Hydrogenation Behavior of Cr-Coated Resistance Upset Welds of E110 Zirconium Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Hydrogenation Tests
2.3. Specimen Characterization
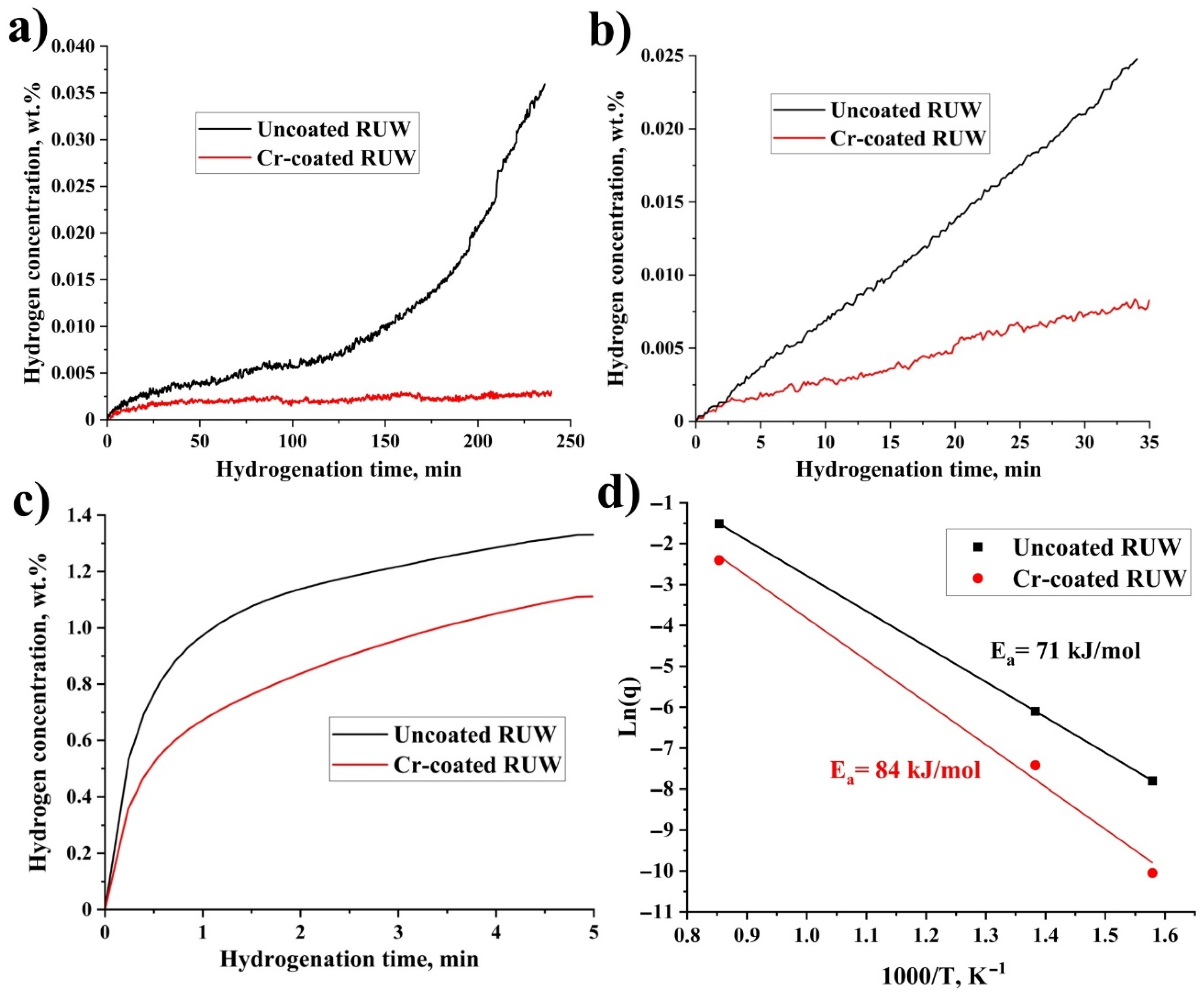
3. Results
3.1. Hydrogenation Tests
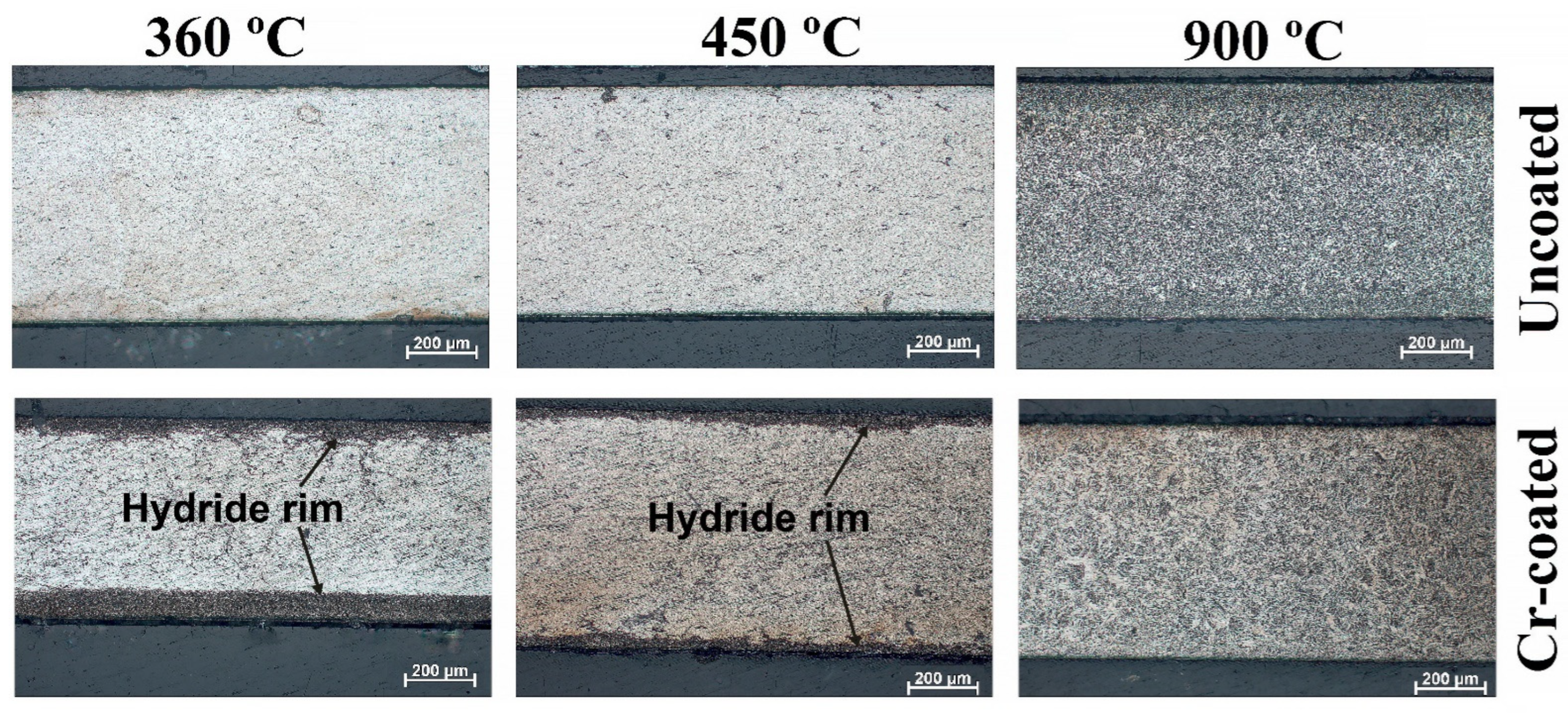
3.2. Phase Composition and Cross-Section Microstructure of RUW Specimens
3.3. Hardness Measurements
4. Discussion
5. Conclusions
- Due to high hydrogen absorption rates of uncoated RUW specimens, a hydride rim can be formed in its outer surface at 360 and 450 °C. Chromium coating can decrease the absorption rate by one order of magnitude and eliminate the growth of the hydride rim during the considered time of hydrogenation tests.
- The influence of Cr coating on the hydrogen absorption rate in RUW specimens is noticeably decreased during hydrogenation at 900 °C. The Cr-coated RUW specimen has a higher residual concentration of hydrogen (3900 ppm) since hydrogen output from the welded alloy is slowed down by the coating during the cooling stage.
- Welding can cause a formation of radially oriented hydrides in the welded zone of RUW specimens during hydrogenation. The coating deposition of Cr limits the formation of hydrides with such an orientation under low-temperature hydriding (360–450 °C).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Charit, I.; Murty, K.L. Creep behavior of niobium-modified zirconium alloys. J. Nucl. Mater. 2008, 374, 354–363. [Google Scholar] [CrossRef]
- Allen, T.R.; Konings, R.J.M.; Motta, A.T. Corrosion of zirconium alloys. Com. Nucl. Mater. 2012, 5, 49–68. [Google Scholar]
- Zinkle, S.J.; Terrani, K.A.; Gehin, J.C.; Ott, L.J.; Snead, L.L. Accident tolerant fuels for LWRs: A perspective. J. Nucl. Mater. 2014, 448, 374–379. [Google Scholar] [CrossRef]
- Kane, K.; Bell, S.; Capps, N.; Garrison, B.; Shapovalov, K.; Jacobsen, G.; Deck, C.; Graening, T.; Koyanagi, T.; Massey, C. The response of accident tolerant fuel cladding to LOCA burst testing: A comparative study of leading concepts. J. Nucl. Mater. 2023, 574, 154152. [Google Scholar] [CrossRef]
- Tang, C.; Stueber, M.; Seifert, H.J.; Steinbrueck, M. Protective coatings on zirconium-based alloys as accident-tolerant fuel (ATF) claddings. J. Corros. Rev. 2017, 35, 3. [Google Scholar]
- Wang, W.; Zhang, G.; Wang, C.; Wang, T.; Li, T. Construction of chromium coatings with (200) preferred orientation and exploration the high-temperature steam oxidation properties. J. Nucl. Mater. 2022, 563, 153660. [Google Scholar] [CrossRef]
- Yueh, K.; Terrani, K.A. Silicon carbide composite for light water reactor fuel assembly applications. J. Nucl. Mater. 2014, 448, 380–388. [Google Scholar] [CrossRef]
- Brachet, J.C.; Urvoy, S.; Rouesne, E.; Nony, G.; Dumerval, M.; Saux, M.L.; Ott, F.; Michau, A.; Schuster, F.; Maury, F. DLI-MOCVD CrxCy coating to prevent Zr-based cladding from inner oxidation and secondary hydriding upon LOCA conditions. J. Nucl. Mater. 2021, 550, 152953. [Google Scholar]
- Dryepondt, S.; Unocic, K.A.; Hoelzer, D.T.; Massey, C.P.; Pint, B.A. Development of low-Cr ODS FeCrAl alloys for accident-tolerant fuel cladding. J. Nucl. Mater. 2018, 501, 59–71. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, C.; Li, Z.; Zhang, W.; Xiang, Y.; He, H.; He, L.; Yang, H.; Liu, N.; Chang, H.; et al. Effect of N content on microstructure and properties of (AlTiCrNiTa)N coatings for accident-tolerant fuels. J. Intermet. 2022, 151, 107728. [Google Scholar] [CrossRef]
- Zeng, S.; Li, P.; Tian, J.; Chen, C.; Meng, Y.; Zhu, C.; Shen, H.; Han, X.; Zhang, H. Influence of Al content on the oxidation behavior of CrAl coating on Zry-4 alloys in 1200 °C steam. Corros. Sci. 2022, 198, 110115. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Liu, S.; Wu, L.; Qin, W.; Wu, X. Enhancement of oxidation resistance of Cr/CrN composite coating on Zr-4 surface by high lattice-matched interfacial Engineering. J. Nucl. Mater. 2023, 574, 154162. [Google Scholar] [CrossRef]
- Wang, X.; Liao, Y.; Xu, C.; Guan, H.; Zhu, M.; Gao, C.; Jin, X.; Pang, P.; Liao, B.; Xue, W. Steam oxidation behavior of ZrO2/Cr-coated pure zirconium prepared by plasma electrolytic oxidation followed by filtered cathodic vacuum arc deposition. J. Alloys Compd. 2021, 883, 160798. [Google Scholar] [CrossRef]
- Syrtanov, M.S.; Kashkarov, E.B.; Abdulmenova, A.V.; Sidelev, D.V. High-temperature oxidation of Zr-1Nb zirconium alloy with protective Cr/Mo coating. Surf. Coat. Technol. 2022, 439, 128459. [Google Scholar] [CrossRef]
- Brachet, J.C.; Idarraga-Trujillo, I.; Le Flem, M.; Le Saux, M.; Vandenberghe, V.; Urvoy, S.; Rouesne, E.; Guilbert, T.; Toffolon-Masclet, C.; Tupin, M.; et al. Early studies on Cr-Coated Zircaloy-4 as enhanced accident tolerant nuclear fuel claddings for light water reactors. J. Nucl. Mater. 2019, 517, 268–285. [Google Scholar]
- Hyun-Gil, K.; Il-Hyun, K.; Yang-Il, J.; Dong-Jun, P.; Jeong-Yong, P.; Yang-Hyun, K. Adhesion property and high-temperature oxidation behavior of Cr-coated Zircaloy-4 cladding tube prepared by 3D laser coating. J. Nucl. Mater. 2015, 465, 531–539. [Google Scholar]
- Motta, A.; Long-Qing, C. Hydride formation in zirconium alloys. JOM 2012, 64, 1403–1408. [Google Scholar] [CrossRef]
- Desquines, J.; Drouan, D.; Guilbert, S.; Lacote, P. Embrittlement of pre-hydrided Zircaloy-4 by steam oxidation under simulated LOCA transients. J. Nucl. Mater. 2016, 469, 20–31. [Google Scholar] [CrossRef]
- Pshenichnikov, A.; Stuckert, J.; Walter, M. Microstructure and mechanical properties of Zircaloy-4 cladding hydrogenated at temperatures typical for loss-of-coolant accident (LOCA) conditions. Nucl. Eng. Des. 2015, 283, 33–39. [Google Scholar] [CrossRef]
- Le Hong, T.; Turque, I.; Brachet, J.C.; Crepin, J.; Andre, G.; Barres, Q.; Guillou, R.; Toffolon-Masclet, C.; Joubert, J.M.; Le Saux, M. Phase transformations during cooling from the βZr phase temperature domain in several hydrogen-enriched zirconium alloys studied by in situ and ex situ neutron diffraction. Acta Mater. 2020, 199, 453–468. [Google Scholar]
- Daub, K.; Nieuwenhove, R.V.; Nordin, H. Investigation of the impact of coatings on corrosion and hydrogen uptake of Zircaloy-4. J. Nucl. Mater. 2015, 467, 260–270. [Google Scholar] [CrossRef]
- Kashkarov, E.B.; Nikitenkov, N.N.; Sutygina, A.N.; Obrosov, A.; Manakhov, A.; Polčák, J.; Weiß, S. Hydrogen absorption by Ti-implanted Zr-1Nb alloy. Int. J. Hydrogen Energy 2018, 43, 2484–2491. [Google Scholar]
- Usui, T.; Sawada, A.; Amaya, M.; Suzuki, A.; Chikada, T.; Terai, T. SiC coating as hydrogen permeation reduction and oxidation resistance for nuclear fuel cladding. J. Nucl. Sci. Technol. 2015, 52, 1318–1322. [Google Scholar] [CrossRef]
- Tang, C.; Grosse, M.K.; Trtik, P.; Steinbrück, M.; Stüber, M.; Seifert, H.J. H2 permeation behavior of Cr2AlC and Ti2AlC max phase coated Zircaloy-4 by neutron radiography. Acta Polytech. 2018, 58, 69–76. [Google Scholar] [CrossRef]
- Kashkarov, E.B.; Kudiiarov, V.N.; Kurdyumov, N.; Krinitcyn, M.G.; Sidelev, D.V. Hydrogenation behavior of Cr-coated laser beam welds of E110 zirconium alloy. J. Nucl. Mater. 2022, 570, 153980. [Google Scholar]
- Sidelev, D.; Ruchkin, S.; Kashkarov, E. High-Temperature Oxidation of Cr-Coated Resistance Upset Welds Made from E110 Alloy. Coatings 2021, 11, 577. [Google Scholar] [CrossRef]
- Sidelev, D.V.; Poltronieri, C.; Bestetti, M.; Krinitcyn, M.G.; Grudinin, V.A.; Kashkarov, E.B. A comparative study on high-temperature air oxidation of Cr-coated E110 zirconium alloy deposited by magnetron sputtering and electroplating. Surf. Coat. Technol. 2022, 433, 128134. [Google Scholar] [CrossRef]
- Kashkarov, E.B.; Nikitenkov, N.N.; Sutygina, A.N.; Syrtanov, M.S.; Vilkhivskaya, O.V.; Pryamushko, T.S.; Kudiiarov, V.N.; Volesky, L. Effect of titanium ion implantation and deposition on hydrogenation behavior of Zr-1Nb alloy. Surf. Coat. Technol. 2016, 308, 2–9. [Google Scholar] [CrossRef]
- Terrani, K.A.; Balooch, M.; Wongsawaeng, D.; Jaiyen, S.; Olander, D.R. The kinetics of hydrogen desorption from and adsorption on zirconium hydride. J. Nucl. Mater. 2010, 397, 61–68. [Google Scholar] [CrossRef]
- Steinbrück, M. Hydrogen absorption by zirconium alloys at high temperatures. J. Nucl. Mater. 2010, 397, 61–68. [Google Scholar] [CrossRef]
- Kurskii, R.A.; Safonov, D.V.; Rozhkov, A.V.; Zabusov, O.O.; Frolov, A.S.; Kuleshova, E.A.; Alekseeva, E.V.; Bragin, A.S.; Vasil’eva, E.A.; Gaiduchenko, A.B.; et al. Reorientation of Hydrides in Unirradiated Clad Tubes Made of Alloy E110 under Conditions Simulating Long-Term Dry Storage of Spent Nuclear Fuel. Phys. Met. Metallogr. 2021, 122, 861–868. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kim, H.-A.; Kang, S.-Y.; Kim, Y.-S. Effects of hydride rim on the ductility of Zircaloy-4 cladding. J. Nucl. Mater. 2019, 523, 283–390. [Google Scholar] [CrossRef]
- Kashkarov, E.B.; Gusev, K.S.; Ashikhmin, D.A.; Abdulmenova, A.V.; Sidelev, D.V. Oxidation and Mechanical Behavior of Cr-Coated Laser Beam Welds Made from E110 Zirconium Alloy. Coatings 2022, 12, 1623. [Google Scholar] [CrossRef]
- Yang, Z.B.; Liao, J.J.; Qiu, S.Y.; Cheng, Z.Q.; Liu, H.; Wu, Z.P.; Qiu, J.; Gao, B. Effect of Final Annealing Temperature on Corrosion Resistance of SZA-6 Zirconium Alloy Cladding Tubes. Mater. Sci. Forum 2019, 944, 488–498. [Google Scholar] [CrossRef]
- Semenov, A.N.; Plyshevskii, M.I.; Melyukov, V.V. Properties of welded joints from alloy Zr-2.5%Nb after electron-beam local thermocycling. Met. Sci. Heat Treat. 2014, 55, 670–674. [Google Scholar] [CrossRef]
- Carpenter, G.J.C. The dilatational misfit of zirconium hydrides precipitated in zirconium. J. Nucl. Mater. 1973, 48, 264–266. [Google Scholar] [CrossRef]
- Wang, S.; Li, S.; Li, R.; Wang, Y.; Xu, N.; Xue, F.; Bai, G.; Wang, Y.-D. Microscopic stress and crystallographic orientation of hydrides precipitated in Zr-1Nb-0.01Cu cladding tube investigated by high-energy X-ray diffraction and EBSD. J. Nucl. Mater. 2020, 542, 152534. [Google Scholar] [CrossRef]
- Chernyayeva, T.P.; Ostapov, A.V. Hydrogen in zirconium part 1. Probl. At. Sci. Technol. 2013, 87, 16–32. [Google Scholar]
- Bischoff, J.; Delafoy, C.; Vauglin, C.; Barberis, P.; Roubeyrie, C.; Perche, D.; Duthoo, D.; Schuster, F.; Brachet, J.-C.; Schweitzer, E.W.; et al. AREVA NP’s enhanced accident-tolerant fuel developments: Focus on Cr-coated M5 cladding. Nucl. Eng. Technol. 2018, 50, 223–228. [Google Scholar] [CrossRef]



| Time, Min | T, °C | Hydrogen Absorption Rate (q), cm3(H2)·cm−2·s−1 | Hydrogen Concentration (CH), ppm | ||
|---|---|---|---|---|---|
| Cr-Coated RUW | Uncoated RUW | Cr-Coated RUW | Uncoated RUW | ||
| 240 | 360 | 2.8·10−5 | 2.1·10−4 | 30 | 240 |
| 35 | 450 | 2.7·10−4 | 2.3·10−3 | 64 | 890 |
| 5 | 900 | 9.8·10−2 | 3.7·10−1 | 3900 | 2700 |
| Specimen | Time, Min | T, °C | CH, ppm | Average Hardness, HV | ||
|---|---|---|---|---|---|---|
| EZ | WZ | TZ | ||||
| As-received Cr-coated | 0 | 0 | 0 | 210 ± 17 | 212 ± 19 | 191 ± 14 |
| As-received uncoated | 0 | 0 | 0 | 208 ± 30 | 213 ± 10 | 178 ± 9 |
| Cr-coated | 240 | 360 | 30 | 208 ± 30 | 220 ± 24 | 207 ± 33 |
| Uncoated | 240 | 360 | 240 | 232 ± 37 | 255 ± 41 | 273 ± 36 |
| Cr-coated | 35 | 450 | 64 | 218 ± 24 | 212 ± 26 | 216 ± 19 |
| Uncoated | 35 | 450 | 890 | 223 ± 37 | 229 ± 32 | 231 ± 50 |
| Cr-coated | 5 | 900 | 3900 | 266 ± 27 | 270 ± 30 | 260 ± 42 |
| Uncoated | 5 | 900 | 2700 | 266 ± 26 | 255 ± 20 | 266 ± 32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashkarov, E.; Gusev, K.; Kudiiarov, V.; Kurdyumov, N.; Sidelev, D. Hydrogenation Behavior of Cr-Coated Resistance Upset Welds of E110 Zirconium Alloy. Coatings 2023, 13, 339. https://doi.org/10.3390/coatings13020339
Kashkarov E, Gusev K, Kudiiarov V, Kurdyumov N, Sidelev D. Hydrogenation Behavior of Cr-Coated Resistance Upset Welds of E110 Zirconium Alloy. Coatings. 2023; 13(2):339. https://doi.org/10.3390/coatings13020339
Chicago/Turabian StyleKashkarov, Egor, Kirill Gusev, Viktor Kudiiarov, Nikita Kurdyumov, and Dmitrii Sidelev. 2023. "Hydrogenation Behavior of Cr-Coated Resistance Upset Welds of E110 Zirconium Alloy" Coatings 13, no. 2: 339. https://doi.org/10.3390/coatings13020339
APA StyleKashkarov, E., Gusev, K., Kudiiarov, V., Kurdyumov, N., & Sidelev, D. (2023). Hydrogenation Behavior of Cr-Coated Resistance Upset Welds of E110 Zirconium Alloy. Coatings, 13(2), 339. https://doi.org/10.3390/coatings13020339









