High Performance and Reusable SAW Sensor Coated with Thiourea-Decorated POSS with Different Functional Groups for DMMP Detection
Abstract
:1. Introduction
2. Experiment
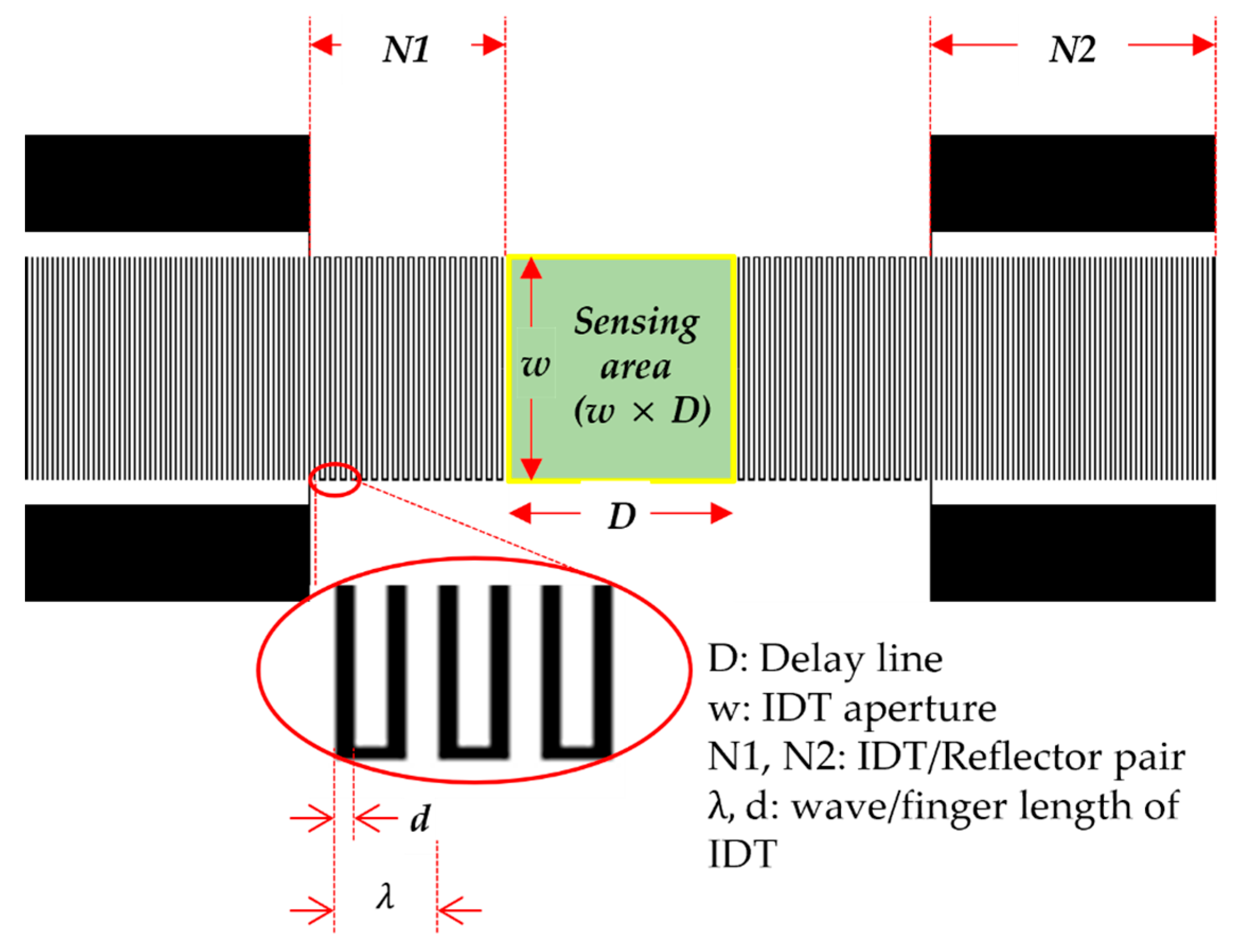
2.1. Design of SAW Sensor
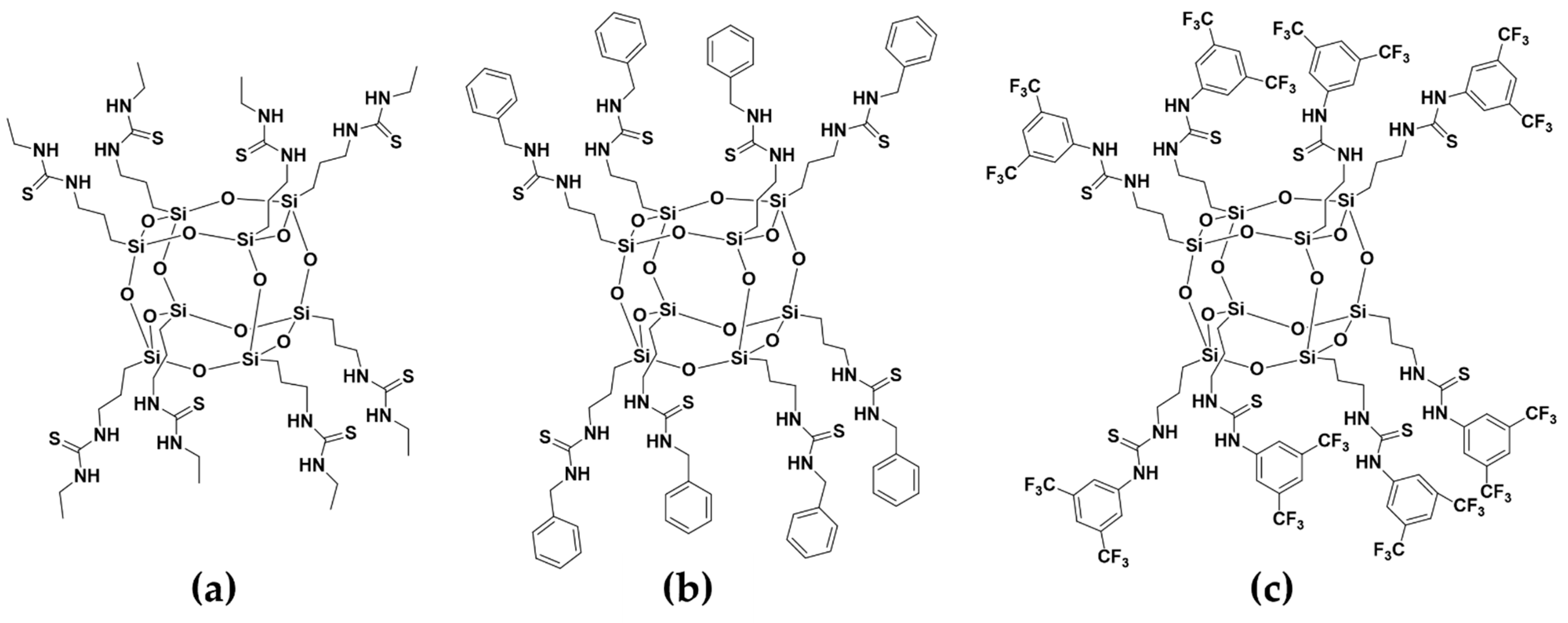
2.2. Sensing Material Synthesis and Coating
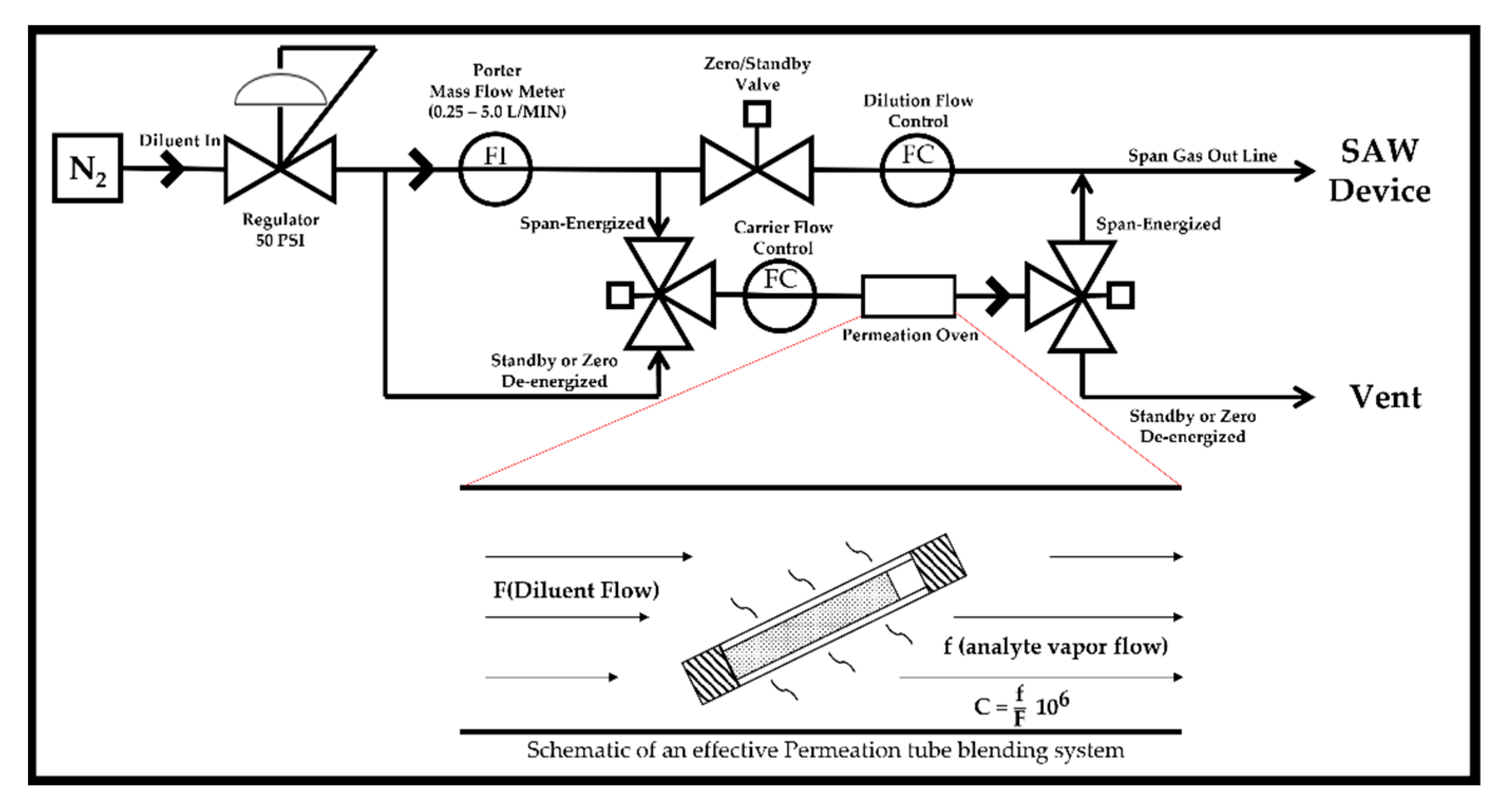
2.3. Experimental Setup
3. Results
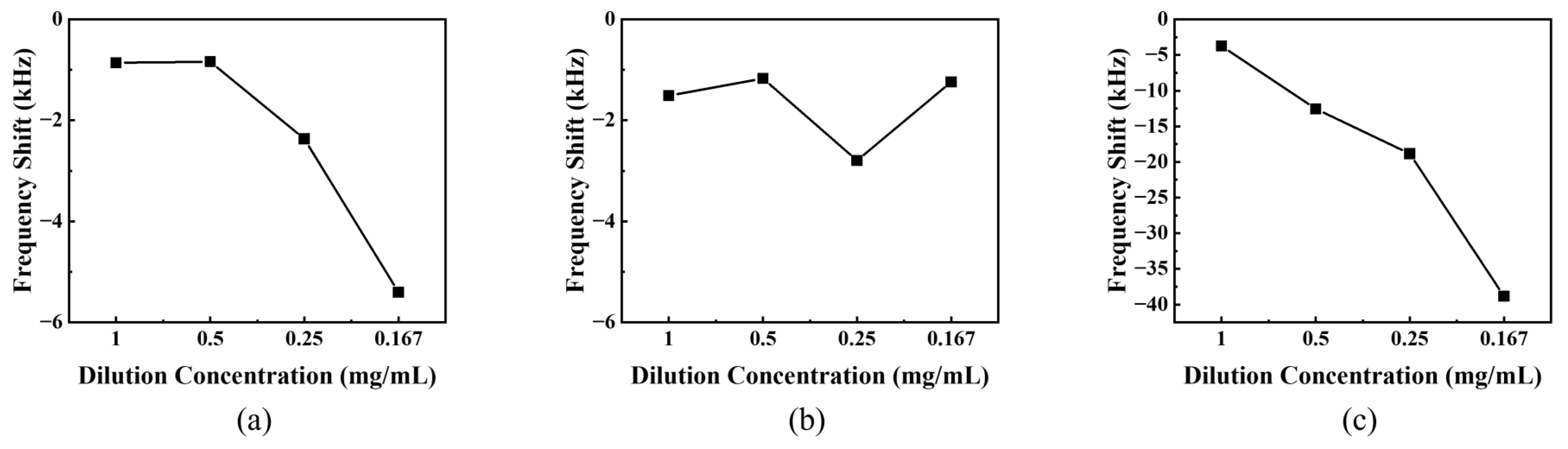
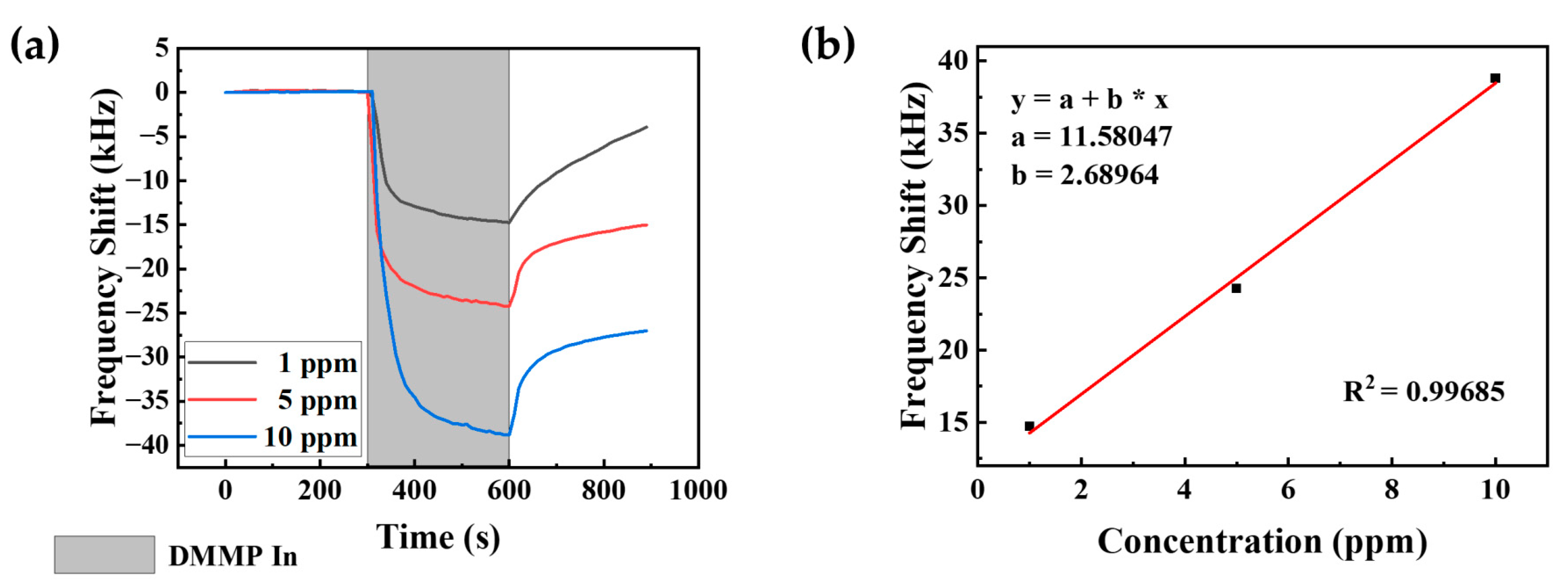
3.1. Sensitivity to DMMP Vapor Due to Coating Concentration
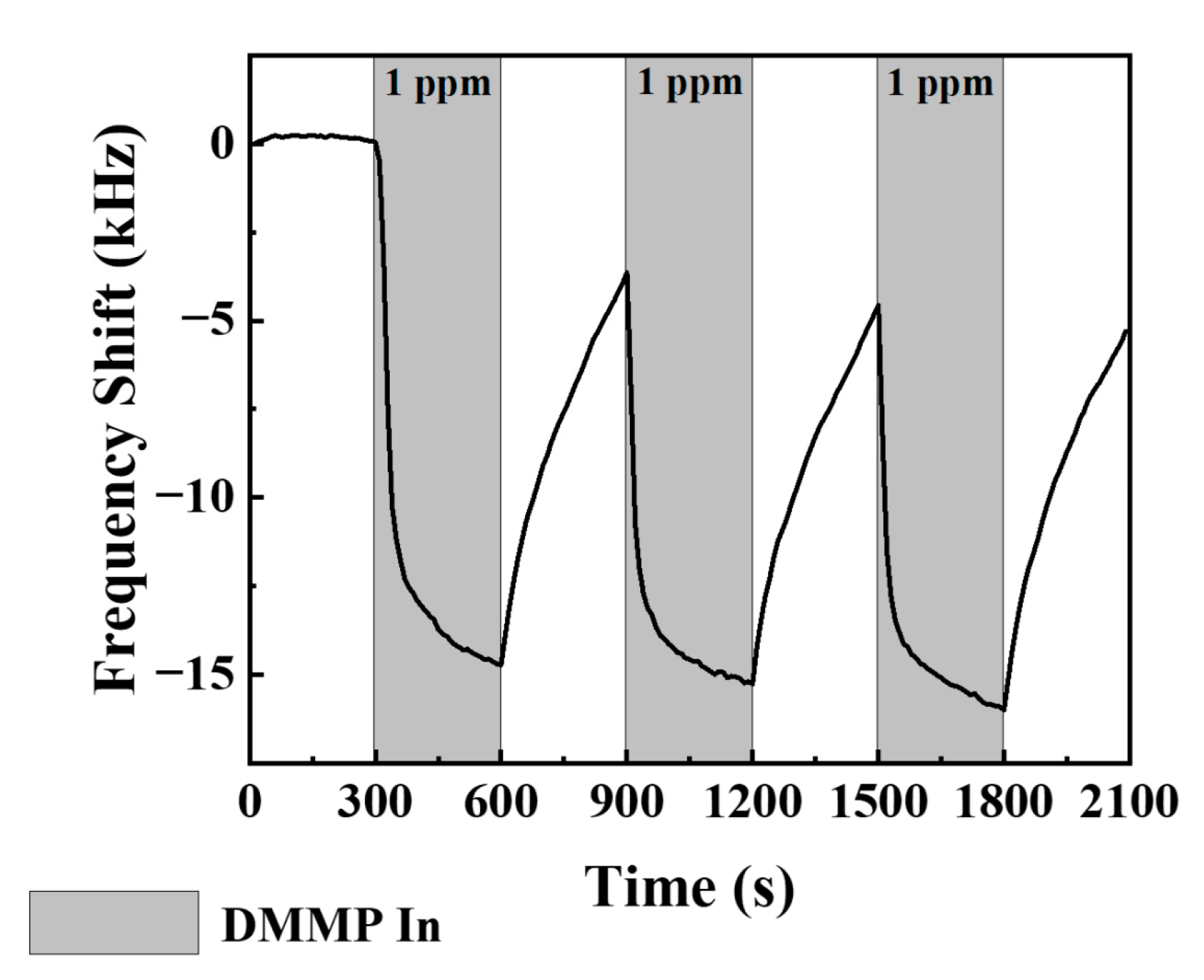
3.2. Sensitivity to Low Concentrations of DMMP Vapor
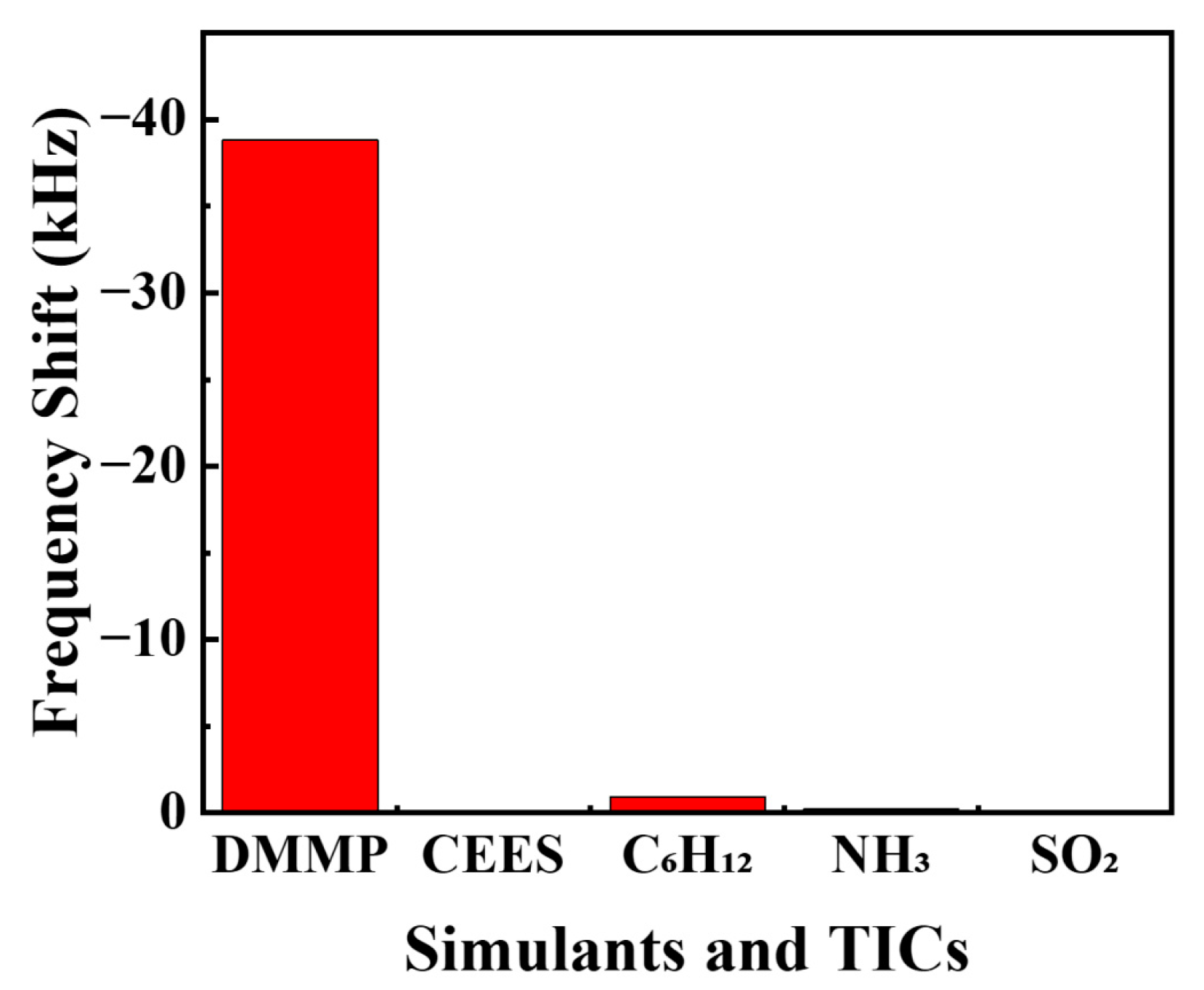
3.3. Response to Other CWAs Simulants and TICs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salem, H.; Sidell, F.R. Nerve agents. In Encyclopedia of Toxicology, 2nd ed.; Wexler, P., Ed.; Elsevier: London, UK, 2005; pp. 201–206. [Google Scholar] [CrossRef]
- Sferopoulos, R. A Review of Chemical Warfare Agent (CWA) Detector Technologies and Commercial-off-the-Shelf Items; Defence Science and Technology Organisation: Canberra, Australia, 2009; pp. 155–219. Available online: https://apps.dtic.mil/sti/citations/ADA502856 (accessed on 3 May 2022).
- Chauhan, S.; D’cruz, R.; Faruqi, S.; Singh, K.K.; Varma, S.; Singh, M.; Karthik, V. Chemical warfare agents. Environ. Toxicol. Pharmacol. 2008, 26, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Sidell, F.R.; Newmark, J.; McDonough, J.H. Chapter 5 Nerve agents. In Medical Aspects of Chemical Warfare; Office of The Surgeon General Department of the Army and US Army Medical Department Center and School: Falls Church, VA, USA, 1997; Volume 130, pp. 155–219. Available online: https://medcoe.army.mil/borden-tb-med-aspects-chem-warfare (accessed on 3 May 2022).
- Pacsial-Ong, E.J.; Aguilar, Z.P. Chemical warfare agent detection: A review of current trends and future perspective. Front. Biosci. -Sch. 2013, 5, 516–543. [Google Scholar] [CrossRef]
- McCafferty, R.R.; Lennarson, P.J. Common chemical agent threats. Neurosurg. Focus. 2002, 12, 1–4. [Google Scholar] [CrossRef]
- Satoh, T.; Kishi, S.; Nagashima, H.; Tachikawa, M.; Kanamori-Kataoka, M.; Nakagawa, T.; Kitagawa, N.; Tokita, K.; Yamamoto, S.; Seto, Y. Ion mobility spectrometric analysis of vaporous chemical warfare agents by the instrument with corona discharge ionization ammonia dopant ambient temperature operation. Anal. Chim. Acta 2015, 865, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Puton, J.; Namieśnik, J. Ion mobility spectrometry: Current status and application for chemical warfare agents detection. TrAC-Trends Anal. Chem. 2016, 85, 10–20. [Google Scholar] [CrossRef]
- Smith, P.; Lepage, C.J.; Koch, D.; Wyatt, H.; Hook, G.; Betsinger, G.; Erickson, R.; Eckenrode, B. Detection of gas-phase chemical warfare agents using field-portable gas chromatography-mass spectrometry systems: Instrument and sampling strategy considerations Trends. Analyt. Chem. 2004, 23, 296–306. [Google Scholar] [CrossRef]
- Contreras, J.; Murray, J.; Tolley, S.; Oliphant, J.; Tolley, H.; Lammert, S.; Lee, E.; Later, D.; Lee, M. Hand-portable gas chromatograph-toroidal ion trap mass spectrometer (GC-TMS) for detection of hazardous compounds. J. Am. Soc. Mass Spectrom. 2008, 19, 1425–1434. [Google Scholar] [CrossRef]
- Logan, T.; Allen, E.; Way, M.; Swift, A.; Soni, S.D.; Koplovitz, I. A method for the analysis of tabun in multisol using gas chromatographic flame photometric detection. Toxicol. Mech. Methods 2006, 16, 359–363. [Google Scholar] [CrossRef]
- Seto, Y.; Kanamori-Kataoka, M.; Tsuge, K.; Ohsawa, I.; Maruko, H.; Sekiguchi, H.; Sano, Y.; Yamashiro, S.; Matsushita, K.; Sekiguchi, H. Development of an on-site detection method for chemical and biological warfare agents. Toxin Rev. 2007, 26, 299–312. [Google Scholar] [CrossRef]
- Kirschner, J. Surface Acoustic Wave Sensors (SAWS). Micromech. Syst. 2010, 6, 1–11. [Google Scholar]
- Bielecki, M.; Witkiewicz, Z.; Rogala, P. Sensors to Detect Sarin Simulant. Crit. Rev. Anal. Chem. 2021, 51, 299–311. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Higgins, B.; Simonson, D.; Houser, E.; Kohl, J.; Andrew Mcgill, R. Synthesis and characterization of a hyperbranched hydrogen bond acidic carbosilane sorbent polymer. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 3000–3009. [Google Scholar] [CrossRef]
- Wang, Y.; Du, X.; Long, Y.; Tang, X.; Tai, H.; Jiang, Y. The response comparison of a hydrogen-bond acidic polymer to sarin, soman and dimethyl methyl phosphonate based on a surface acoustic wave sensor. Anal. Methods 2014, 6, 1951–1955. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, J.; Kim, J.; Yun, J.; Jang, W. Detection of Dimethyl Methylphosphonate (DMMP) Using Polyhedral Oilgomeric Silsesquioxane (POSS). J. Nanosci. Nanotechnol. 2018, 18, 6565–6569. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, H.; Kim, J.; Seo, B.; Kim, J. SAW Chemical Array Device Coated with Polymeric Sensing Materials for the Detection of Nerve Agents. Sensors 2020, 20, 7028. [Google Scholar] [CrossRef]
- Chung, Y.; Ha, S.; Woo, T.; Kim, Y.; Song, C.; Kim, S. Binding thiourea derivatives with dimethyl methylphosphonate for sensing nerve agents. RSC Adv. 2019, 9, 10693–10701. [Google Scholar] [CrossRef]
- Song, S.; Ha, S.; Cho, H.; Lee, M.; Jung, D.; Han, J.; Song, C. Single-walled carbon-nanotube-based chemocapacitive sensors with molecular receptors for selective detection of chemical warfare agents. ACS Appl. Nano Mater. 2018, 2, 109–117. [Google Scholar] [CrossRef]
- Drafts, B. Acoustic wave technology sensors. IEEE Trans. Microw. Theory Tech. 2001, 49, 795–802. [Google Scholar] [CrossRef]
- Devkota, J.; Ohodnicki, P.; Greve, D. SAW sensors for chemical vapors and gases. Sensors 2017, 17, 801. [Google Scholar] [CrossRef] [Green Version]
- Mujahid, A.; Dickert, F. Surface acoustic wave (SAW) for chemical sensing applications of recognition layers. Sensors 2017, 17, 2716. [Google Scholar] [CrossRef] [PubMed]
- Xiaofeng, X.; Xiaotao, Z.; Dongyi, A.; Jingxia, Y.; Jingxia, Y.; Xia, X.; Wanfeng, X.; Yongliang, T.; Sean, L.; Yongqing, F. NH3-Sensing Mechanism Using Surface Acoustic Wave Sensor with AlO(OH) Film. Nanomaterials 2019, 9, 1732. [Google Scholar] [CrossRef]
- Sheng, X.; Rui, Z.; Junpeng, C.; Tao, L.; Xiuli, S.; Meng, H.; Fu, Z.; Xiaoguang, H. Surface Acoustic Wave DMMP Gas Sensor with a Porous Graphene/PVDF Molecularly Imprinted Sensing Membrane. Micromachines 2021, 12, 552. [Google Scholar] [CrossRef]
- Bo, L.; Xiao, C.; Hualin, C.; Mohammad, M.A.; Xiangguang, T.; Luqi, T.; Yi, Y.; Tianling, R. Surface acoustic wave devices for sensor applications. J. Semicond. 2016, 37, 021001. [Google Scholar] [CrossRef]
- Go, D.; Atashbar, M.; Ramshani, Z.; Chang, H. Surface acoustic wave devices for chemical sensing and microfluidics: A review and perspective. Anal. Methods 2017, 9, 4112–4134. [Google Scholar] [CrossRef] [PubMed]
- Sayago, I.; Fernandez, M.; Fontecha, J.; Horrillo, M.; Terrado, E.; Seral-Ascaso, A.; Munoz, E. Carbon nanotube-based SAW sensors. In Proceedings of the 2013 Spanish Conference on Electron Devices, Valladolid, Spain, 12–14 February 2013; IEEE: New York, NY, USA, 2013; pp. 127–130. [Google Scholar] [CrossRef]
- Penza, M.; Antolini, F.; Vittori, M. Carbon nanotubes as SAW chemical sensors materials. Sens. Actuators B Chem. 2004, 100, 47–59. [Google Scholar] [CrossRef]
- Kumar, M.K.; Ramaprabhu, S. Nanostructured Pt functionlized multiwalled carbon nanotube-based hydrogen sensor. J. Phys. Chem. B 2006, 110, 11291–11298. [Google Scholar] [CrossRef] [PubMed]
- Joo, B.S.; Huh, J.S.; Lee, D.D. Fabrication of polymer SAW sensor array to classify chemical warfare agents. Sens. Actuators B Chem. 2007, 121, 47–53. [Google Scholar] [CrossRef]
- Hartmann-Thompson, C.; Hu, J.; Kaganove, S.; Keinath, S.; Keeley, D.; Dvornic, P. Hydrogen-bond acidic hyperbranched polymers for surface acoustic wave (SAW) sensors. Chem. Mater. 2004, 16, 5357–5364. [Google Scholar] [CrossRef]
- Le, X.; Liu, Y.; Peng, L.; Peng, J.; Xu, Z.; Gao, C.; Xie, J. Surface acoustic wave humidity sensors based on uniform and thickness controllable graphene oxide thin films formed by surface tension. Microsyst. Nanoeng. 2019, 5, 36. [Google Scholar] [CrossRef]
- Lama, S.; Kim, J.; Ramesh, S.; Lee, Y.; Kim, J.; Kim, J. Highly Sensitive Hybrid Nanostructures for Dimethyl Methyl Phosphonate Detection. Micromachines 2021, 12, 648. [Google Scholar] [CrossRef] [PubMed]
- Connelly, N.G.; William, E.G. Chemical redox agents for organometallic chemistry. Chem. Rev. 1996, 96, 877–910. [Google Scholar] [CrossRef] [PubMed]
- McKinley, J.; Majors, R. The preparation of calibration standards for volatile organic compounds—A question of traceability. Lc Gc N. Am. 2000, 18, 1024–1033. [Google Scholar]
- McKinley, J. Permeation Tubes: A Simple Path to very Complex Gas Mixtures. Available online: https://kin-tek.com/wp-content/uploads/2015/12/Gases-Instruments-Jan-2008.pdf (accessed on 3 May 2022).
- Matatagui, D.; Martí, J.; Fernández, M.; Fontecha, J.; Gutiérrez, J.; Gràcia, I.; Cané, C.; Horrillo, M. Chemical warfare agents simulants detection with an optimized SAW sensor array. Sens. Actuators B Chem. 2011, 154, 199–205. [Google Scholar] [CrossRef]
- Kim, J.; Kim, E.; Kim, J.; Kim, J.-H.; Ha, S.; Song, C.; Jang, W.J.; Yun, J. Four-Channel Monitoring System with Surface Acoustic Wave Sensors for Detection of Chemical Warfare Agents. J. Nanosci. Nanotechnol. 2020, 20, 7151–7157. [Google Scholar] [CrossRef]



| CWAs | Examples |
|---|---|
| Nerve agents | Sarin (GB), Soman (GD), Tabun (GA) |
| Blister agents | Mustard gas, Nitrogen mustard |
| Blood agents | Arsine, Cyanogen chloride, Hydrogen cyanide |
| Choking agents | Chlorine, Hydrogen chloride, Phosgene |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, B.-G.; Jang, H.-C.; Choi, H.-S.; Lee, Y.-J.; Kim, J.-H. High Performance and Reusable SAW Sensor Coated with Thiourea-Decorated POSS with Different Functional Groups for DMMP Detection. Coatings 2023, 13, 348. https://doi.org/10.3390/coatings13020348
Bae B-G, Jang H-C, Choi H-S, Lee Y-J, Kim J-H. High Performance and Reusable SAW Sensor Coated with Thiourea-Decorated POSS with Different Functional Groups for DMMP Detection. Coatings. 2023; 13(2):348. https://doi.org/10.3390/coatings13020348
Chicago/Turabian StyleBae, Bong-Gyu, Hee-Chan Jang, Hyeong-Seon Choi, Young-Jun Lee, and Joo-Hyung Kim. 2023. "High Performance and Reusable SAW Sensor Coated with Thiourea-Decorated POSS with Different Functional Groups for DMMP Detection" Coatings 13, no. 2: 348. https://doi.org/10.3390/coatings13020348




