Oxidation Behavior of NiCoCrAlY Coatings Deposited by Vacuum Plasma Spraying and High-Velocity Oxygen Fuel Processes
Abstract
:1. Introduction
2. Materials and Method
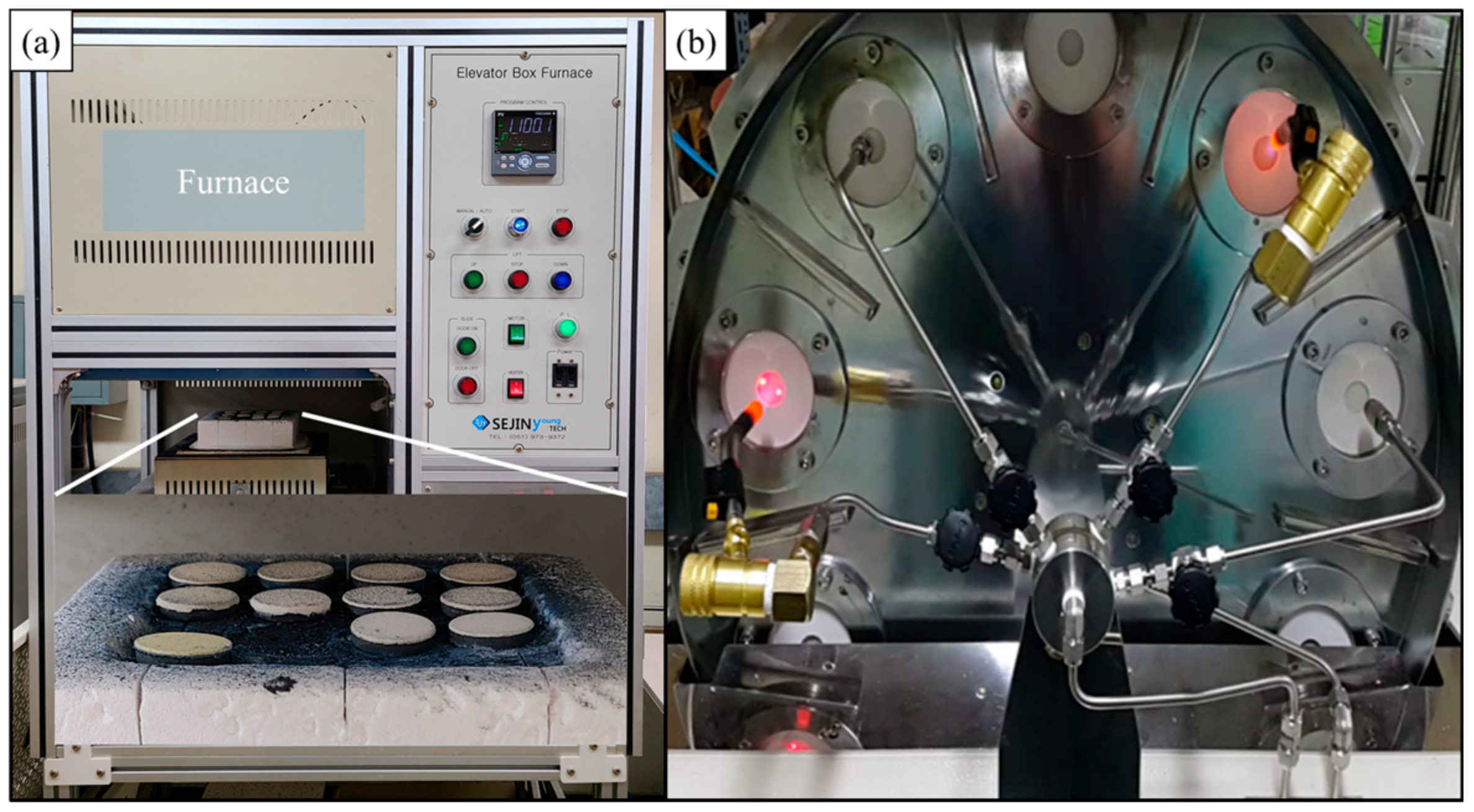
2.1. Preparation of the Coating Layer
2.2. Thermal Durability Evaluation and Preparation of Analysis Specimens
2.3. Characterization
3. Results
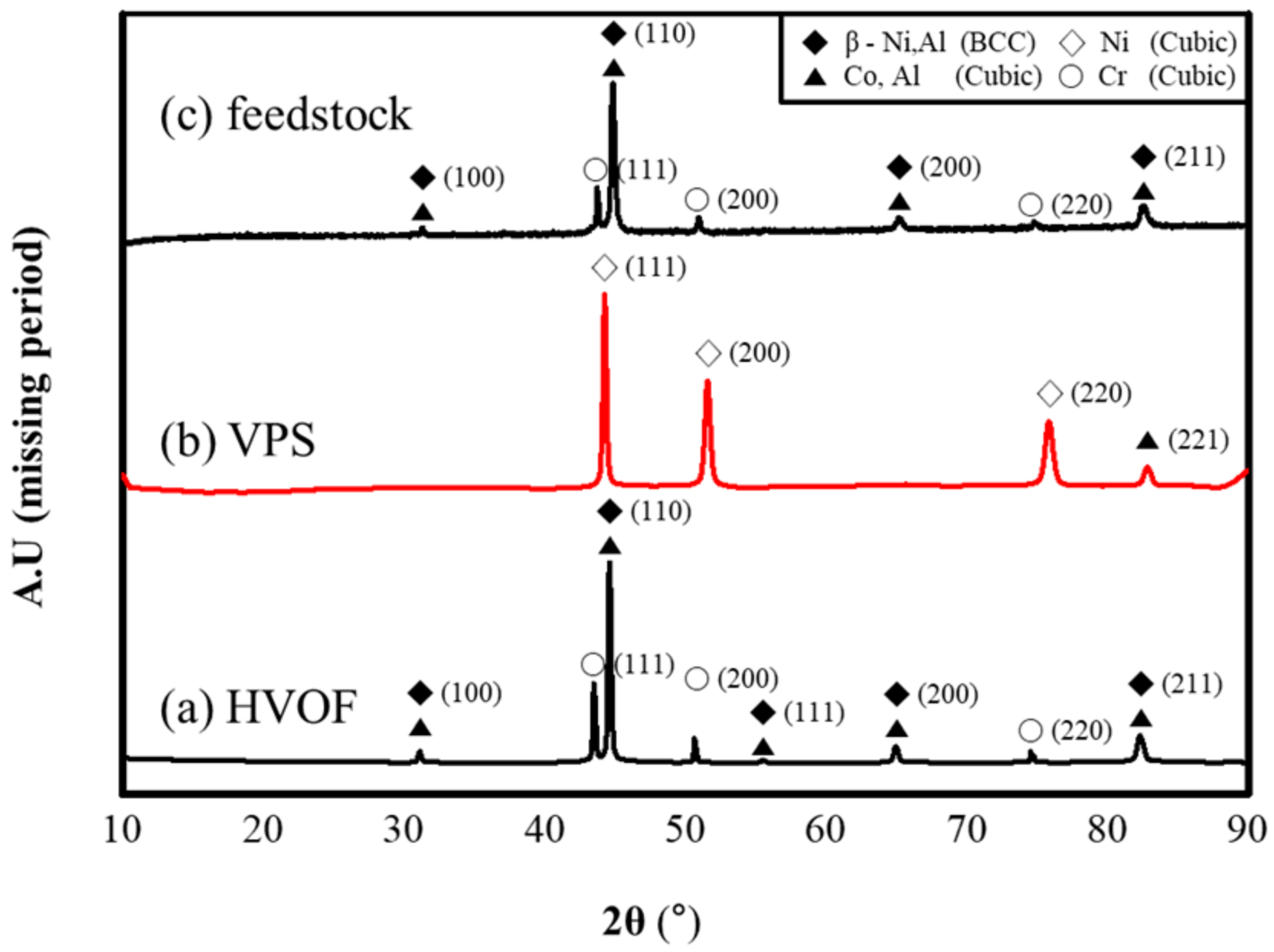
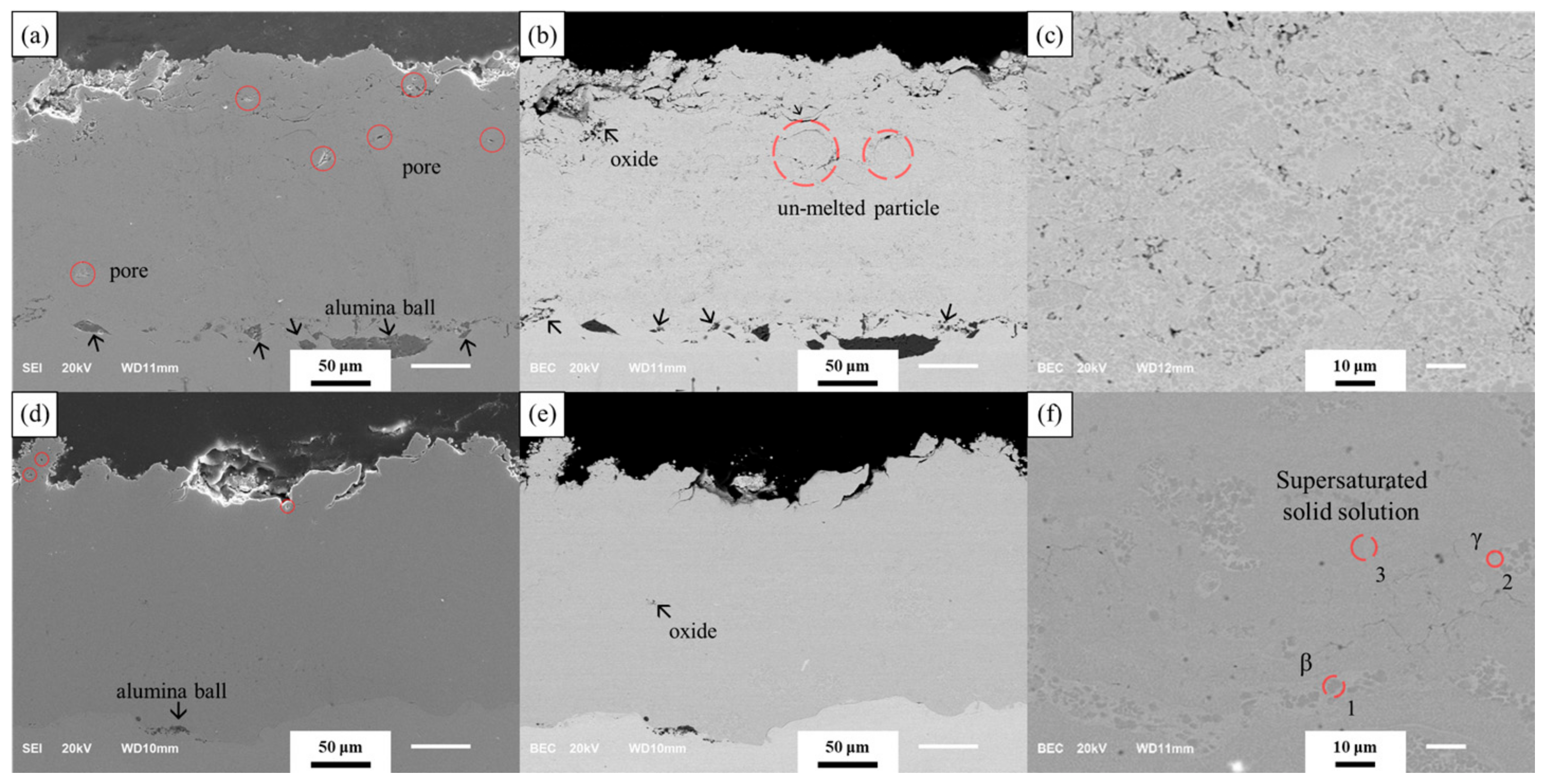
3.1. Analysis of As-Coated Samples
3.2. Evaluation Result of JETS Test
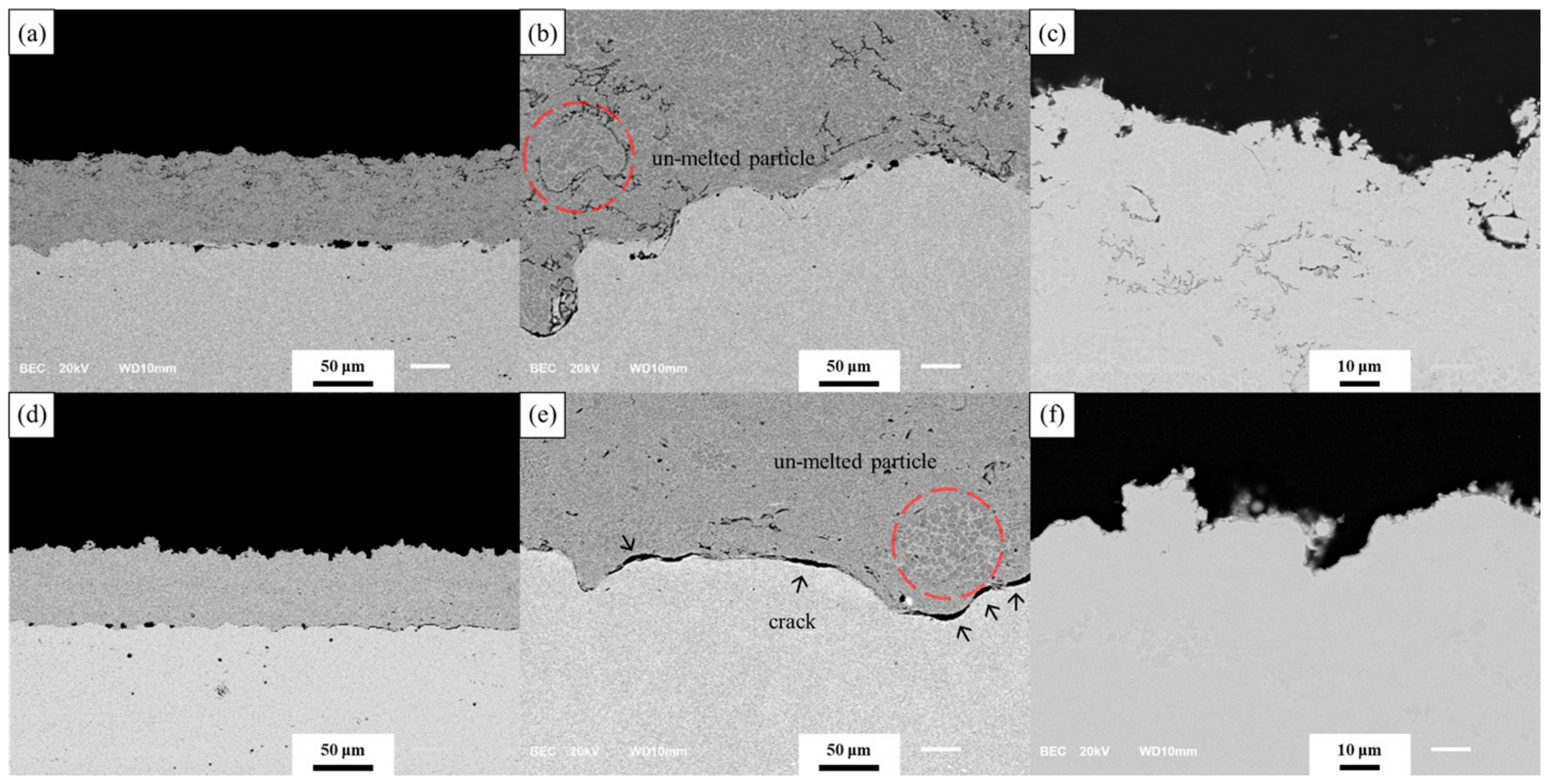
3.3. Evaluation Result of FCT
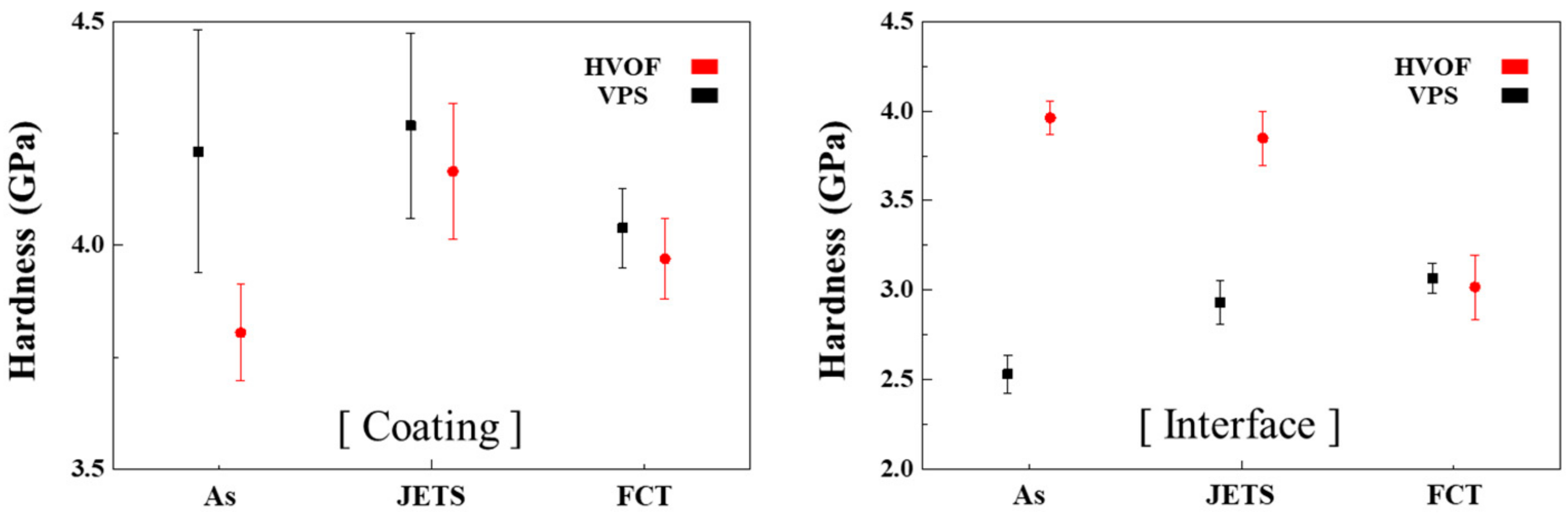
3.4. Vickers Hardness of Each Coating
4. Discussion
4.1. Analysis of As-Coated Samples
4.2. Microstructure Analysis after FCT
4.3. Oxidation Behavior of Each Coating after FCT
4.4. Analysis of Vickers Hardness
4.5. Applicability and Development Direction of HVOF
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Padture, N.P.; Gell, M.; Jordan, E.H. Thermal Barrier Coatings for Gas-Turbine Engine Applications. Science 2002, 296, 280–284. [Google Scholar] [CrossRef]
- Beele, W.; Marijnissen, G.; Van Lieshout, A. The Evolution of Thermal Barrier Coatings—Status and Upcoming Solutions for Today’s Key Issues. Surf. Coat. Technol. 1999, 120–121, 61–67. [Google Scholar] [CrossRef]
- Padture, N.P.; Gell, M.; Klemens, P.G. Ceramic Materials for Thermal Barrier Coatings 2000. J. Eur. Ceram. Soc. 2004, 24, 1–10. [Google Scholar]
- Padture, N.P. Advanced Structural Ceramics in Aerospace Propulsion. Nat. Mater. 2016, 15, 804–809. [Google Scholar] [CrossRef]
- Smialek, J.L.; Miller, R.A. Revisiting the Birth of 7YSZ Thermal Barrier Coatings: Stephan Stecura. Coatings 2018, 8, 255. [Google Scholar] [CrossRef]
- Haynes, J.A. Elevated Temperature Coatings. Sci. Technol. 1999, 185–196. [Google Scholar]
- Worch, H.P. Kofstad, High Temperature Corrosion; Elsevier Applied Science: London, UK, 1988; ISBN 1-85166-154-91989. [Google Scholar]
- Stiger, M.J.; Yanar, N.M.; Topping, M.G.; Pettit, F.S.; Meier, G.H. Thermal Barrier Coatings for the 21st Century. Z. Fur Met. 1999, 90, 1069–1078. [Google Scholar]
- Kawakita, J.; Kuroda, S.; Fukushima, T.; Kodama, T. Development of Dense Corrosion Resistant Coatings by an Improved HVOF Spraying Process. Sci. Technol. Adv. Mater. 2003, 4, 281–289. [Google Scholar] [CrossRef]
- Gudmundsson, B.; Jacobson, B.E.; Gruner, H. The Influence of Substrate Temperature on the Microstructure and Hardness of Vacuum-Plasma-Sprayed Co Ni Cr Al Si Zr Y and Co Ni Cr Al Y Alloys. Mater. Sci. Eng. A 1989, 108, 105–115. [Google Scholar] [CrossRef]
- Noguchi, K.; Nishida, M.; Chiba, A. Transmission Electron Microscopy of Low Pressure Plasma Sprayed CoNiCrAlY Coating. Scr. Mater. 1996, 35, 1359–1364. [Google Scholar] [CrossRef]
- Lugscheider, E.; Herbst, C.; Zhao, L. Parameter Studies on High-Velocity Oxy-Fuel Spraying of MCrAlY Coatings. Surf. Coat. Technol. 1998, 108, 16–23. [Google Scholar] [CrossRef]
- Toma, D.; Brandl, W.; Köster, U. Studies on the Transient Stage of Oxidation of VPS and HVOF Sprayed MCrAlY Coatings. Surf. Coat. Technol. 1999, 120, 8–15. [Google Scholar] [CrossRef]
- Ajdelsztajn, L.; Picas, J.A.; Kim, G.E.; Bastian, F.L.; Schoenung, J.; Provenzano, V. Oxidation Behavior of HVOF Sprayed Nanocrystalline NiCrAlY Powder. Mater. Sci. Eng. A 2002, 338, 33–43. [Google Scholar] [CrossRef]
- Brandl, W.; Toma, D.; Krüger, J.; Grabke, H.J.; Matthäus, G. The Oxidation Behaviour of HVOF Thermal-Sprayed MCrAlY Coatings. Surf. Coat. Technol. 1997, 94, 21–26. [Google Scholar] [CrossRef]
- DeMasi-Marcin, J.T.; Gupta, D.K. Protective Coatings in the Gas Turbine Engine. Surf. Coat. Technol. 1994, 68–69, 1–9. [Google Scholar] [CrossRef]
- Myoung, S.-W.; Kim, J.-H.; Lee, W.-R.; Jung, Y.-G.; Lee, K.-S.; Paik, U. Microstructure Design and Mechanical Properties of Thermal Barrier Coatings with Layered Top and Bond Coats. Surf. Coat. Technol. 2010, 205, 1229–1235. [Google Scholar] [CrossRef]
- Haynes, J.A.; Unocic, K.A.; Pint, B.A. Effect of Water Vapor on the 1100 C Oxidation Behavior of Plasma-Sprayed TBCs with HVOF NiCoCrAlX Bond Coatings. Surf. Coat. Technol. 2013, 215, 39–45. [Google Scholar] [CrossRef]
- Brindley, W.J.; Miller, R.A. Thermal Barrier Coating Life and Isothermal Oxidation of Low-Pressure Plasma-Sprayed Bond Coat Alloys. Surf. Coat. Technol. 1990, 43, 446–457. [Google Scholar] [CrossRef]
- Saeidi, S.; Voisey, K.T.; McCartney, D.G. The Effect of Heat Treatment on the Oxidation Behavior of HVOF and VPS CoNiCrAlY Coatings. J. Therm. Spray Technol. 2009, 18, 209–216. [Google Scholar] [CrossRef]
- Li, C.-J.; Li, W.-Y. Effect of Sprayed Powder Particle Size on the Oxidation Behavior of MCrAlY Materials during High Velocity Oxygen-Fuel Deposition. Surf. Coatings Technol. 2003, 162, 31–41. [Google Scholar] [CrossRef]
- Lipkin, D.M.; Clarke, D.R. Measurement of the Stress in Oxide Scales Formed by Oxidation of Alumina-Forming Alloys. Oxid. Met. 1996, 45, 267–280. [Google Scholar] [CrossRef]
- Schlichting, K.W.; Padture, N.P.; Jordan, E.H.; Gell, M. Failure Modes in Plasma-Sprayed Thermal Barrier Coatings. Mater. Sci. Eng. A 2003, 342, 120–130. [Google Scholar] [CrossRef]
- Wagner, C. Reaktionstypen Bei Der Oxydation von Legierungen. Z. Für Elektrochem. Ber. Der Bunsenges. Für Phys. Chem. 1959, 63, 772–782. [Google Scholar] [CrossRef]
- Tang, F.; Ajdelsztajn, L.; Schoenung, J.M. Characterization of Oxide Scales Formed on HVOF NiCrAlY Coatings with Various Oxygen Contents Introduced during Thermal Spraying. Scr. Mater. 2004, 51, 25–29. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, H.K.; Choi, H.S.; Ahn, H.S. Phase Transformation and Bond Coat Oxidation Behavior of Plasma-Sprayed Zirconia Thermal Barrier Coating. Surf. Coat. Technol. 2000, 124, 1–12. [Google Scholar] [CrossRef]
- Brandl, W.; Toma, D.; Grabke, H.J. The Characteristics of Alumina Scales Formed on HVOF-Sprayed MCrAlY Coatings. Surf. Coat. Technol. 1998, 108, 10–15. [Google Scholar] [CrossRef]
- Raza, A.; Ahmad, F.; Badri, T.M.; Raza, M.R.; Malik, K. An Influence of Oxygen Flow Rate and Spray Distance on the Porosity of HVOF Coating and Its Effects on Corrosion—A Review. Materials 2022, 15, 6329. [Google Scholar] [CrossRef]
- Lyu, G.; Song, D.; Kim, J.; Lu, Z.; Yang, B.; Yang, S.; Byeun, Y.; Jung, Y.-G. Multidisciplinary Approach for Investigating the Hot Corrosion Behavior of Thermal Barrier Coatings. J. Eur. Ceram. Soc. 2021, 41, 324–333. [Google Scholar] [CrossRef]
- Li, M.H.; Sun, X.F.; Li, J.G.; Zhang, Z.Y.; Jin, T.; Guan, H.R.; Hu, Z.Q. Oxidation Behavior of a Single-Crystal Ni-Base Superalloy in Air. I: At 800 and 900 C. Oxid. Met. 2003, 59, 591–605. [Google Scholar] [CrossRef]
- Giese, S.; Neumeier, S.; Bergholz, J.; Naumenko, D.; Quadakkers, W.J.; Vaßen, R.; Göken, M. Influence of Different Annealing Atmospheres on the Mechanical Properties of Freestanding MCrAlY Bond Coats Investigated by Micro-Tensile Creep Tests. Metals 2019, 9, 692. [Google Scholar] [CrossRef]
- Kanesund, J.; Moverare, J.; Johansson, S. The Deformation and Damage Mechanisms during Thermomechanical Fatigue (TMF) in IN792. Procedia Eng. 2011, 10, 189–194. [Google Scholar] [CrossRef]
- Murthy, G.V.S.; Sridhar, G.; Kumar, A.; Jayakumar, T. Characterization of Intermetallic Precipitates in a Nimonic Alloy by Ultrasonic Velocity Measurements. Mater. Charact. 2009, 60, 234–239. [Google Scholar] [CrossRef]
- Miller, R.A. Oxidation-based Model for Thermal Barrier Coating Life. J. Am. Ceram. Soc. 1984, 67, 517–521. [Google Scholar] [CrossRef]
- Michels, D.; Hadeler, J.; Lienhard, V.J.H. High-Heat-Flux Resistance Heaters from VPS and HVOF Thermal Spraying. Exp. HEAT Transf. Int. J. 1998, 11, 341–359. [Google Scholar] [CrossRef]
- Huntz, A.M. Influence of Active Elements on the Oxidation Mechanism of M Cr Al Alloys. Mater. Sci. Eng. 1987, 87, 251–260. [Google Scholar] [CrossRef]
- Stott, F.H.; Wood, G.C. Growth and Adhesion of Oxide Scales on Al2O3-Forming Alloys and Coatings. Mater. Sci. Eng. 1987, 87, 267–274. [Google Scholar] [CrossRef]
- Smeggil, J.G. Some Comments on the Role of Yttrium in Protective Oxide Scale Adherence. Mater. Sci. Eng. 1987, 87, 261–265. [Google Scholar] [CrossRef]
- Volenik, K.; Novak, V.; Dubský, J.; Chraska, P.; Neufuss, K. Properties of Alloy Steel Coatings Oxidized during Plasma Spraying. Mater. Sci. Eng. A 1997, 234, 493–496. [Google Scholar] [CrossRef]
- Wei, T.; Fan, Z.; Luo, G.; Zheng, C.; Xie, D. A Rapid and Efficient Method to Prepare Exfoliated Graphite by Microwave Irradiation. Carbon N. Y. 2009, 47, 337–339. [Google Scholar] [CrossRef]
- Mumm, D.R.; Evans, A.G. On the Role of Imperfections in the Failure of a Thermal Barrier Coating Made by Electron Beam Deposition. Acta Mater. 2000, 48, 1815–1827. [Google Scholar] [CrossRef]
- Hou, P.Y.; Stringer, J. Room Temperature Strains in Cr2O3 Scales Formed at Elevated Temperatures on Ni-25 wt% Cr and Y-and Al-Doped Ni-25 wt% Cr. Acta Metall. Mater. 1991, 39, 841–849. [Google Scholar] [CrossRef]
- Perez, P. Influence of the Alloy Grain Size on the Oxidation Behaviour of PM2000 Alloy. Corros. Sci. 2002, 44, 1793–1808. [Google Scholar] [CrossRef]
- Brandl, W.; Grabke, H.J.; Toma, D.; Krüger, J. The Oxidation Behaviour of Sprayed MCrAlY Coatings. Surf. Coat. Technol. 1996, 86, 41–47. [Google Scholar] [CrossRef]
- Clarke, D.R.; Pompe, W. Critical Radius for Interface Separation of a Compressively Stressed Film from a Rough Surface. Acta Mater. 1999, 47, 1749–1756. [Google Scholar] [CrossRef]
- Sampath, S. Microstructural Characteristics of Plasma Spray Consolidated Amorphous Powders. Mater. Sci. Eng. A 1993, 167, 1–10. [Google Scholar] [CrossRef]
- Levi, C.G.; Hutchinson, J.W.; Vidal-Sétif, M.-H.; Johnson, C.A. Environmental Degradation of Thermal-Barrier Coatings by Molten Deposits. MRS Bull. 2012, 37, 932–941. [Google Scholar] [CrossRef]
- Raza, A.; Ahmad, F.; Badri, T.M.; Raza, M.R.; Malik, K.; Ali, S. Selection of Materials Based on Thermo-Mechanical Properties of Thermal Barrier Coatings and Their Failures—A Review. Adv. Mater. Sci. Eng. 2023, 255–264. [Google Scholar]
- Messaoudi, K.; Huntz, A.M.; Lesage, B. Diffusion and Growth Mechanism of Al2O3 Scales on Ferritic Fe-Cr-Al Alloys. Mater. Sci. Eng. A 1998, 247, 248–262. [Google Scholar] [CrossRef]
- Evans, A.G.; Mumm, D.R.; Hutchinson, J.W.; Meier, G.H.; Pettit, F.S. Mechanisms Controlling the Durability of Thermal Barrier Coatings. Prog. Mater. Sci. 2001, 46, 505–553. [Google Scholar] [CrossRef]
- Brandes, E.A.; Brook, G.B. Smithells Metals Reference Book; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 0080517307. [Google Scholar]
- Ambrico, J.M.; Begley, M.R.; Jordan, E.H. Stress and Shape Evolution of Irregularities in Oxide Films on Elastic–Plastic Substrates Due to Thermal Cycling and Film Growth. Acta Mater. 2001, 49, 1577–1588. [Google Scholar] [CrossRef]
- Karlsson, A.M.; Evans, A.G. A Numerical Model for the Cyclic Instability of Thermally Grown Oxides in Thermal Barrier Systems. Acta Mater. 2001, 49, 1793–1804. [Google Scholar]


| Composition (wt. %) | Ni | Co | Cr | Al | Y | Hf | Si | Ta | W | Mo | Ti | Fe | C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amdry 386 [31] | Bal. | 19.0 | 16.5 | 23.0 | 0.36 | 0.08 | 0.79 | - | - | - | - | - | - |
| Inconel 792 [32] | Bal. | 8.9 | 12.4 | 3.5 | - | - | - | 4.1 | 4.0 | 1.8 | 4.95 | - | 0.8 |
| Nimonic 263 [33] | Bal. | 19.6 | 20.1 | 0.4 | - | - | 0.4 | - | - | 6.0 | 2.0 | 0.7 | 0.06 |
| Spectra | Phase | O | Al | Ni | Co | Cr | Y | Hf | Si | Total (at. %) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | β (BCC) | 2.53 | 29.54 | 39.22 | 15.80 | 12.09 | - | - | 0.82 | 100 |
| 2 | γ (FCC) | 3.16 | 15.35 | 38.00 | 20.75 | 21.48 | - | - | 1.25 | |
| 3 | supersaturated solid solution | 3.75 | 23.14 | 38.91 | 17.73 | 15.41 | - | 0.11 | 0.95 |
| Spectra | Phase | O | Al | Ni | Co | Cr | Y | Hf | Si | Total (at. %) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | γ (FCC) | - | 9.78 | 39.43 | 26.89 | 22.64 | 0.25 | 0.04 | 0.98 | 100 |
| 2 | 0.36 | 9.99 | 38.80 | 27.02 | 22.41 | 0.47 | 0.01 | 0.94 | ||
| 9 | 0.19 | 7.98 | 38.77 | 25.07 | 26.85 | 0.26 | - | 0.88 | ||
| 10 | 0.21 | 9.01 | 39.96 | 24.52 | 25.31 | 0.17 | - | 0.81 | ||
| 3 | β (BCC) | - | 34.14 | 49.02 | 11.20 | 5.20 | 0.05 | - | 0.38 | |
| 4 | 0.23 | 34.29 | 48.68 | 11.01 | 4.99 | 0.20 | 0.10 | 0.52 | ||
| 11 | - | 34.20 | 50.02 | 9.60 | 5.72 | 0.03 | - | 0.43 | ||
| 12 | 0.75 | 33.72 | 50.24 | 9.51 | 5.25 | 0.10 | - | 0.44 | ||
| 5 | Al2O3 | 60.52 | 37.32 | 0.73 | 0.51 | 0.56 | 0.34 | 0.02 | - | |
| 6 | 58.71 | 38.19 | 1.17 | 077 | 0.82 | 0.32 | 0.01 | - | ||
| 13 | 60.87 | 38.23 | 0.22 | 0.09 | 0.11 | 0.32 | 0.15 | - | ||
| 14 | 60.98 | 37.28 | 0.32 | 0.17 | 0.18 | 0.80 | 0.27 | - | ||
| 7 | Spinel TGO | 62.11 | 26.43 | 6.32 | 3.77 | 1.36 | 0.01 | - | - | |
| 8 | 64.46 | 16.27 | 4.44 | 3.50 | 10.60 | 0.11 | 0.01 | 0.6 | ||
| 15 | 64.65 | 17.88 | 4.63 | 4.70 | 7.38 | 0.23 | 0.07 | 0.45 | ||
| 16 | 60.79 | 18.51 | 1.25 | 1.34 | 16.33 | 0.77 | 0.02 | 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Pyeon, J.; Kim, B.-G.; Khadaa, T.; Choi, H.; Zhe, L.; Dube, T.; Zhang, J.; Yang, B.-i.; Jung, Y.-g.; et al. Oxidation Behavior of NiCoCrAlY Coatings Deposited by Vacuum Plasma Spraying and High-Velocity Oxygen Fuel Processes. Coatings 2023, 13, 319. https://doi.org/10.3390/coatings13020319
Kim J, Pyeon J, Kim B-G, Khadaa T, Choi H, Zhe L, Dube T, Zhang J, Yang B-i, Jung Y-g, et al. Oxidation Behavior of NiCoCrAlY Coatings Deposited by Vacuum Plasma Spraying and High-Velocity Oxygen Fuel Processes. Coatings. 2023; 13(2):319. https://doi.org/10.3390/coatings13020319
Chicago/Turabian StyleKim, Junseong, Janghyeok Pyeon, Bong-Gu Kim, Tserendorj Khadaa, Hyeryang Choi, Lu Zhe, Tejesh Dube, Jing Zhang, Byung-il Yang, Yeon-gil Jung, and et al. 2023. "Oxidation Behavior of NiCoCrAlY Coatings Deposited by Vacuum Plasma Spraying and High-Velocity Oxygen Fuel Processes" Coatings 13, no. 2: 319. https://doi.org/10.3390/coatings13020319






