Electrochemical Synthesis of Functional Coatings and Nanomaterials in Molten Salts and Their Application
Abstract
:1. Introduction
- electrolysis of melts using pulsed and reversible currents provides the ability to easily adjust the structure, thickness, porosity, roughness, grain size and texture of electroplated coatings and materials;
- a large number of environments for synthesis (both aqueous and non-aqueous) used for various purposes;
- electrodeposition parameters determined at a laboratory scale can be transferred to the industrial scale;
- high purity of the obtained coatings and materials even if low-quality initial reagents are used, since metals are refined in the process of electrolysis;
- low operating costs and low costs of electrochemical equipment.
2. Materials and Methods
3. Results and Discussions
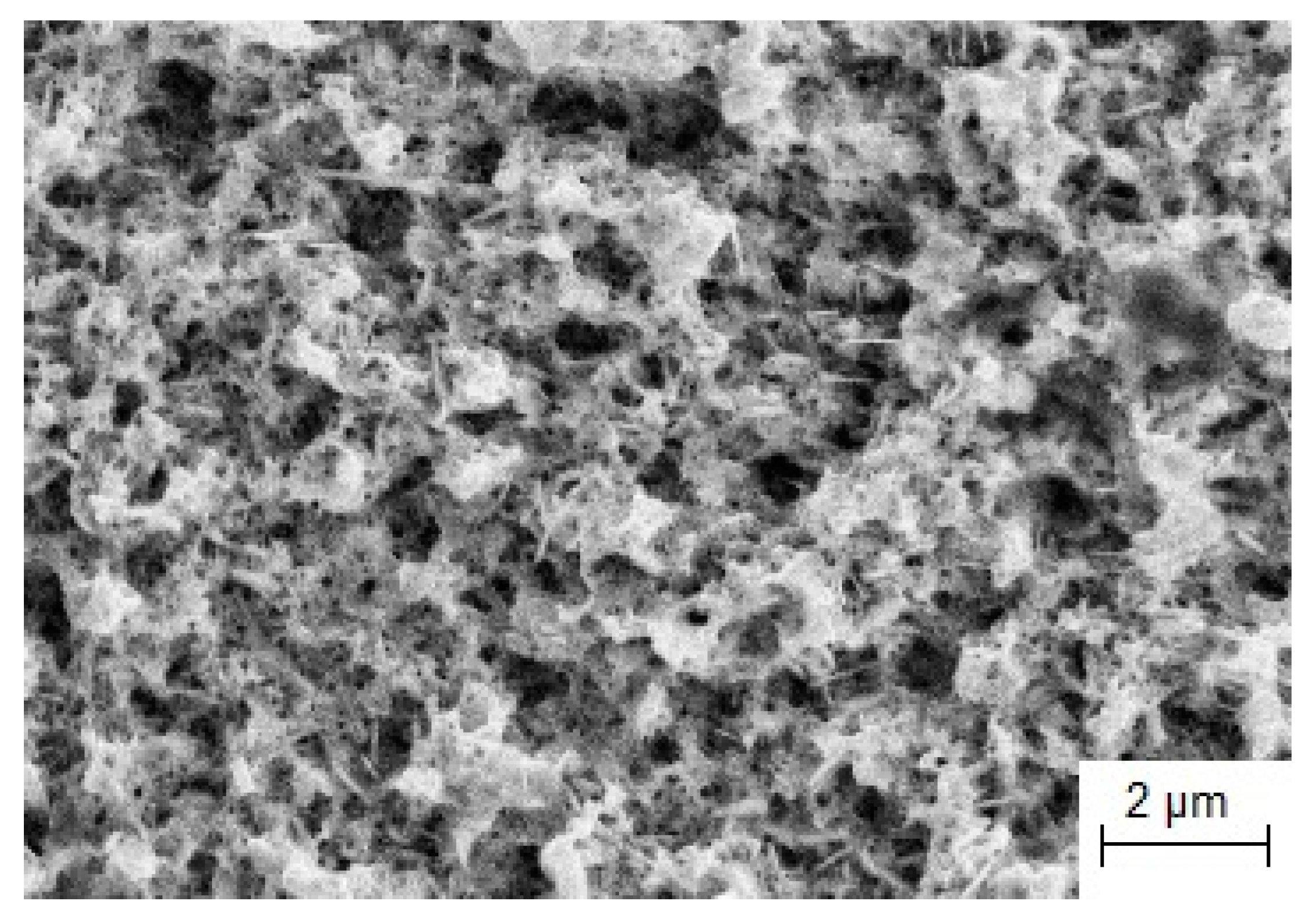
3.1. Nanostructured Catalytic Coatings Mo2C
3.1.1. Electrochemical Synthesis
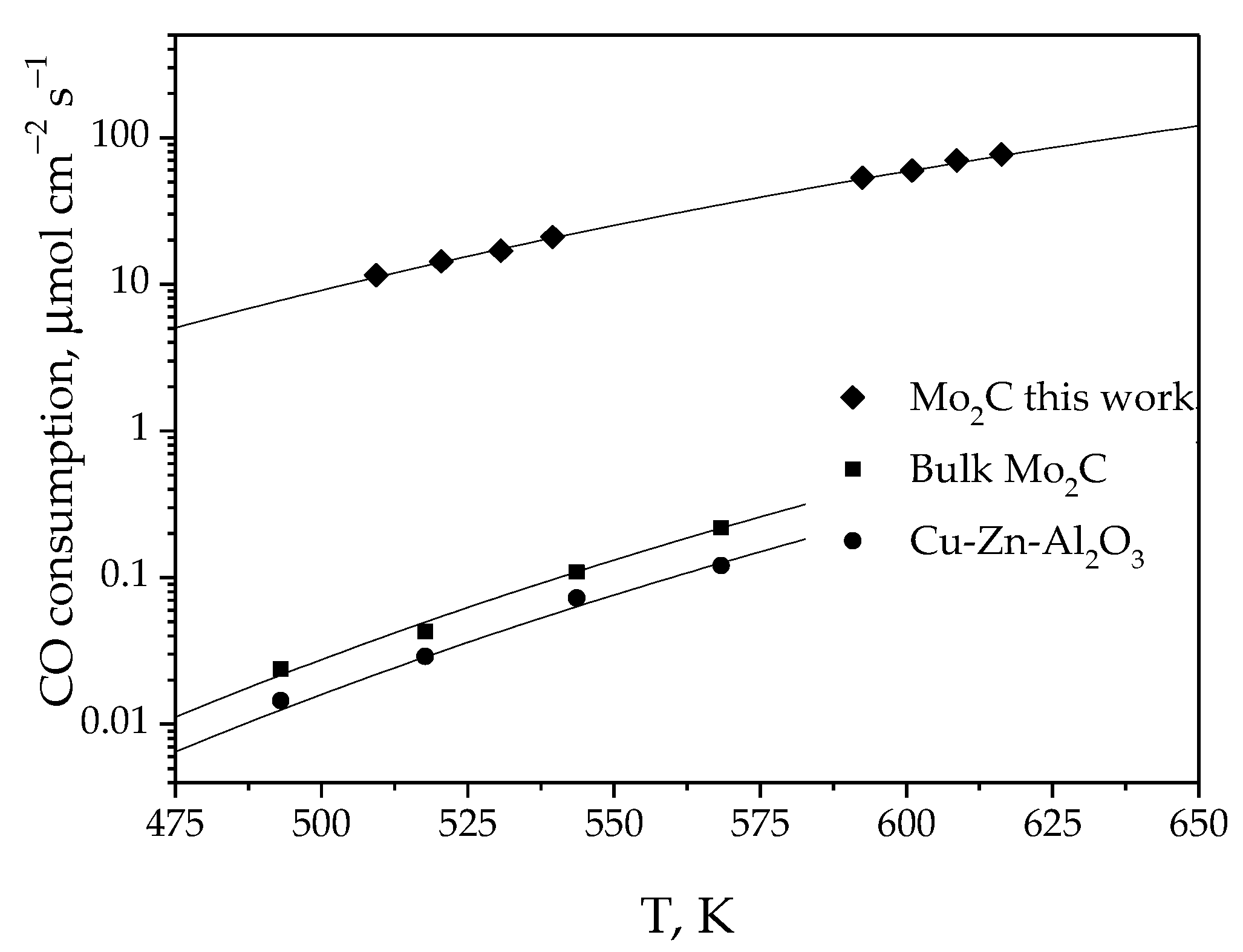
3.1.2. Catalytic Activity
3.2. Wear and Corrosion Resistence Coatings on Steels
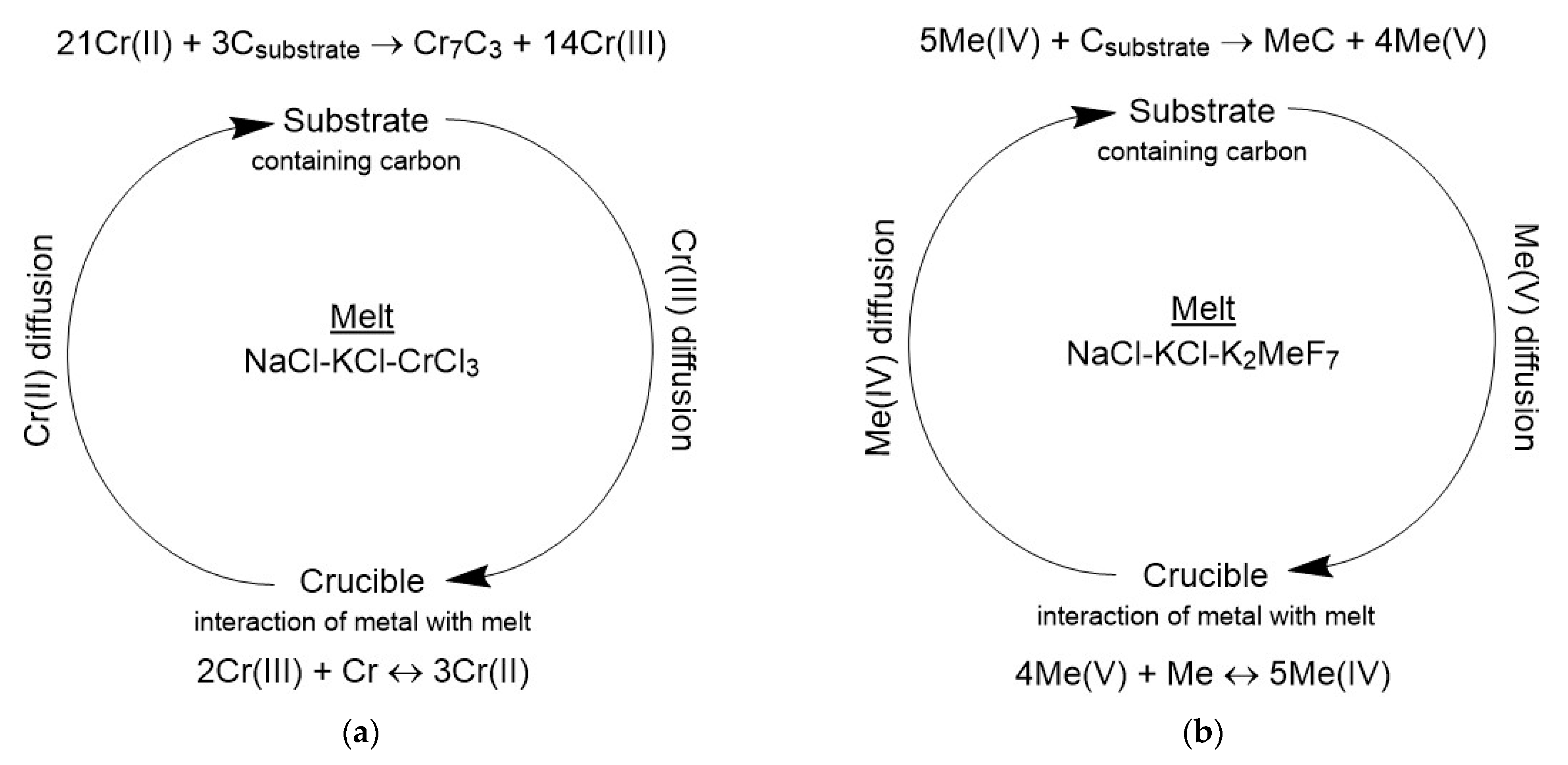
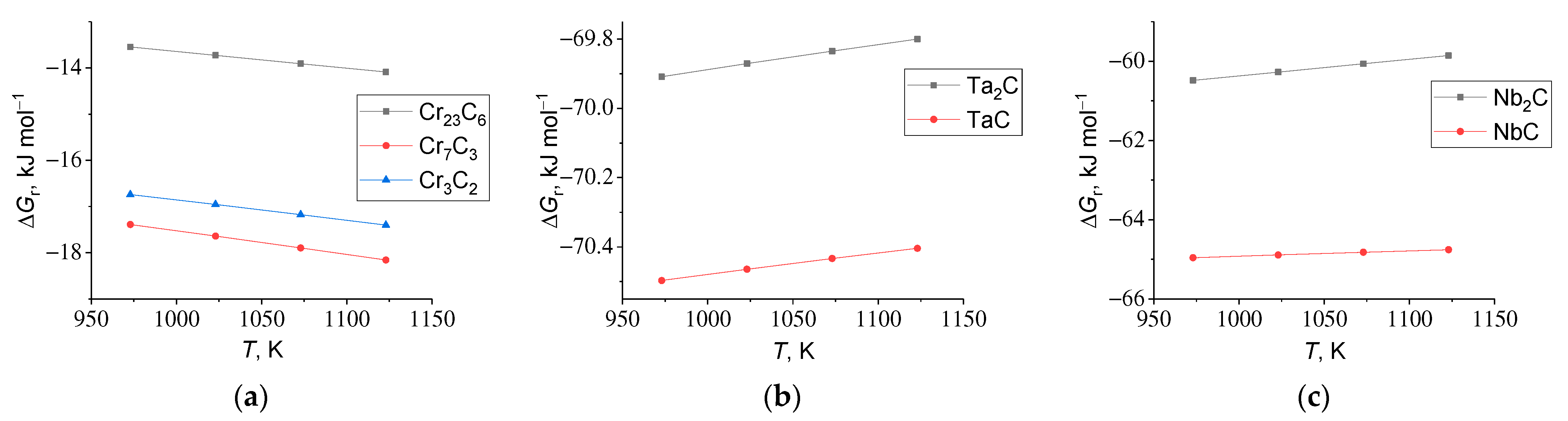
3.2.1. Currentless Transfer: A Simple Way to Obtain Refractory Carbide Coatings
3.2.2. Protective Properties of Refractory Carbide Coatings
3.3. Electrocatalytic Compositions Carbon Fiber/Refractory Metal Carbide
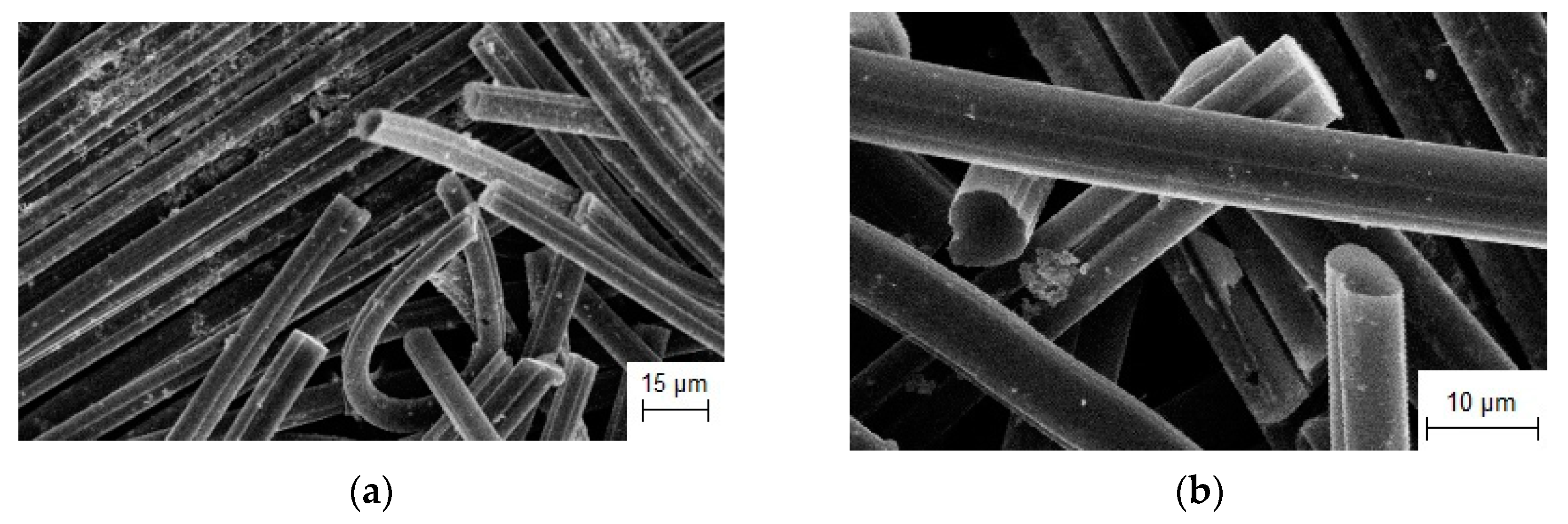
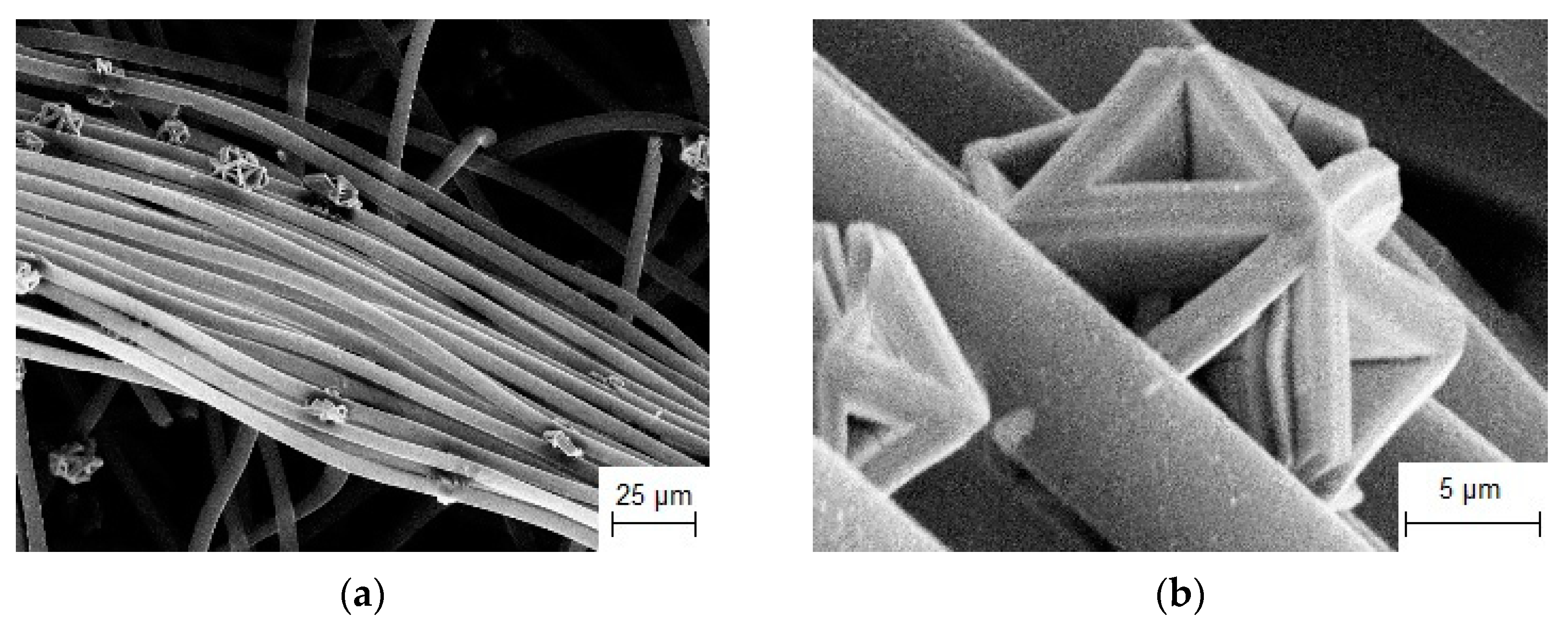
3.3.1. Synthesis of Refractory Metal Carbide Coatings on Carbon Fibers
3.3.2. Investigation of the Electrocatalytic Activity of Composites Based on Refractory Metal Carbide on Carbon Fiber
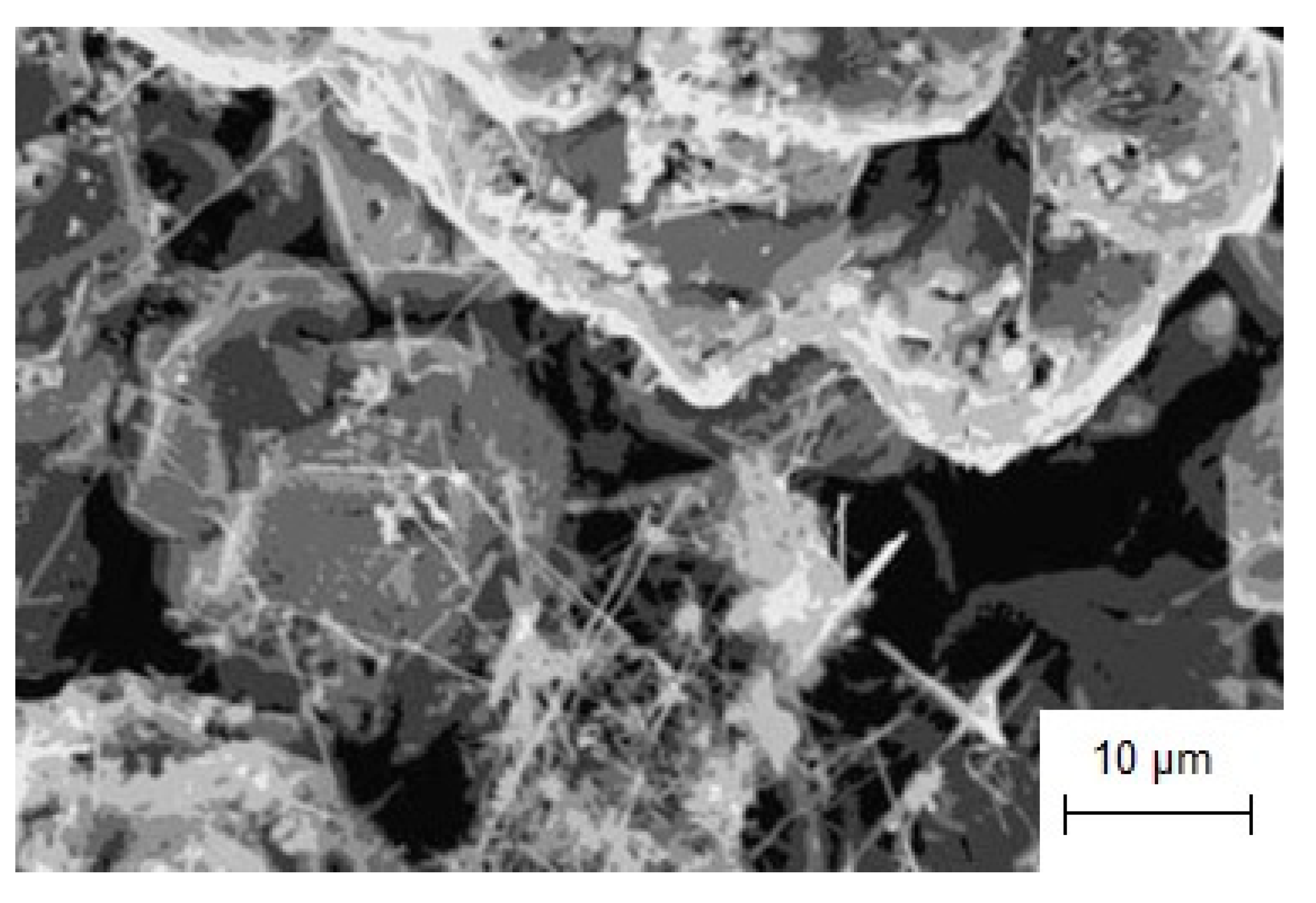
3.4. Electrochemical Synthesis of Gadolinium Borides Nanorods in Molten Salts
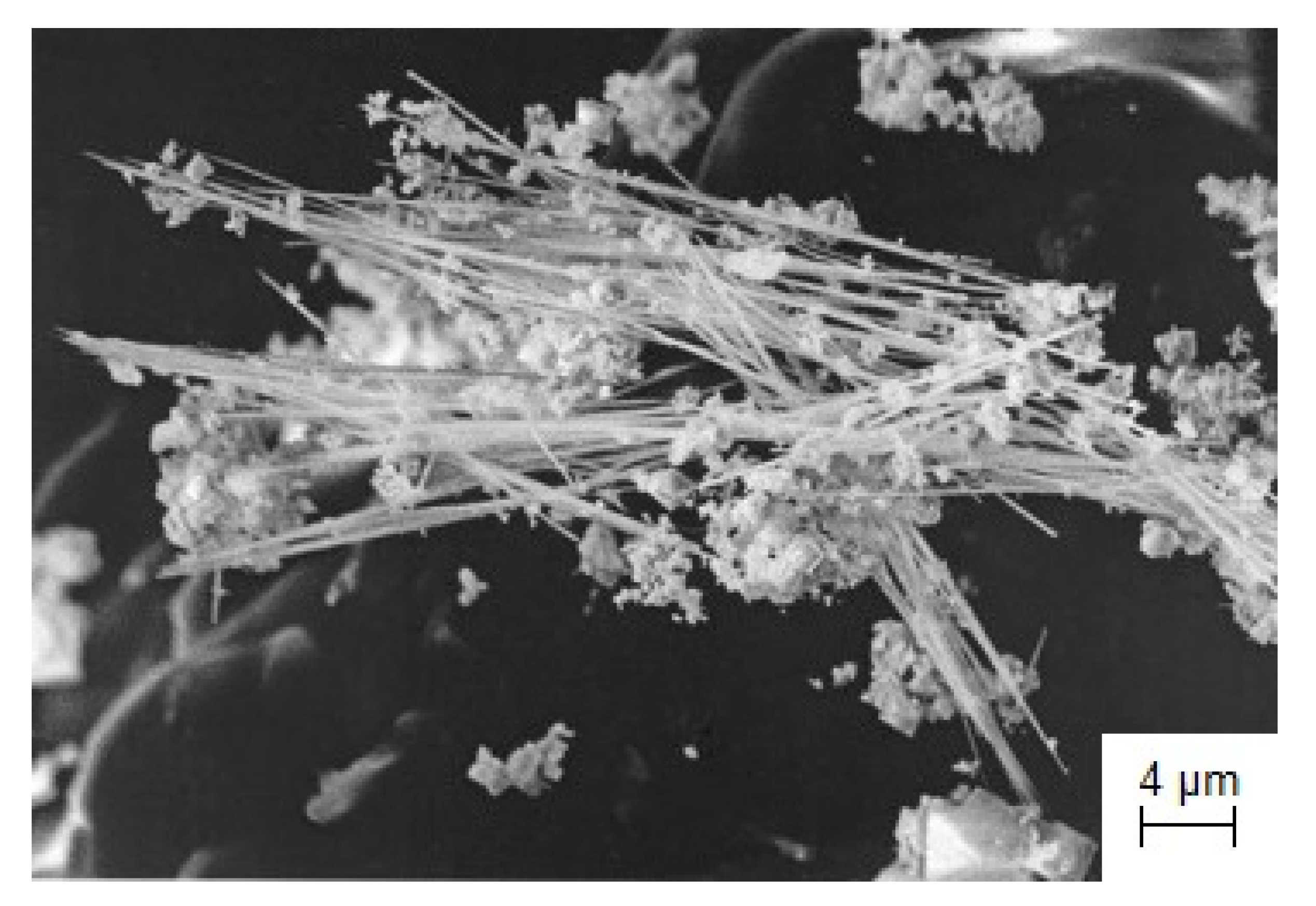
3.5. Synthesis of One-Dimensional Nanostructures: Si and TaO Nanoneedles
3.5.1. Electrochemical Synthesis of Si-Nanoneedles in Molten Salts
3.5.2. Obtaining of TaO Nanoneedles by Electroreduction of K3TaOF6 in Molten Salts
4. Conclusions
- By electrochemical synthesis in molten salts, a new Mo2C/Mo-based catalytic system for a low-temperature steam reforming reaction was obtained. The catalytic activity of this composition, produced by the simultaneous reduction of electroactive MoO42− and CO32− species, was three orders of magnitude higher than that of the bulk Mo2C phase and commercial Cu-ZnO-Al2O3 catalyst.
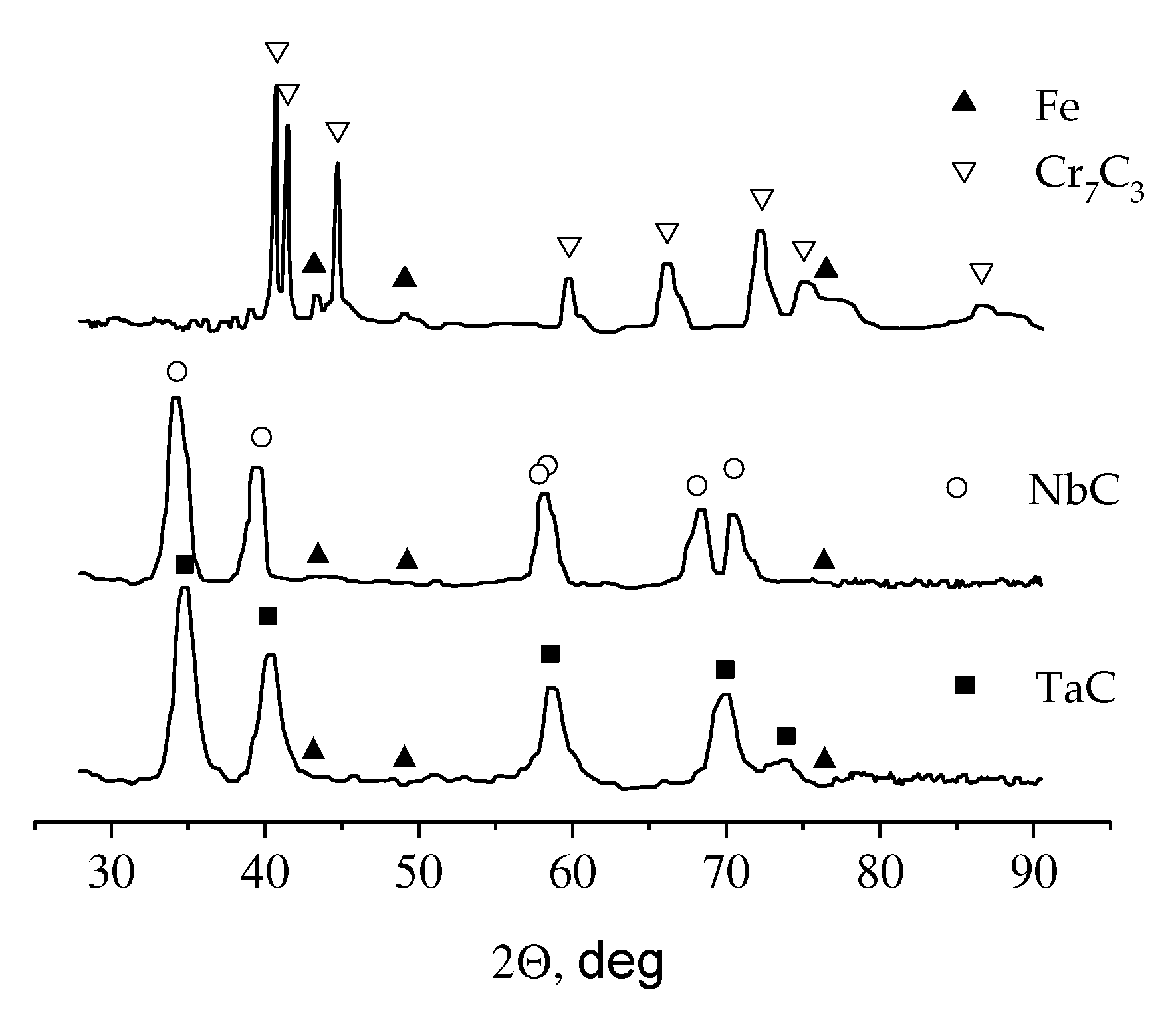
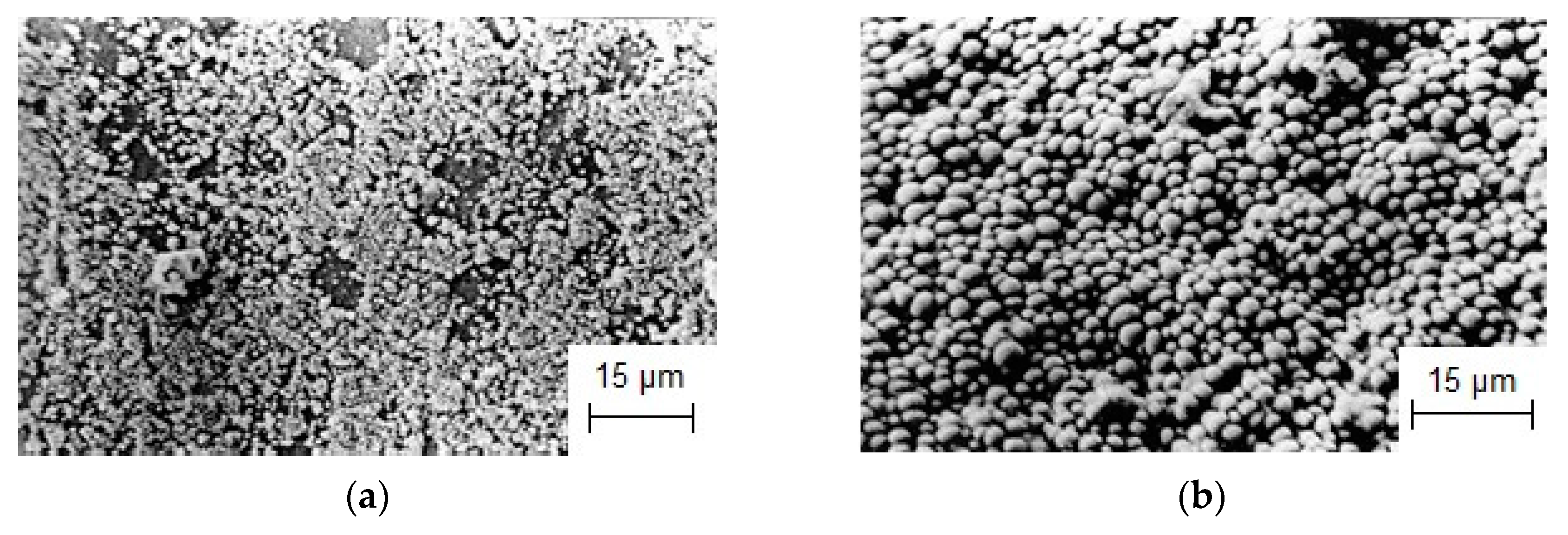
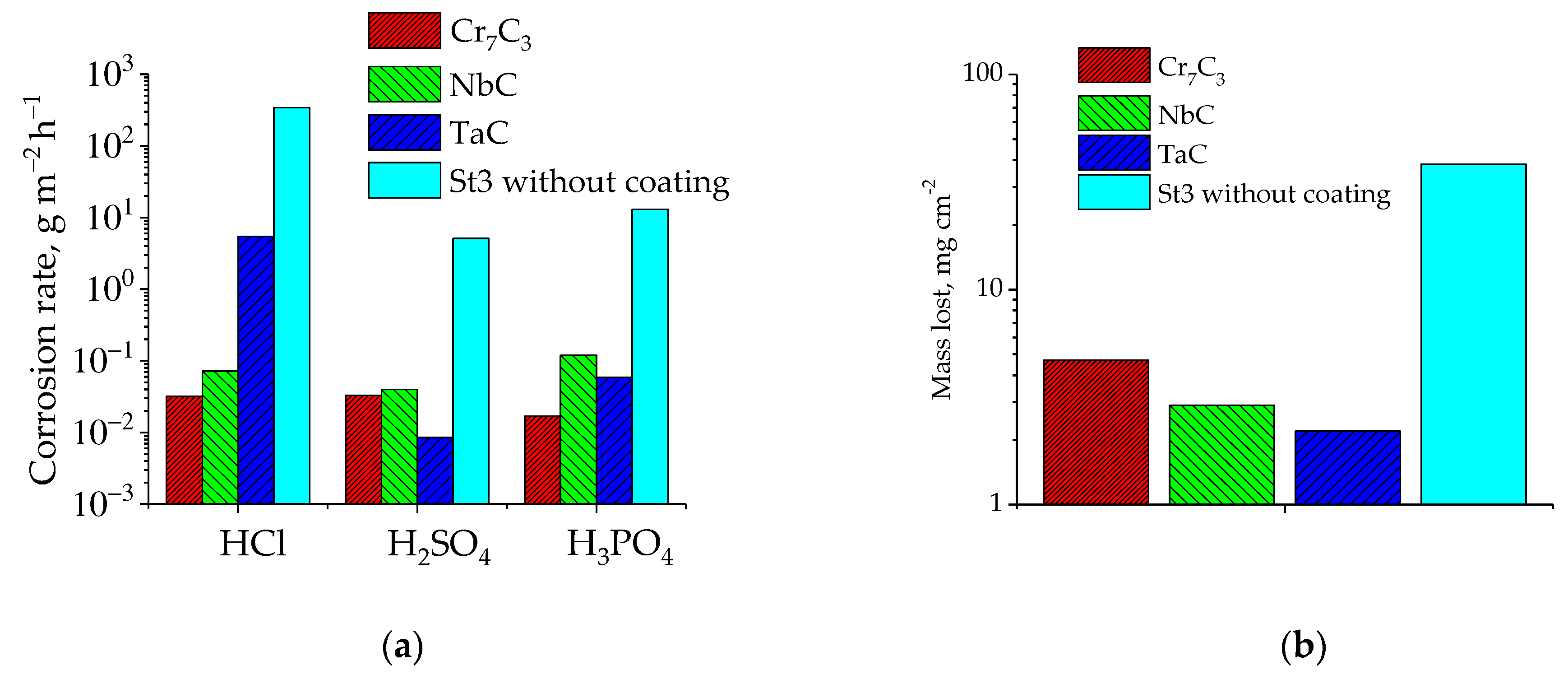
- The currentless transfer for the synthesis of nanoscale coatings of carbide refractory metals on substrates containing carbon was studied. It was shown that these coatings on steels increase corrosion resistance by several orders of magnitude and increase wear resistance by 3–5 times. Tests carried out by industrial facilities showed that the coatings of Cr7C3 or TaC on rubber-cutting knifes made of St3 can improve their wear resistance and increase a tool lifetime by 2.0 (for Cr7C3) and 2.5 (for TaC) times.
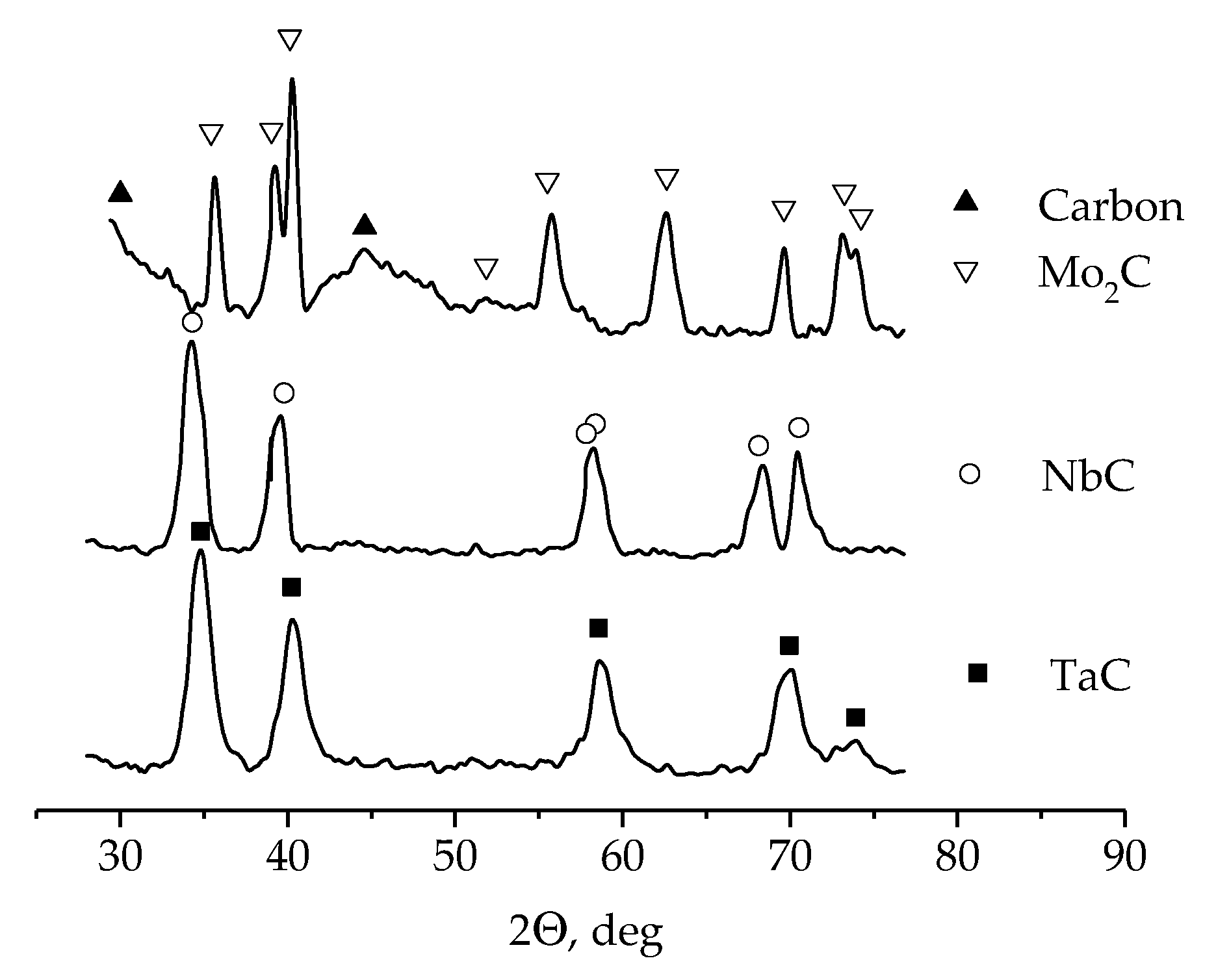
- It was found that NbC, TaC, and Mo2C carbides deposited on carbon fibers by currentless transfer in molten salts can be used as highly active electrocatalysts for hydrogen peroxide decomposition. The kinetics of the electrocatalytic decomposition of H2O2 were studied and the following series of electrocatalytic activity was established: Mo2C < TaC < Pt < Cu < NbC.
- Using potentiostatic electrolysis, GdB6 nanorods for different applications were synthesized in the KCl-NaCl-NaF(10 wt.%)-GdF3-KBF4 melt.
- The synthesis of one-dimensional nanomaterials based on Si and TaO for application in modern electronic devices was discussed. Silicon nanoneedles were synthesized by potentiostatic electrolysis in the NaCl–KCl–NaF(10 wt.%)-K2SiF6 melt. The possibility to synthesize TaO using the CsCl-K3TaOF6 melt was shown. TaO crystallized at the cathode as nanoneedles, together with other tantalum compounds. It was found that TaO can be obtained by the electrolysis of molten salts only at temperatures below 1173 K.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gleiter, H. Nanostructured Materials: Basic Concepts and Microstructure. Acta Mater. 2000, 48, 1–29. [Google Scholar] [CrossRef]
- Nazar, L.F.; Goward, G.; Leroux, F.; Duncan, M.; Huang, H.; Kerr, T.; Gaubicher, J. Nanostructured Materials for Energy Storage. Int. J. Inorg. Mater. 2001, 3, 191–200. [Google Scholar] [CrossRef]
- Hone, F.G.; Tegegne, N.A.; Andoshe, D.M. Advanced Materials for Energy Storage Devices. In Electrode Materials for Energy Storage and Conversion; CRC Press: Boca Raton, FL, USA, 2021; pp. 71–107. [Google Scholar] [CrossRef]
- Kebede, M.A.; Ezema, F.I. Electrode Materials for Energy Storage and Conversion. In Electrode Materials for Energy Storage and Conversion; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar] [CrossRef]
- Aoki, Y.; Tominaga, H.; Nagai, M. Hydrogenation of CO on Molybdenum and Cobalt Molybdenum Carbide Catalysts—Mass and Infrared Spectroscopy Studies. Catal. Today 2013, 215, 169–175. [Google Scholar] [CrossRef]
- Ren, H.; Yu, W.; Salciccioli, M.; Chen, Y.; Huang, Y.; Xiong, K.; Vlachos, D.G.; Chen, J.G. Selective Hydrodeoxygenation of Biomass-Derived Oxygenates to Unsaturated Hydrocarbons Using Molybdenum Carbide Catalysts. ChemSusChem 2013, 6, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, N.M.; Schaidle, J.A.; Ezekoye, O.K.; Pan, X.; Linic, S.; Thompson, L.T. High Activity Carbide Supported Catalysts for Water Gas Shift. J. Am. Chem. Soc. 2011, 133, 2378–2381. [Google Scholar] [CrossRef]
- Malyshev, V.; Uskova, N.; Shakhnin, D.; Lukashenko, T.; Antsibor, V.; Ustundag, Z. High-Temperature Electrochemical Synthesis of Nanostructured Coatings of Molybdenum (Tungsten)–Nickel (Cobalt) Alloys and Intermetallic Compounds. In Selected Proceedings of the 5th International Conference Nanotechnology and Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2018; pp. 165–176. ISBN 9783319925660. [Google Scholar]
- Zhang, W.; Hu, Y.; Ma, L.; Zhu, G.; Wang, Y.; Xue, X.; Chen, R.; Yang, S.; Jin, Z. Progress and Perspective of Electrocatalytic CO2 Reduction for Renewable Carbonaceous Fuels and Chemicals. Adv. Sci. 2018, 5, 1700275. [Google Scholar] [CrossRef]
- Hanus, M.J.; Harris, A.T. Nanotechnology Innovations for the Construction Industry. Prog. Mater. Sci. 2013, 58, 1056–1102. [Google Scholar] [CrossRef]
- Zhai, W.; Srikanth, N.; Kong, L.B.; Zhou, K. Carbon Nanomaterials in Tribology. Carbon N. Y. 2017, 119, 150–171. [Google Scholar] [CrossRef]
- Pottathara, Y.B.; Thomas, S.; Kalarikkal, N.; Grohens, Y.; Kokol, V. Nanomaterials Synthesis; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128157510. [Google Scholar]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Chen, J.S.; Lou, X.W. SnO2-Based Nanomaterials: Synthesis and Application in Lithium-Ion Batteries. Small 2013, 9, 1877–1893. [Google Scholar] [CrossRef]
- Kolahalam, L.A.; Kasi Viswanath, I.V.; Diwakar, B.S.; Govindh, B.; Reddy, V.; Murthy, Y.L.N. Review on Nanomaterials: Synthesis and Applications. Mater. Today Proc. 2019, 18, 2182–2190. [Google Scholar] [CrossRef]
- Sriondee, M.; Dungsuwan, W.; Thountom, S. Synthesis and Characterization of Bi0.5(Na1-XKx)0.5TiO3 Powders by Sol–Gel Combustion Method with Glycine Fuel. Ceram. Int. 2018, 44, S168–S171. [Google Scholar] [CrossRef]
- Hao, S.; Lin, T.; Ning, S.; Qi, Y.; Deng, Z.; Wang, Y. Research on Cracking of SiO2 Nanofilms Prepared by the Sol-Gel Method. Mater. Sci. Semicond. Process. 2019, 91, 181–187. [Google Scholar] [CrossRef]
- Kasi Viswanath, I.V.; Murthy, Y.L.N.; Tata, K.R.; Singh, R. Synthesis and Characterization of Nano Ferrites by Citrate Gel Method. Int. J. Chem. Sci. 2013, 11, 64–72. [Google Scholar]
- Ikesue, A.; Kinoshita, T.; Kamata, K.; Yoshida, K. Fabrication and Optical Properties of High-Performance Polycrystalline Nd:YAG Ceramics for Solid-State Lasers. J. Am. Ceram. Soc. 1995, 78, 1033–1040. [Google Scholar] [CrossRef]
- Yu, S.; Jing, W.; Tang, M.; Xu, T.; Yin, W.; Kang, B. Fabrication of Nd:YAG Transparent Ceramics Using Powders Synthesized by Citrate Sol-Gel Method. J. Alloys Compd. 2019, 772, 751–759. [Google Scholar] [CrossRef]
- Holland, B.T.; Blanford, C.F.; Do, T.; Stein, A. Synthesis of Highly Ordered, Three-Dimensional, Macroporous Structures of Amorphous or Crystalline Inorganic Oxides, Phosphates, and Hybrid Composites. Chem. Mater. 1999, 11, 795–805. [Google Scholar] [CrossRef]
- Diwald, O.; Thompson, T.L.; Zubkov, T.; Goralski, E.G.; Walck, S.D.; Yates, J.T. Photochemical Activity of Nitrogen-Doped Rutile TiO2(110) in Visible Light. J. Phys. Chem. B 2004, 108, 6004–6008. [Google Scholar] [CrossRef]
- Sasaki, T.; Watanabe, M.; Hashizume, H.; Yamada, H.; Nakazawa, H. Macromolecule-like Aspects for a Colloidal Suspension of an Exfoliated Titanate. Pairwise Association of Nanosheets and Dynamic Reassembling Process Initiated from It. J. Am. Chem. Soc. 1996, 118, 8329–8335. [Google Scholar] [CrossRef]
- Schlüter, M.; Hentzel, T.; Suarez, C.; Koch, M.; Lorenz, W.G.; Böhm, L.; Düring, R.A.; Koinig, K.A.; Bunge, M. Synthesis of Novel Palladium(0) Nanocatalysts by Microorganisms from Heavy-Metal-Influenced High-Alpine Sites for Dehalogenation of Polychlorinated Dioxins. Chemosphere 2014, 117, 462–470. [Google Scholar] [CrossRef]
- Anderson, S.H.; Chung, D.D.L. Exfoliation of Single Crystal Graphite and Graphite Fibers Intercalated with Halogens. Synth. Met. 1983, 8, 343–349. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, V.; Sharma, S.K.; Sachdev, K. Gas Sensing Behaviour of Green Synthesized Reduced Graphene Oxide (RGO) for H2 and NO. Mater. Lett. 2019, 236, 444–447. [Google Scholar] [CrossRef]
- Kadam, R.H.; Desai, K.; Shinde, V.S.; Hashim, M.; Shirsath, S.E. Influence of Gd3+ Ion Substitution on the MnCrFeO4 for Their Nanoparticle Shape Formation and Magnetic Properties. J. Alloys Compd. 2016, 657, 487–494. [Google Scholar] [CrossRef]
- Shi, Y.; Li, H.; Li, L.J. Recent Advances in Controlled Synthesis of Two-Dimensional Transition Metal Dichalcogenides via Vapour Deposition Techniques. Chem. Soc. Rev. 2015, 44, 2744–2756. [Google Scholar] [CrossRef] [PubMed]
- Ismach, A.; Druzgalski, C.; Penwell, S.; Schwartzberg, A.; Zheng, M.; Javey, A.; Bokor, J.; Zhang, Y. Direct Chemical Vapor Deposition of Graphene on Dielectric Surfaces. Nano Lett. 2010, 10, 1542–1548. [Google Scholar] [CrossRef]
- Kim, K.K.; Hsu, A.; Jia, X.; Kim, S.M.; Shi, Y.; Hofmann, M.; Nezich, D.; Rodriguez-Nieva, J.F.; Dresselhaus, M.; Palacios, T.; et al. Synthesis of Monolayer Hexagonal Boron Nitride on Cu Foil Using Chemical Vapor Deposition. Nano Lett. 2012, 12, 161–166. [Google Scholar] [CrossRef]
- Saito, T.; Chiba, H.; Ito, T.; Ogino, T. Growth of Carbon Hybrid Materials by Grafting on Pre-Grown Carbon Nanotube Surfaces. Carbon N. Y. 2010, 48, 1305–1311. [Google Scholar] [CrossRef]
- Xie, W.; Pang, Z.; Fan, J.; Song, H.; Jiang, F.; Yuan, H.; Li, J.; Ji, Z.; Han, S. Structural Properties of Alq3 Nanocrystals Prepared by Physical Vapor Deposition and Facile Solution Method. Int. J. Mod. Phys. B 2015, 29, 1542042. [Google Scholar] [CrossRef]
- Maleki, A. Green Oxidation Protocol: Selective Conversions of Alcohols and Alkenes to Aldehydes, Ketones and Epoxides by Using a New Multiwall Carbon Nanotube-Based Hybrid Nanocatalyst via Ultrasound Irradiation. Ultrason. Sonochem. 2018, 40, 460–464. [Google Scholar] [CrossRef]
- Xu, H.; Suslick, K.S. Sonochemical Synthesis of Highly Fluorescent Ag Nanoclusters. ACS Nano 2010, 4, 3209–3214. [Google Scholar] [CrossRef] [PubMed]
- Morel, A.L.; Nikitenko, S.I.; Gionnet, K.; Wattiaux, A.; Lai-Kee-Him, J.; Labrugere, C.; Chevalier, B.; Deleris, G.; Petibois, C.; Brisson, A.; et al. Sonochemical Approach to the Synthesis of Fe3O4 @SiO2 Core—Shell Nanoparticles with Tunable Properties. ACS Nano 2008, 2, 847–856. [Google Scholar] [CrossRef]
- Gedanken, A. Using Sonochemistry for the Fabrication of Nanomaterials. Ultrason. Sonochem. 2004, 11, 47–55. [Google Scholar] [CrossRef]
- Bang, J.H.; Suslick, K.S. Applications of Ultrasound to the Synthesis of Nanostructured Materials. Adv. Mater. 2010, 22, 1039–1059. [Google Scholar] [CrossRef]
- Zuo, P.; Lu, X.; Sun, Z.; Guo, Y.; He, H. A Review on Syntheses, Properties, Characterization and Bioanalytical Applications of Fluorescent Carbon Dots. Microchim. Acta 2016, 183, 519–542. [Google Scholar] [CrossRef]
- Gawande, M.B.; Shelke, S.N.; Zboril, R.; Varma, R.S. Microwave-Assisted Chemistry: Synthetic Applications for Rapid Assembly of Nanomaterials and Organics. Acc. Chem. Res. 2014, 47, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Baghbanzadeh, M.; Carbone, L.; Cozzoli, P.D.; Kappe, C.O. Microwave-Assisted Synthesis of Colloidal Inorganic Nanocrystals. Angew. Chem.-Int. Ed. 2011, 50, 11312–11359. [Google Scholar] [CrossRef]
- Bilecka, I.; Niederberger, M. Microwave Chemistry for Inorganic Nanomaterials Synthesis. Nanoscale 2010, 2, 1358–1374. [Google Scholar] [CrossRef] [PubMed]
- Ustarroz, J.; Hammons, J.A.; Altantzis, T.; Hubin, A.; Bals, S.; Terryn, H. A Generalized Electrochemical Aggregative Growth Mechanism. J. Am. Chem. Soc. 2013, 135, 11550–11561. [Google Scholar] [CrossRef]
- Yin, Y.; Jin, Z.; Hou, F. Enhanced Solar Water-Splitting Efficiency Using Core/Sheath Heterostructure CdS/TiO2 Nanotube Arrays. Nanotechnology 2007, 18, 495608. [Google Scholar] [CrossRef]
- Zhu, T.; Chong, M.N.; Chan, E.S. Nanostructured Tungsten Trioxide Thin Films Synthesized for Photoelectrocatalytic Water Oxidation: A Review. ChemSusChem 2014, 7, 2974–2997. [Google Scholar] [CrossRef] [PubMed]
- Mudring, A.V.; Tang, S. Ionic Liquids for Lanthanide and Actinide Chemistry. Eur. J. Inorg. Chem. 2010, 2010, 2569–2581. [Google Scholar] [CrossRef]
- Chou, S.; Cheng, F.; Chen, J. Electrodeposition Synthesis and Electrochemical Properties of Nanostructured γ-MnO2 Films. J. Power Sources 2006, 162, 727–734. [Google Scholar] [CrossRef]
- Logeeswaran, V.J.; Kobayashi, N.P.; Islam, M.S.; Wu, W.; Chaturvedi, P.; Fang, N.X.; Wang, S.Y.; Williams, R.S. Ultrasmooth Silver Thin Films Deposited with a Germanium Nucleation Layer. Nano Lett. 2009, 9, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Tepavcevic, S.; Xiong, H.; Stamenkovic, V.R.; Zuo, X.; Balasubramanian, M.; Prakapenka, V.B.; Johnson, C.S.; Rajh, T. Nanostructured Bilayered Vanadium Oxide Electrodes for Rechargeable Sodium-Ion Batteries. ACS Nano 2012, 6, 530–538. [Google Scholar] [CrossRef]
- Abo-Hamad, A.; Hayyan, M.; AlSaadi, M.A.H.; Hashim, M.A. Potential Applications of Deep Eutectic Solvents in Nanotechnology. Chem. Eng. J. 2015, 273, 551–567. [Google Scholar] [CrossRef]
- Shiddiky, M.J.A.; Torriero, A.A.J. Application of Ionic Liquids in Electrochemical Sensing Systems. Biosens. Bioelectron. 2011, 26, 1775–1787. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, A. Nanostructured Materials in Electrochemistry; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Krishtalik, L.I. Electrode Reactions. Mechanism of Elementary Act; Nauka: Moscow, Russia, 1982. [Google Scholar]
- Islam, M.M.; Abdellaoui, I.; Moslah, C.; Sakurai, T.; Ksibi, M.; Hamzaoui, S.; Akimoto, K. Electrodeposition and Characterization of Silicon Films Obtained through Electrochemical Reduction of SiO2 Nanoparticles. Thin Solid Film. 2018, 654, 1–10. [Google Scholar] [CrossRef]
- Zou, X.; Ji, L.; Ge, J.; Sadoway, D.R.; Yu, E.T.; Bard, A.J. Electrodeposition of Crystalline Silicon Films from Silicon Dioxide for Low-Cost Photovoltaic Applications. Nat. Commun. 2019, 10, 5772. [Google Scholar] [CrossRef]
- Weng, W.; Xiao, W. Electrodeposited Silicon Nanowires from Silica Dissolved in Molten Salts as a Binder-Free Anode for Lithium-Ion Batteries. ACS Appl. Energy Mater. 2019, 2, 804–813. [Google Scholar] [CrossRef]
- Laptev, M.V.; Isakov, A.V.; Grishenkova, O.V.; Vorob’ev, A.S.; Khudorozhkova, A.O.; Akashev, L.A.; Zaikov, Y.P. Electrodeposition of Thin Silicon Films from the KF-KCl-KI-K2SiF6 Melt. J. Electrochem. Soc. 2020, 167, 042506. [Google Scholar] [CrossRef]
- Zou, X.; Ji, L.; Pang, Z.; Xu, Q.; Lu, X. Continuous Electrodeposition of Silicon and Germanium Micro⁄nanowires from Their Oxides Precursors in Molten Salt. J. Energy Chem. 2020, 44, 147–153. [Google Scholar] [CrossRef]
- Yasko, O.; Shakhnin, D.B.; Gab, A.; Malyshev, V.; Gaune-Escard, M. Electrodeposition of Nanostructured Silicon Coatings onto Different Materials from Halide and Halide–Oxide Melts. In Springer Proceedings in Physics; Springer Science and Business Media Deutschland GmbH: Berlin, Germany, 2021; Volume 246, pp. 231–235. ISBN 9783030519049. [Google Scholar]
- Gevel, T.; Zhuk, S.; Leonova, N.; Leonova, A.; Trofimov, A.; Suzdaltsev, A.; Zaikov, Y. Electrochemical Synthesis of Nano-Sized Silicon from KCl–K2SiF6 Melts for Powerful Lithium-Ion Batteries. Appl. Sci. 2021, 11, 10927. [Google Scholar] [CrossRef]
- Wang, F.; Ma, Y.; Li, P.; Peng, C.; Yin, H.; Li, W.; Wang, D. Electrochemical Conversion of Silica Nanoparticles to Silicon Nanotubes in Molten Salts: Implications for High-Performance Lithium-Ion Battery Anode. ACS Appl. Nano Mater. 2021, 4, 7028–7036. [Google Scholar] [CrossRef]
- Jing, S.; Xiao, J.; Shen, Y.; Hong, B.; Gu, D.; Xiao, W. Silicate-Mediated Electrolytic Silicon Nanotube from Silica in Molten Salts. Small 2022, 18, 2203251. [Google Scholar] [CrossRef]
- Rezaei, A.; Kamali, A.R. Green Production of Carbon Nanomaterials in Molten Salts, Mechanisms and Applications. Diam. Relat. Mater. 2018, 83, 146–161. [Google Scholar] [CrossRef]
- Hu, L.; Song, Y.; Ge, J.; Zhu, J.; Jiao, S. Capture and Electrochemical Conversion of CO2 to Ultrathin Graphite Sheets in CaCl2-Based Melts. J. Mater. Chem. A 2015, 3, 21211–21218. [Google Scholar] [CrossRef]
- Wu, H.; Li, Z.; Ji, D.; Liu, Y.; Li, L.; Yuan, D.; Zhang, Z.; Ren, J.; Lefler, M.; Wang, B.; et al. One-Pot Synthesis of Nanostructured Carbon Materials from Carbon Dioxide via Electrolysis in Molten Carbonate Salts. Carbon N. Y. 2016, 106, 208–217. [Google Scholar] [CrossRef]
- Novoselova, I.A.; Oliynyk, N.F.; Volkov, S.V. Electrolytic Production of Carbon Nano-Tubes in Chloride-Oxide Melts under Carbon Dioxide Pressure. In Hydrogen Materials Science and Chemistry of Carbon Nanomaterials; Springer: Dordrecht, The Netherlands, 2007; pp. 459–465. ISBN 1402055129. [Google Scholar]
- Ijije, H.V.; Lawrence, R.C.; Chen, G.Z. Carbon Electrodeposition in Molten Salts: Electrode Reactions and Applications. RSC Adv. 2014, 4, 35808–35817. [Google Scholar] [CrossRef]
- Mao, X.; Yan, Z.; Sheng, T.; Gao, M.; Zhu, H.; Xiao, W.; Wang, D. Characterization and Adsorption Properties of the Electrolytic Carbon Derived from CO2 Conversion in Molten Salts. Carbon N. Y. 2017, 111, 162–172. [Google Scholar] [CrossRef]
- Kushkhov, K.B.; Ligidova, M.N.; Ali, J.Z.; Khotov, A.A.; Tlenkopachev, M.R.; Karatsukova, R.K. Electrochemical Processes in Molten Alkaline Metal Carbonates under Carbon Dioxide Overpressure. Russ. Metall. 2021, 2021, 141–150. [Google Scholar] [CrossRef]
- Wang, M.; Kim, Y.; Zhang, L.; Seong, W.K.; Kim, M.; Chatterjee, S.; Wang, M.; Li, Y.; Bakharev, P.V.; Lee, G.; et al. Controllable Electrodeposition of Ordered Carbon Nanowalls on Cu(111) Substrates. Mater. Today 2022, 57, 75–83. [Google Scholar] [CrossRef]
- Kumar, K.; Mondal, S. Fabrication and Characterisation of Carbon Nanotube Reinforced Copper Matrix Nanocomposites. Can. Metall. Q. 2022, 61, 77–84. [Google Scholar] [CrossRef]
- Groult, H.; Le Van, K.; Lantelme, F. Electrodeposition of Carbon-Metal Powders in Alkali Carbonate Melts. J. Electrochem. Soc. 2014, 161, D3130–D3138. [Google Scholar] [CrossRef]
- Hu, L.; Song, Y.; Ge, J.; Zhu, J.; Han, Z.; Jiao, S. Electrochemical Deposition of Carbon Nanotubes from CO2 in CaCl2 –NaCl-Based Melts. J. Mater. Chem. A 2017, 5, 6219–6225. [Google Scholar] [CrossRef]
- Xu, Q.; Schwandt, C.; Chen, G.Z.; Fray, D.J. Electrochemical Investigation of Lithium Intercalation into Graphite from Molten Lithium Chloride. J. Electroanal. Chem. 2002, 530, 16–22. [Google Scholar] [CrossRef]
- Kamali, A.R. Nanocatalytic Conversion of CO2 into Nanodiamonds. Carbon N. Y. 2017, 123, 205–215. [Google Scholar] [CrossRef]
- Yasko, O.; Malyshev, V.; Gab, A.; Lukashenko, T. Joint Electroreduction of Carbonate and Tungstate Ions as the Base for Tungsten Carbide Nanopowders Synthesis in Ionic Melts. In Springer Proceedings in Physics; Springer Science and Business Media, LLC: Berlin, Germany, 2019; Volume 221, pp. 397–402. ISBN 9783030177584. [Google Scholar]
- Malyshev, V.; Gab, A.; Shakhnin, D.; Lukashenko, T.; Ishtvanik, O.; Gaune-Escard, M. High-Temperature Electrochemical Synthesis of Nanopowders of Tungsten Carbide in Ionic Melts. In Springer Proceedings in Physics; Springer Science and Business Media, LLC: Berlin, Germany, 2018; Volume 214, pp. 311–321. ISBN 9783319925660. [Google Scholar]
- Kushkhov, K.B.; Kardanov, A.L.; Adamokova, M.N. Electrochemical Synthesis of Binary Molybdenum-Tungsten Carbides (Mo,W)2C from Tungstate-Molybdate-Carbonate Melts. Russ. Metall. 2013, 2013, 79–85. [Google Scholar] [CrossRef]
- Bukatova, G.A.; Kuznetsov, S.A. Electrosynthesis of Gadolinium Hexaboride Nanotubes. Electrochem. Commun. 2005, 7, 637–641. [Google Scholar] [CrossRef]
- Kuznetsov, S.A.; Kremenetsky, V.G.; Popova, A.V.; Kremenetskaya, O.V.; Kalinnikov, V.T. Unusual Effect of the Second Coordination Sphere on the Standard Charge Transfer Rate Constants for the Nb(V)/Nb(IV) Redox Couple in Chloride—Fluoride Melts. Dokl. Phys. Chem. 2009, 428, 209–212. [Google Scholar] [CrossRef]
- Grinevitch, V.V.; Kuznetsov, S.A.; Arakcheeva, A.A.; Olyunina, T.V.; Schønleber, A.; Gaune-Escard, M. Electrode and Chemical Reactions during Electrodeposition of Tantalum Products in CsCl Melt. Electrochim. Acta 2006, 51, 6563–6571. [Google Scholar] [CrossRef]
- Kuznetsov, S.A.; Rebrov, E.V.; Mies, M.J.M.; de Croon, M.H.J.M.; Schouten, J.C. Synthesis of Protective Mo–Si–B Coatings in Molten Salts and Their Oxidation Behavior in an Air–Water Mixture. Surf. Coat. Technol. 2006, 201, 971–978. [Google Scholar] [CrossRef]
- Grinevitch, V.V.; Arakcheeva, A.V.; Kuznetsov, S.A. Tantalum Metal of Peculiar Cristal Lattice (b-Ta) as Creation of Elec-Trocrystallization from Molten Salts. In International Symposium on Ionic Liquids; DTU Research Database: Carry le Rouet, France, 2003; pp. 277–285. [Google Scholar]
- Dubrovskiy, A.R.; Makarova, O.V.; Kuznetsov, S.A. Effect of the Molybdenum Substrate Shape on Mo2C Coating Electrodeposition. Coatings 2018, 8, 442. [Google Scholar] [CrossRef]
- Dubrovskii, A.R.; Kuznetsov, S.A.; Rebrov, E.V.; Schouten, J.C. Catalytic Coatings of New Generation Based on Mo2C and a Microstructured Reactor for Steam Conversion of Carbon Monoxide. Russ. J. Appl. Chem. 2014, 87, 601–607. [Google Scholar] [CrossRef]
- Dubrovskiy, A.R.; Okunev, M.A.; Makarova, O.V.; Kuznetsov, S.A. Superconducting Niobium Coatings Deposited on Spherical Substrates in Molten Salts. Coatings 2018, 8, 213. [Google Scholar] [CrossRef]
- Patt, J.; Moon, D.J.; Phillips, C.; Thompson, L. Molybdenum Carbide Catalysts for Water-Gas Shift. Catal. Lett. 2000, 65, 193–195. [Google Scholar] [CrossRef]
- Derakhshandeh, M.R.; Eshraghi, M.J.; Razavi, M. Recent Developments in the New Generation of Hard Coatings Applied on Cemented Carbide Cutting Tools. Int. J. Refract. Met. Hard Mater. 2023, 111, 106077. [Google Scholar] [CrossRef]
- Lee, C.; Danon, Y.; Mulligan, C. Characterization of Niobium, Tantalum and Chromium Sputtered Coatings on Steel Using Eddy Currents. Surf. Coat. Technol. 2005, 200, 2547–2556. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, T.H.; Kim, D.Y.; Nersisyan, H.H.; Han, M.H.; Kang, K.S.; Bae, K.K.; Shin, Y.J.; Lee, J.H. Microstructural and Corrosion Characteristics of Tantalum Coatings Prepared by Molten Salt Electrodeposition. Surf. Coat. Technol. 2013, 235, 819–826. [Google Scholar] [CrossRef]
- Emsley, J. Nature’s Building Blocks: An A-Z Guide to the Elements; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Baklanova, N.I.; Zima, T.M.; Boronin, A.I.; Kosheev, S.V.; Titov, A.T.; Isaeva, N.V.; Graschenkov, D.V.; Solntsev, S.S. Protective Ceramic Multilayer Coatings for Carbon Fibers. Surf. Coat. Technol. 2006, 201, 2313–2319. [Google Scholar] [CrossRef]
- Wang, S.-C.; Lin, H.-T.; Nayak, P.K.; Chang, S.-Y.; Huang, J.-L. Carbothermal Reduction Process for Synthesis of Nanosized Chromium Carbide via Metal-Organic Vapor Deposition. Thin Solid Film. 2010, 518, 7360–7365. [Google Scholar] [CrossRef]
- Sen, S. Influence of Chromium Carbide Coating on Tribological Performance of Steel. Mater. Des. 2006, 27, 85–91. [Google Scholar] [CrossRef]
- Gómez, M.A.; Romero, J.; Lousa, A.; Esteve, J. Tribological Performance of Chromium/Chromium Carbide Multilayers Deposited by r.f. Magnetron Sputtering. Surf. Coat. Technol. 2005, 200, 1819–1824. [Google Scholar] [CrossRef]
- Cintho, O.M.; Favilla, E.A.P.; Capocchi, J.D.T. Mechanical–Thermal Synthesis of Chromium Carbides. J. Alloys Compd. 2007, 439, 189–195. [Google Scholar] [CrossRef]
- Esteve, J.; Romero, J.; Gómez, M.; Lousa, A. Cathodic Chromium Carbide Coatings for Molding Die Applications. Surf. Coat. Technol. 2004, 188–189, 506–510. [Google Scholar] [CrossRef]
- Shatinsky, V.D.; Nesterenko, F.I. Protecting Diffusional Coatings; Naukova Dumka: Kiev, Ukraine, 1988. [Google Scholar]
- Stulov, Y.V.; Kuznetsov, S.A. Synthesis of Chromium Carbide Coatings on Carbon Steels in Molten Salts and Their Properties. Glas. Phys. Chem. 2014, 40, 324–328. [Google Scholar] [CrossRef]
- Ilyushchenko, N.G.; Afinogenov, A.I.; Shurov, N.I. Metal Interactions in Ionic Melts; Nauka: Moscow, Russia, 1991. [Google Scholar]
- Novokreshchenov, Y.V.; Petenev, O.S.; Kositsyn, Y.N. Influence of anodic polarization on spontaneous transfer of metals from salt melts onto articles for protection by thermal chemical treatment. J. Appl. Chem. USSR 1986, 59, 2492–2494. [Google Scholar]
- Malyshev, V.V.; Shakhnin, D.B.; Hab, A.; Kublanovs’kyi, V.S.; Schuster, D. Synthesis of Chromium Silicides in Ionic Melts. Mater. Sci. 2020, 55, 745–757. [Google Scholar] [CrossRef]
- Kovalevskii, A.V.; El’Kin, O.V. The Electrochemical Properties of LiCl-KCl Melt Held in Contact with Samarium. Russ. J. Phys. Chem. A 2011, 85, 499–502. [Google Scholar] [CrossRef]
- Stulov, Y.V.; Dolmatov, V.S.; Dubrovskii, A.R.; Kuznetsov, S.A. Coatings by Refractory Metal Carbides: Deposition from Molten Salts, Properties, Application. Russ. J. Appl. Chem. 2017, 90, 676–683. [Google Scholar] [CrossRef]
- Böhm, H. Adsorption Und Anodische Oxydation von Wasserstoff an Wolframcarbid. Electrochim. Acta 1970, 15, 1273–1280. [Google Scholar] [CrossRef]
- Goti, A.; Cardona, F. Hydrogen Peroxide in Green Oxidation Reactions: Recent Catalytic Processes. In Green Chemical Reactions; Springer: Dordrecht, The Netherlands, 2008; pp. 191–212. [Google Scholar] [CrossRef]
- Bloom, H. Electrochemistry: The Past Thirty and the Next Thirty Years; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Dolmatov, V.S.; Kuznetsov, S.A. Synthesis of Refractory Metal Carbides on Carbon Fibers in Molten Salts and Their Electrocatalytic Properties. J. Electrochem. Soc. 2021, 168, 122501. [Google Scholar] [CrossRef]
- Guan, A.; Yang, C.; Quan, Y.; Shen, H.; Cao, N.; Li, T.; Ji, Y.; Zheng, G. One-Dimensional Nanomaterial Electrocatalysts for CO2 Fixation. Chem.-An Asian J. 2019, 14, 3969–3980. [Google Scholar] [CrossRef]
- Peng, S.; Li, L.; Kong Yoong Lee, J.; Tian, L.; Srinivasan, M.; Adams, S.; Ramakrishna, S. Electrospun Carbon Nanofibers and Their Hybrid Composites as Advanced Materials for Energy Conversion and Storage. Nano Energy 2016, 22, 361–395. [Google Scholar] [CrossRef]
- Wang, H.G.; Yuan, S.; Ma, D.L.; Zhang, X.B.; Yan, J.M. Electrospun Materials for Lithium and Sodium Rechargeable Batteries: From Structure Evolution to Electrochemical Performance. Energy Environ. Sci. 2015, 8, 1660–1681. [Google Scholar] [CrossRef]
- Jin, T.; Han, Q.; Wang, Y.; Jiao, L.; Jin, T.; Han, Q.; Wang, Y.; Jiao, L. 1D Nanomaterials: Design, Synthesis, and Applications in Sodium–Ion Batteries. Small 2018, 14, 1703086. [Google Scholar] [CrossRef] [PubMed]
- Kohmura, Y.; Zhakhovsky, V.; Takei, D.; Suzuki, Y.; Takeuchi, A.; Inoue, I.; Inubushi, Y.; Inogamov, N.; Ishikawa, T.; Yabashi, M. Nano-Structuring of Multi-Layer Material by Single x-Ray Vortex Pulse with Femtosecond Duration. Appl. Phys. Lett. 2018, 112, 123103. [Google Scholar] [CrossRef]
- Salih, O.S.; Al-Akkam, E.J. Microneedles as A Magical Technology to Facilitate Transdermal Drug Delivery: A Review Article. Int. J. Drug Deliv. Technol. 2022, 12, 896–901. [Google Scholar] [CrossRef]
- Pan, J.; Low, K.L.; Ghosh, J.; Jayavelu, S.; Ferdaus, M.M.; Lim, S.Y.; Zamburg, E.; Li, Y.; Tang, B.; Wang, X.; et al. Transfer Learning-Based Artificial Intelligence-Integrated Physical Modeling to Enable Failure Analysis for 3 Nanometer and Smaller Silicon-Based CMOS Transistors. ACS Appl. Nano Mater. 2021, 4, 6903–6915. [Google Scholar] [CrossRef]
- Kuznetsova, S.V.; Dolmatov, V.S.; Kuznetsov, S.A. Voltammetric Study of Electroreduction of Silicon Complexes in a Chloride-Fluoride Melt. Russ. J. Electrochem. 2009, 45, 742–748. [Google Scholar] [CrossRef]
- Nicholson, R.S.; Shain, I. Theory of Stationary Electrode Polarography: Single Scan and Cyclic Methods Applied to Reversible, Irreversible, and Kinetic Systems. Anal. Chem. 1964, 36, 706–723. [Google Scholar] [CrossRef]
- Nakamura, Y.; Asano, I.; Hiratani, M.; Saito, T.; Goto, H. Oxidation-Resistant Amorphous TaN Barrier for MIM-Ta2O5 Capacitors in Giga-Bit DRAMs. In Proceedings of the 2001 Symposium on VLSI Technology. Digest of Technical Papers, Kyoto, Japan, 12–14 June 2001; pp. 39–40. [Google Scholar] [CrossRef]
- Matsui, Y.; Hiratani, M.; Kimura, S.; Asano, I. Combining Ta2O5 and Nb2O5 in Bilayered Structures and Solid Solutions for Use in MIM Capacitors. J. Electrochem. Soc. 2005, 152, F54. [Google Scholar] [CrossRef]
- Fairbrother, F. The Chemistry of Niobium and Tantalum; Elsevier Publishing Company: Amsterdam, The Netherlands, 1967; ISBN 978-0444402059. [Google Scholar]
- Mozalev, A.; Sakairi, M.; Takahashi, H. Structure, Morphology, and Dielectric Properties of Nanocomposite Oxide Films Formed by Anodizing of Sputter-Deposited Ta-Al Bilayers. J. Electrochem. Soc. 2004, 151, F257. [Google Scholar] [CrossRef]
- Zainulin, Y.G.; Alyamovskii, S.I.; Shveikin, G.P.; Popova, S.V. Possibility of Preparing Zirconium and Tantalum Monoxides at High Pressures and Temperatures. Zh. Neorg. Khim. 1978, 23, 1155–1157. [Google Scholar]
- Lantelme, F.; Barhoun, A.; Li, G.; Besse, J. Electrodeposition of Tantalum in NaCl–KCl–K2TaF7 Melts. J. Electrochem. Soc. 1992, 139, 1249–1255. [Google Scholar] [CrossRef]
- Chamelot, P.; Palau, P.; Massot, L.; Savall, A.; Taxil, P. Electrodeposition Processes of Tantalum(V) Species in Molten Fluorides Containing Oxide Ions. Electrochim. Acta 2002, 47, 3423–3429. [Google Scholar] [CrossRef]








| Molten Salt System | Deposition Regime | Experimental Condition | Substrate | Product | Possible Application |
|---|---|---|---|---|---|
| NaCl-KCl-Li2CO3-Na2MoO4 | Galvanostatic electrolysis | i = 5 mA cm−2 τ = 7 h T = 1123 K | Mo plate | Mo2C nanostructured coating [84] | Catalyst for steam reforming reaction |
| NaCl-KCl-CrCl3-Cr | Currentless transfer | τ = 8 h T = 1173 K | Steel St.3 | Cr7C3 coating [98,103] | Protective corrosion- and wear-resistant coating |
| NaCl-KCl-K2TaF7-Ta | Currentless transfer | τ = 8 h T = 1173 K | Steel St.3 | TaC coating [103] | Protective corrosion- and wear-resistant coating |
| NaCl-KCl-K2NbF7-Nb | Currentless transfer | τ = 8 h T = 1173 K | Steel St.3 | NbC coating [103] | Protective corrosion- and wear-resistant coating |
| NaCl-KCl-K2TaF7-Ta | Currentless transfer | τ = 24 h T = 1123 K | Carbon fibers | TaC coating [107] | Electrocatalyst for H2O2 decomposition |
| NaCl-KCl-K2NbF7-Nb | Currentless transfer | τ = 24 h T = 1123 K | Carbon fibers | NbC coating [107] | Electrocatalyst for H2O2 decomposition |
| NaCl-KCl-Na2MoO4-Mo | Currentless transfer | τ = 1 h T = 1123 K | Carbon fibers | Mo2C crystals [107] | Electrocatalyst for H2O2 decomposition |
| NaCl-KCl-NaF-GdF3-KBF4 | Potentiostatic electrolysis | E = −1.8 V vs. GC τ = 7 h T = 1023 K | Ag rod | GdB6 nanorods and nanowires [78] | Material for point electron emitters; Material for the neutron adsorption |
| NaCl-KCl-NaF-K2SiF6 | Potentiostatic electrolysis | E = −0.75 V vs. GC τ = 2 h T = 1023 K | Ag rod | Si nanoneedles [115] | Material for Li-ion batteries; Drug delivery into living cells |
| CsCl-K3TaOF6 | Galvanostatic electrolysis | i = 0.15 A cm−2 τ = 1 h T = 1023 K | Mo rod | TaO nanoneedles [80] | Material for high-density electric capacitors |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stulov, Y.; Dolmatov, V.; Dubrovskiy, A.; Kuznetsov, S. Electrochemical Synthesis of Functional Coatings and Nanomaterials in Molten Salts and Their Application. Coatings 2023, 13, 352. https://doi.org/10.3390/coatings13020352
Stulov Y, Dolmatov V, Dubrovskiy A, Kuznetsov S. Electrochemical Synthesis of Functional Coatings and Nanomaterials in Molten Salts and Their Application. Coatings. 2023; 13(2):352. https://doi.org/10.3390/coatings13020352
Chicago/Turabian StyleStulov, Yuriy, Vladimir Dolmatov, Anton Dubrovskiy, and Sergey Kuznetsov. 2023. "Electrochemical Synthesis of Functional Coatings and Nanomaterials in Molten Salts and Their Application" Coatings 13, no. 2: 352. https://doi.org/10.3390/coatings13020352
APA StyleStulov, Y., Dolmatov, V., Dubrovskiy, A., & Kuznetsov, S. (2023). Electrochemical Synthesis of Functional Coatings and Nanomaterials in Molten Salts and Their Application. Coatings, 13(2), 352. https://doi.org/10.3390/coatings13020352






