Atmospheric-Pressure Plasma Jet-Induced Graft Polymerization of Composite Hydrogel on 3D-Printed Polymer Surfaces for Biomedical Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pretreatment of Materials
2.2. Atmospheric-Pressure Plasma Jet Activation Pre-Treatment
2.3. Composite Hydrogel on 3D-Printed Polymer Surfaces by UV Light Surface Graft Polymerization
2.3.1. Wettability (Surface Hydrophobicity/Hydrophilicity) Test
2.3.2. Surface Characterization
2.3.3. Swelling Studies of the Treatment Composite Hydrogel on 3D-Printed Polymer Surfaces
2.3.4. In Vitro Degradation Study of the Treatment Composite Hydrogel on 3D-Printed Polymer Surfaces
2.3.5. In Vitro Cell Culture of the Treatment Composite Hydrogel on 3D-Printed Polymer Surfaces
3. Results and Discussion
3.1. Wettability of Surface-Modified 3D-Printed PLA Samples
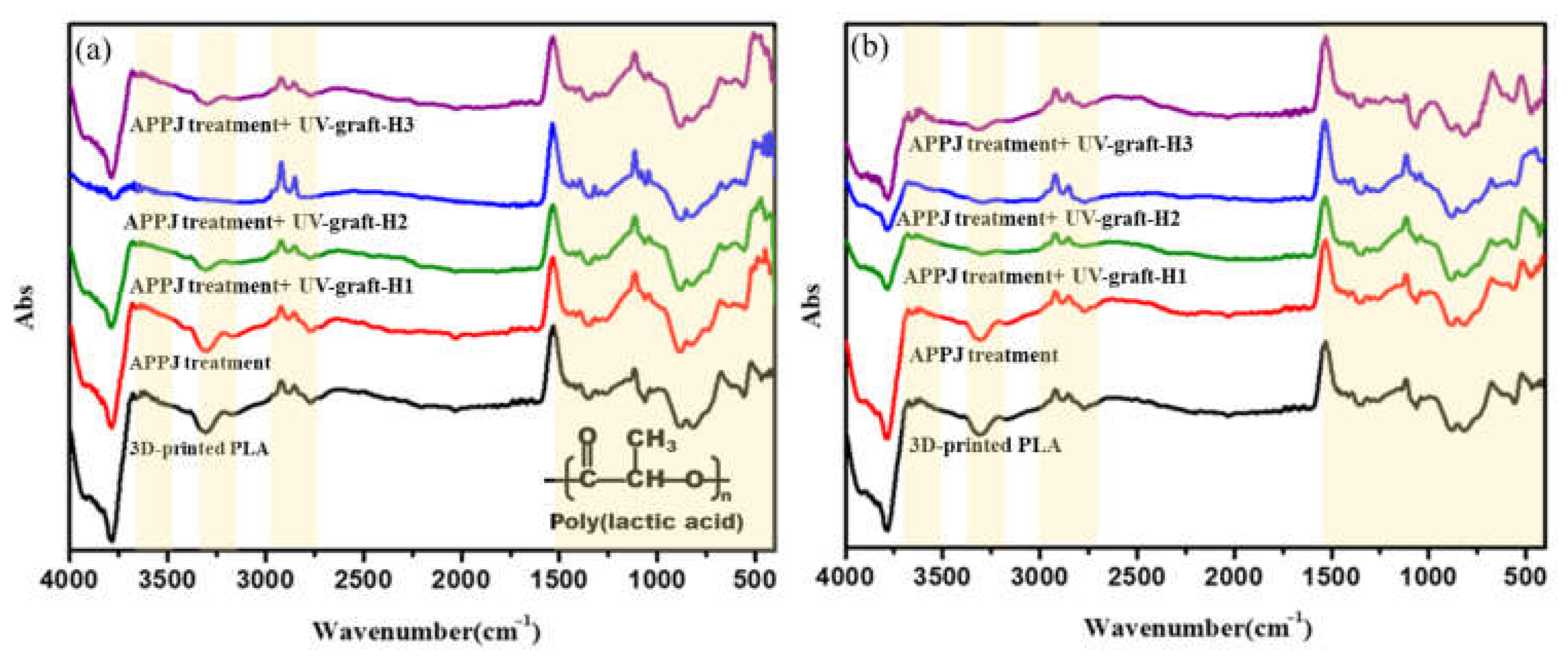
3.2. FTIR Characterization of Surface-Modified 3D-Printed PLA Samples
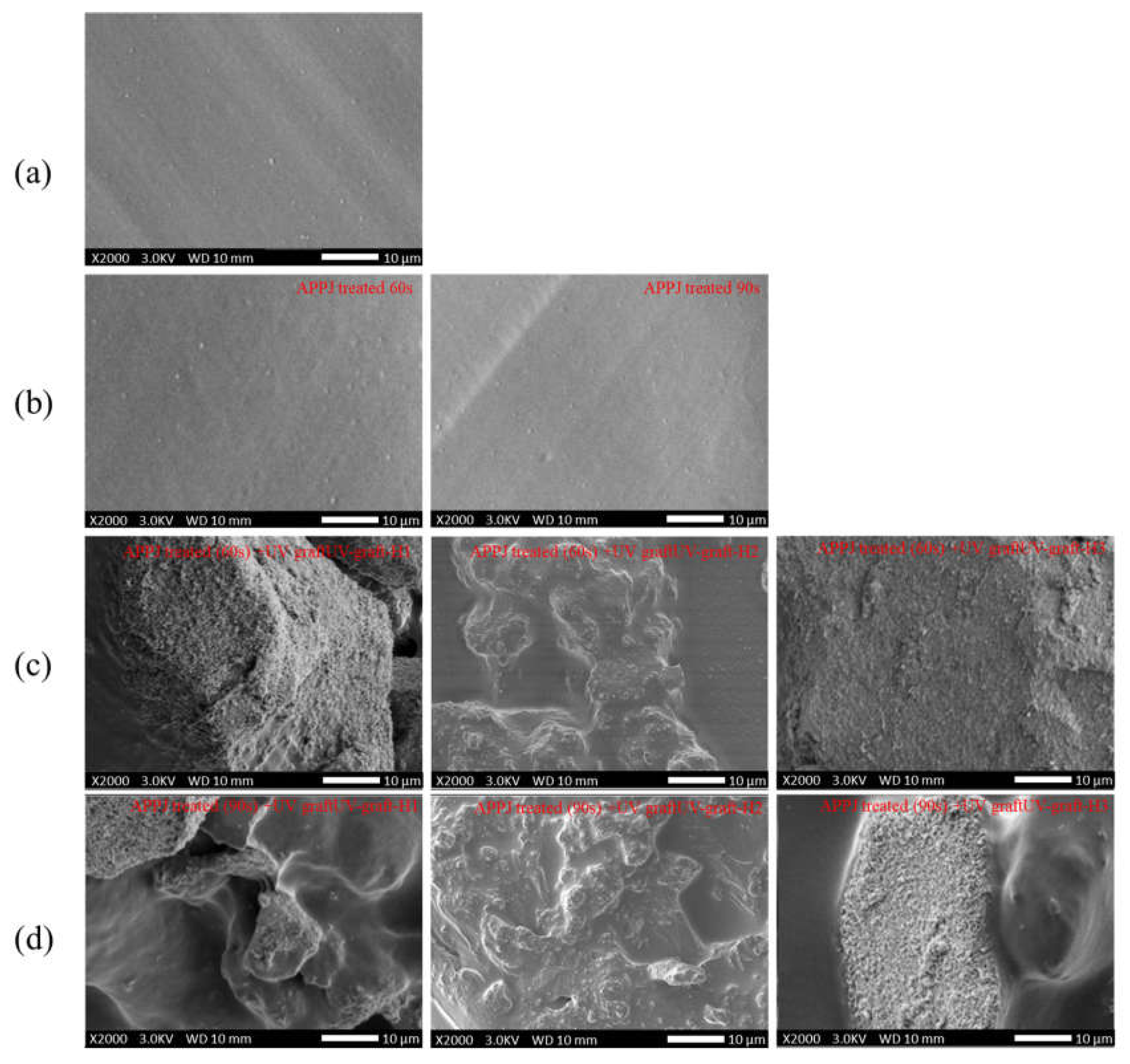
3.3. Surface Morphology of Surface-Modified 3D-Printed PLA Samples
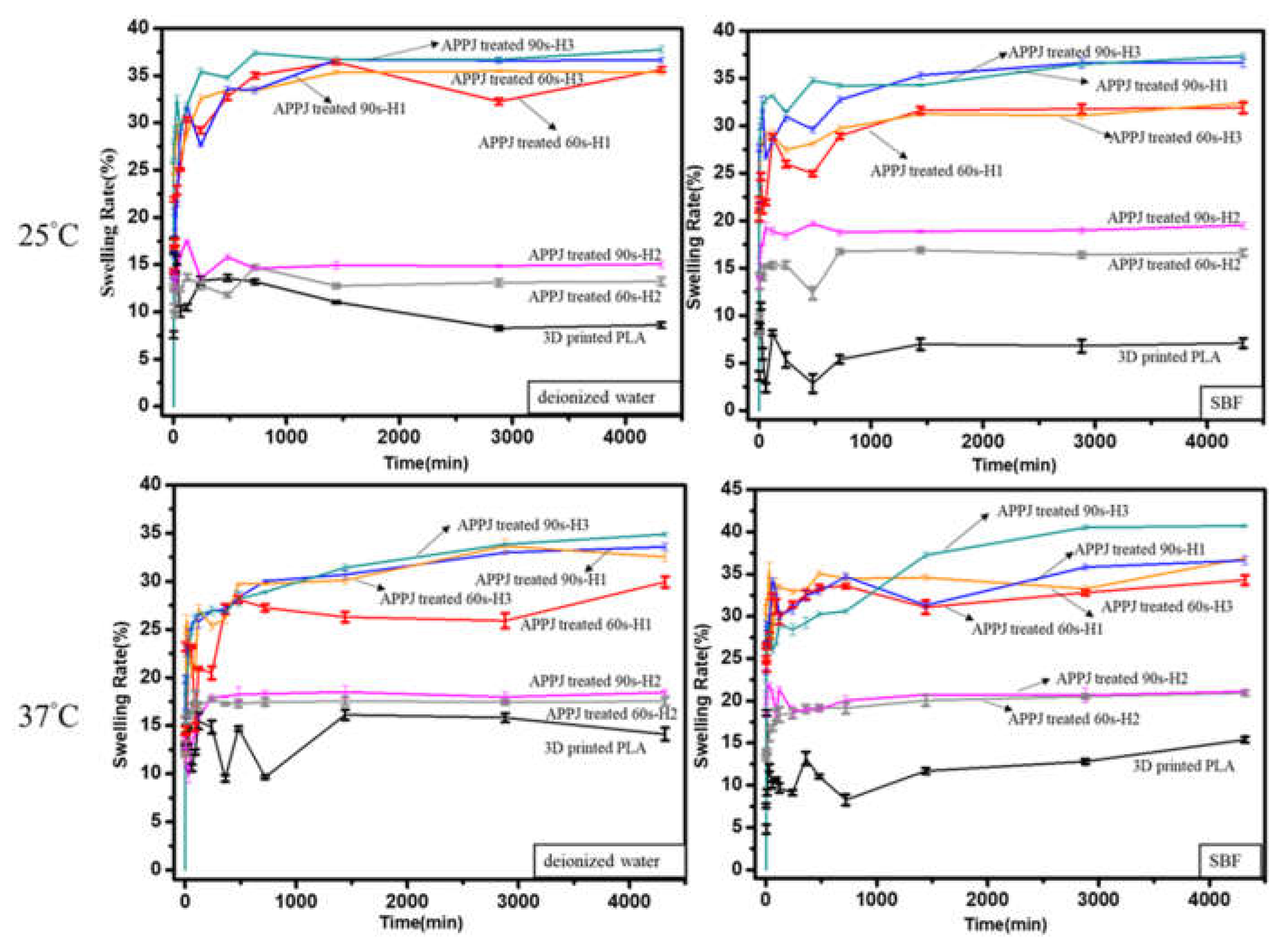
3.4. Swelling Ratio of Surface-Modified 3D-Printed PLA Samples
3.5. In Vitro Degradation of Surface-Modified 3D-Printed PLA Samples
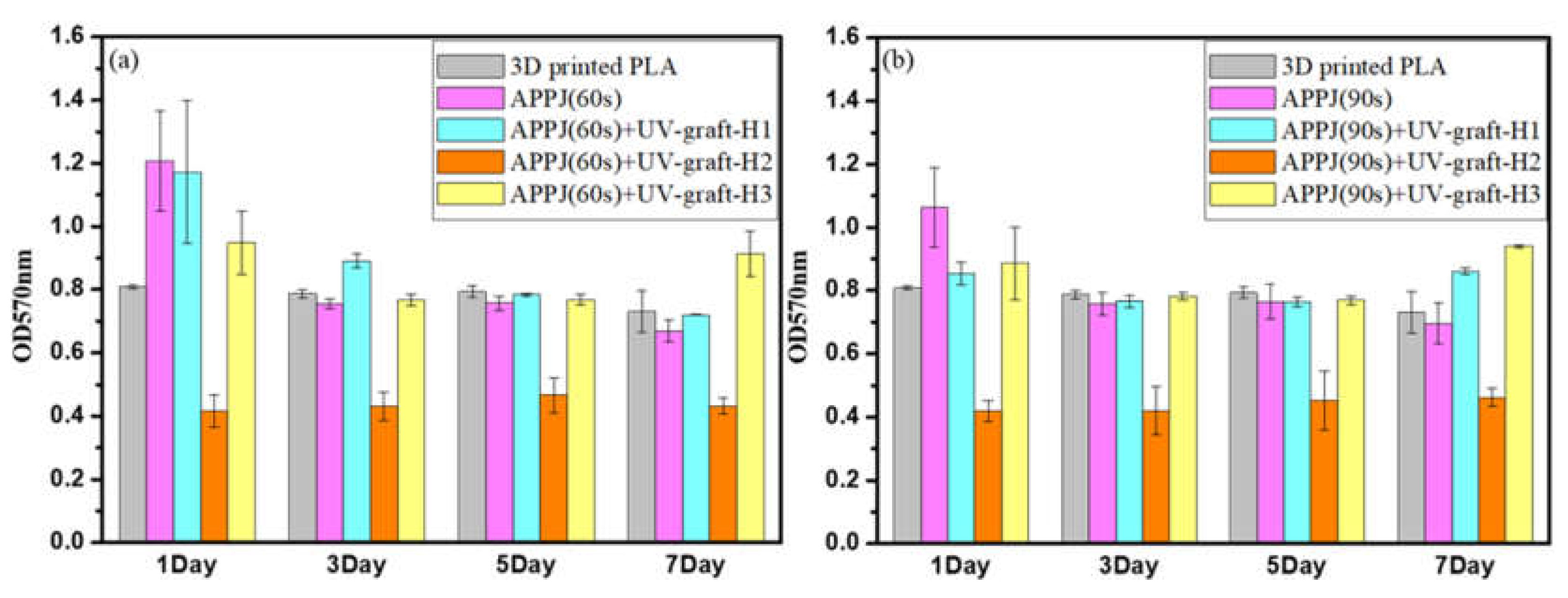
3.6. In Vitro Cytocompatibility Assay of Surface-Modified 3D-Printed PLA Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saxena, K. Materials for 3D printing in Medicine. In Additive Manufacturing with Medical Applications; Banga, K.H., Ed.; CRC Press: Boca Raton, FL, USA, 2022; pp. 97–110. [Google Scholar]
- Horvath, J. A brief history of 3D printing. In Mastering 3D Printing; Apress: Berkeley, CA, USA, 2014; pp. 3–10. [Google Scholar]
- Sun, Z. 3D printing in Medical Applications. Curr. Med. Imaging Former. Curr. Med. Imaging Rev. 2021, 17, 811–813. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Rastogi, V.; Ginn, M. A review on 3D printing techniques for medical applications. Curr. Opin. Chem. Eng. 2020, 28, 152–157. [Google Scholar] [CrossRef]
- Ballarre, J.; Desimone, M.; Katunar, M.R.; Baca, M.; Orellano, J.C.; Ceré, S.M. Coated stainless steel permanent implants with bioactive surface: Bone quality as success parameter. Bone 2015, 71, 258. [Google Scholar] [CrossRef]
- Dick, J.C.; Bourgeault, C.A. Notch sensitivity of titanium alloy, commercially pure titanium, and stainless steel spinal implants. Spine 2001, 26, 1668–1672. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.S.; Chhibber, R.; Mehta, R. Evaluation of mechanical properties of peek hap bio-composite used in load bearing bone implants. Mater. Sci. Forum 2017, 909, 193–198. [Google Scholar] [CrossRef]
- Kumar, S.S.; Chhibber, R.; Mehta, R. Peek composite scaffold preparation for load bearing bone implants. Mater. Sci. Forum 2018, 911, 77–82. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.; Farina, I. On the 3D printing of recycled ABS, PLA and hips thermoplastics for structural applications. PSU Res. Rev. 2018, 2, 115–137. [Google Scholar] [CrossRef]
- Tümer, E.H.; Erbil, H.Y. Extrusion-based 3D printing applications of PLA Composites: A review. Coatings 2021, 11, 390. [Google Scholar] [CrossRef]
- Marianna, C.; Bruna, T.; Daniel, K.; Rossana Mara, T. Structural evaluation of PLA scaffolds obtained by 3D printing via Fused Deposition Modeling (FDM) technique for applications in tissue engineering. Front. Bioeng. Biotechnol. 2016, 4, 995–997. [Google Scholar] [CrossRef]
- Joseph, S.S.; Aju, D. Three-dimensional reconstruction and digital printing of medical objects in purview of clinical applications. In Machine Learning and Deep Learning in Medical Data Analytics and Healthcare Applications; Taylor and Francis: Abingdon, UK, 2022; pp. 39–64. [Google Scholar]
- Lim, L.-T.; Cink, K.; Vanyo, T. Processing of poly(lactic acid). Poly (Lact. Acid) 2010, 189–215. [Google Scholar] [CrossRef]
- Anderson, J.M.; Shive, M.S. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 2012, 64, 72–82. [Google Scholar] [CrossRef]
- Herrero-Herrero, M.; Alberdi-Torres, S.; González-Fernández, M.L.; Vilariño-Feltrer, G.; Rodríguez-Hernández, J.C.; Vallés-Lluch, A.; Villar-Suárez, V. Influence of chemistry and fiber diameter of Electrospun PLA, PCL and their blend membranes, intended as cell supports, on their biological behavior. Polym. Test. 2021, 103, 107364. [Google Scholar] [CrossRef]
- Grémare, A.; Guduric, V.; Bareille, R.; Heroguez, V.; Latour, S.; L’heureux, N.; Fricain, J.-C.; Catros, S.; Le Nihouannen, D. Characterization of printed PLA scaffolds for Bone Tissue Engineering. J. Biomed. Mater. Res. Part A 2017, 106, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Shah Mohammadi, M.; Bureau, M.N.; Nazhat, S.N. Polylactic acid (PLA) biomedical foams for Tissue Engineering. In Biomedical Foams for Tissue Engineering Applications; Woodhead Publishing: Sawston, UK, 2014; pp. 313–334. [Google Scholar]
- Sinha, S.K. Additive manufacturing (AM) of medical devices and scaffolds for tissue engineering based on 3D and 4D printing. In 3D and 4D Printing of Polymer Nanocomposite Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 119–160. [Google Scholar]
- Büyük, N.İ.; Aksu, D.; Torun Köse, G. Effect of different pore sizes of 3D printed PLA-based scaffold in bone tissue engineering. Int. J. Polym. Mater. Polym. Biomater. 2022, 1–11. [Google Scholar] [CrossRef]
- Kaviani-Samani, S.; Bagheri-Khoulenjani, S.; Mirzadeh, H.; Dariushi, S. Biodegradable 3D printed scaffolds based on PLA for Bone Tissue Engineering. In Eco-Friendly and Smart Polymer Systems; Springer: Berlin/Heidelberg, Germany, 2020; pp. 83–86. [Google Scholar] [CrossRef]
- Hassanajili, S.; Karami-Pour, A.; Oryan, A.; Talaei-Khozani, T. Preparation and characterization of PLA/PCL/HA composite scaffolds using indirect 3D printing for Bone Tissue Engineering. Mater. Sci. Eng. C 2019, 104, 109960. [Google Scholar] [CrossRef]
- Algarni, M.; Ghazali, S. Comparative study of the sensitivity of PLA, ABS, Peek, and PETG’s mechanical properties to FDM printing process parameters. Crystals 2021, 11, 995. [Google Scholar] [CrossRef]
- Kuwabara, A.; Kuroda S-ichi Kubota, H. Polymer surface treatment by atmospheric pressure low temperature surface discharge plasma: Its characteristics and comparison with low pressure oxygen plasma treatment. Plasma Sci. Technol. 2007, 9, 181–189. [Google Scholar] [CrossRef]
- Jung, S.H.; Park, S.M.; Park, S.H.; Kim, S.D. Surface modification of fine powders by atmospheric pressure plasma in a circulating fluidized bed reactor. Ind. Eng. Chem. Res. 2004, 43, 5483–5488. [Google Scholar] [CrossRef]
- Chen, K.-S.; Liao, S.-C.; Tsao, S.-H.; Inagaki, N.; Wu, H.-M.; Chou, C.-Y.; Chen, W.-Y. Deposition of tetramethylsilane on the glass by plasma-enhanced chemical vapor deposition and atmospheric pressure plasma treatment. Surf. Coat. Technol. 2013, 228, S33–S36. [Google Scholar] [CrossRef]
- Patel, D.; Bonova, L.; Jeckell, Z.; Barlaz, D.E.; Chaudhuri, S.; Krogstad, D.V.; Ruzic, D.N. Deposition of zirconium oxide using atmospheric pressure plasma enhanced chemical vapor deposition with various precursors. Thin Solid Film. 2021, 733, 138815. [Google Scholar] [CrossRef]
- Bakhshzadmahmoudi, M.; Jamali, S.; Ahmadi, E. Wettability modification of polystyrene surface by cold atmospheric pressure plasma jet. Colloid Polym. Sci. 2022, 300, 103–110. [Google Scholar] [CrossRef]
- Gomathi, N.; Chanda, A.K.; Neogi, S. Atmospheric plasma treatment of polymers for biomedical applications. In Atmospheric Pressure Plasma Treatment of Polymers; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 199–215. [Google Scholar]
- Kasza, G.; Gyulai, G.; Ábrahám, Á.; Szarka, G.; Iván, B.; Kiss, É. Amphiphilic hyperbranched polyglycerols in a new role as highly efficient multifunctional surface active stabilizers for poly(lactic/glycolic acid) nanoparticles. RSC Adv. 2017, 7, 4348–4352. [Google Scholar] [CrossRef]
- Turicek, J.; Ratts, N.; Kaltchev, M.; Masoud, N. Investigation of a helium tubular cold atmospheric pressure plasma source and polymer surface treatment application. Plasma Sources Sci. Technol. 2021, 30, 025005. [Google Scholar] [CrossRef]
- Laroussi, M. Cold plasma in medicine and Healthcare: The New Frontier in low temperature plasma applications. Front. Phys. 2020, 8, 74. [Google Scholar] [CrossRef]
- Kuo, Y.-L.; Chang, K.-H.; Hung, T.-S.; Chen, K.-S.; Inagaki, N. Atmospheric-pressure plasma treatment on polystyrene for the photo-induced grafting polymerization of N-isopropylacrylamide. Thin Solid Film. 2010, 518, 7568–7573. [Google Scholar] [CrossRef]
- Vesel, A.; Primc, G. Investigation of surface modification of polystyrene by a direct and remote atmospheric-pressure plasma jet treatment. Materials 2020, 13, 2435. [Google Scholar] [CrossRef]
- Zaplotnik, R.; Vesel, A. Effect of VUV radiation on surface modification of polystyrene exposed to atmospheric pressure plasma jet. Polymers 2020, 12, 1136. [Google Scholar] [CrossRef]
- Liu, S.-J.; Liao, S.-C. Surface modification of bamboo charcoal by O2 plasma treatment and UV-grafted thermo-sensitive AgNPs hydrogel to improve antibacterial properties in biomedical application. Nanomaterials 2021, 11, 2697. [Google Scholar] [CrossRef]
- Şenol, Ş.; Akyol, E. Study on the preparation and drug release property of modified PEGDA based hydrogels. J. Turk. Chem. Soc. Sect. A Chem. 2019, 6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Senol, S.; Akyol, E. Synthesis and characterization of hydrogels based on poly(2-hydroxyethyl methacrylate) for drug delivery under UV irradiation. J. Mater. Sci. 2018, 53, 14953–14963. [Google Scholar] [CrossRef]
- Chen, K.-S.; Chang, S.-J.; Feng, C.-K.; Lin, W.-L.; Liao, S.-C. Plasma deposition and UV light induced surface grafting polymerization of NIPAAM on stainless steel for enhancing corrosion resistance and its drug delivery property. Polymers 2018, 10, 1009. [Google Scholar] [CrossRef]
- Taaca, K.L.; Prieto, E.I.; Vasquez, M.R. Current trends in biomedical hydrogels: From traditional crosslinking to plasma-assisted synthesis. Polymers 2022, 14, 2560. [Google Scholar] [CrossRef]
- Augustine, R.; Alhussain, H.; Zahid, A.A.; Raza Ur Rehman, S.; Ahmed, R.; Hasan, A. Crosslinking strategies to develop hydrogels for biomedical applications. In Gels Horizons: From Science to Smart Materials; Springer: Berlin/Heidelberg, Germany, 2021; pp. 21–57. [Google Scholar]
- Micutz, M.; Lungu, R.M.; Circu, V.; Ilis, M.; Staicu, T. Hydrogels obtained via γ-irradiation based on poly(acrylic acid) and its copolymers with 2-hydroxyethyl methacrylate. Appl. Sci. 2020, 10, 4960. [Google Scholar] [CrossRef]
- Justin, G.; Guiseppi-Elie, A. Electroconductive blends of poly(HEMA-co-PEGMA-co-HMMAco-SPMA) and poly(py-co-pyBA): In Vitro biocompatibility. J. Bioact. Compat. Polym. 2009, 25, 121–140. [Google Scholar] [CrossRef]
- Cui, X.; Murakami, T.; Hoshino, Y.; Miura, Y. Anti-biofouling phosphorylated HEMA and PEGMA block copolymers show high affinity to hydroxyapatite. Colloids Surf. B Biointerfaces 2017, 160, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Doğan, D.; Ulu, A.; Sel, E.; Köytepe, S.; Ateş, B. α-amylase immobilization on p(HEMA-co-PEGMA) hydrogels: Preparation, characterization, and catalytic investigation. Starch Stärke 2021, 73, 2000217. [Google Scholar] [CrossRef]
- Cools, P.; De Geyter, N.; Morent, R.; Uday Kumar, S.; Kumar, V.; Gopinath, P.; Jaganathan, S.K.; Deshmukh, R.R. Atmospheric pressure non-thermal plasma assisted polymerization of poly (ethylene glycol) methylether methacrylate (PEGMA) on low density polyethylene (LDPE) films for enhancement of Biocompatibility. Surf. Coat. Technol. 2017, 329, 55–67. [Google Scholar]
- Padmanabhan, V.P.; Kulandaivelu, R.; Santhana Panneer, D.; Vivekananthan, S.; Sagadevan, S.; Anita Lett, J. Microwave synthesis of hydroxyapatite encumbered with ascorbic acid intended for drug leaching studies. Mater. Res. Innov. 2019, 24, 171–178. [Google Scholar] [CrossRef]
- Thangavel, M.; Elsen Selvam, R. Review of physical, mechanical, and biological characteristics of 3D-printed bioceramic scaffolds for bone tissue engineering applications. ACS Biomater. Sci. Eng. 2022, 8, 5060–5093. [Google Scholar] [CrossRef] [PubMed]
- Dussault, A.; Pitaru, A.A.; Weber, M.H.; Haglund, L.; Rosenzweig, D.H.; Villemure, I. Optimizing design parameters of PLA 3D-printed scaffolds for bone defect repair. Surgeries 2022, 3, 162–174. [Google Scholar] [CrossRef]
- Choudhary, R.; Bulygina, I.; Lvov, V.; Zimina, A.; Zhirnov, S.; Kolesnikov, E.; Leybo, D.; Anisimova, N.; Kiselevskiy, M.; Kirsanova, M.; et al. Mechanical, structural, and biological characteristics of polylactide/wollastonite 3D printed scaffolds. Polymers 2022, 14, 3932. [Google Scholar] [CrossRef] [PubMed]
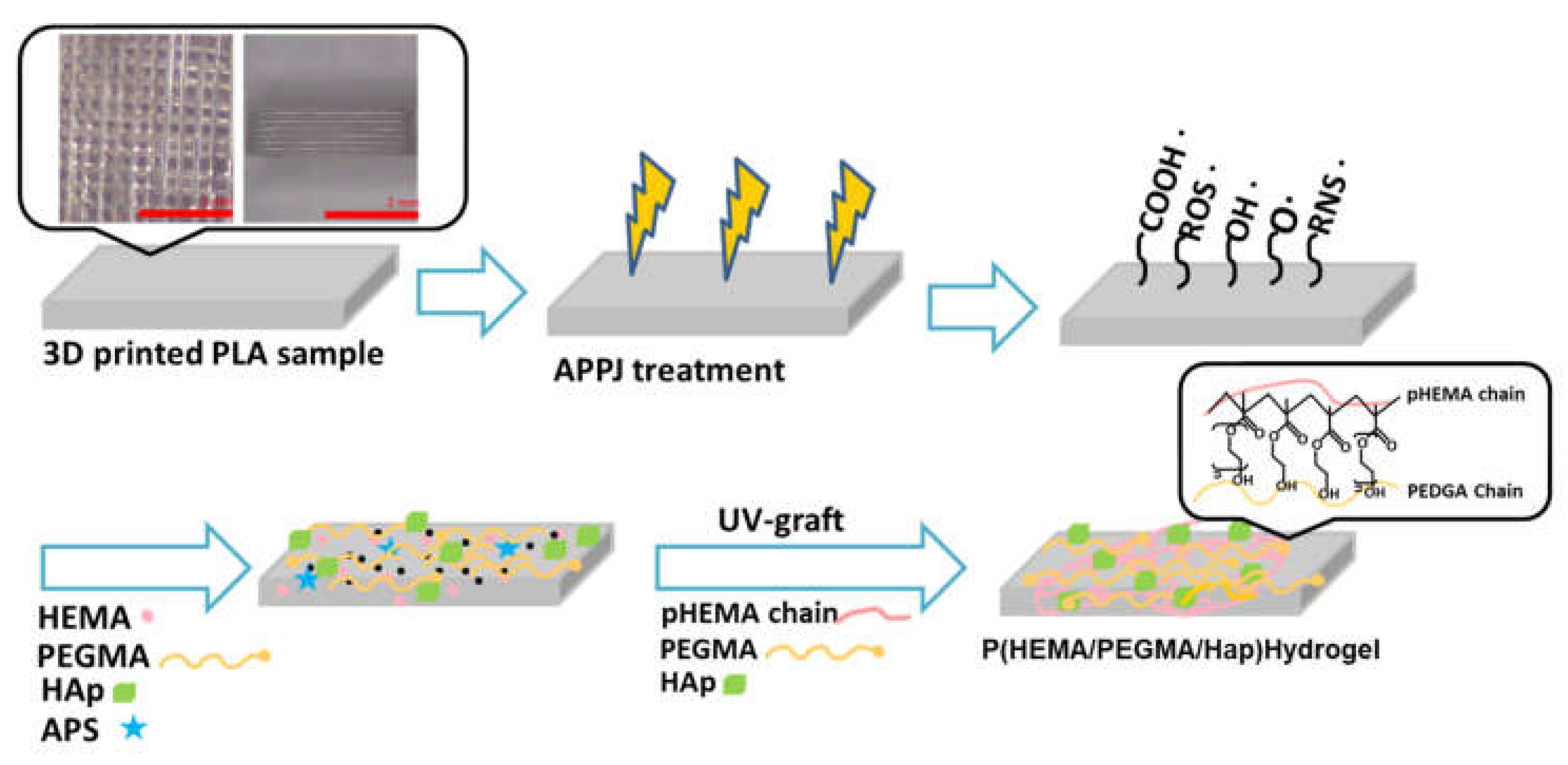
| Hydrogels | HEMA (mL) | PEGMA (mL) | APS (mol%) | Hap (0.1 g) |
|---|---|---|---|---|
| H1 | 15 | 15 | 1 | 0.1 |
| H2 | 10 | 20 | 1 | 0.1 |
| H3 | 20 | 10 | 1 | 0.1 |
| Contact Angle (°) | Untreated | Treatment A | Treatment B |
|---|---|---|---|
| θH2O | 62.2° ± 3.2° | 31.4° ± 3.2° | 22.5° ± 2.7° |
| Contact Angle (°) | Treatment A-H1 | Treatment A-H2 | Treatmen A-H3 | Treatment B-H1 | Treatment B-H2 | Treatment B-H3 |
|---|---|---|---|---|---|---|
| θH2O | 9.8° ± 2° | 13.9° ± 3.8° | 11.5° ± 3° | 10.2° ± 1.7° | 9.5° ± 2.3° | 0° |
| Temperature | Experimental Parameters | Solution | |
|---|---|---|---|
| Deionized Water | SBF | ||
| 25 °C | 3D-printed PLA | 8.61 ± 0.31 | 7.08 ± 0.49 |
| APPJ-treated 60 s-H1 | 35.6 ± 0.28 | 31.8 ± 0.56 | |
| APPJ-treated 60 s-H2 | 13.2 ± 0.43 | 16.6 ± 0.36 | |
| APPJ-treated 60 s-H3 | 35.3 ± 0.28 | 32.3 ± 0.28 | |
| APPJ-treated 90 s-H1 | 36.6 ± 0.27 | 36.6 ± 0.46 | |
| APPJ-treated 90 s-H2 | 15.0 ± 0.37 | 19.4 ± 0.34 | |
| APPJ-treated 90 s-H3 | 37.7 ± 0.36 | 37.3 ± 0.35 | |
| 37 °C | 3D-printed PLA | 14.1 ± 0.67 | 15.3 ± 0.3.5 |
| APPJ-treated 60 s-H1 | 29.9 ± 0.56 | 34.3 ± 0.54 | |
| APPJ-treated 60 s-H2 | 17.5 ± 0.43 | 20.9 ± 0.32 | |
| APPJ-treated 60 s-H3 | 33.5 ± 0.37 | 36.9 ± 0.21 | |
| APPJ-treated 90 s-H1 | 32.5 ± 0.43 | 36.6 ± 0.52 | |
| APPJ-treated 90 s-H2 | 18.4 ± 0.26 | 21.1 ± 0.24 | |
| APPJ-treated 90 s-H3 | 34.8 ± 0.21 | 40.7 ± 0.16 | |
| Experimental Parameters | Degradation (%) | |
|---|---|---|
| 7 days | 14 days | |
| APPJ-treated 60 s-H1 | 33.6 ± 1.21 | 36.4 ± 1.78 |
| APPJ-treated 60 s-H2 | 27.1 ± 0.78 | 27.3 ± 0.89 |
| APPJ-treated 60 s-H3 | 28.6 ± 0.89 | 33.6 ± 0.76 |
| APPJ-treated 90 s-H1 | 35.8 ± 1.07 | 37.3 ± 1.03 |
| APPJ-treated 90 s-H2 | 18.3 ± 1.34 | 18.9 ± 0.65 |
| APPJ-treated 90 s-H3 | 39.2 ± 0.98 | 40.3 ± 0.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, S.-C.; Wu, Y.-D.; Siao, J.-K. Atmospheric-Pressure Plasma Jet-Induced Graft Polymerization of Composite Hydrogel on 3D-Printed Polymer Surfaces for Biomedical Application. Coatings 2023, 13, 367. https://doi.org/10.3390/coatings13020367
Liao S-C, Wu Y-D, Siao J-K. Atmospheric-Pressure Plasma Jet-Induced Graft Polymerization of Composite Hydrogel on 3D-Printed Polymer Surfaces for Biomedical Application. Coatings. 2023; 13(2):367. https://doi.org/10.3390/coatings13020367
Chicago/Turabian StyleLiao, Shu-Chuan, Yu-De Wu, and Jhong-Kun Siao. 2023. "Atmospheric-Pressure Plasma Jet-Induced Graft Polymerization of Composite Hydrogel on 3D-Printed Polymer Surfaces for Biomedical Application" Coatings 13, no. 2: 367. https://doi.org/10.3390/coatings13020367
APA StyleLiao, S.-C., Wu, Y.-D., & Siao, J.-K. (2023). Atmospheric-Pressure Plasma Jet-Induced Graft Polymerization of Composite Hydrogel on 3D-Printed Polymer Surfaces for Biomedical Application. Coatings, 13(2), 367. https://doi.org/10.3390/coatings13020367






