Characterization and Growth of TiO2/ZnO on PTFE Substrates at Different Volumetric Ratios Using Chemical Bath Deposition
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials and Chemicals
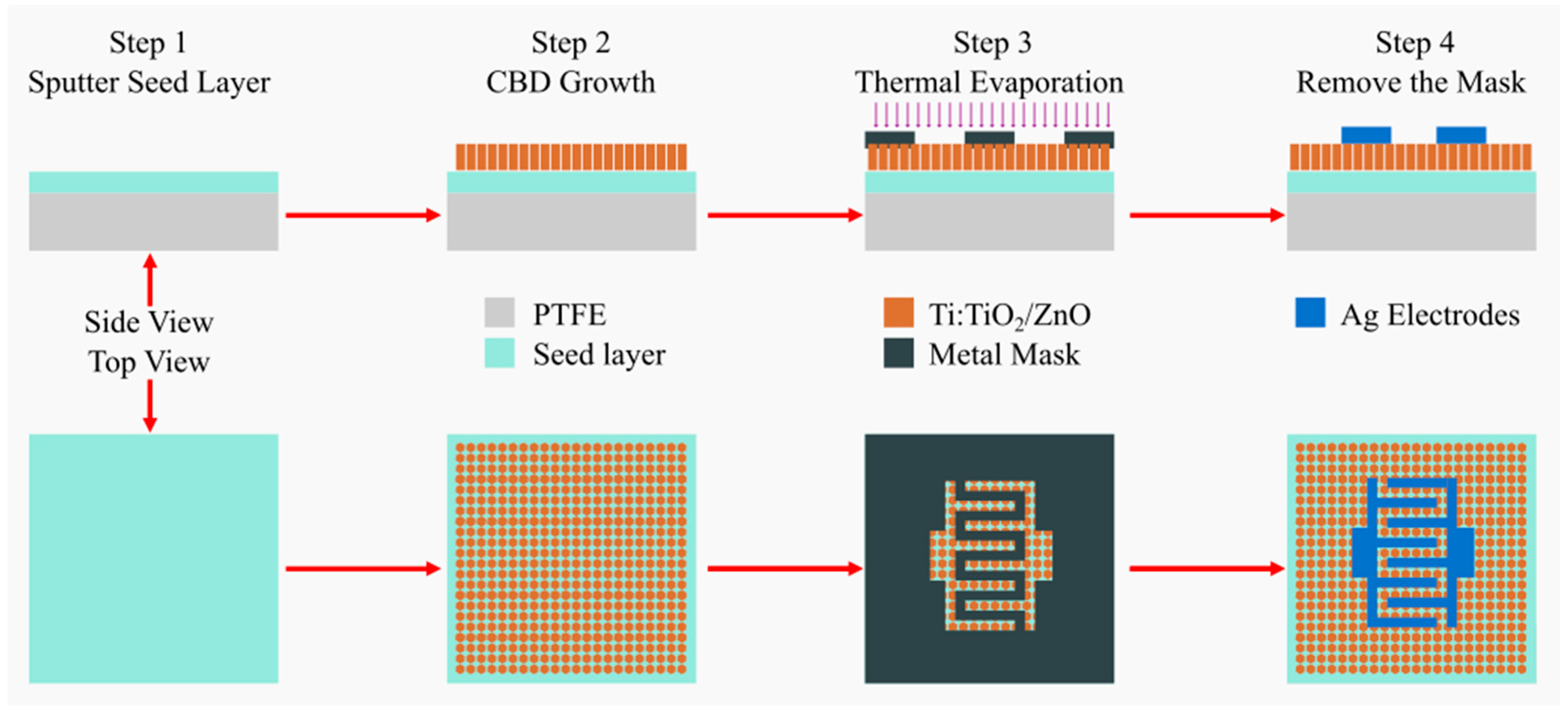
2.2. Synthesis of (Ti:TiO2/ZnO)@ZnO TFs
2.3. Fabrication of MSM Photodetectors
2.4. Characterization
3. Results and Discussion
3.1. Formation Mechanisms of ZnO and Ti:TiO2/ZnO
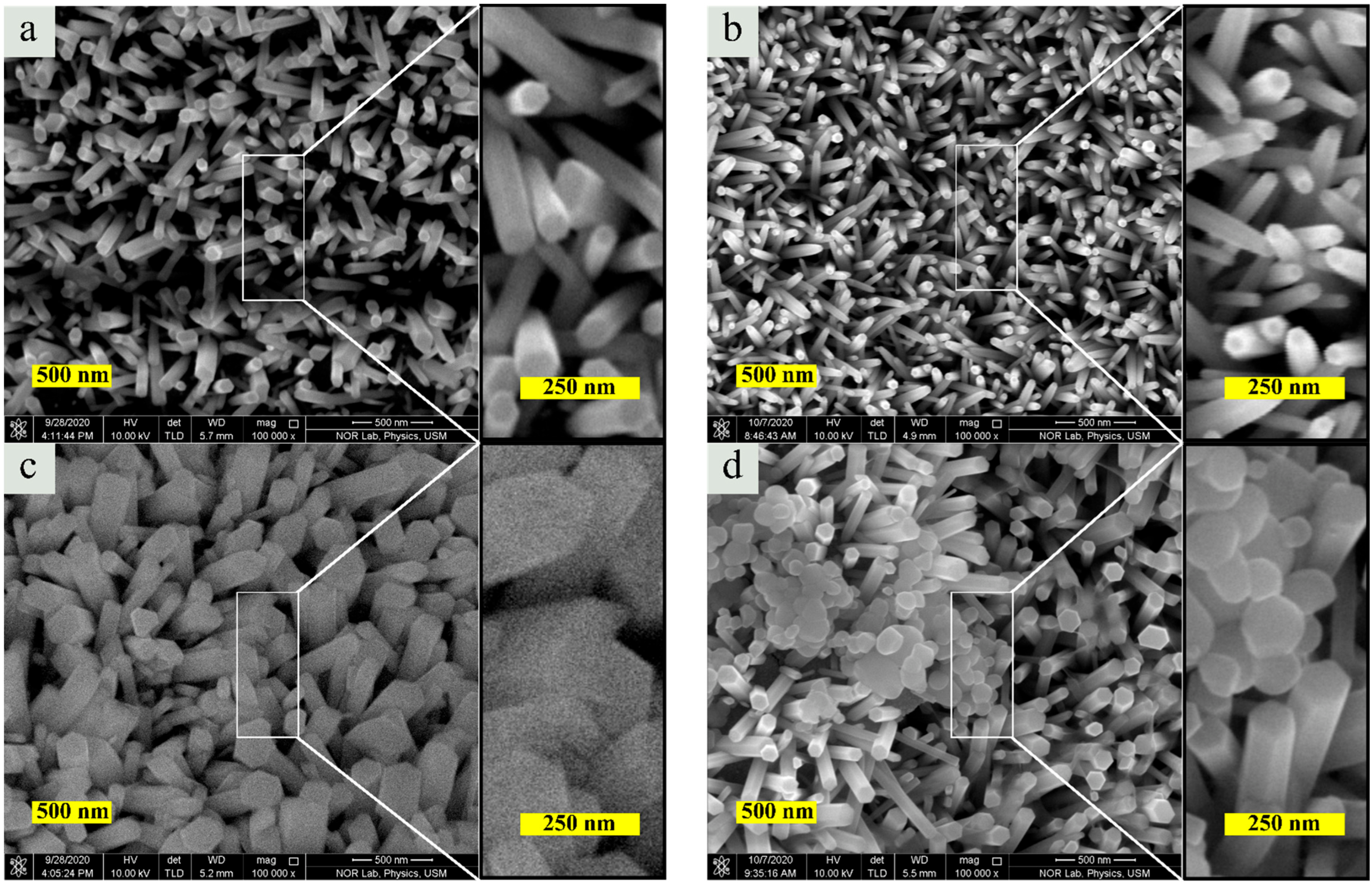
3.2. Morphology and Elemental Composition
3.3. Atomic Force Microscopy (AFM)
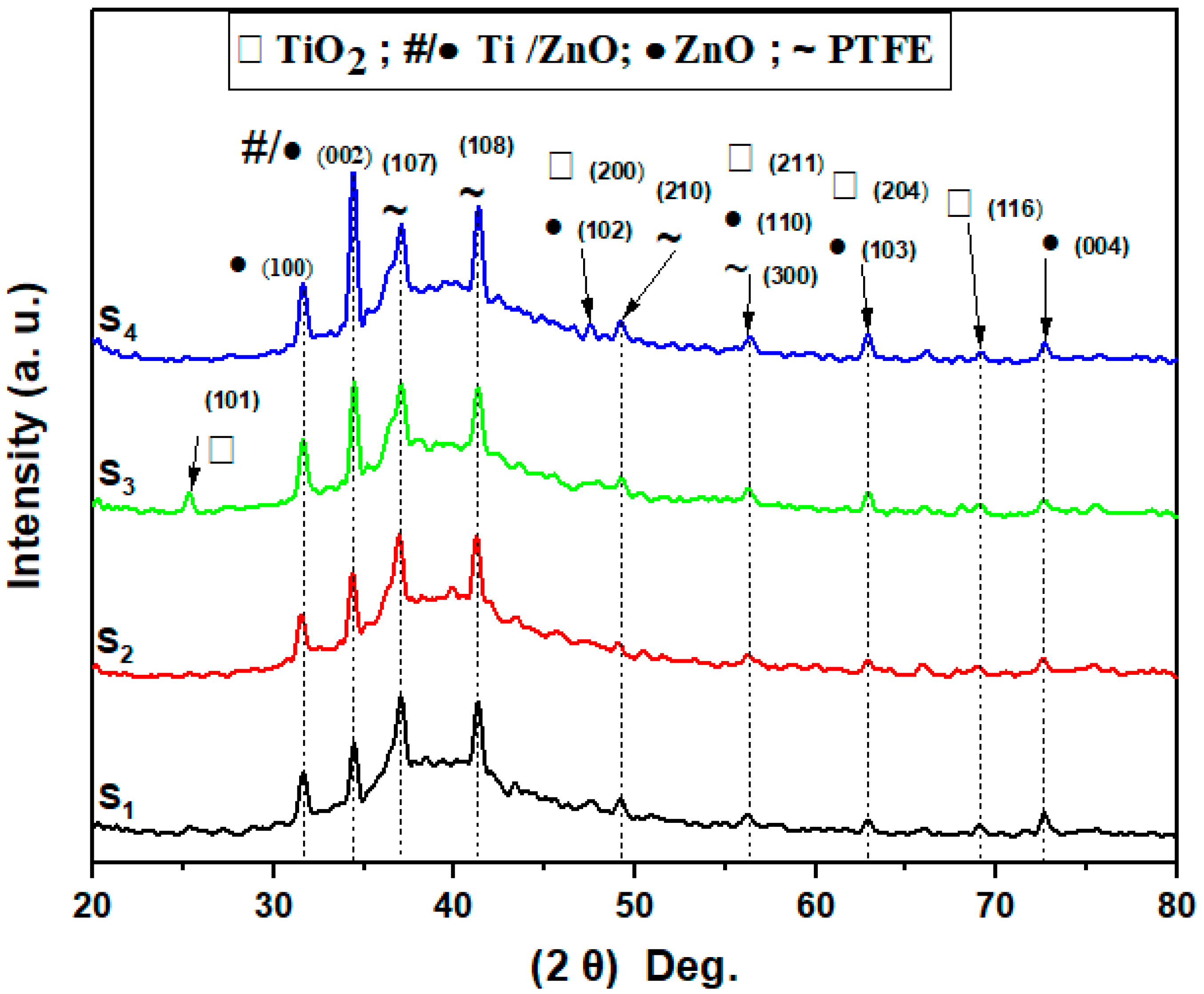
3.4. XRD Analysis
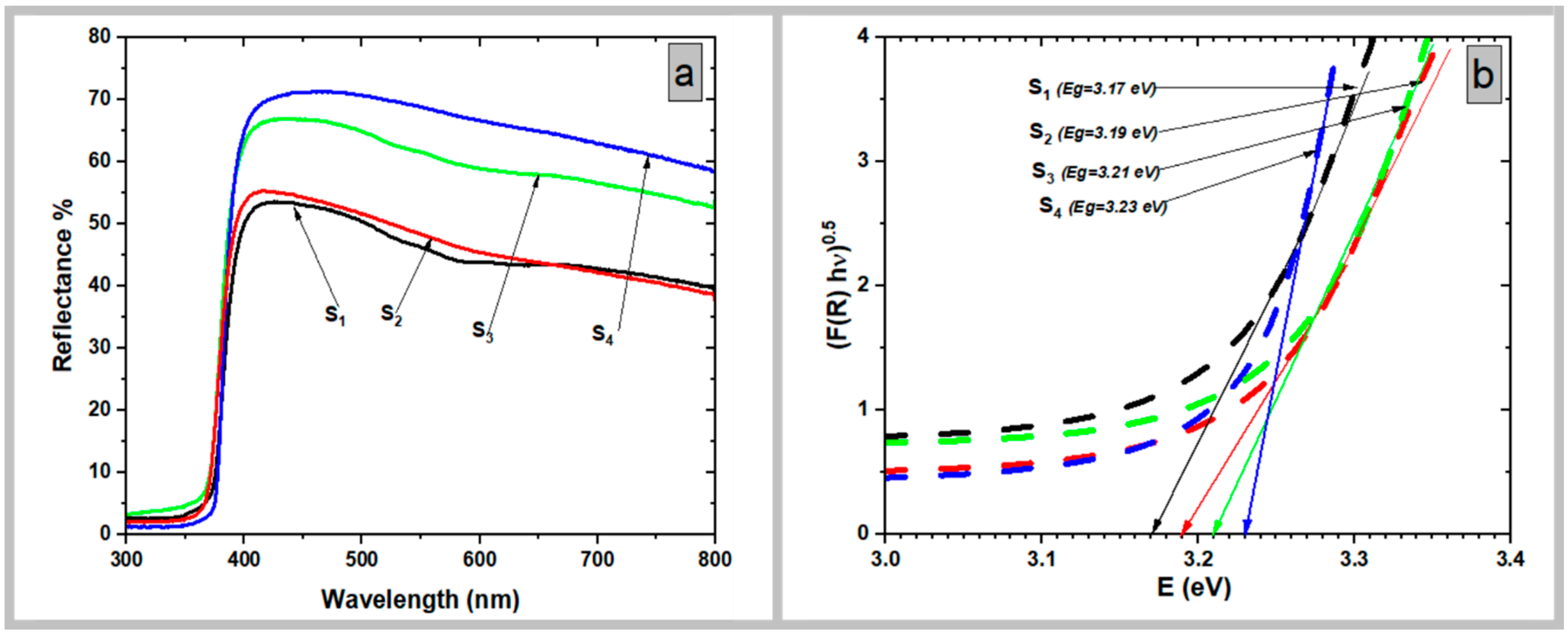
3.5. UV–Vis Diffuse Reflectance Spectra
3.6. FTIR Analysis
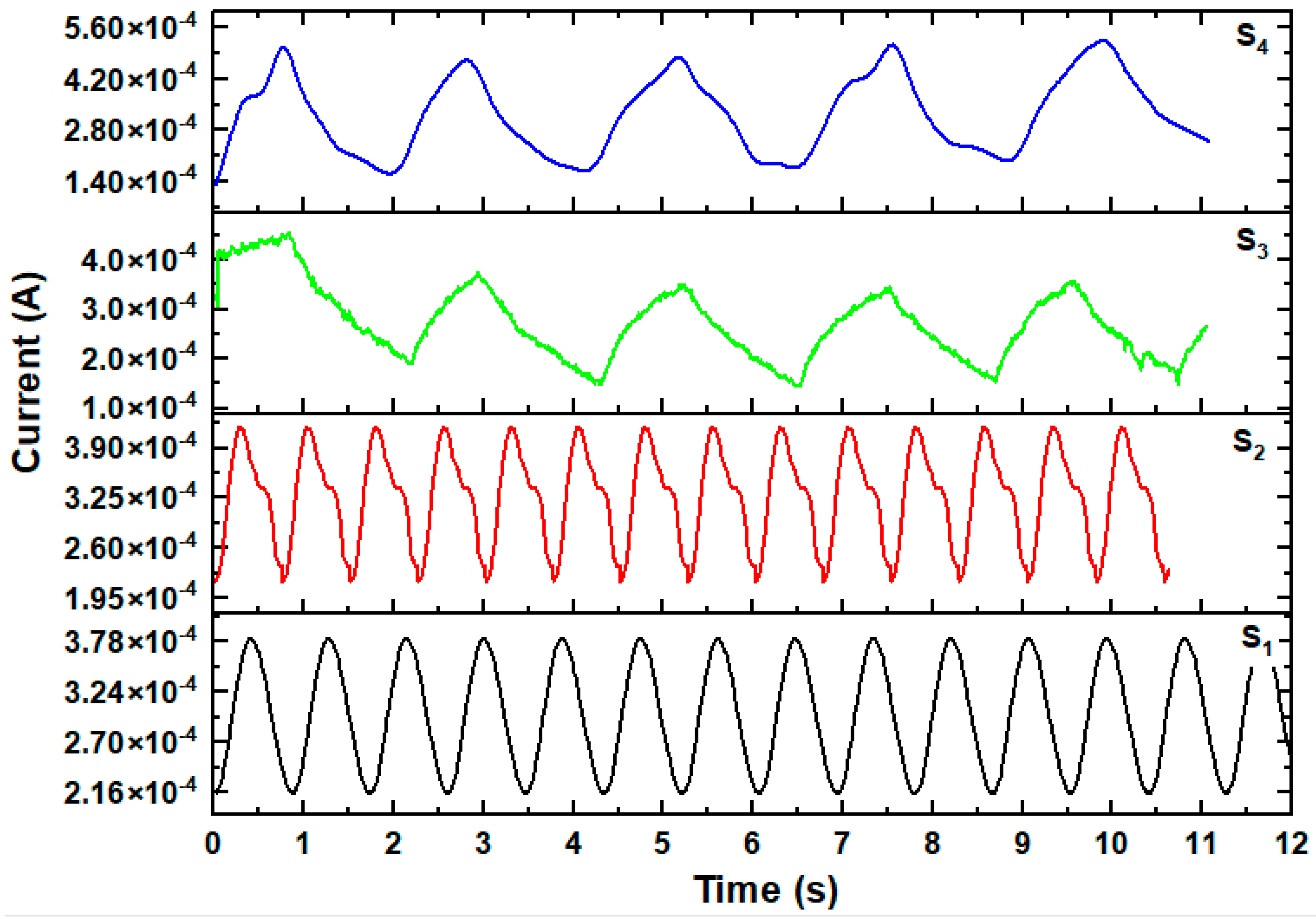
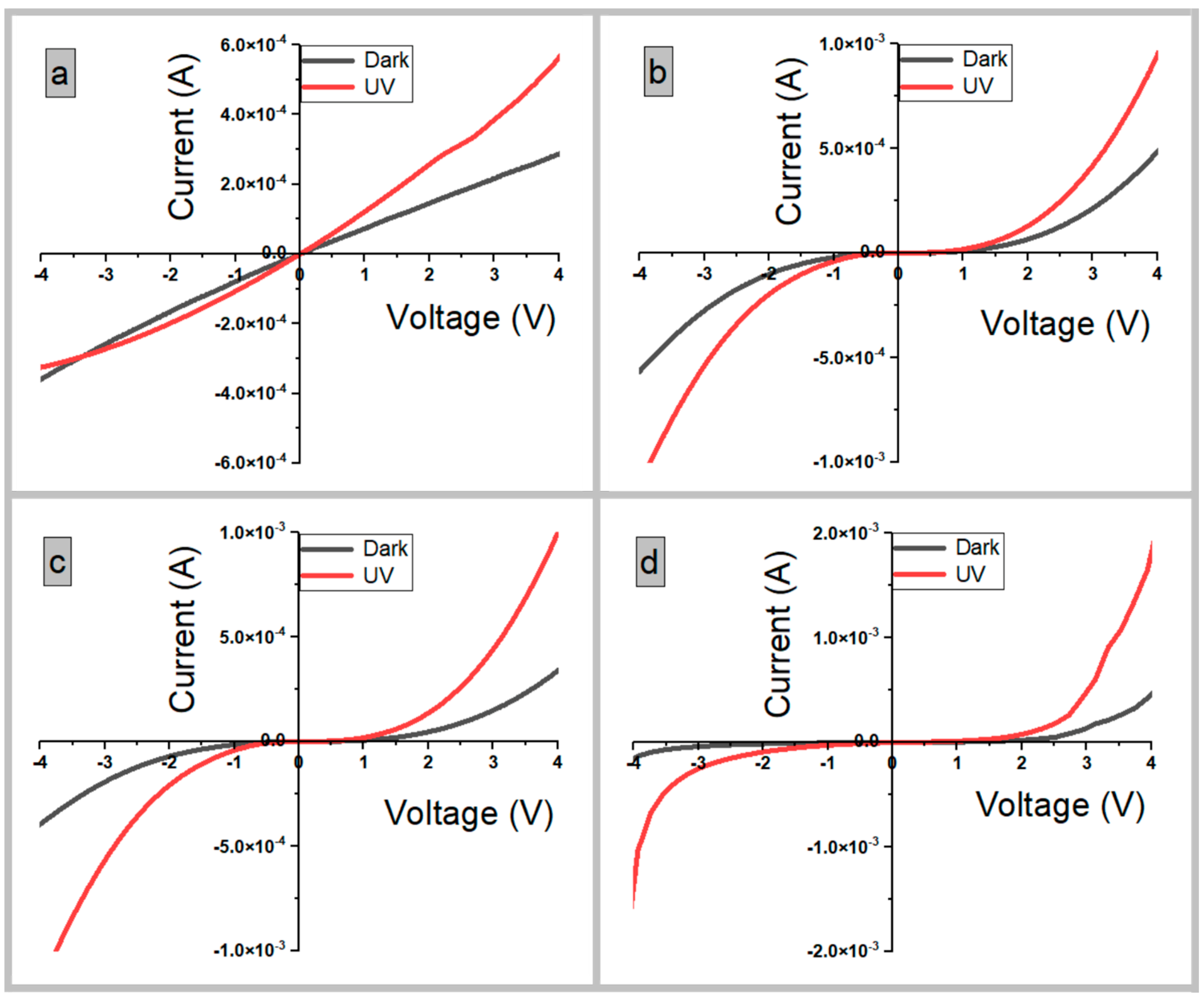
3.7. Characteristics of the UV Photodetectors
3.7.1. Photo-Response Properties
3.7.2. Current–Voltage Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shaheera, M.; Girija, K.G.; Kaur, M.; Geetha, V.; Debnath, A.K.; Vatsa, R.K.; Muthe, K.P.; Gadkari, S.C. Elucidation of Structural, Morphological, Optical and Photoluminescence Properties of Single and (In, Ga) Co-Doped ZnO Nanocrystalline Thin Films. Bull. Mater. Sci. 2019, 42, 266. [Google Scholar] [CrossRef]
- Epie, E.N.; Chu, W.K. Ionoluminescence Study of Zn—and O—Implanted ZnO Crystals: An Additional Perspective. Appl. Surf. Sci. 2016, 371, 28–34. [Google Scholar] [CrossRef]
- Saikia, L.; Bhuyan, D.; Saikia, M.; Malakar, B.; Dutta, D.K.; Sengupta, P. Photocatalytic Performance of ZnO Nanomaterials for Self Sensitized Degradation of Malachite Green Dye under Solar Light. Appl. Catal. A Gen. 2015, 490, 42–49. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, C. TiO2 Coated Zno Nanorods by Mist Chemical Vapor Deposition for Application as Photoanodes for Dye-Sensitized Solar Cells. Nanomaterials 2019, 9, 1339. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Weng, Y. Band Alignment and Controllable Electron Migration between Rutile and Anatase TiO2. Sci. Rep. 2015, 5, 11482. [Google Scholar] [CrossRef] [PubMed]
- Bidier, S.A.; Hashim, M.R.; Bououdina, M. Structural and Optical Characteristics of Ti-Doped ZnO Nanorods Deposited by Simple Chemical Bath Deposition. J. Mater. Sci. Mater. Electron. 2017, 28, 11178–11185. [Google Scholar] [CrossRef]
- Ilkhechi, N.N.; Mozammel, M.; Khosroushahi, A.Y. Antifungal Effects of ZnO, TiO2 and ZnO-TiO2 Nanostructures on Aspergillus Flavus. Pestic. Biochem. Physiol. 2021, 176, 104869. [Google Scholar] [CrossRef]
- Sahrin, N.T.; Nawaz, R.; Chong, F.K.; Lee, S.L.; Wirzal, M.D.H. Current Perspectives of Anodized TiO2 Nanotubes towards Photodegradation of Formaldehyde: A Short Review. Environ. Technol. Innov. 2021, 22, 101418. [Google Scholar] [CrossRef]
- Gupta, T.; Samriti; Cho, J.; Prakash, J. Hydrothermal Synthesis of TiO2 Nanorods: Formation Chemistry, Growth Mechanism, and Tailoring of Surface Properties for Photocatalytic Activities. Mater. Today Chem. 2021, 20, 100428. [Google Scholar] [CrossRef]
- Seshan, K. Handbook of Thin Film Deposition Techniques Principles, Methods, Equipment and Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2002; ISBN 9780429076749. [Google Scholar]
- Enríquez, J. Influence of the Thickness on Structural, Optical and Electrical Properties of Chemical Bath Deposited CdS Thin Films. Sol. Energy Mater. Sol. Cells 2003, 76, 313–322. [Google Scholar] [CrossRef]
- Lopez Garcia, A.J.; Sico, G.; Montanino, M.; Defoor, V.; Pusty, M.; Mescot, X.; Loffredo, F.; Villani, F.; Nenna, G.; Ardila, G. Low-temperature Growth of Zno Nanowires from Gravure-Printed Zno Nanoparticle Seed Layers for Flexible Piezoelectric Devices. Nanomaterials 2021, 11, 1430. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, M.; Bhatt, V.; Mukherjee, S.; Kumar, S.; Sharma, H.; Yadav, M.K.; Tomar, S.; Yun, J.H.; Choubey, R.K. Highly Responsive and Low-Cost Ultraviolet Sensor Based on ZnS/p-Si Heterojunction Grown by Chemical Bath Deposition. Sens. Actuators A Phys. 2021, 331, 112988. [Google Scholar] [CrossRef]
- Poornajar, M.; Marashi, P.; Haghshenas Fatmehsari, D.; Kolahdouz Esfahani, M. Synthesis of ZnO Nanorods via Chemical Bath Deposition Method: The Effects of Physicochemical Factors. Ceram. Int. 2016, 42, 173–184. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, Y.; Qin, F.; Ding, Y. DC Sputtering Assisted Nano-Branched Core-Shell TiO 2 /ZnO Electrodes for Application in Dye-Sensitized Solar Cells. Appl. Surf. Sci. 2016, 376, 10–15. [Google Scholar] [CrossRef]
- Pawar, V.; Kumar, M.; Dubey, P.K.; Singh, M.K.; Sinha, A.S.K.; Singh, P. Influence of Synthesis Route on Structural, Optical, and Electrical Properties of TiO2. Appl. Phys. A Mater. Sci. Process. 2019, 125, 657. [Google Scholar] [CrossRef]
- Kolodziejczak-Radzimska, A.; Jesionowski, T. Zinc Oxide—From Synthesis to Application: A Review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef]
- Muhammad, A.; Mohammad, S.M.; Hassan, Z.; Rajamanickam, S.; Abed, S.M.; Ashiq, M.G.B. Fabrication of Fluorine and Silver Co-Doped ZnO Photodetector Using Modified Hydrothermal Method. Microelectron. Int. 2022, 40. [Google Scholar] [CrossRef]
- Wisz, G.; Sawicka-Chudy, P.; Wal, A.; Potera, P.; Bester, M.; Płoch, D.; Sibiński, M.; Cholewa, M.; Ruszała, M. TiO2:ZnO/CuO Thin Film Solar Cells Prepared via Reactive Direct-Current (DC) Magnetron Sputtering. Appl. Mater. Today 2022, 29, 101673. [Google Scholar] [CrossRef]
- Huang, J.Y.; Wang, Y.; Fei, G.T.; Xu, S.H.; Zeng, Z.; Wang, B. TiO2/ZnO Double-Layer Broadband Antireflective and Down-Shifting Coatings for Solar Applications. Ceram. Int. 2022. [Google Scholar] [CrossRef]
- Alfakes, B.; Garlisi, C.; Villegas, J.; Al-Hagri, A.; Tamalampudi, S.; Rajput, N.S.; Lu, J.Y.; Lewin, E.; Sá, J.; Almansouri, I.; et al. Enhanced Photoelectrochemical Performance of Atomic Layer Deposited Hf-Doped ZnO. Surf. Coatings Technol. 2020, 385, 125352. [Google Scholar] [CrossRef]
- Ahmad, H.; Naderi, N. Enhanced Optoelectrical Performance of Ultraviolet Detectors Based on Self-Assembled Porous Zinc Oxide Nanostructures. Ceram. Int. 2022. [Google Scholar] [CrossRef]
- Dhanumalayan, E.; Joshi, G.M. Performance Properties and Applications of Polytetrafluoroethylene (PTFE)—A Review. Adv. Compos. Hybrid Mater. 2018, 1, 247–268. [Google Scholar] [CrossRef]
- Edinger, S.; Bansal, N.; Bauch, M.; Wibowo, R.A.; Hamid, R.; Trimmel, G.; Dimopoulos, T. Comparison of Chemical Bath-Deposited ZnO Films Doped with Al, Ga and In. J. Mater. Sci. 2017, 52, 9410–9423. [Google Scholar] [CrossRef]
- Farhat, O.F.; Halim, M.M.; Ahmed, N.M.; Oglat, A.A.; Abuelsamen, A.A.; Bououdina, M.; Qaeed, M.A. A Study of the Effects of Aligned Vertically Growth Time on ZnO Nanorods Deposited for the First Time on Teflon Substrate. Appl. Surf. Sci. 2017, 426, 906–912. [Google Scholar] [CrossRef]
- Farhat, O.F.; Halim, M.M.; Abdullah, M.J.; Ali, M.K.M.; Ahmed, N.M.; Allam, N.K. Growth of Vertically Aligned ZnO Nanorods on Teflon as a Novel Substrate for Low-Power Flexible Light Sensors. Appl. Phys. A Mater. Sci. Process. 2015, 119, 1197–1201. [Google Scholar] [CrossRef]
- Farhat, O.F.; Halim, M.M.; Ahmed, N.M.; Qaeed, M.A. ZnO Nanofiber (NFs) Growth from ZnO Nanowires (NWs) by Controlling Growth Temperature on Flexible Teflon Substrate by CBD Technique for UV Photodetector. Superlattices Microstruct. 2016, 100, 1120–1127. [Google Scholar] [CrossRef]
- Ade, R.; Kumar, S.S.; Valanarasu, S.; Kumar, S.S.; Sasikumar, S.; Ganesh, V.; Bitla, Y.; Algarni, H.; Yahia, I.S. Enhanced Optoelectronic Properties of Ti-Doped ZnO Nanorods for Photodetector Applications. Ceram. Int. 2021, 47, 24031–24038. [Google Scholar] [CrossRef]
- Dao, T.D.; Dang, C.T.T.; Han, G.; Hoang, C.V.; Yi, W.; Narayanamurti, V.; Nagao, T. Chemically Synthesized Nanowire TiO2/ZnO Core-Shell P-N Junction Array for High Sensitivity Ultraviolet Photodetector. Appl. Phys. Lett. 2013, 103, 193119. [Google Scholar] [CrossRef]
- Shaikh, S.K.; Ganbavle, V.V.; Inamdar, S.I.; Rajpure, K.Y. Multifunctional Zinc Oxide Thin Films for High-Performance UV Photodetectors and Nitrogen Dioxide Gas Sensors. RSC Adv. 2016, 6, 25641–25650. [Google Scholar] [CrossRef]
- Nguyen, X.H.; Luong, H.N.; Pham, H.A.; Nguyen, N.M.; Dang, V.Q. Visible Photodetector Based on Transition Metal-Doped ZnO NRs/PEDOT:PSS Hybrid Materials. RSC Adv. 2021, 11, 36340–36347. [Google Scholar] [CrossRef]
- Zhou, M.; Wu, B.; Zhang, X.; Cao, S.; Ma, P.; Wang, K.; Fan, Z.; Su, M. Preparation and UV Photoelectric Properties of Aligned ZnO-TiO2and TiO2-ZnO Core-Shell Structured Heterojunction Nanotubes. ACS Appl. Mater. Interfaces 2020, 12, 38490–38498. [Google Scholar] [CrossRef] [PubMed]
- Alwadai, N.; Mitra, S.; Hedhili, M.N.; Alamoudi, H.; Xin, B.; Alaal, N.; Roqan, I.S. Enhanced-Performance Self-Powered Solar-Blind UV-C Photodetector Based on N-ZnO Quantum Dots Functionalized by P-CuO Micro-Pyramids. ACS Appl. Mater. Interfaces 2021, 13, 33335–33344. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.M.; Babar, H.; Shah, T.R.; Sajid, M.U.; Qasim, M.A.; Javed, S. Preparation Techniques of TiO2 Nanofluids and Challenges: A Review. Appl. Sci. 2018, 8, 587. [Google Scholar] [CrossRef]
- Tiple, A.; Sinhmar, P.S.; Gogate, P.R. Improved Direct Synthesis of TiO2 Catalyst Using Sonication and Its Application for the Desulfurization of Thiophene. Ultrason. Sonochem. 2021, 73, 105547. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Tovar, R.; Blasco-Tamarit, E.; Fernández-Domene, R.M.; Villanueva-Pascual, M.; García-Antón, J. Electrochemical Formation of Novel TiO2-ZnO Hybrid Nanostructures for Photoelectrochemical Water Splitting Applications. Surf. Coat. Technol. 2020, 388, 125605. [Google Scholar] [CrossRef]
- Truong, D.C.; Thaowonkaew, S.; Muthitamongkol, P.; Horprathum, M.; Kumar, M.; Le, T.B.N.; Pham, A.T.T.; Seetawan, T.; Vora-ud, A.; Phan, T.B. Relaxation of Residual Stress-Controlled Thermopower Factor in Transparent-Flexible Ti-Doped ZnO Thin Films. Ceram. Int. 2022, 48, 2605–2613. [Google Scholar] [CrossRef]
- Fu, R.; Wang, Q.; Gao, S.; Wang, Z.; Huang, B.; Dai, Y.; Lu, J. Effect of Different Processes and Ti/Zn Molar Ratios on the Structure, Morphology, and Enhanced Photoelectrochemical and Photocatalytic Performance of Ti3+ Self-Doped Titanium-Zinc Hybrid Oxides. J. Power Sources 2015, 285, 449–459. [Google Scholar] [CrossRef]
- Na, S.; Seo, S.; Lee, H. Recent Developments of Advanced Ti3+-Self-Doped TiO2 for Efficient Visible-Light-Driven Photocatalysis. Catalysts 2020, 10, 679. [Google Scholar] [CrossRef]
- Watanabe, H.; Yamada, N.; Okaji, M. Linear Thermal Expansion Coefficient of Silicon from 293 to 1000 K. Int. J. Thermophys. 2004, 25, 221–236. [Google Scholar] [CrossRef]
- Lima, M.F.S.; Villeti, M.; Samios, D. Thermodynamical Behaviour of Deformed Semicrystalline Polymers in Temperatures between Tg and Tm. J. Polym. Eng. 1997, 17, 75–94. [Google Scholar] [CrossRef]
- Jamil, A.; Fareed, S.; Tiwari, N.; Li, C.; Cheng, B.; Xu, X.; Rafiq, M.A. Effect of Titanium Doping on Conductivity, Density of States and Conduction Mechanism in ZnO Thin Film. Appl. Phys. A Mater. Sci. Process. 2019, 125, 238. [Google Scholar] [CrossRef]
- Hsiang, H.I.; Liu, Y.L. Electrical Properties of Copper and Titanium-Codoped Zinc Ferrites. J. Alloys Compd. 2009, 472, 516–520. [Google Scholar] [CrossRef]
- Lou, Z.; Deng, J.; Wang, L.; Wang, R.; Fei, T.; Zhang, T. A Class of Hierarchical Nanostructures: ZnO Surface-Functionalized TiO 2 with Enhanced Sensing Properties. RSC Adv. 2013, 3, 3131–3136. [Google Scholar] [CrossRef]
- Tayel, A.; Ramadan, A.R.; El Seoud, O.A. Titanium Dioxide/graphene and Titanium Dioxide/graphene Oxide Nanocomposites: Synthesis, Characterization and Photocatalytic Applications for Water Decontamination. Catalysts 2018, 8, 491. [Google Scholar] [CrossRef]
- Butalid, R.J.B.; Cristobal, A.P.S.; Montallana, A.D.S.; Vasquez, M.R. Stability of TiO2 -Coated ZnO Photocatalytic Thin Films for Photodegradation of Methylene Blue. J. Vac. Sci. Technol. B 2020, 38, 62205. [Google Scholar] [CrossRef]
- Mokkapati, S.; Beck, F.J.; De Waele, R.; Polman, A.; Catchpole, K.R. Resonant Nano-Antennas for Light Trapping in Plasmonic Solar Cells. J. Phys. D Appl. Phys. 2011, 44, 185101. [Google Scholar] [CrossRef]
- Martínez, L.; García-Salgado, G.; Morales-Morales, F.; Campillo, B.; Hernández, A.G.; Karthik, T.V.K.; Jiménez-Vivanco, M.R.; Campos-álvarez, J. Zno Films Incorporation Study on Macroporous Silicon Structure. Materials 2021, 14, 3697. [Google Scholar] [CrossRef]
- Al-Ruqeishi, M.S.; Mohiuddin, T. Growth of Single-Sided ZnO nanocombs/ML Graphene Heterostructures. Arab. J. Chem. 2019, 12, 2774–2781. [Google Scholar] [CrossRef]
- Praveen, P.; Viruthagiri, G.; Mugundan, S.; Shanmugam, N. Structural, Optical and Morphological Analyses of Pristine Titanium Di-Oxide Nanoparticles—Synthesized via Sol-Gel Route. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 117, 622–629. [Google Scholar] [CrossRef]
- Surmenev, R.A.; Surmeneva, M.A.; Grubova, I.Y.; Chernozem, R.V.; Krause, B.; Baumbach, T.; Loza, K.; Epple, M. RF Magnetron Sputtering of a Hydroxyapatite Target: A Comparison Study on Polytetrafluorethylene and Titanium Substrates. Appl. Surf. Sci. 2017, 414, 335–344. [Google Scholar] [CrossRef]
- Dobromir, M.; Konrad-Soare, C.T.; Stoian, G.; Semchenko, A.; Kovalenko, D.; Luca, D. Surface Wettability of Zno-Loaded TiO2 Nanotube Array Layers. Nanomaterials 2020, 10, 1901. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Marín, P.; Angelats-Silva, L.; Noriega-Diaz, E.; Chavez-Bacilio, M.; Verde-Vera, R. Understanding the Phenomenon of X-ray Diffraction by Crystals and Related Concepts. Eur. J. Phys. 2020, 41, 045501. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the Anatase to Rutile Phase Transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Bidier, S.A.; Hashim, M.R.; Al-Diabat, A.M.; Bououdina, M. Effect of Growth Time on Ti-Doped ZnO Nanorods Prepared by Low-Temperature Chemical Bath Deposition. Phys. E Low-Dimens. Syst. Nanostruct. 2017, 88, 169–173. [Google Scholar] [CrossRef] [PubMed]
- John, K.I.; Adenle, A.A.; Adeleye, A.T.; Onyia, I.P.; Amune-Matthews, C.; Omorogie, M.O. Unravelling the Effect of Crystal Dislocation Density and Microstrain of Titanium Dioxide Nanoparticles on Tetracycline Removal Performance. Chem. Phys. Lett. 2021, 776, 138725. [Google Scholar] [CrossRef]
- Garcia, V.J.; Pelicano, C.M.; Yanagi, H. Low Temperature-Processed ZnO Nanorods-TiO2 Nanoparticles Composite as Electron Transporting Layer for Perovskite Solar Cells. Thin Solid Film. 2018, 662, 70–75. [Google Scholar] [CrossRef]
- Koutu, V.; Ojhab, P.; Shastri, L.; Malik, M.M. Study of the Effect of Temperature Gradient on the Thermal and Electrical Properties of ZnO Nanoparticles. AIP Conf. Proc. 2018, 1953, 030278. [Google Scholar] [CrossRef]
- Premkumar, S.; Nataraj, D.; Bharathi, G.; Ramya, S.; Thangadurai, T.D. Highly Responsive Ultraviolet Sensor Based on ZnS Quantum Dot Solid with Enhanced Photocurrent. Sci. Rep. 2019, 9, 18704. [Google Scholar] [CrossRef]
- López, R.; Gómez, R. Band-Gap Energy Estimation from Diffuse Reflectance Measurements on Sol-Gel and Commercial TiO2: A Comparative Study. J. Sol-Gel Sci. Technol. 2012, 61, 1–7. [Google Scholar] [CrossRef]
- Muhamad, S.; Kamari, H.M.; Al-Hada, N.M.; Abdullah, C.A.C.; Nidzam, N.N.S. Fabrication of Binary (ZnO)x(TiO2)1−x Nanoparticles via Thermal Treatment Route and Evaluating the Impact of Various Molar Concentrations on the Structure and Optical Behaviors. Appl. Phys. A Mater. Sci. Process. 2020, 126, 587. [Google Scholar] [CrossRef]
- Sharma, S.; Periasamy, C.; Chakrabarti, P. Thickness Dependent Study of RF Sputtered ZnO Thin Films for Optoelectronic Device Applications. Electron. Mater. Lett. 2015, 11, 1093–1101. [Google Scholar] [CrossRef]
- Mofokeng, S.J.; Kumar, V.; Kroon, R.E.; Ntwaeaborwa, O.M. Structure and Optical Properties of Dy3+ Activated Sol-Gel ZnO-TiO2 Nanocomposites. J. Alloys Compd. 2017, 711, 121–131. [Google Scholar] [CrossRef]
- Norouzzadeh, P.; Mabhouti, K.; Golzan, M.M.; Naderali, R. Investigation of Structural, Morphological and Optical Characteristics of Mn Substituted Al-Doped ZnO NPs: A Urbach Energy and Kramers-Kronig Study. Optik 2020, 204, 164227. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, Y.H.; Zheng, J.C.; Zhu, Z.Z. Direct to Indirect Band Gap Transition in Ultrathin ZnO Nanowires under Uniaxial Compression. Appl. Phys. Lett. 2009, 94, 113114. [Google Scholar] [CrossRef]
- Padmanabhan, S.C.; Pillai, S.C.; Colreavy, J.; Balakrishnan, S.; McCormack, D.E.; Perova, T.S.; Gun’ko, Y.; Hinder, S.J.; Kellys, J.M. A Simple Sol—Gel Processing for the Development of High-Temperature Stable Photoactive Anatase Titania. Chem. Mater. 2007, 19, 4474–4481. [Google Scholar] [CrossRef]
- Wang, H.; Wen, Y.; Peng, H.; Zheng, C.; Li, Y.; Wang, S.; Sun, S.; Xie, X.; Zhou, X. Grafting Polytetrafluoroethylene Micropowder via In Situ Electron Beam Irradiation-Induced Polymerization. Polymers 2018, 10, 503. [Google Scholar] [CrossRef]
- Tsiourvas, D.; Tsetsekou, A.; Arkas, M.; Diplas, S.; Mastrogianni, E. Covalent Attachment of a Bioactive Hyperbranched Polymeric Layer to Titanium Surface for the Biomimetic Growth of Calcium Phosphates. J. Mater. Sci. Mater. Med. 2011, 22, 85–96. [Google Scholar] [CrossRef]
- Kim, K.H.; Umakoshi, T.; Abe, Y.; Kawamura, M.; Kiba, T. Morphological Properties of Al-Doped ZnO Nano/Microstructures. Superlattices Microstruct. 2016, 91, 188–192. [Google Scholar] [CrossRef]
- Pant, H.R.; Pant, B.; Sharma, R.K.; Amarjargal, A.; Kim, H.J.; Park, C.H.; Tijing, L.D.; Kim, C.S. Antibacterial and Photocatalytic Properties of Ag/TiO2/ZnO Nano-Flowers Prepared by Facile One-Pot Hydrothermal Process. Ceram. Int. 2013, 39, 1503–1510. [Google Scholar] [CrossRef]
- Chougala, L.S.; Yatnatti, M.S.; Linganagoudar, R.K.; Kamble, R.R.; Kadadevarmath, J.S. A Simple Approach on Synthesis of TiO2 Nanoparticles and Its Application in Dye Sensitized Solar Cells. J. Nano Electron. Phys. 2017, 9, 04005. [Google Scholar] [CrossRef]
- Shooshtari, M.; Salehi, A.; Vollebregt, S. Effect of Temperature and Humidity on the Sensing Performance of TiO2 Nanowire-Based Ethanol Vapor Sensors. Nanotechnology 2021, 32, 325501. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Bandog, Y.; Lee, C.J. Growth of Self-Organized Hierarchical ZnO Nanoarchitectures by a Simple In/In2S3 Controlled Thermal Evaporation Process. J. Phys. Chem. B 2005, 109, 10779–10785. [Google Scholar] [CrossRef] [PubMed]
- Hao, B.; Guo, J.; Zhang, L.; Ma, H. Cr-Doped TiO2/CuO Photocatalytic Nanofilms Prepared by Magnetron Sputtering for Wastewater Treatment. Ceram. Int. 2022, 48, 7106–7116. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chen, M.W.; Hsu, C.Y.; Lien, D.H.; Chen, M.J.; He, J.H. Enhanced Recovery Speed of Nanostructured ZnO Photodetectors Using Nanobelt Networks. IEEE J. Sel. Top. Quantum Electron. 2012, 18, 1807–1811. [Google Scholar] [CrossRef]
- Pawong, W.; Wasapinyokul, K. Impressive Response of Spin-Coated ZnO Nanoparticle UV-Sensitive Devices with Various Thicknesses under Different UV Intensities. J. Electron. Mater. 2021, 50, 5375–5386. [Google Scholar] [CrossRef]
- Saleh Al-Khazali, S.M.; Al-Salman, H.S.; Hmood, A. Low Cost Flexible Ultraviolet Photodetector Based on ZnO Nanorods Prepared Using Chemical Bath Deposition. Mater. Lett. 2020, 277, 128177. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Alex, K.V.; Latha, M.B.; Sathish, K.K.S.; Sekhar, K.C. Effect of the Thickness on the Photocatalytic and the Photocurrent Properties of ZnO Films Deposited by Spray Pyrolysis. Discov. Mater. 2022, 2, 10. [Google Scholar] [CrossRef]
- Lahewil, A.S.Z.; Zyoud, S.H.; Ahmed, N.M.; Omar, A.F.; Azman, N.Z.N. Synthesis ZnO Nanoclusters Micro Active Area Using Continues Wave Blue Laser-Assisted Chemical Bath Deposition Based on UV Photodetector. Optik 2022, 260, 169099. [Google Scholar] [CrossRef]
- Abd-Alghafour, N.M.; Kadhim, I.H.; Naeem, G.A. UV Detector Characteristics of ZnO Thin Film Deposited on Corning Glass Substrates Using Low-Cost Fabrication Method. J. Mater. Sci. Mater. Electron. 2021, 33, 23888–23899. [Google Scholar] [CrossRef]
- Low, K.T.; Yam, F.K.; Beh, K.P.; Abd Manaf, A.; Beh, K.K. Characteristics and Sensing of Sol-Gel Derived Titanium Dioxide-Based Ultraviolet Photodetector on Flame Retardant-4 Board. Sens. Actuators A Phys. 2021, 323, 112654. [Google Scholar] [CrossRef]
- Hammed, N.A.; Aziz, A.A.; Usman, A.I.; Qaeed, M.A. The Sonochemical Synthesis of Vertically Aligned ZnO Nanorods and Their UV Photodetection Properties: Effect of ZnO Buffer Layer. Ultrason. Sonochem. 2019, 50, 172–181. [Google Scholar] [CrossRef]
- Shewale, P.S.; Lee, N.K.; Lee, S.H.; Kang, K.Y.; Yu, Y.S. Ti Doped ZnO Thin Film Based UV Photodetector: Fabrication and Characterization. J. Alloys Compd. 2015, 624, 251–257. [Google Scholar] [CrossRef]
- Gayen, R.N.; Paul, R.; Biswas, S. Schottky Enabled Enhanced UV Detection by Graphene Oxide Composited Transparent ZnO Thin Films. Appl. Surf. Sci. 2020, 533, 147149. [Google Scholar] [CrossRef]
- Soumya, K.; Selvam, I.P.; Vinod, C.P.; Potty, S.N. Tuning of Work Function of ZnO by Doping and Co-Doping: An Investigation Using X-ray Photoelectron Spectroscopy. Thin Solid Film. 2022, 761, 139538. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, P.; Wan, D.; Xue, C.; Zhao, J.; Shao, G. Direct Evidence of 2D/1D Heterojunction Enhancement on Photocatalytic Activity through Assembling MoS2 Nanosheets onto Super-Long TiO2 Nanofibers. Appl. Surf. Sci. 2020, 504, 144361. [Google Scholar] [CrossRef]
- Anjum, F.; Nasir, H.; Lee, J.; Kim, H.J. Fabrication and Characterization of Silver Polycrystalline Photocathode. Optik 2022, 269, 169906. [Google Scholar] [CrossRef]
- Nair, V.G.; Jayakrishnan, R.; John, J.; Salam, J.A.; Anand, A.M.; Raj, A. Anomalous Photoconductivity in Chemical Spray Pyrolysis Deposited Nano-Crystalline ZnO Thin Films. Mater. Chem. Phys. 2020, 247, 122849. [Google Scholar] [CrossRef]
- Chinnasamy, M.; Balasubramanian, K. Enhanced UV Photodetection Behavior of Cr Doped Wurtzite ZnO Crystalline Nanorods. Opt. Mater. 2020, 110, 110492. [Google Scholar] [CrossRef]



| Samples | Atomic% | ||
|---|---|---|---|
| Zn% | O% | Ti% | |
| S1 | 45.27 | 53.7 | 1.03 |
| S2 | 52.58 | 45.02 | 2.4 |
| S3 | 56.28 | 40.08 | 3.64 |
| S4 | 45.43 | 50.56 | 4.01 |
| Samples | Rq (nm) |
|---|---|
| S1 | 66.8 |
| S2 | 76.7 |
| S3 | 88.1 |
| S4 | 107 |
| Peak no. | Nanocomposite Peak | 2 | (hkl) | FWHM (°) | D (nm) | C (nm) | % |
|---|---|---|---|---|---|---|---|
| 1 | ZnO | 31.25 | 100 | 0.1968 | 41.9 | 0.572 | 9.79 |
| 2 | Ti/ZnO | 34.43 | 002 | 0.1476 | 56.4 | 0.5206 | −0.12 |
| 3 | TiO2/ZnO | 55.8 | 211/110 | 0.09 | 99.9 | (ZnO) = 0.3292 | (ZnO) = −36.8 |
| 4 | TiO2/ZnO | 62.9 | 204/103 | 0.3936 | 23.7 | (ZnO) = 0.2952 | (ZnO) = −43.3 |
| 5 | TiO2 | 69.2 | 116 | 0.09 | 107.2 | - | - |
| 6 | ZnO | 72.5 | 004 | 0.72 | 13.7 | (ZnO) = 0.2605 | (ZnO) = −50 |
| 7 (c) | TiO2 | 25.24 | 101 | 0.09 | 90.5 | - | - |
| 8 (d) | TiO2/ZnO | 47.2 | 200/102 | 0.01 | 867 | (ZnO) = 0.3848 | (ZnO) = −26.1 |
| Samples | Atomic Relative Ratios | ||
|---|---|---|---|
| S1 | 0.021 | 1.069 | 0.023 |
| S2 | 0.039 | 1.42 | 0.055 |
| S3 | 0.063 | 1.634 | 0.103 |
| S4 | 0.088 | 0.911 | 0.08 |
| Photodetector Device | τr/τd (s) | R (AW−1) | S % | G | D* × 108 (Jones) | EQE% |
|---|---|---|---|---|---|---|
| S1 | 0.17/0.27 | 0.236 | 76.04 | 1.76 | 1.40 | 80 |
| S2 | 0.23/0.36 | 0.29 | 94.86 | 1.95 | 1.74 | 98.8 |
| S3 | 0.52/0.85 | 0.419 | 195.33 | 2.95 | 3.00 | 142.76 |
| S4 | 0.63/0.99 | 0.495 | 247.14 | 3.47 | 3.68 | 168.59 |
| Materials | Bias Voltage (V) | (s) | R (AW−1) | S % | G | D* × 108 (jones) | EQE % | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| ZnO NC’s | 196 | 30 | 395 | 0.84/0.66 | 0.216 | 100.6 | 2.06 | 0.714 | 68 | [79] |
| ZnO thin film | 1.32 | 5 | 380 | 3.7/5.3 | 4.2 | 43 | - | - | - | [80] |
| TiO2 | - | 5 | 365 | 100–182/ 122–170 | 0.006614 | 2764 | - | - | - | [81] |
| ZnO NRs/Ti/ZnO/Zn | 360 | 5 | 365 | 25.74/36.73 | 0.878 | - | 3 | - | - | [82] |
| Ti-doped ZnO | 112.68 | 5 | 365 | -/~135 | 0.051 | - | - | - | - | [83] |
| Ti (5%) doped ZnO NRs | 0.53 | 5 | 365 | 1/2.5 | 0.094 | - | 8.5 | 210 | 32 | [28] |
| (Ti:TiO2/ZnO)@ZnO TFs | 346 | 3 | 365 | 0.63/0.99 | 0.495 | 247.14 | 3.47 | 3.68 | 168.59 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elzawiei, Y.S.M.; Hashim, M.R.; Halim, M.M.; Abdulhameed, A. Characterization and Growth of TiO2/ZnO on PTFE Substrates at Different Volumetric Ratios Using Chemical Bath Deposition. Coatings 2023, 13, 379. https://doi.org/10.3390/coatings13020379
Elzawiei YSM, Hashim MR, Halim MM, Abdulhameed A. Characterization and Growth of TiO2/ZnO on PTFE Substrates at Different Volumetric Ratios Using Chemical Bath Deposition. Coatings. 2023; 13(2):379. https://doi.org/10.3390/coatings13020379
Chicago/Turabian StyleElzawiei, Youssif S M, Md Roslan Hashim, Mohd Mahadi Halim, and Abdullah Abdulhameed. 2023. "Characterization and Growth of TiO2/ZnO on PTFE Substrates at Different Volumetric Ratios Using Chemical Bath Deposition" Coatings 13, no. 2: 379. https://doi.org/10.3390/coatings13020379





