Bi-Phase NiCo2S4-NiS2/CFP Nanocomposites as a Highly Active Catalyst for Oxygen Evolution Reaction
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents
2.2. Treatment of CFP
2.3. Synthesis of NiCo2S4-NiS2/CFP, Ni3S4-NiS2/CFP and Co2S/CFP
2.4. Physical Characterization
2.5. Electrochemical Testing
3. Results and Discussion
3.1. Synthesis and Characterization
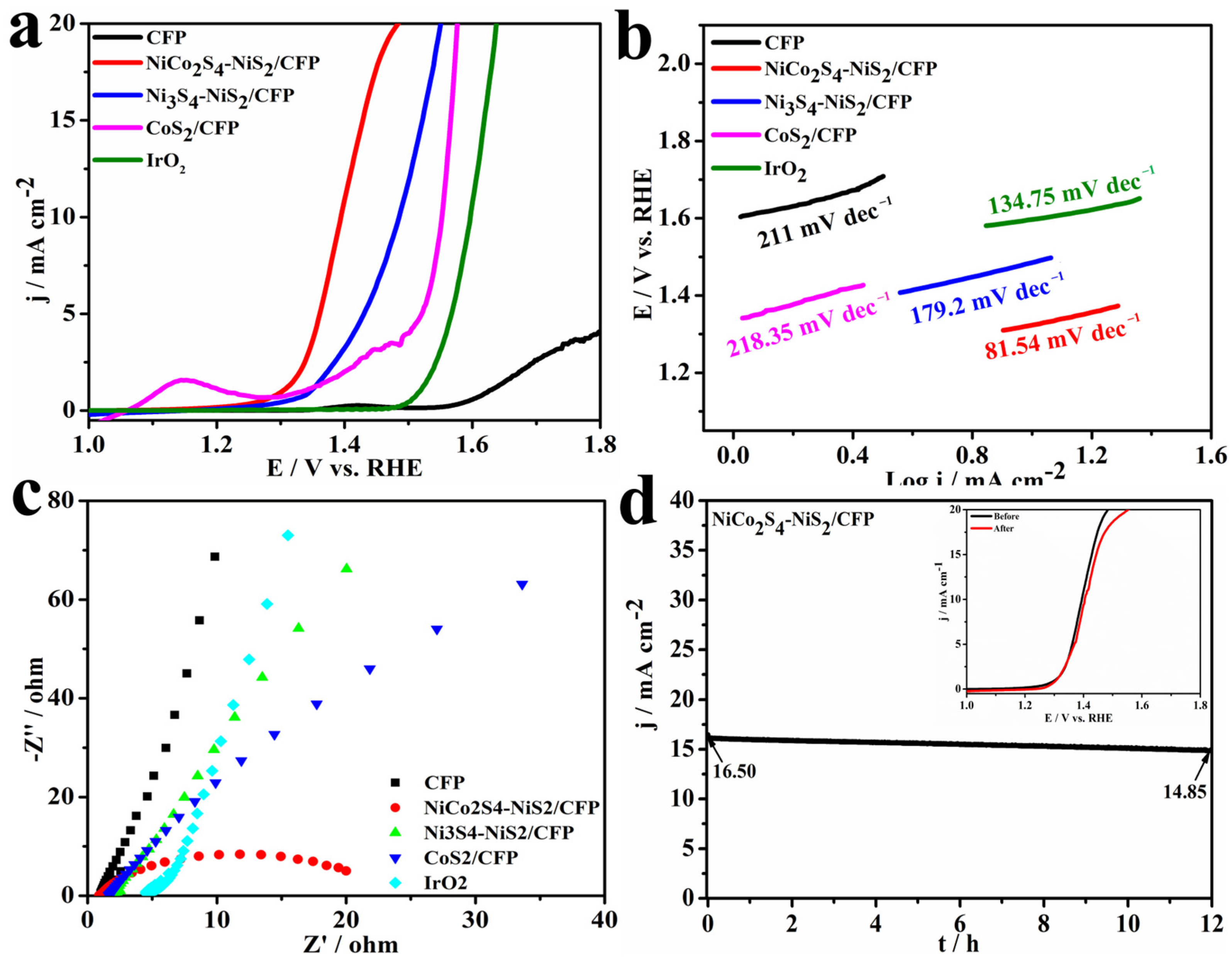
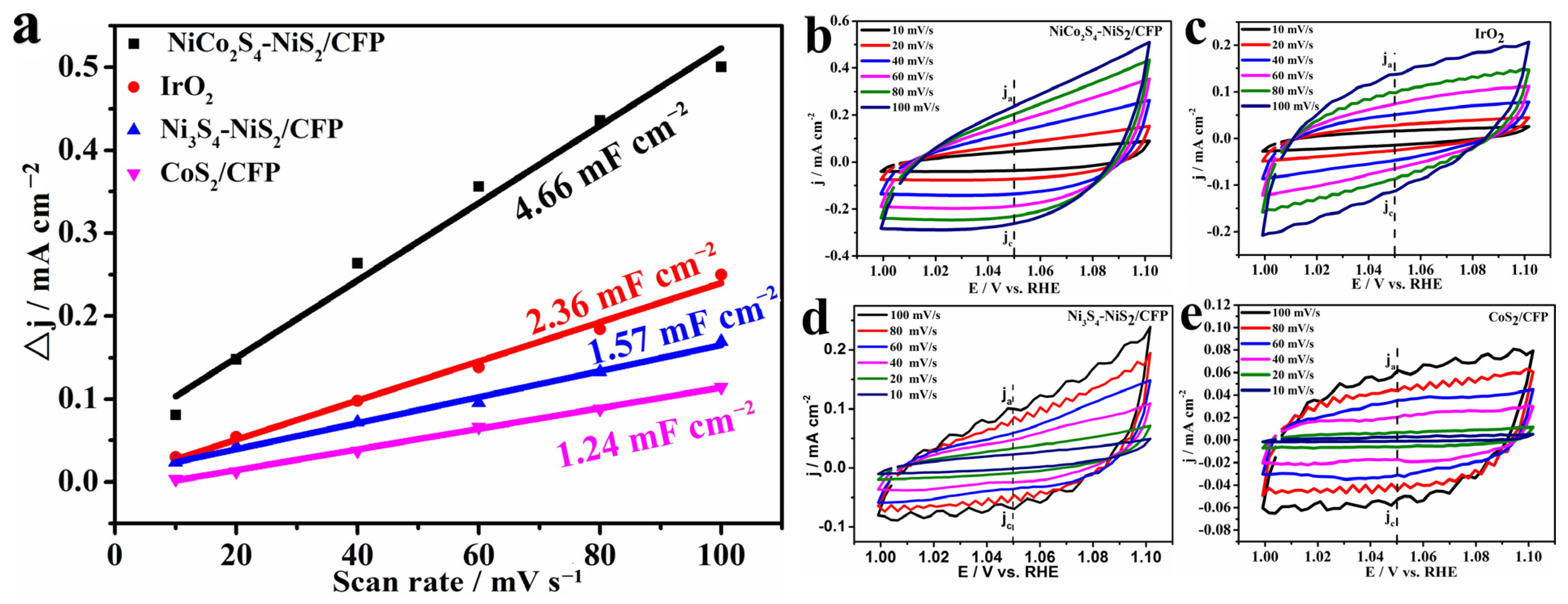
3.2. Electrocatalytic Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, T.L.; Xia, Y.J.; Mao, T.L.; Ding, Q.W.; Wang, Z.J.; Hong, Z.Y.; Han, J.J.; Peng, D.L.; Yue, G.H. Phosphorus Vacancies and Heterojunction Interface as Effective Lithium-Peroxide Promoter for Long-Cycle Life Lithium–Oxygen Batteries. Adv. Funct. Mater. 2022, 32, 2209876. [Google Scholar] [CrossRef]
- Dutta, S.; Indra, A.; Feng, Y.; Han, H.; Song, T. Promoting electrocatalytic overall water splitting with nanohybrid of transition metal nitride-oxynitride. Appl. Catal. B Environ. 2019, 241, 521–527. [Google Scholar] [CrossRef]
- Hu, C.; Dai, L. Multifunctional Carbon-Based Metal-Free Electrocatalysts for Simultaneous Oxygen Reduction, Oxygen Evolution, and Hydrogen Evolution. Adv. Mater. 2017, 29, 1604942. [Google Scholar] [CrossRef]
- Sathiskumar, C.; Ramakrishnan, S.; Vinothkannan, M.; Karthikeyan, S.; Yoo, D.J.; Kim, A.R. Nitrogen-Doped Porous Carbon Derived from Biomass Used as Trifunctional Electrocatalyst toward Oxygen Reduction, Oxygen Evolution and Hydrogen Evolution Reactions. Nanomaterials 2019, 10, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, C.G.; Chen, X.Y.; Dai, Q.B.; Wang, M.; Qu, L.T.; Dai, L.M. Earth-Abundant Carbon Catalysts for Renewable Generation of Clean Energy from Sunlight and Water. Nano Energy 2017, 41, 367–376. [Google Scholar] [CrossRef]
- Xia, G.; Li, D.; Chen, X.; Tan, Y.; Tang, Z.; Guo, Z.; Liu, H.; Liu, Z.; Yu, X. Carbon-Coated Li3 N Nanofibers for Advanced Hydrogen Storage. Adv. Mater. 2013, 25, 6238–6244. [Google Scholar] [CrossRef] [PubMed]
- Koper, M.T.M. Thermodynamic theory of multi-electron transfer reactions: Implications for electrocatalysis. J. Electroanal. Chem. 2011, 660, 254–260. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Design of Electrocatalysts for Oxygen- and Hydrogen-Involving Energy Conversion Reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef]
- Zhao, H.H.; Yang, Y.; Dai, X.P.; Qiao, H.Y.; Yong, J.X.; Luan, X.B.; Yu, L.; Luan, C.L.; Wang, Y.; Zhang, X. Nico-Dh Nanodots Anchored on Amorphous Nico-Sulfide Sheets as Efficient Electrocatalysts for Oxygen Evolution Reaction. Electrochim. Acta 2019, 295, 1085–1092. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Liu, Y.P.; Ren, T.Z.; Yuan, Z.Y. Self-Supported Cobalt Phosphide Mesoporous Nanorod Arrays: A Flexible and Bifunctional Electrode for Highly Active Electrocatalytic Water Reduction and Oxidation. Adv. Funct. Mater. 2015, 25, 7337–7347. [Google Scholar] [CrossRef]
- Xu, W.; Lu, Z.; Lei, X.; Li, Y.; Sun, X. A Hierarchical Ni-Co-O@Ni-Co-S Nanoarray as an Advanced Oxygen Evolution Reaction Electrode. Phys. Chem. Chem. Phys. 2014, 16, 20402–20405. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, Y.; Liu, J.; Gong, P.; Li, X.; Zhao, Y.; Yue, G.; Zhou, Z. Highly Hierarchical Porous Structures Constructed from Nio Nanosheets Act as Li Ion and O(2) Pathways in Long Cycle Life, Rechargeable Li-O(2) Batteries. Chem. Commun. 2016, 52, 11772–11774. [Google Scholar] [CrossRef]
- Alburaih, H.A.; Ansari, M.Z.; Abid, A.G.; Khosa, R.Y.; Ashiq, M.N.; Manzoor, S.; Aman, S.; Chaudhry, H.; Waheed, M.S.; Taha, T.A. Study on active sites of Mn-doped iron selenide on pencil electrode for electrocatalytic water splitting. J. Sol-Gel Sci. Technol. 2022, 11, 1–9. [Google Scholar] [CrossRef]
- Khan, M.T.N.; Ahmed, F.; Houda, S.; Manzoor, S.; Hasnain, K.; Zahra, M.; Hussain, R.; Ansari, M.Z.; Hegazy, H.H.; Ashiq, M.N. Facile synthesis of novel Ag@cerium zirconate heterostructure for efficient oxygen evolution reaction. Surf. Interfaces 2022, 35, 102410–102420. [Google Scholar] [CrossRef]
- Owidah, Z.O.; Aman, S.; Abdullah, M.; Manzoor, S.; Fallatah, A.M.; Ibrahim, M.M.; Elnasr, T.A.S.; Ansari, M.Z. Metal oxide/carbon nanosheet arrays derivative of stacked metal organic frameworks for triggering oxygen evolution reaction. Ceram. Int. 2022, 49, 5936–5943. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J.; Zhang, L.; Wan, H.; Qi, T.; Xia, D. Highly conductive NiCo2S4 urchin-like nanostructures for high-rate pseudocapacitors. Nanoscale 2013, 5, 8879–8883. [Google Scholar] [CrossRef]
- Jing, F.; Lv, Q.Y.; Xiao, J.; Wang, Q.J.; Wang, S. Highly Active and Dual-Function Self-Supported Multiphase Nis-Nis2-Ni3s2/Nf Electrodes for Overall Water Splitting. J. Mater. Chem. A 2018, 6, 14207–14214. [Google Scholar] [CrossRef]
- Chinnadurai, D.; Manivelan, N.; Prabakar, K. Modulating the Intrinsic Electrocatalytic Activity of Copper Sulfide by Silver Doping for Electrocatalytic Overall Water Splitting. Chemelectrochem 2022, 9, e202200254. [Google Scholar] [CrossRef]
- Viswanathan, V.; Pickrahn, K.L.; Luntz, A.C.; Bent, S.F.; Norskov, J.K. Nanoscale Limitations in Metal Oxide Electrocatalysts for Oxygen Evolution. Nano Lett. 2014, 14, 5853–5857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chauhan, M.; Reddy, K.P.; Gopinath, C.S.; Deka, S. Copper Cobalt Sulfide Nanosheets Realizing a Promising Electrocatalytic Oxygen Evolution Reaction. ACS Catal. 2017, 7, 5871–5879. [Google Scholar] [CrossRef]
- Jacobs, J.P.; Maltha, A.; Reintjes, J.G.H.; Drimal, J.; Ponec, V.; Brongersma, H.H. The Surface of Catalytically Active Spinels. J. Catal. 1994, 147, 294–300. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, C.; Lyu, M.; Lin, Y.; Cai, W.; Huang, P.; Tong, W.; Zou, Y.; Xie, Y. Ultrathin Co3S4 Nanosheets that Synergistically Engineer Spin States and Exposed Polyhedra that Promote Water Oxidation under Neutral Conditions. Angew. Chem. Int. Ed. 2015, 54, 11231–11235. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Lu, Q.; Luo, Y.; Sun, X.; Asiri, A.M. NiCo2S4 nanowires array as an efficient bifunctional electrocatalyst for full water splitting with superior activity. Nanoscale 2015, 7, 15122–15126. [Google Scholar] [CrossRef]
- Liu, Q.; Jin, J.; Zhang, J. NiCo2S4@Graphene as a Bifunctional Electrocatalyst for Oxygen Reduction and Evolution Reactions. ACS Appl. Mater. Interfaces 2013, 5, 5002–5008. [Google Scholar] [CrossRef]
- Li, F.; Xu, R.C.; Li, Y.M.; Liang, F.; Zhang, D.F.; Fu, W.F.; Lv, X.J. N-doped carbon coated NiCo2S4 hollow nanotube as bifunctional electrocatalyst for overall water splitting. Carbon 2019, 145, 521–528. [Google Scholar] [CrossRef]
- Feng, X.; Jiao, Q.; Cui, H.; Yin, M.; Li, Q.; Zhao, Y.; Li, H.; Zhou, W.; Feng, C. One-Pot Synthesis of NiCo2S4 Hollow Spheres via Sequential Ion-Exchange as an Enhanced Oxygen Bifunctional Electrocatalyst in Alkaline Solution. ACS Appl. Mater. Interfaces 2018, 10, 29521–29531. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Guo, H.J.; Wu, J.; Sun, X.; Zhang, Z.; Liao, Q.L.; Zhang, S.C.; Si, H.N.; Wu, P.W.; Wang, L.; et al. Engineering an Earth-Abundant Element-Based Bifunctional Electrocatalyst for Highly Efficient and Durable Overall Water Splitting. Adv. Funct. Mater. 2019, 29, 1807031. [Google Scholar] [CrossRef]
- Khan, Z.; Senthilkumar, B.; O Park, S.; Park, S.; Yang, J.; Lee, J.H.; Song, H.K.; Kim, Y.; Kwak, S.K.; Ko, H. Carambola-shaped VO2 nanostructures: A binder-free air electrode for an aqueous Na–air battery. J. Mater. Chem. A 2017, 5, 2037–2044. [Google Scholar] [CrossRef]
- Ma, T.T.; Dai, Z.; Shen, X.R.; Jiao, Q.Z.; Zhao, Y.; Li, H.S.; Feng, C.H. Three-Dimensional Porous MnCo2S4 Microrugby Balls Supported on Carbon Cloth for Efficient Oxygen Evolution Reaction. Chemelectrochem 2022, 9, e202200552. [Google Scholar] [CrossRef]
- Zhong, M.X.; Song, N.; Li, C.M.; Wang, C.; Chen, W.; Lu, X.F. Controllable Growth of Fe-Doped Nis2 on Nife-Carbon Nanofibers for Boosting Oxygen Evolution Reaction. J. Colloid Interface Sci. 2022, 614, 556–565. [Google Scholar] [CrossRef]
- Zhou, X.C.; Yang, H.; Rao, D.W.; Yan, X.H. Morphology Controllable NiCo2S4 Nanostructure on Carbon Cloth for Enhanced Electrocatalytic Water Oxidation. J. Alloys Compd. 2022, 897, 163152. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Shim, J.-J. In situ growth of hierarchical mesoporous NiCo2S4@MnO2 arrays on nickel foam for high-performance supercapacitors. Electrochim. Acta 2015, 166, 302–309. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Zhao, Y.C.; Wang, C.G.; Li, X.; Liu, J.D.; Yue, G.H.; Zhou, Z.D. Facile synthesis of hollow urchin-like NiCo2O4 microspheres for high-performance sodium-ion batteries. J. Mater. Sci. 2016, 51, 9296–9305. [Google Scholar] [CrossRef]
- Knözinger, H.; Ratnasamy, P. Catalytic Aluminas: Surface Models and Characterization of Surface Sites. Catal. Rev. 1978, 17, 31–70. [Google Scholar] [CrossRef]
- Toby, B.H. Expgui, a Graphical User Interface for Gsas. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.Q.; Lu, C.J.; Xia, Z.P.; Zhou, Y.; Luo, Z. X-ray diffraction patterns of graphite and turbostratic carbon. Carbon 2007, 45, 1686–1695. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1290. [Google Scholar] [CrossRef]
- Su, Y.Z.; Xiao, K.; Li, N.; Liu, Z.Q.; Qiao, S.Z. Amorphous Ni(OH)2 @ three-dimensional Ni core–shell nanostructures for high capacitance pseudocapacitors and asymmetric supercapacitors. J. Mater. Chem. A 2014, 2, 13845–13853. [Google Scholar] [CrossRef]
- Wu, Z.C.; Wang, X.; Huang, J.S.; Gao, F. A Co-doped Ni–Fe mixed oxide mesoporous nanosheet array with low overpotential and high stability towards overall water splitting. J. Mater. Chem. A 2018, 6, 167–178. [Google Scholar] [CrossRef]
- Shuai, C.; Mo, Z.L.; Niu, X.H.; Yang, X.; Liu, G.G.; Wang, J.; Liu, N.J.; Guo, R.B. Hierarchical NiCo2S4 nanosheets grown on graphene to catalyze the oxygen evolution reaction. J. Mater. Sci. 2020, 55, 1627–1636. [Google Scholar] [CrossRef]
- Wu, X.Y.; Li, S.M.; Wang, B.; Liu, J.H.; Yu, M. Graphene Foam Supported Multilevel Network-Like NiCo2S4 Nanoarchitectures for Robust Lithium Storage and Efficient Orr Catalysis. New J. Chem. 2017, 41, 115–125. [Google Scholar] [CrossRef]
- Yin, J.L.; Zhang, H.; Luo, J.Q.; Yao, M.Q.; Hu, W.C. High-boiling-point solvent synthesis of mesoporous NiCo2S4 with high specific surface area as supercapacitor electrode material. J. Mater. Sci. Mater. Electron. 2017, 28, 2093–2099. [Google Scholar] [CrossRef]
- Du, W.M.; Wang, Z.Y.; Zhu, Z.Q.; Hu, S.; Zhu, X.Y.; Shi, Y.F.; Pang, H.; Qian, X.F. Facile Synthesis and Superior Electrochemical Performances of Coni2s4/Graphene Nanocomposite Suitable for Supercapacitor Electrodes. J. Mater. Chem. A 2014, 2, 9613–9619. [Google Scholar] [CrossRef]
- Liu, N.; Guo, Y.; Yang, X.; Lin, H.; Yang, L.; Shi, Z.; Zhong, Z.; Wang, S.; Tang, Y.; Gao, Q. Microwave-Assisted Reactant-Protecting Strategy toward Efficient Mos2 Electrocatalysts in Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2015, 7, 23741–23749. [Google Scholar] [CrossRef]
- Jiang, J.Y.; Yan, C.Y.; Zhao, X.H.; Luo, H.X.; Xue, Z.M.; Mu, T.C. A PEGylated deep eutectic solvent for controllable solvothermal synthesis of porous NiCo2S4 for efficient oxygen evolution reaction. Green Chem. 2017, 19, 3023–3031. [Google Scholar] [CrossRef]
- Yuan, F.F.; Wei, J.D.; Qin, G.X.; Ni, Y.H. Carbon Cloth Supported Hierarchical Core-Shell NiCo2s4@Coni-Ldh Nanoarrays as Catalysts for Efficient Oxygen Evolution Reaction in Alkaline Solution. J. Alloys Compd. 2020, 830, 154658. [Google Scholar] [CrossRef]
- Liu, Y.; Bai, Y.; Han, Y.; Yu, Z.; Zhang, S.; Wang, G.; Wei, J.; Wu, Q.; Sun, K. Self-Supported Hierarchical Feconi-Lth/NiCo2O4/Cc Electrodes with Enhanced Bifunctional Performance for Efficient Overall Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 36917–36926. [Google Scholar] [CrossRef]
- Hamdani, M.; Singh, R.N.; Chartier, P. Co3o4 and Co- Based Spinel Oxides Bifunctional Oxygen Electrodes. Int. J. Electrochem. Sci. 2010, 5, 556–577. [Google Scholar]
- McCrory, C.C.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking Heterogeneous Electrocatalysts for the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef]
- Trasatti, S.; Petrii, O.A. Real Surface Area Measurements in Electrochemistry. J. Electroanal. Chem. 1992, 327, 353–376. [Google Scholar] [CrossRef]
- Zhan, T.R.; Liu, X.L.; Lu, S.S.; Hou, W.G. Nitrogen doped NiFe layered double hydroxide/reduced graphene oxide mesoporous nanosphere as an effective bifunctional electrocatalyst for oxygen reduction and evolution reactions. Appl. Catal. B Environ. 2017, 205, 551–558. [Google Scholar] [CrossRef]
- Darband, G.B.; Aliofkhazraei, M.; Hyun, S.; Rouhaghdam, A.S.; Shanmugam, S. Electrodeposition of Ni-Co-Fe mixed sulfide ultrathin nanosheets on Ni nanocones: A low-cost, durable and high performance catalyst for electrochemical water splitting. Nanoscale 2019, 11, 16621–16634. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.R.; Mhin, S.; Kim, K.M.; Han, W.S.; Choi, H.; Ali, G.; Chung, K.Y.; Lee, H.J.; Moon, S.I.; Dutta, S.; et al. Electrochemically activated cobalt nickel sulfide for an efficient oxygen evolution reaction: Partial amorphization and phase control. J. Mater. Chem. A 2019, 7, 3592–3602. [Google Scholar] [CrossRef]
- Zou, H.; He, B.; Kuang, P.; Yu, J.; Fan, K. Metal-Organic Framework-Derived Nickel-Cobalt Sulfide on Ultrathin Mxene Nanosheets for Electrocatalytic Oxygen Evolution. ACS Appl. Mater. Interfaces 2018, 10, 22311–22319. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yang, H.; Sun, J.; Xia, M.; Guo, W.; Yu, L.; Yan, J.; Zheng, J.; Chang, L.; Gao, F. Phase-pure Pentlandite Ni4.3Co4.7S8 Binary Sulfide as Efficient Bifunctional Electrocatalyst for Oxygen Evolution and Hydrogen Evolution. Nanoscale 2018, 10, 10459–10466. [Google Scholar] [CrossRef] [PubMed]
- Qin, K.; Wang, L.; Wen, S.; Diao, L.; Liu, P.; Li, J.; Ma, L.; Shi, C.; Zhong, C.; Hu, W.; et al. Designed synthesis of NiCo-LDH and derived sulfide on heteroatom-doped edge-enriched 3D rivet graphene films for high-performance asymmetric supercapacitor and efficient OER. J. Mater. Chem. A 2018, 6, 8109–8119. [Google Scholar] [CrossRef]
- Zhao, C.; Li, P.; Shao, D.; Zhang, R.; Wang, S.; Zhu, Z.; Zhao, C. Phytic acid-derived Co2-xNixP2O7-C/RGO and its superior OER electrocatalytic performance. Int. J. Hydrog. Energy 2019, 44, 844–852. [Google Scholar] [CrossRef]
- Yu, Z.; Bai, Y.; Zhang, S.; Liu, Y.; Zhang, N.; Sun, K. MOF-directed templating synthesis of hollow nickel-cobalt sulfide with enhanced electrocatalytic activity for oxygen evolution. Int. J. Hydrog. Energy 2018, 43, 8815–8823. [Google Scholar] [CrossRef]





| Sample | Thiourea (mol) | Co2+ (mmol) | Ni2+ (mmol) | SDS (mmol) | DI Water (mL) |
|---|---|---|---|---|---|
| NiCo2S4-NiS2/CFP | 0.1 | 1.42 | 2.84 | 0.6 | 60 |
| Ni3S4-NiS2/CFP | 0.1 | / | 4.26 | 0.6 | 60 |
| Co2S/CFP | 0.1 | 4.26 | / | 0.6 | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Xia, Y.; Luo, X.; Mao, T.; Wang, Z.; Hong, Z.; Yue, G. Bi-Phase NiCo2S4-NiS2/CFP Nanocomposites as a Highly Active Catalyst for Oxygen Evolution Reaction. Coatings 2023, 13, 313. https://doi.org/10.3390/coatings13020313
Li J, Xia Y, Luo X, Mao T, Wang Z, Hong Z, Yue G. Bi-Phase NiCo2S4-NiS2/CFP Nanocomposites as a Highly Active Catalyst for Oxygen Evolution Reaction. Coatings. 2023; 13(2):313. https://doi.org/10.3390/coatings13020313
Chicago/Turabian StyleLi, Jintang, Yongji Xia, Xianrui Luo, Tianle Mao, Zhenjia Wang, Zheyu Hong, and Guanghui Yue. 2023. "Bi-Phase NiCo2S4-NiS2/CFP Nanocomposites as a Highly Active Catalyst for Oxygen Evolution Reaction" Coatings 13, no. 2: 313. https://doi.org/10.3390/coatings13020313





