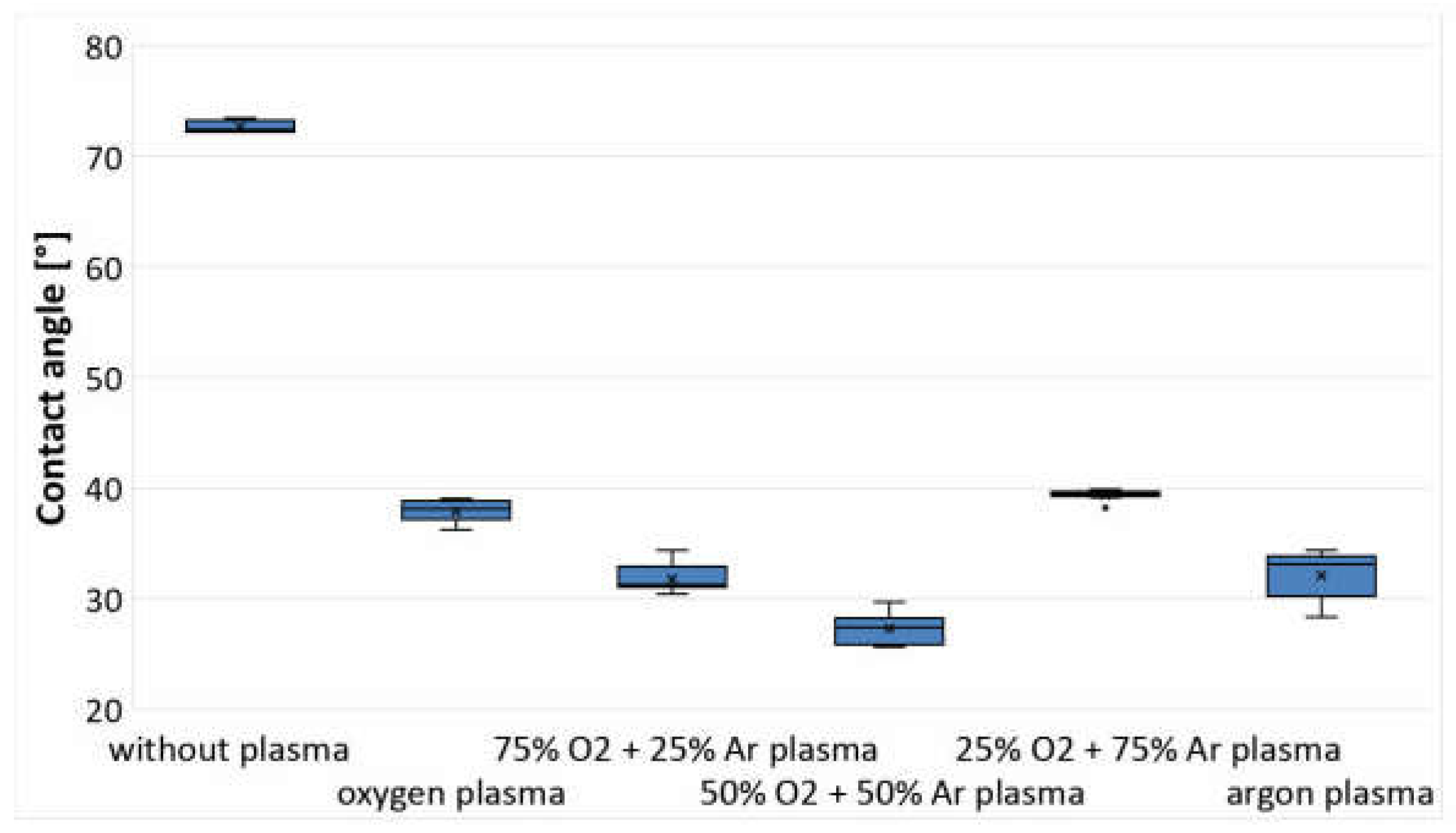
3.1. Analysis of Changes in Water Contact Angle and Surface Roughness
3.2. Analysis of Surface Free Energy and Its Components
3.3. Surface Chemistry Analysis
3.4. Analysis of Changes in Mass and Strength Properties
4. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kost, B.; Basko, M.; Bednarek, M.; Socka, M.; Kopka, B.; Łapienis, G.; Biela, T.; Kubisa, P.; Brzeziński, M. The influence of the functional end groups on the properties of polylactide-based materials. Prog. Poly. Sci. 2022, 130, 101556. [Google Scholar] [CrossRef]
- Shlush, E.; Davidovich-Pinhas, M. Bioplastics for food packaging. Trends Food Sci. Technol. 2022, 125, 66–80. [Google Scholar] [CrossRef]
- Khan, N.A.; Khan, A.H.; López-Maldonado, E.A.; Alam, S.S.; López López, J.R.; Méndez Herrera, P.F.; Mohamed, B.A.; Mahmoud, A.E.D.; Abutaleb, A.; Singh, L. Microplastics: Occurrences, treatment methods, regulations and foreseen environmental impacts. Environ. Res. 2022, 215, 114224. [Google Scholar] [CrossRef] [PubMed]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef] [PubMed]
- Vroman, I.; Tighzert, L. Biodegradable Polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef] [Green Version]
- Jasim Ahmed, J.; Mulla, M.Z.; Al-Zuwayed, S.A.; Joseph, A.; Auras, R. Morphological, barrier, thermal, and rheological properties of high-pressure treated co-extruded polylactide films and the suitability for food packaging. Food Packag. 2022, 32, 100812. [Google Scholar] [CrossRef]
- Škrlová, K.; Rybková, Z.; Stachurová, T.; Zagora, J.; Malachová, K.; Měřinská, D.; Gabor, R.; Havlíček, M.; Muñoz-Bonilla, A.; Fernández-García, M.; et al. Long-term antimicrobial effect of polylactide-based composites suitable for biomedical use. Polym. Test. 2022, 116, 107760. [Google Scholar] [CrossRef]
- Wang, M.; Favi, P.; Cheng, X.; Golshan, N.H.; Ziemer, K.S.; Keidar, M.; Webster, T.J. Cold atmospheric plasma (CAP) surface nanomodified 3D printed polylactic acid (PLA) scaffolds for bone regeneration. Acta Biomater. 2016, 46, 256–265. [Google Scholar] [CrossRef]
- Chavan, Y.R.; Tambe, S.M.; Jain, D.D.; Khairnar, S.V.; Amin, P.D. Redefining the importance of polylactide-co-glycolide acid (PLGA) in drug delivery. Ann. Pharm. Françaises 2022, 80, 603–616. [Google Scholar] [CrossRef]
- Nampoothiri, K.M.; Nair, N.R.; John, R.P. An overview of the recent developments in polylactide (PLA) research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef]
- Luque-Agudo, V.; Hierro-Oliva, M.; Gallardo-Moreno, A.M.; González-Martín, M.L. Effect of plasma treatment on the surface properties of polylactic acid films. Polym. Test. 2021, 96, 107097. [Google Scholar] [CrossRef]
- Spyrides, S.M.M.; Alencastro, F.S.; Guimaraes, E.F.; Bastian, F.L.; Simao, R.A. Mechanism of oxygen and argon low pressure plasma etching on polyethylene (UHMWPE). Surf. Coat. Technol. 2019, 378, 124990. [Google Scholar] [CrossRef]
- Izdebska, J. Chapter 1—Printing on Polymers: Theory and Practice. In Printing on Polymers, 1st ed.; Izdebska, J., Thomas, S., Eds.; William Andrew Publishing: Oxford, UK, 2016; pp. 1–20. [Google Scholar]
- Izdebska-Podsiadły, J. Chapter 6—Application of Plasma in Printed Surfaces and Print Quality. In Non-Thermal Plasma Technology for Polymeric Materials; Thomas, S., Mozetič, M., Cvelbar, U., Špatenka, P., Praveen, K.M., Eds.; Elsevier: Oxford, UK, 2019; pp. 159–191. [Google Scholar]
- Izdebska-Podsiadły, J. Effect of Plasma Surface Modification on Print Quality of Biodegradable PLA Films. Appl. Sci. 2021, 11, 8245. [Google Scholar] [CrossRef]
- Ma, C.; Nikiforov, A.; Hegemann, D.; De Geyter, N.; Morent, R.; Ostrikov, K. Plasma-controlled surface wettability: Recent advances and future applications. Int. Mater. Rev. 2022, 67, 1–38. [Google Scholar] [CrossRef]
- Tyczkowski, J.; Kierzkowska-Pawlak, H.; Sielski, J.; Krawczyk-Kłys, I. Low-Temperature Plasma Modification of Styrene–Butadiene Block Copolymer Surfaces for Improved Adhesion—A Kinetic Approach. Polymers 2020, 12, 935. [Google Scholar] [CrossRef]
- Stepczyńska, M. Surface Modification by Low Temperature Plasma: Sterilization of Biodegradable Materials. Plasma Process. Polym. 2016, 13, 1080–1088. [Google Scholar] [CrossRef]
- Vishnuvarthanan, M.; Rajeswari, N. Effect of mechanical, barrier and adhesion properties on oxygen plasma surface modified PP. Innov. Food Sci. Emerg. Technol. 2015, 30, 119–126. [Google Scholar] [CrossRef]
- De Geyter, N.; Morent, R. Cold plasma surface modification of biodegradable polymer biomaterials. Ch.7. In Biomaterials for Bone Regeneration; Dubruel, P., Van Vlierberghe, S., Eds.; Elsevier: Oxford, UK, 2014. [Google Scholar]
- Nageswaran, G.; Jothi, L.; Jagannathan, S. Chapter 4—Plasma Assisted Polymer Modifications. In Non-Thermal Plasma Technology for Polymeric Materials, 1st ed.; Thomas, S., Mozetič, M., Cvelbar, U., Špatenka, P., Praveen, K.M., Eds.; Elsevier: Oxford, UK, 2019; pp. 95–127. [Google Scholar] [CrossRef]
- Moraczewski, K.; Stepczyńska, M.; Malinowski, R.; Rytlewski, P.; Jagodziński, B.; Żenkiewicz, M. Stability studies of plasma modification effects of polylactide and polycaprolactone surface layers. Appl. Surf. Sci. 2016, 337, 228–237. [Google Scholar] [CrossRef]
- Hirotsu, T.; Nakayama, K.; Tsujisaka, T.; Mas, A.; Schue, F. Plasma surface treatments of melt-extruded sheets of poly(L-lactic acid). Polym. Eng. Sci. 2002, 42, 299–306. [Google Scholar] [CrossRef]
- Song, A.Y.; Oh, Y.A.; Roh, S.H.; Kim, J.K.; Min, S.C. Cold Oxygen Plasma Treatments for the Improvement of the Physicochemical and Biodegradable Properties of Polylactic Acid Films for Food Packaging. J. Food Sci. 2016, 81, E86–E96. [Google Scholar] [CrossRef] [PubMed]
- Izdebska-Podsiadły, J.; Dörsam, E. Storage stability of the oxygen plasma-modified PLA film. Bull. Mater. Sci. 2021, 44, 79. [Google Scholar] [CrossRef]
- Inagaki, N.; Narushima, K.; Tsutsui, Y.; Ohyama, Y. Surface modification and degradation of poly(lactic acid) films by Ar-plasma. J. Adhes. Sci. Technol. 2002, 16, 1041–1054. [Google Scholar] [CrossRef]
- Izdebska-Podsiadły, J.; Dörsam, E. Effects of argon low temperature plasma on PLA film surface and aging behaviors. Vacuum 2017, 145, 278–284. [Google Scholar] [CrossRef]
- Murphy, A.B.; Arundell, C.J. Transport coefficients of argon, nitrogen, oxygen, argon-nitrogen, and argon-oxygen plasmas. Plasma Chem. Plasma Process. 1994, 14, 451–490. [Google Scholar] [CrossRef]
- Kim, B.K.; Kim, K.S.; Park, C.E.; Ryu, C.M. Improvement of wettability and reduction of aging effect by plasma treatment of low-density polyethylene with argon and oxygen mixtures. J. Adhes. Sci. Technol. 2002, 16, 509–521. [Google Scholar] [CrossRef]
- Karam, L.; Jama, C.; Nuns, N.; Mamede, A.-S.; Dhulster, P.; Chihib, N.-E. Nisin adsorption on hydrophilic and hydrophobic surfaces: Evidence of its interactions and antibacterial activity. J. Pept. Sci. 2013, 19, 1075–2617. [Google Scholar] [CrossRef]
- Fang, Z.; Wang, X.; Shao, T.; Zhang, C. Influence of Oxygen Content on Argon/Oxygen Dielectric Barrier Discharge Plasma Treatment of Polyethylene Terephthalate Film. IEEE Trans Plasma Sci. 2017, 45, 310–317. [Google Scholar] [CrossRef]
- Saxena, N.; Prabhavathy, C.; De, S.; DasGupta, S. Flux enhancement by argon–oxygen plasma treatment of polyethersulfone membranes. Sep. Purif. Technol. 2009, 70, 160–165. [Google Scholar] [CrossRef]
- Izdebska-Podsiadły, J. Impact of low temperature plasma treatment parameters on wettability and printability of PLA film. IC J. 2020, 12, 1–7. [Google Scholar]
- Mortazavi, M.; Nosonovsky, M. A model for diffusion-driven hydrophobic recovery in plasma treated polymers. Appl. Surf. Sci. 2012, 258, 6876–6883. [Google Scholar] [CrossRef]
- Luna, P.; Mariño, A.; Lizarazo-Marriaga, J.; Beltrán, O. Dry etching plasma applied to fique fibers: Influence on their mechanical properties and surface appearance. Procedia Eng. 2017, 200, 141–147. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, L.; Yang, K.; Liu, T.; Liao, W.; Zhang, C.; Zhang, L.; Liu, Y.; Jiang, X. Kinetic etch front instability responsible for roughness formation in plasma etching. Appl. Surf. Sci. 2021, 543, 148862. [Google Scholar] [CrossRef]
- Grace, J.M.; Gerenser, L.J. Plasma Treatment of Polymers. J. Dispers. Sci. Technol. 2003, 24, 305–341. [Google Scholar] [CrossRef]
- Primc, G.; Mozetič, M. Hydrophobic Recovery of Plasma-Hydrophilized Polyethylene Terephthalate Polymers. Polymers 2022, 14, 2496. [Google Scholar] [CrossRef] [PubMed]
- Hebbar, R.S.; Isloor, A.M.; Ismail, A.F. Chapter 12—Contact Angle Measurements. In Membrane Characterization; Hilal, N., Ismail, A.F., Matsuura, T., Oatley-Radcliffe, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 219–255. [Google Scholar] [CrossRef]
- Akbari, R.; Antonini, C. Contact angle measurements: From existing methods to an open-source tool. Adv. Colloid Interface Sci. 2021, 294, 102470. [Google Scholar] [CrossRef]
- Kubiak, K.J.; Wilson, M.C.T.; Mathia, T.G.; Carval, P. Wettability versus roughness of engineering surfaces. Wear 2011, 271, 523–528. [Google Scholar] [CrossRef] [Green Version]
- Busscher, H.J.; Weerkamp, A.H.; van Der Mei, H.C.; van Pelt, A.W.; de Jong, H.P.; Arends, J. Measurement of the Surface Free Energy of Bacterial Cell Surfaces and Its Relevance for Adhesion. Appl. Environ. Microbiol. 1984, 48, 980–983. [Google Scholar] [CrossRef] [Green Version]
- Żenkiewicz, M. Analysis of the most important methods of investigations of polymeric materials’ surface free energy. Polimery 2007, 52, 760–768. [Google Scholar] [CrossRef] [Green Version]
- Liber-Kneć, A.; Łagan, S. Surface Testing of Dental Biomaterials—Determination of Contact Angle and Surface Free Energy. Materials 2021, 14, 2716. [Google Scholar] [CrossRef]
- Żołek-Tryznowska, Z.; Prica, M.; Pavlović, Ž.; Cveticanin, L.; Annusik, T. The influence of aging on surface free energy of corona treated packaging films. Polym. Test. 2020, 89, 1–8. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Rudawska, A.; Jacniacka, E. Analysis for determining surface free energy uncertainty by the Owen–Wendt method. Int. J. Adhes. Adhes. 2009, 29, 451–457. [Google Scholar] [CrossRef]
- Kiss, É.; Bertóti, I.; Vargha-Butler, E.I. XPS and Wettability Characterization of Modified Poly(lactic acid) and Poly(lactic/glycolic acid) Films. J. Colloid Interface Sci. 2002, 245, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Jordá-Vilaplana, A.; Fombuena, V.; García-García, D.; Samper, M.D.; Sánchez-Nácher, L. Surface modification of polylactic acid (PLA) by air atmospheric plasma treatment. Eur. Polym. J. 2014, 58, 23–33. [Google Scholar] [CrossRef]
- Rider, A.N.; Brack, N.; Andres, S.; Pigram, P.J. The influence of hydroxyl group concentration on epoxy–aluminium bond durability. J. Adhes. Sci. Technol. 2004, 18, 1123–1152. [Google Scholar] [CrossRef]
- Marcuello, C.; Foulon, L.; Chabbert, B.; Molinari, M.; Aguié-Béghin, V. Langmuir-Blodgett Procedure to Precisely Control the Coverage of Functionalized AFM Cantilevers for SMFS Measurements: Application with Cellulose Nanocrystals. Langmuir 2018, 34, 9376–9386. [Google Scholar] [CrossRef] [PubMed]
- Berzin, F.; Lemkhanter, L.; Marcuello, C.; Chabbert, B.; Aguié-Béghin, V.; Molinari, M.; Castellani, R.; Vergnes, B. Influence of the polarity of the matrix on the breakage mechanisms of lignocellulosic fibers during twin-screw extrusion. Polym. Compos. 2020, 41, 1106–1117. [Google Scholar] [CrossRef]
- Yousifa, E.; Ahmedb, D.; Zainulabdeena, K.; Jawad, A. Photo-physical and Morphological Study of Polymers: A Review. Phys. Chem. Res. 2023, 11, 409–424. [Google Scholar] [CrossRef]








| PLA Films | C1s Components (%) | O1s Components (%) | ||||
|---|---|---|---|---|---|---|
| C-C/C-H 285 eV | C-O 286.3 eV | C=O 288 eV | O-C=O 289.1 eV | O=C 532.25 eV | O-C 533.66 eV | |
| Without plasma | 39.9 | 24.6 | 13.8 | 21.7 | 34.1 | 65.9 |
| 50% O2 + 50% Ar plasma | 15.6 | 53.3 | 0 | 31.1 | 34.8 | 65.2 |
| 100% Ar plasma | 14.6 | 44.8 | 9.9 | 30.7 | 29.2 | 70.8 |
| 100% O2 plasma | 23.8 | 28.5 | 22.8 | 25.0 | 50.1 | 49.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izdebska-Podsiadły, J. Study of Argon and Oxygen Mixtures in Low Temperature Plasma for Improving PLA Film Wettability. Coatings 2023, 13, 279. https://doi.org/10.3390/coatings13020279
Izdebska-Podsiadły J. Study of Argon and Oxygen Mixtures in Low Temperature Plasma for Improving PLA Film Wettability. Coatings. 2023; 13(2):279. https://doi.org/10.3390/coatings13020279
Chicago/Turabian StyleIzdebska-Podsiadły, Joanna. 2023. "Study of Argon and Oxygen Mixtures in Low Temperature Plasma for Improving PLA Film Wettability" Coatings 13, no. 2: 279. https://doi.org/10.3390/coatings13020279
APA StyleIzdebska-Podsiadły, J. (2023). Study of Argon and Oxygen Mixtures in Low Temperature Plasma for Improving PLA Film Wettability. Coatings, 13(2), 279. https://doi.org/10.3390/coatings13020279






