The Effect of Heat Treatment on the Structural-Phase State and Abrasive Wear Resistance of a Hard-Anodized Layer on Aluminum Alloy 1011
Abstract
:1. Introduction
2. Materials and Methods
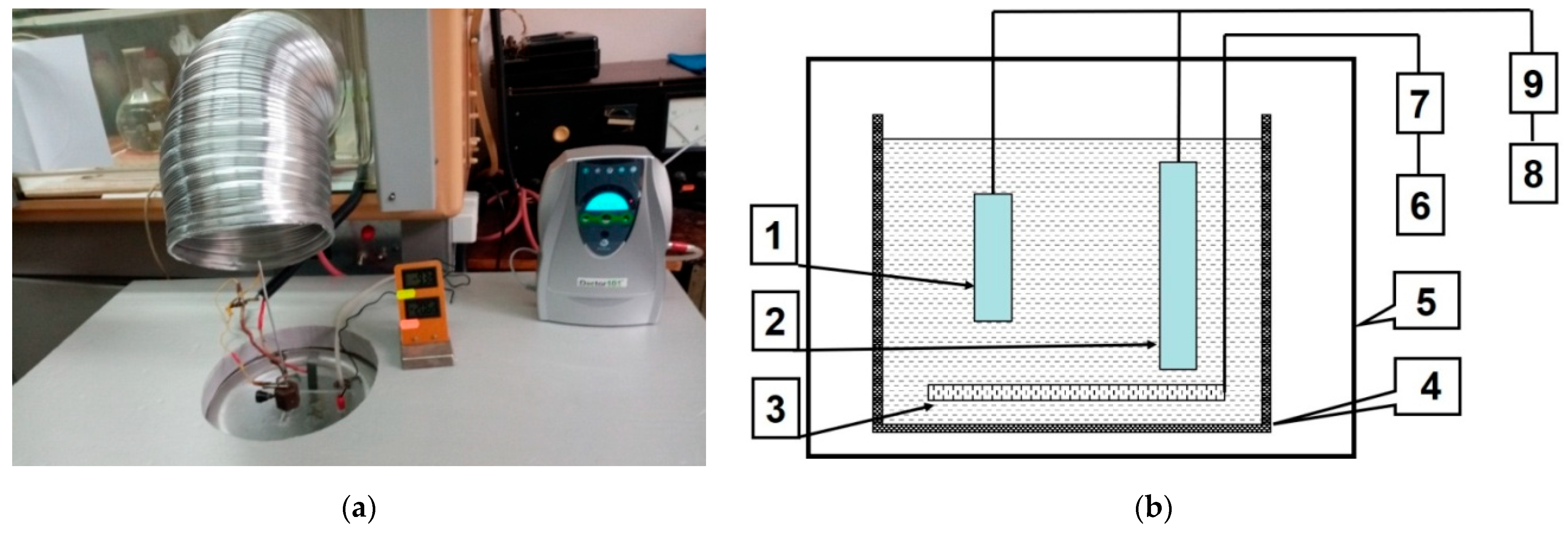
2.1. The Method of Syntheses of Hard-Anodized Layers
2.2. Alternative Surface Hardening Methods for Aluminum Alloys Used in the Article to Rank HAL Properties
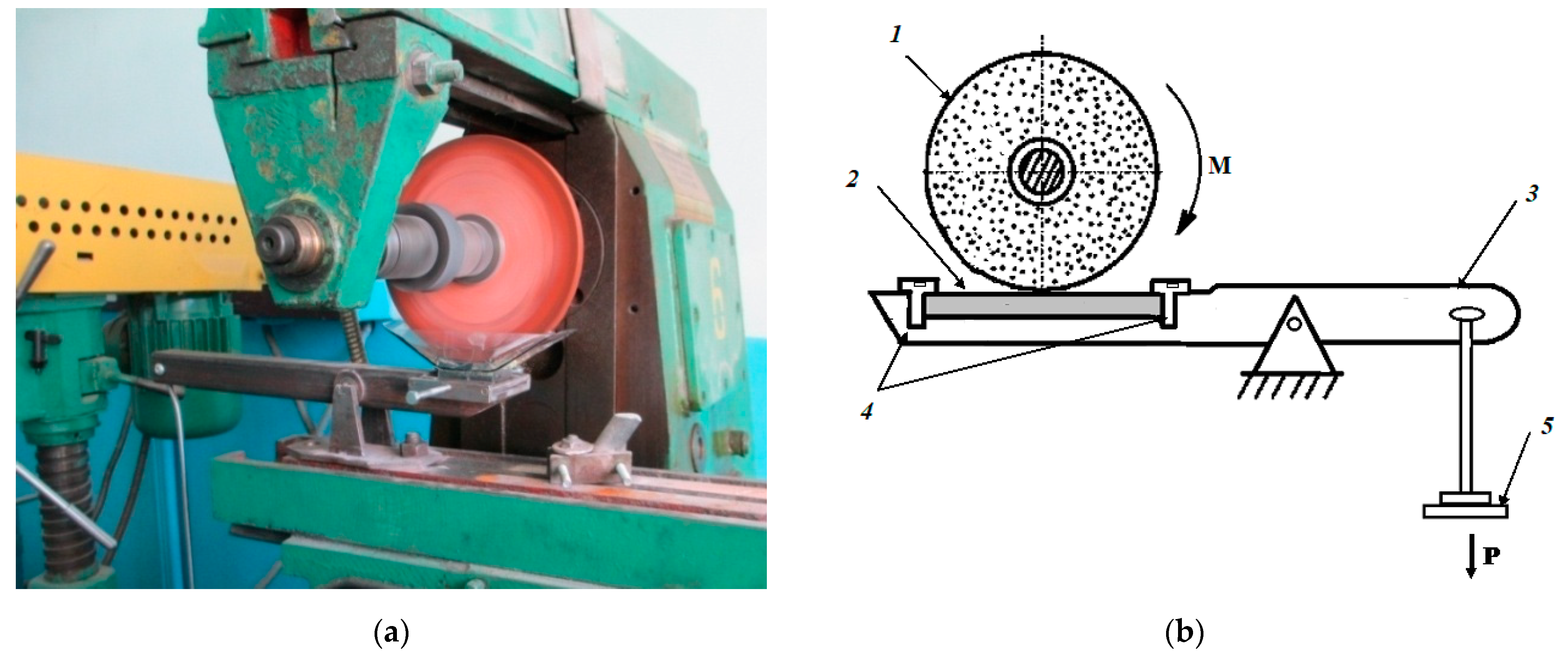
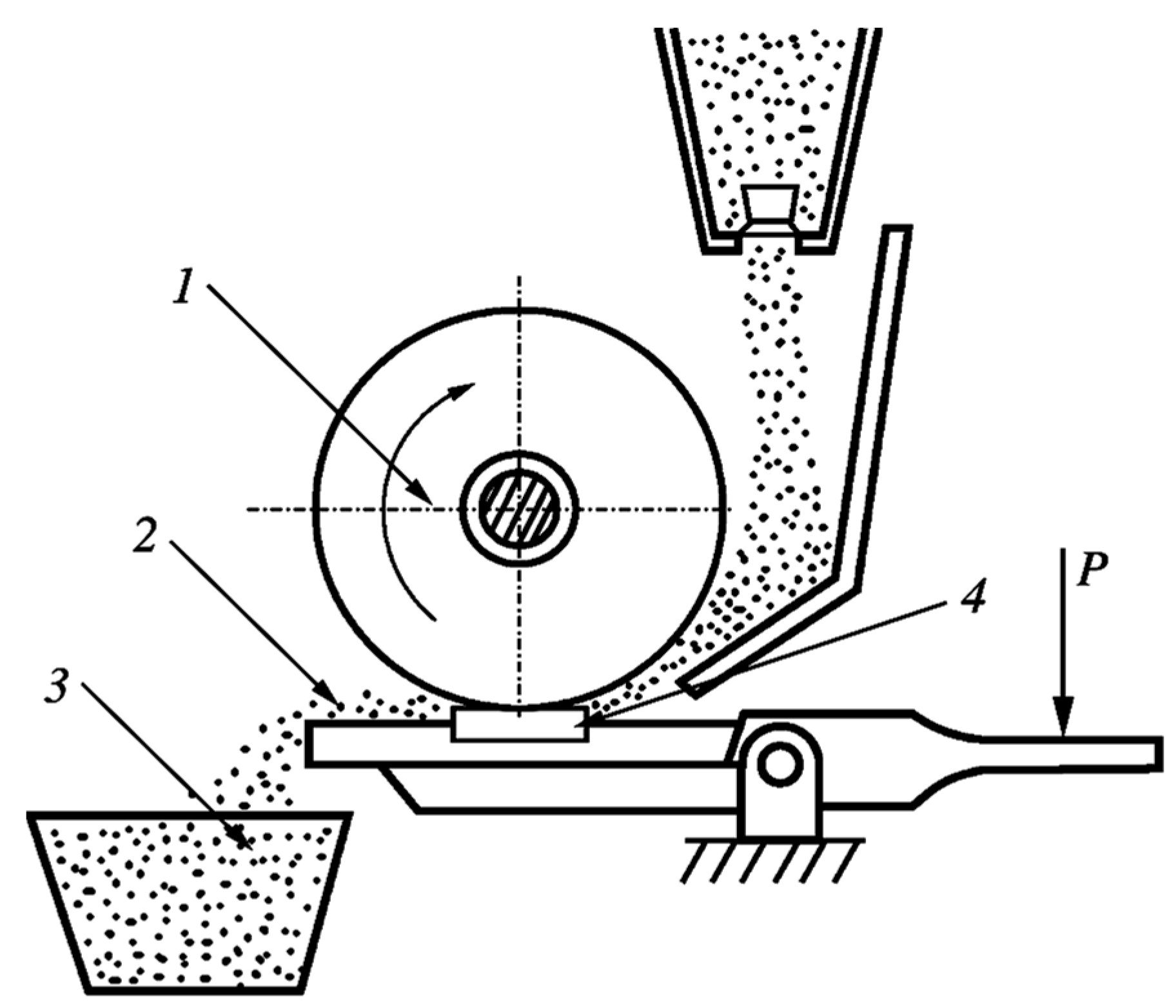
2.3. Test Method for Determining the Abrasive Wear Resistance of Hard-Anodized Layers
3. Results
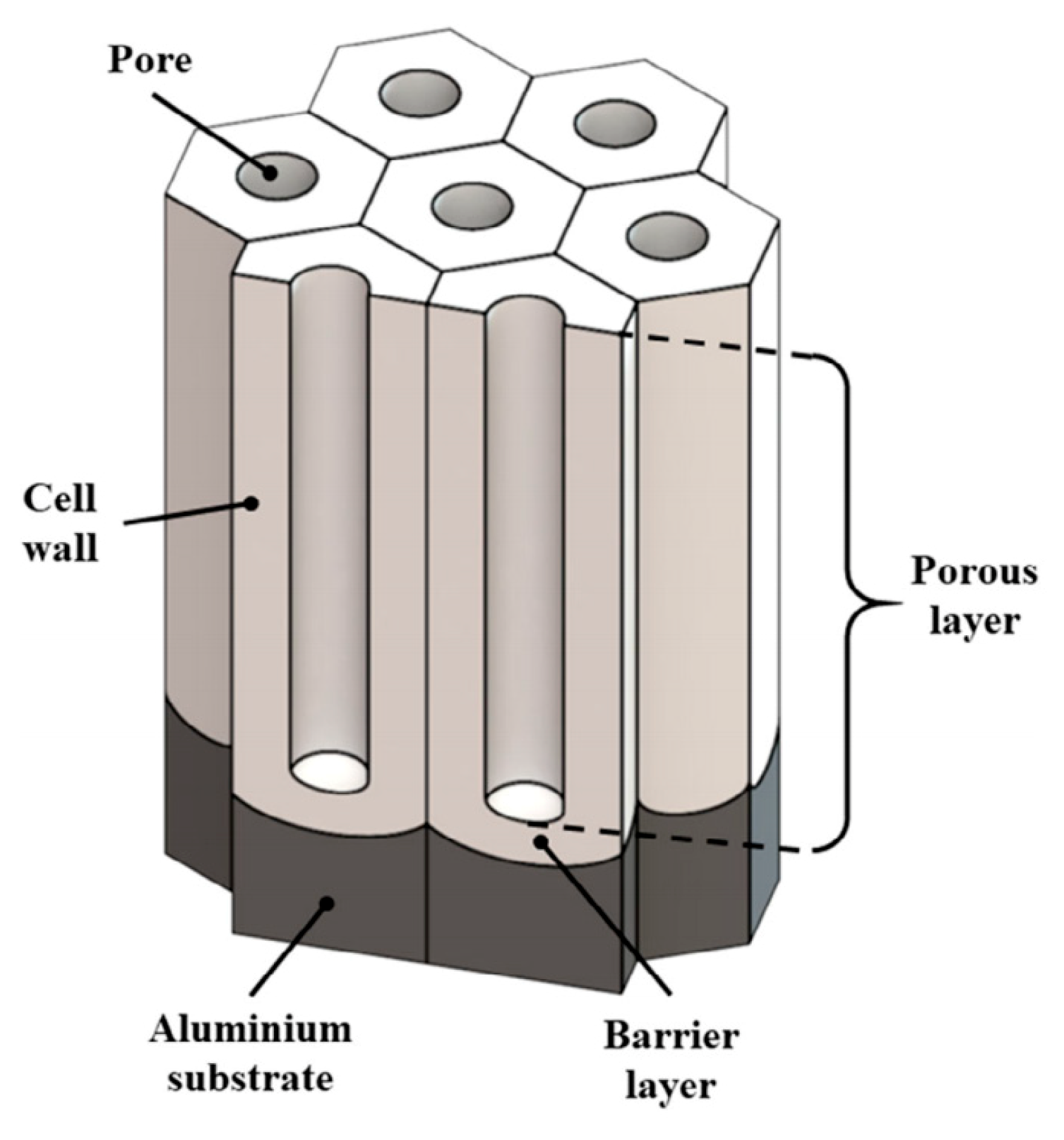
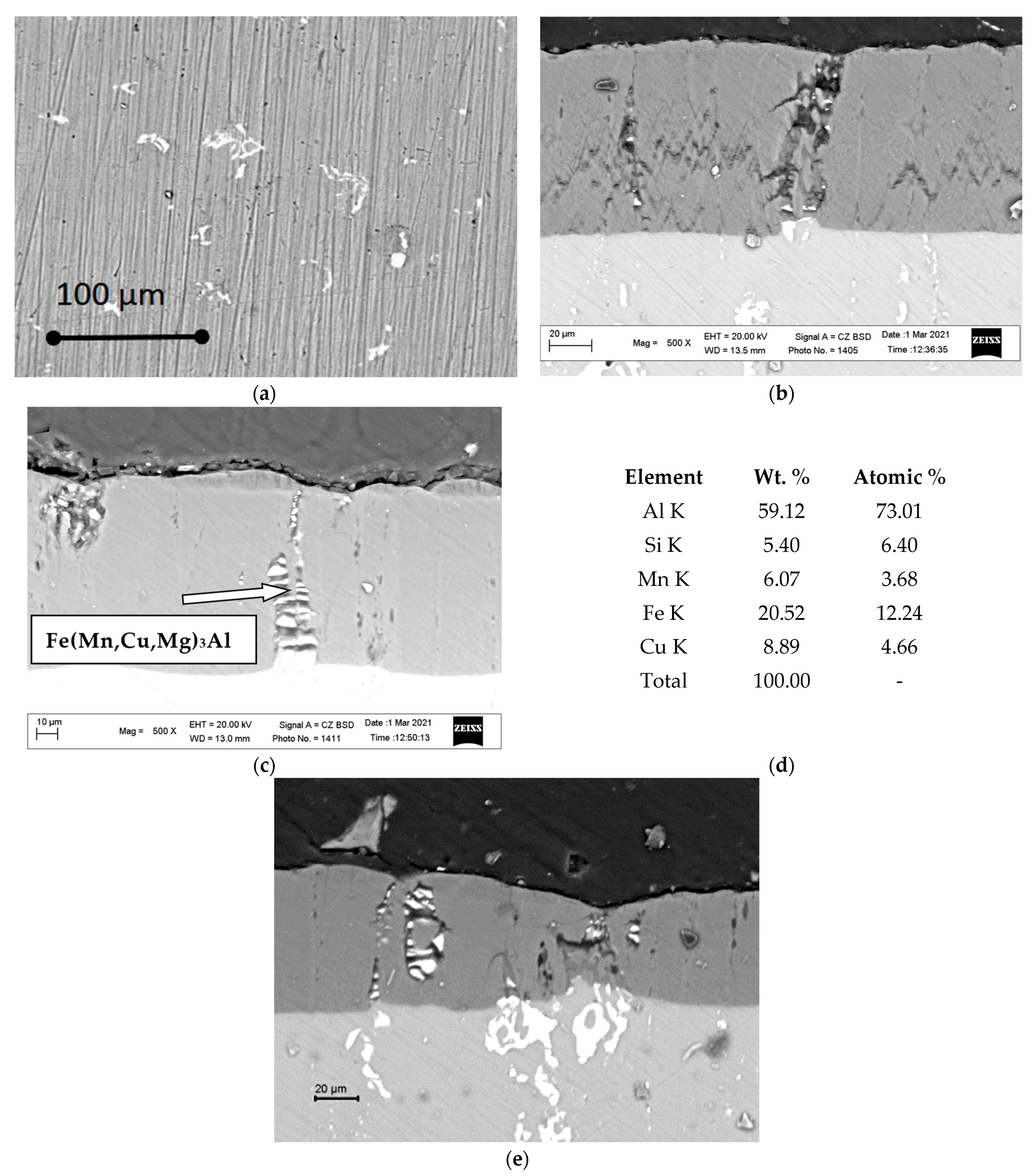
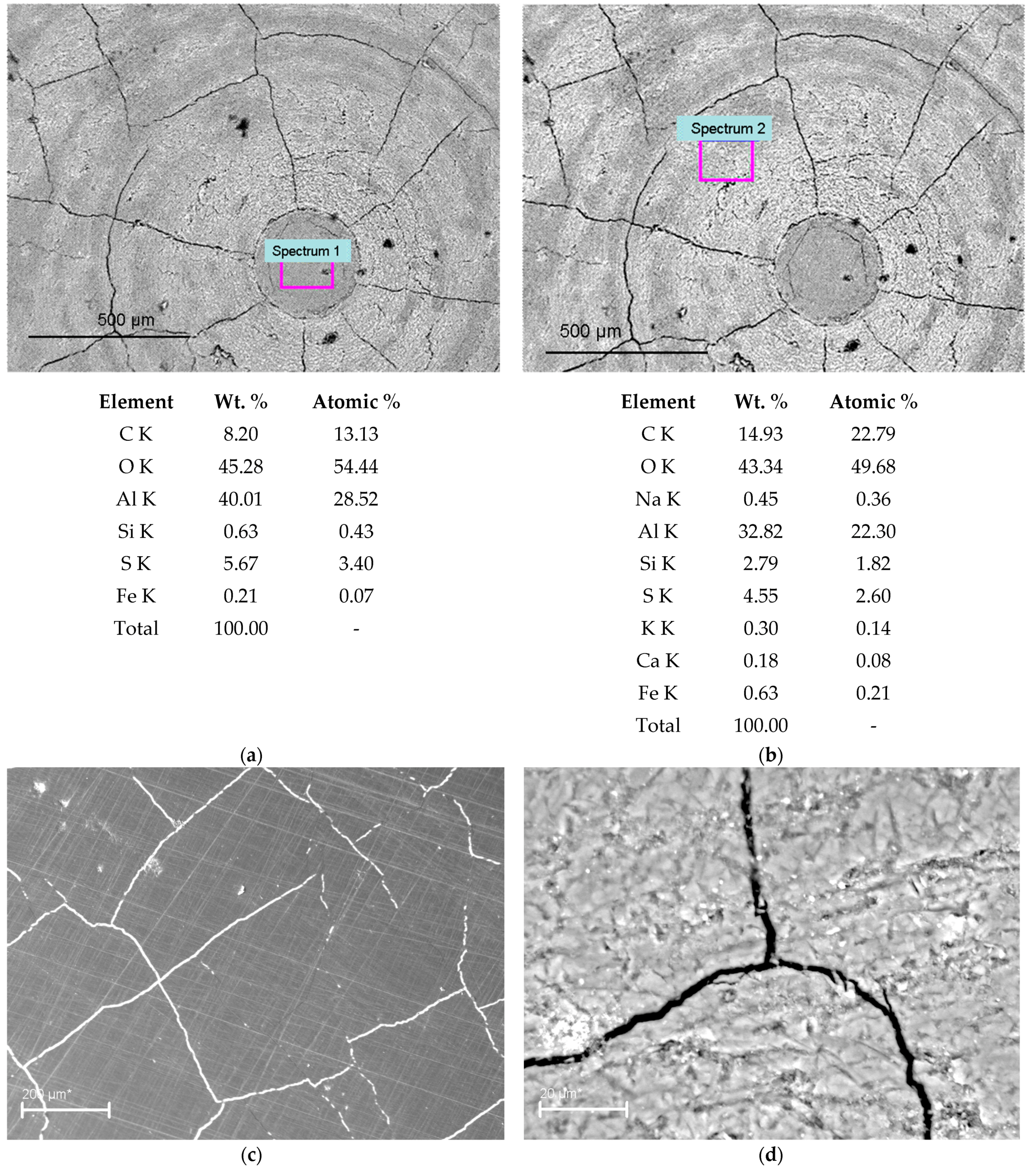
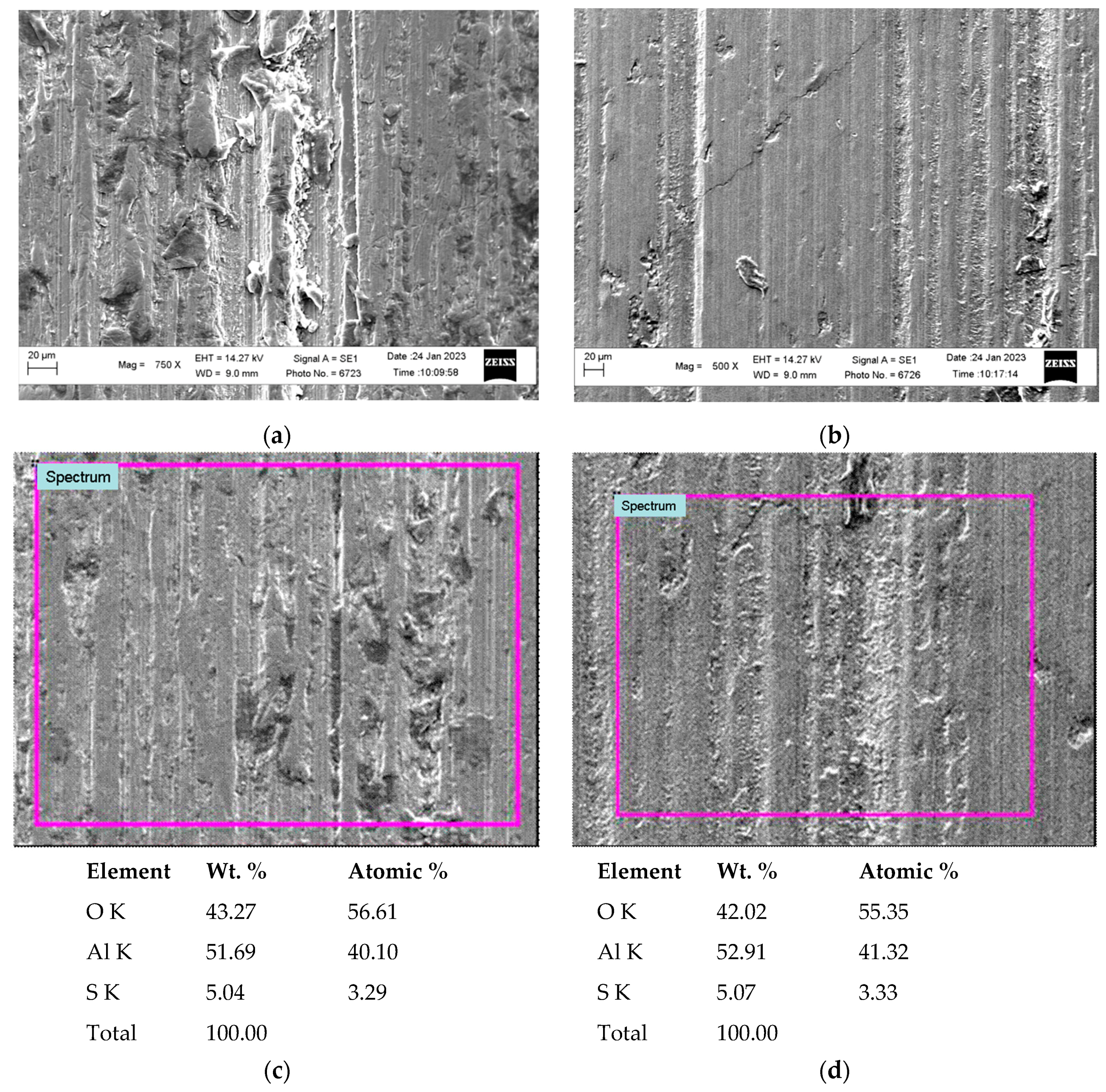
3.1. Metallographic Analysis of the Structure of Hard Anodized Layers
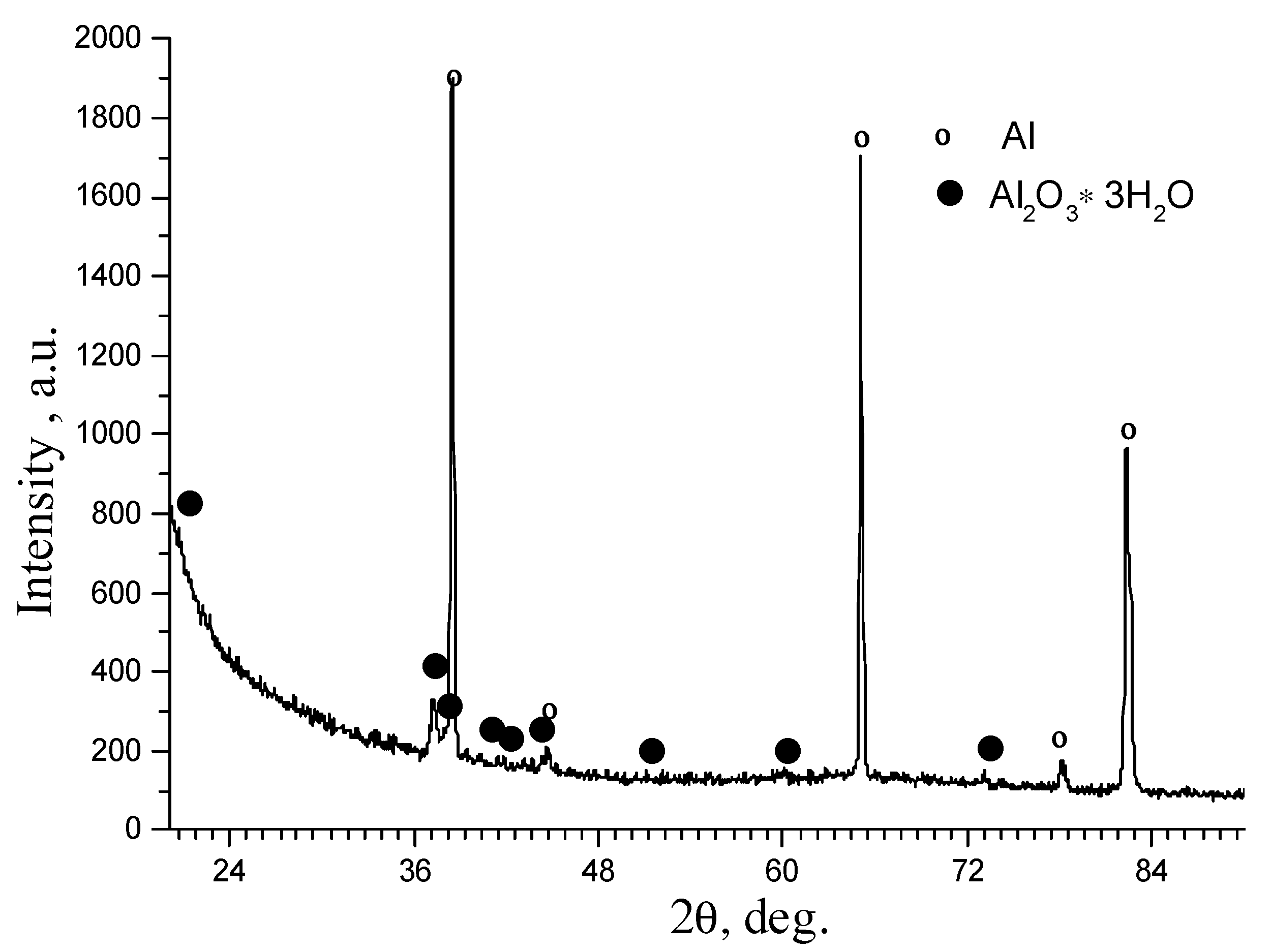
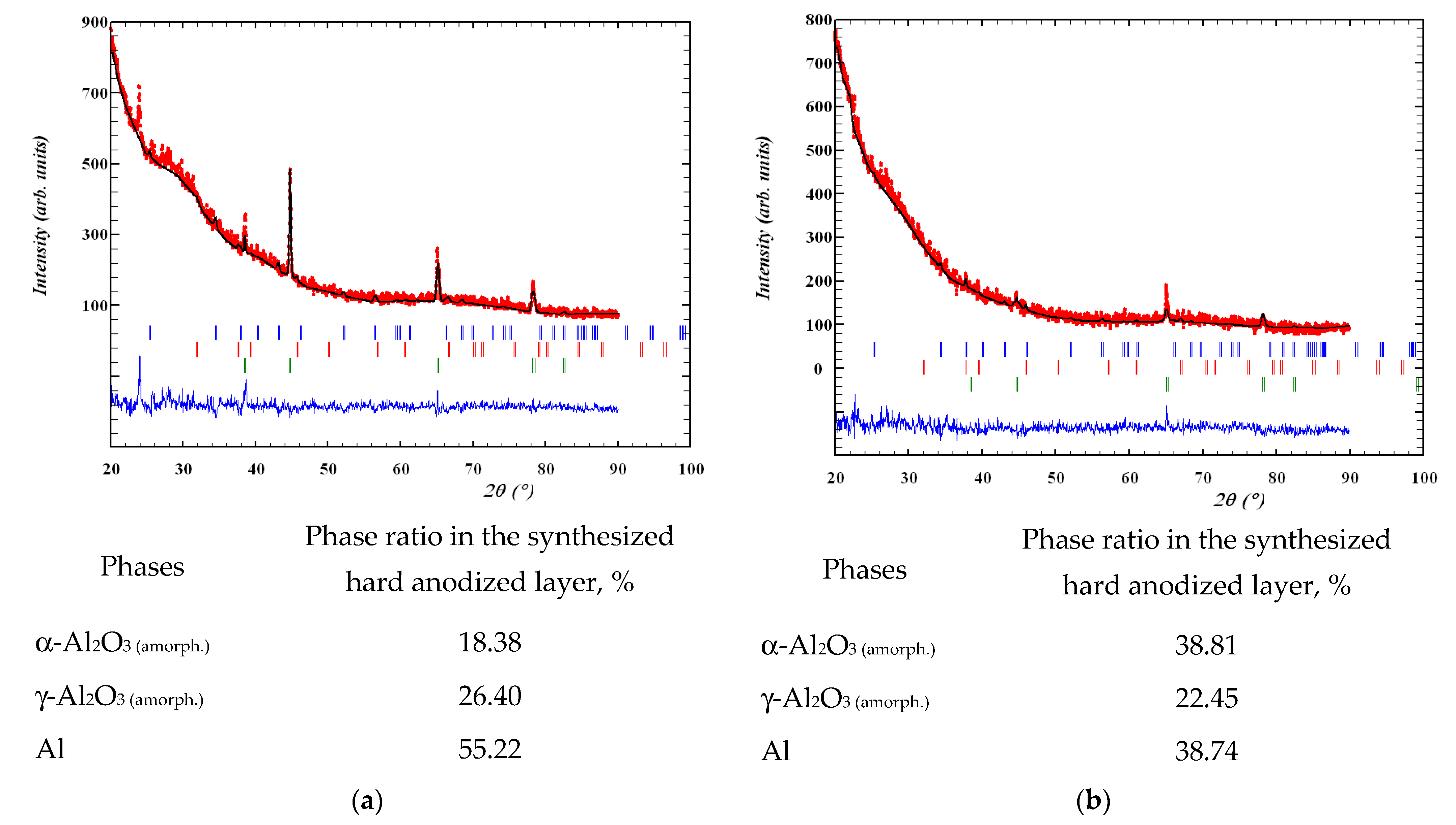
3.2. Phase Analysis of Anodized Layers Synthesized on the Surface of 1011 Aluminum Alloy
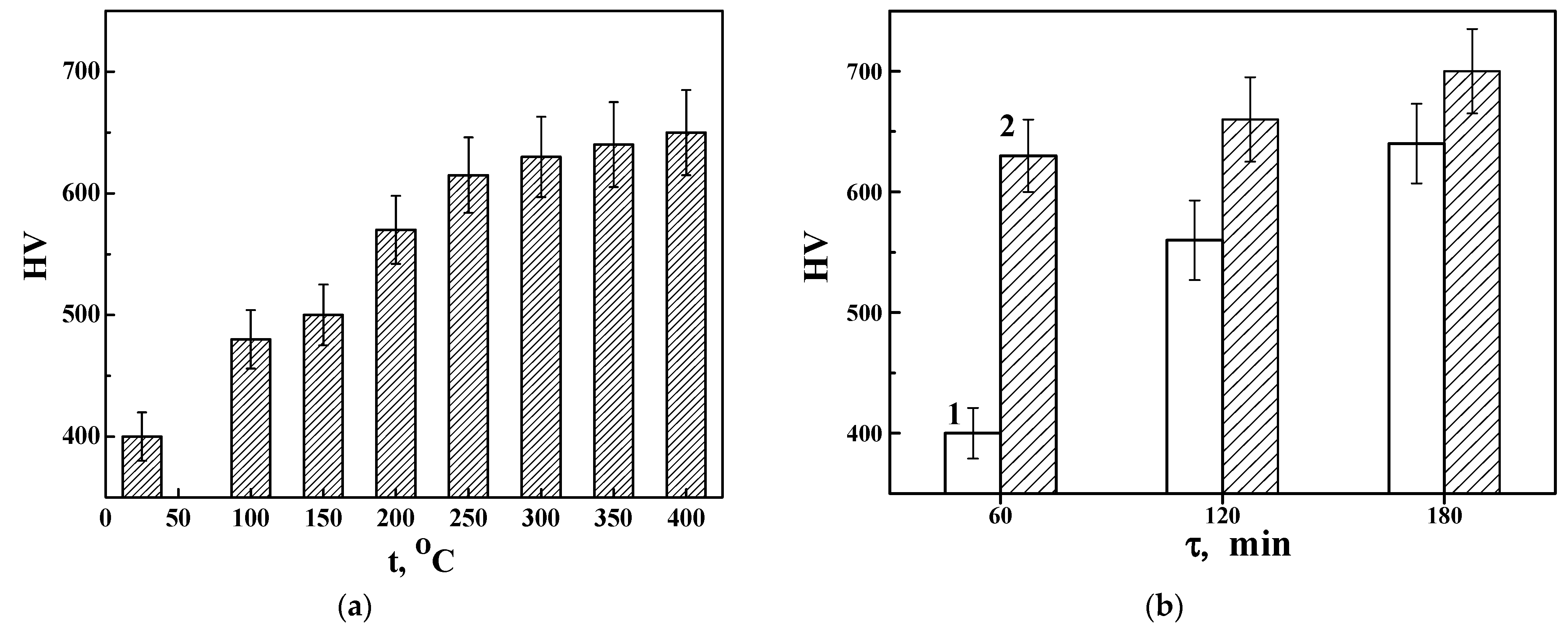
3.3. The Hardness HV of Hard Anodized Layers on the Surface of Aluminum Alloy 1011
3.4. Negative Manifestations of Heat Treatment on Hard Anodized Layers
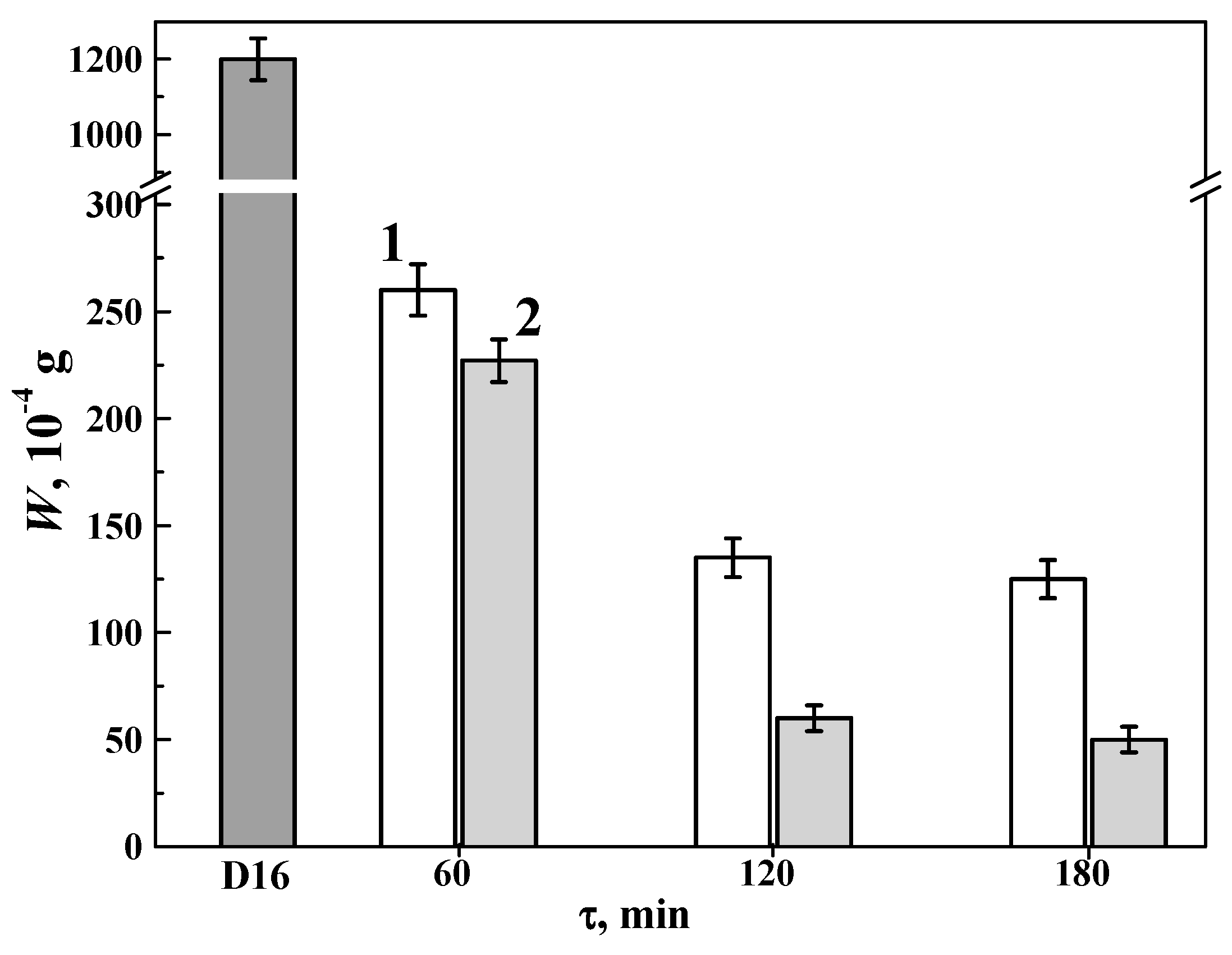
3.5. Abrasive Wear Resistance of 1011 Aluminum Alloy with Hard Anodized Layers on Its Surface
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peltier, F.; Thierry, D. Review of Cr-free coatings for the corrosion protection of aluminum aerospace alloys. Coatings 2022, 12, 518. [Google Scholar] [CrossRef]
- Hutsaylyuk, V.; Student, M.; Zadorozhna, K.; Student, O.; Veselivska, H.; Gvosdetskii, V.; Maruschak, P.; Pokhmurska, H. Improvement of wear resistance of aluminum alloy by HVOF method. J. Mater. Res. Technol. 2020, 9, 16367. [Google Scholar] [CrossRef]
- Student, M.; Gvozdetsky, V.; Student, O.; Prentkovskis, O.; Maruschak, P.; Olenyuk, O.; Titova, L. The Effect of Increasing the Air Flow Pressure on the Properties of Coatings during the Arc Spraying of Cored Wires. Stroj. Čas. J. Mech. Eng. 2019, 69, 133–146. [Google Scholar] [CrossRef]
- Dolgov, N.A.; Romashin, S.N.; Frolenkova, L.Y.; Shorkin, V.S. A model of contact of elastic bodies with account for their adhesion. Int. J. Nanomech. Sci. Technol. 2015, 6, 117–133. [Google Scholar] [CrossRef]
- Peng, Z.; Xu, H.; Liu, S.; Qi, Y.; Liang, J. Wear and corrosion resistance of plasma electrolytic oxidation coatings on 6061 Al alloy in electrolytes with aluminate and phosphate. Materials 2021, 14, 4037. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Famiyeh, L. Plasma electrolytic oxidation coatings on aluminum alloys: Microstructures, properties, and applications. Mod. Concept Mater. Sci. 2019, 2. [Google Scholar] [CrossRef]
- Treviño, M.; Garza-Montes-de-Oca, N.F.; Pérez, A.; Hernández-Rodríguez, M.A.L.; Juárez, A.; Colás, R. Wear of an aluminium alloy coated by plasma electrolytic oxidation. Surf. Coat. Technol. 2012, 206, 2213–2219. [Google Scholar] [CrossRef]
- Aydin, H.; Bayram, A.; Uguz, A. Abrasive and sliding wear characteristics of Al-Si cast alloys before and after coating by plasma electrolytic oxidation process. Mater. Test. 2008, 50, 318–325. [Google Scholar] [CrossRef]
- Sieber, M.; Simchen, F.; Morgenstern, R.; Scharf, I.; Lampke, T. Plasma electrolytic oxidation of high-strength aluminium alloys—Substrate effect on wear and corrosion performance. Metals 2018, 8, 356. [Google Scholar] [CrossRef]
- Pritchard, C.; Robinson, P.R. Abrasion resistance of hard anodised aluminium. Wear 1969, 13, 361–368. [Google Scholar] [CrossRef]
- Chiang, M.-H.; Yeh, C.-C.; Lee, C.-L. Improvement in abrasive wear resistance of an aluminum alloy casting for a continuously-variable transmission using heat treatment and pulsed anodizing. Wear 2020, 442–443, 203137. [Google Scholar] [CrossRef]
- Kwolek, P. Hard anodic coatings on aluminium alloys. Adv. Manuf. Sci. Technol. 2007, 41, 3. [Google Scholar] [CrossRef]
- Bazaluk, O.; Dubei, O.; Ropyak, L.; Shovkoplias, M.; Pryhorovska, T.; Lozynskyi, V. Strategy of compatible use of jet and plunger pump with chrome parts in oil well. Energies 2022, 15, 83. [Google Scholar] [CrossRef]
- Dubei, O.Y.; Tutko, T.F.; Ropyak, L.Y.; Shovkoplias, M.V. Development of Analytical Model of Threaded Connection of Tubular Parts of Chrome-Plated Metal Structures. Metallofiz. Noveishie Tekhnol. 2022, 44, 251–272. [Google Scholar] [CrossRef]
- Clyne, T.W.; Troughton, S.C. A review of recent work on discharge characteristics during plasma electrolytic oxidation of various metals. Int. Mater. Rev. 2019, 64, 127–162. [Google Scholar] [CrossRef]
- Simchen, F.; Sieber, M.; Kopp, A.; Lampke, T. Introduction to Plasma Electrolytic Oxidation—An Overview of the Process and Applications. Coatings 2020, 10, 628. [Google Scholar] [CrossRef]
- Sikdar, S.; Menezes, P.V.; Maccione, R.; Jacob, T.; Menezes, P.L. Plasma Electrolytic Oxidation (PEO) Process—Processing, Properties, and Applications. Nanomaterials 2021, 11, 1375. [Google Scholar] [CrossRef]
- Ropyak, L.Y.; Velychkovych, A.S.; Vytvytskyi, V.S.; Shovkoplias, M.V. Analytical study of “crosshead–slide rail” wear effect on pump rod stress state. J. Phys. Conf. Ser. 2021, 1741, 012039. [Google Scholar] [CrossRef]
- Wu, Z.; Xia, Y.; Li, G.; Xu, F. Structure and mechanical properties of ceramic coatings fabricated by plasma electrolytic oxidation on aluminized steel. Appl. Surf. Sci. 2007, 253, 8398–8403. [Google Scholar] [CrossRef]
- Wielage, B.; Meyer, D.; Pokhmurska, H.; Alisch, G.; Grund, T. Plasma electrolytic oxidation of arc sprayed aluminium coatings [Plasmaelektrolytische Oxidation Thermisch Gespritzter Aluminiumschichten]. Materialwissenschaft Werkstofftechnik 2008, 39, 920–923. [Google Scholar] [CrossRef]
- Lampke, T.; Meyer, D.; Alisch, G.; Wielage, B.; Pokhmurska, H.; Klapkiv, M.; Student, M. Corrosion and wear behavior of alumina coatings obtained by various methods. Mater. Sci. 2011, 46, 591–598. [Google Scholar] [CrossRef]
- Durdu, S.; Aktuğ, S.L.; Korkmaz, K. Characterization and mechanical properties of the duplex coatings produced on steel by electro-spark deposition and micro-arc oxidation. Surf. Coat. Technol. 2013, 236, 303–308. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, X.; Lu, S.; Zhang, Y.; Hu, H.; Lu, K.; Zhang, J. Investigation of Stray Radiation Suppression in Infrared Imaging System Using a Novel Broadband and High-Absorption Ceramic Coating. Appl. Sci. 2021, 11, 4952. [Google Scholar] [CrossRef]
- Bembenek, M.; Makoviichuk, M.; Shatskyi, I.; Ropyak, L.; Pritula, I.; Gryn, L.; Belyakovskyi, V. Optical and Mechanical Properties of Layered Infrared Interference Filters. Sensors 2022, 22, 8105. [Google Scholar] [CrossRef]
- Hutsaylyuk, V.; Student, M.; Posuvailo, V.; Student, O.; Sirak, Y.; Hvozdets'kyi, V.; Maruschak, P.; Veselivska, H. The properties of oxide-ceramic layers with Cu and Ni inclusions synthesizing by PEO method on top of the gas-spraying coatings on aluminium alloys. Vacuum 2020, 179, 109514. [Google Scholar] [CrossRef]
- Student, M.; Shmyrko, V.; Klapkiv, M.; Lyasota, M.; Dobrovol’ska, L. Evaluation of the mechanical properties of combined metal-oxide-ceramic layers on aluminum alloys. Mater. Sci. 2014, 50, 290–295. [Google Scholar] [CrossRef]
- Student, M.M.; Dovhunyk, V.M.; Klapkiv, M.D.; Posuvailo, V.M.; Shmyrko, V.V.; Kytsya, A.P. Tribological Properties of combined metal-oxide–ceramic layers on light alloys. Mater. Sci. 2012, 48, 180–190. [Google Scholar] [CrossRef]
- Lu, X.; Mohedano, M.; Blawert, C.; Matykina, E.; Arrabal, R.; Kainer, K.U.; Zheludkevich, M.L. Plasma electrolytic oxidation coatings with particle additions—A review. Surf. Coat. Technol. 2016, 307, 1165–1182. [Google Scholar] [CrossRef]
- Student, M.M.; Pohrelyuk, I.M. Modification of the Surfaces of Aluminum and Titanium Alloys Aimed at the Improvement of Their Wear Resistance and Tribological Characteristics. Mater. Sci. 2021, 57, 377–386. [Google Scholar] [CrossRef]
- Student, M.M.; Pohrelyuk, I.M.; Hvozdetskyi, V.M.; Veselivska, H.H.; Zadorozhna, K.R.; Mardarevych, R.S.; Dzioba, Y.V. Influence of the Composition of Electrolyte for Hard Anodizing of Aluminum on the Characteristics of Oxide Layer. Mater. Sci. 2021, 57, 240–247. [Google Scholar] [CrossRef]
- Remešová, M.; Tkachenko, S.; Kvarda, D.; Ročňáková, I.; Gollas, B.; Menelaou, M.; Čelko, L.; Kaiser, J. Effects of anodizing conditions and the addition of Al2O3/PTFE particles on the microstructure and the mechanical properties of porous anodic coatings on the AA1050 aluminium alloy. Appl. Surface Sci. 2020, 513, 145780. [Google Scholar] [CrossRef]
- Kwolek, P.; Drapała, D.; Krupa, K.; Obłój, A.; Tokarski, T.; Sieniawski, J. Mechanical properties of a pulsed anodised 5005 aluminium alloy. Surface Coat. Technol. 2020, 383, 125233. [Google Scholar] [CrossRef]
- Student, M.M.; Pohrelyuk, I.M.; Chumalo, H.V.; Hvozdetskyi, V.M. Improvement of the functional characteristics of coatings obtained by the method of hard anodizing of aluminum alloys. Mater. Sci. 2021, 56, 820–829. [Google Scholar] [CrossRef]
- Roshani, M.; Sabour Rouhaghdam, A.; Aliofkhazraei, M.; Heydari Astaraee, A. Optimization of mechanical properties for pulsed anodizing of aluminum. Surf. Coat. Technol. 2017, 310, 17–24. [Google Scholar] [CrossRef]
- Lämmel, C.; Schneider, M.; Heubner, C.; Beckert, W.; Michaelis, A. Investigations of burning phenomena during the hard anodising of aluminium by local in-operando temperature measurements. Electrochim. Acta 2017, 249, 271–277. [Google Scholar] [CrossRef]
- Sheasby, P.G.; Pinner, R. The Surface Treatment and Finishing of Aluminium and Its Alloys, 6th ed.; ASM International: Novelty, OH, USA, 2001; Volume 1, ISBN 0-904477-21-5. Volume 2, ISBN 0-904477-22-3. [Google Scholar]
- Li, F.; Zhang, L.; Metzger, R.M. On the growth of highly ordered pores in anodized aluminum oxide. Chem. Mater. 1998, 10, 2470–2480. [Google Scholar] [CrossRef]
- Mohammadi, I.; Ahmadi, S.; Afshar, A. Effect of pulse current parameters on the mechanical and corrosion properties of anodized nanoporous aluminum coatings. Mater. Chem. Phys. 2016, 183, 490–498. [Google Scholar] [CrossRef]
- Aerts, T.; Dimogerontakis, T.; De Graeve, I.; Fransaer, J.; Terryn, H. Influence of the anodizing temperature on the porosity and the mechanical properties of the porous anodic oxide film. Surf. Coat. Technol. 2007, 201, 7310–7317. [Google Scholar] [CrossRef]
- Mohammadi, I.; Afshar, A. Modification of nanostructured anodized aluminum coatings by pulse current mode. Surf. Coat. Technol. 2015, 278, 48–55. [Google Scholar] [CrossRef]
- Bargui, M.; Elleuch, K.; Wery, M.; Ayedia, H.F. Optimization of mechanical and tribological properties of anodized 5754 aluminium alloy. Surf. Eng. Appl. Electrochem. 2017, 53, 371–382. [Google Scholar] [CrossRef]
- Niedźwiedź, M.; Skoneczny, W.; Bara, M. The influence of anodic alumina coating nanostructure produced on EN AW-5251 alloy on type of tribological wear process. Coatings 2020, 10, 105. [Google Scholar] [CrossRef]
- Kwolek, P.; Krupa, K.; Obłój, A.; Kocurek, P.; Wierzbińska, M.; Sieniawski, J. Tribological properties of the oxide coatings produced onto 6061-T6 aluminum alloy in the hard anodizing process. J. Mater. Eng. Perform. 2018, 27, 3268–3275. [Google Scholar] [CrossRef]
- Rodriguez, L.; Vieu, A.; Balsarin, M.; Combes, P.; Alexis, J.; Esvan, J.; Lesko, S.; Denape, J.; Paris, J.-Y.; Delbé, K. Physico-chemical characterisation and tribological behaviour of ground micro-arc oxidation coating on aluminium alloy—Comparison with hard anodised oxidation. Wear 2023, 516–517, 204591. [Google Scholar] [CrossRef]
- Hu, N.; Ge, S.; Fang, L. Tribological properties of nano-porous anodic aluminum oxide template. J. Cent. South Univ. Technol. 2011, 18, 1004–1008. [Google Scholar] [CrossRef]
- Kolenchin, N.F.; Kuskov, V.N. Application of ozone-air mixture for intensification of anodizing processes of aluminum alloy AK74. Strength. Technol. Coat. 2013, 2, 6–8. (In Russian) [Google Scholar]
- Bozza, A.; Giovanardi, R.; Manfredini, T.; Mattioli, P. Pulsed current effect on hard anodizing process of 7075-T6 aluminium alloy. Surf. Coat. Technol. 2015, 270, 139–144. [Google Scholar] [CrossRef]
- Bononi, M.; Giovanardi, R.; Bozza, A. Pulsed current hard anodizing of heat treated aluminum alloys: Frequency and current amplitude influence. Surf. Coat. Technol. 2016, 307, 861–870. [Google Scholar] [CrossRef]
- Forn, A.; Picas, J.A.; Baile, M.T.; Martin, E. Microstructure and tribological properties of anodic oxide layer formed on Al–Si alloy produced by semisolid processing. Surf. Coat. Technol. 2007, 202, 1139–1143. [Google Scholar] [CrossRef]
- Available online: https://en.wikipedia.org/wiki/Gibbsite (accessed on 20 December 2022).
- Available online: https://liv-unikon.com.ua/gidroksyd-alyuminiyu-i-jogo-riznovydy.html (accessed on 20 December 2022).
- Hutsaylyuk, V.; Student, M.; Posuvailo, V.; Student, O.; Hvozdets'kyi, V.; Maruschak, P.; Zakiev, V. The role of hydrogen in the formation of oxide-ceramic layers on aluminum alloys during their plasma-electrolytic oxidation. J. Mater. Res. Technol. 2021, 14, 1682–1696. [Google Scholar] [CrossRef]
- Abd-Elnaiem, A.M.; Gaber, A. Parametric study on the anodization of pure aluminum thin film used in fabricating nano-pores Template. Int. J. Electrochem. 2013, 8, 9741–9751. [Google Scholar]
- Scampone, G.; Timelli, G. Anodizing Al–Si foundry alloys: A critical review. Adv. Eng. Mater. 2022, 24, 2101480. [Google Scholar] [CrossRef]
- Van Gog, H. First-principles study of dehydration interfaces between diaspore and corundum, gibbsite and boehmite, and boehmite and γ-Al2O3: Energetic stability, interface charge effects, and dehydration defects. Appl. Surf. Sci. 2021, 541, 148501. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, W.; Wang, W.; Wang, L.; Xue, Q. Novel anodic oxide film with self-sealing layer showing excellent corrosion resistance. Sci. Rep. 2017, 7, 1344. [Google Scholar] [CrossRef] [PubMed]



| Spraying Methods | Relative Abrasive Wear Resistance * of Specimens Obtained During Tests | Microhardness at Loading 50 g, HV | Relative Energy Costs ** for Forming a Hardened Layer with a Thickness of 100 Microns on an Area of 1 m2 | Ecological Advantages and Disadvantages of the Analyzed Processes | |
|---|---|---|---|---|---|
| with a Rigidly Fixed Abrasive | with Non-Fixed Abrasive | ||||
| Standart for comparison: aluminium alloy D16 (2024 ISO) | 1 | 1 | 110 | − | − |
| 100Cr6 steel (SAE 52100) (HRC 64) | 20 | 3 | 800 | − | CO2 emissions to the atmosphere |
| Galvanic chrome plating | 35 | 5 | 1000 | 3 | Carcinogenic electrolytes |
| HVOF spraying by VC carbides | 75–85 | 7–10 | 1100, with carbide VC microhardness to 2500 HV | 7 | Noise level: 130 dB; dust from micron-sized particles |
| PEO | 70–90 | 8–10 | 1900 | 7 | Eco-friendly electrolytes |
| HAL | 20 | 3 | 700 | 2 | Eco-friendly electrolytes |
| ASC | 30 | 5 | 750, with oxides microhardness to 800–2000 HV | 1 | Noise level: 120 dB; dust from micron-sized particles |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Student, M.; Pohrelyuk, I.; Padgurskas, J.; Hvozdets’kyi, V.; Zadorozna, K.; Chumalo, H.; Student, O.; Kovalchuk, I. The Effect of Heat Treatment on the Structural-Phase State and Abrasive Wear Resistance of a Hard-Anodized Layer on Aluminum Alloy 1011. Coatings 2023, 13, 391. https://doi.org/10.3390/coatings13020391
Student M, Pohrelyuk I, Padgurskas J, Hvozdets’kyi V, Zadorozna K, Chumalo H, Student O, Kovalchuk I. The Effect of Heat Treatment on the Structural-Phase State and Abrasive Wear Resistance of a Hard-Anodized Layer on Aluminum Alloy 1011. Coatings. 2023; 13(2):391. https://doi.org/10.3390/coatings13020391
Chicago/Turabian StyleStudent, Mykhailo, Iryna Pohrelyuk, Juozas Padgurskas, Volodymyr Hvozdets’kyi, Khrystyna Zadorozna, Halyna Chumalo, Oleksandra Student, and Ihor Kovalchuk. 2023. "The Effect of Heat Treatment on the Structural-Phase State and Abrasive Wear Resistance of a Hard-Anodized Layer on Aluminum Alloy 1011" Coatings 13, no. 2: 391. https://doi.org/10.3390/coatings13020391
APA StyleStudent, M., Pohrelyuk, I., Padgurskas, J., Hvozdets’kyi, V., Zadorozna, K., Chumalo, H., Student, O., & Kovalchuk, I. (2023). The Effect of Heat Treatment on the Structural-Phase State and Abrasive Wear Resistance of a Hard-Anodized Layer on Aluminum Alloy 1011. Coatings, 13(2), 391. https://doi.org/10.3390/coatings13020391







