Reconstructed Cd(0001) Surface Induced by Adsorption of Triphenyl Bismuth
Abstract
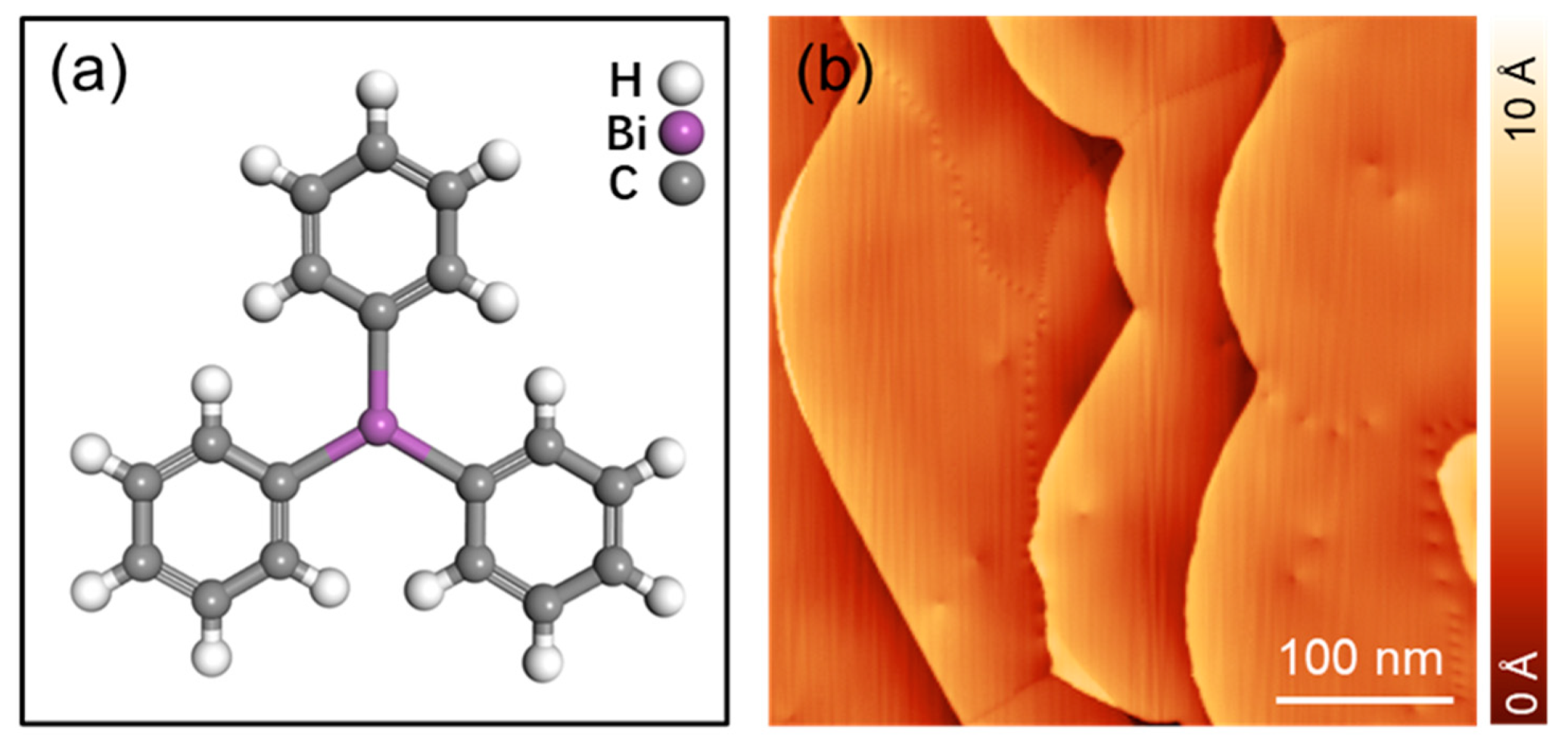
:1. Introduction
2. Experimental Method
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Goronzy, D.P.; Ebrahimi, M.; Rosei, F.; Arramel; Fang, Y.; De Feyter, S.; Tait, S.L.; Wang, C.; Beton, P.H.; Wee, A.T.S.; et al. Supramolecular Assemblies on Surfaces: Nanopatterning, Functionality, and Reactivity. ACS Nano 2018, 12, 7445–7481. [Google Scholar] [CrossRef] [PubMed]
- Gimzewski, J.K.; Modesti, S.; Schlittler, R.R. Cooperative Self-Assembly of Au Atoms and C60 on Au(110) Surfaces. Phys. Rev. Lett. 1994, 72, 1036–1039. [Google Scholar] [CrossRef] [PubMed]
- Rosei, F.; Schunack, M.; Naitoh, Y.; Jiang, P.; Gourdon, A.; Laegsgaard, E.; Stensgaard, I.; Joachim, C.; Besenbacher, F. Properties of Large Organic Molecules on Metal Surfaces. Prog. Surf. Sci. 2003, 71, 95–146. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, Q.; Zhang, Y.; Hou, S.; Wang, Y. Ordered Patterns of Copper Phthalocyanine Nanoflowers Grown around Fe Islands on Au(111). J. Clust. Sci. 2022, 33, 2393–2397. [Google Scholar] [CrossRef]
- Klappenberger, F. Two-Dimensional Functional Molecular Nanoarchitectures—Complementary Investigations with Scanning Tunneling Microscopy and X-ray Spectroscopy. Prog. Surf. Sci. 2014, 89, 1–55. [Google Scholar] [CrossRef]
- Weckesser, J.; Cepek, C.; Fasel, R.; Barth, J.V.; Baumberger, F.; Greber, T.; Kern, K. Binding and Ordering of C60 on Pd(110): Investigations at the Local and Mesoscopic Scale. J. Chem. Phys. 2001, 115, 9001–9009. [Google Scholar] [CrossRef]
- Hsu, C.L.; Pai, W.W. Aperiodic Incommensurate Phase of a C-60 Monolayer on Ag(100). Phys. Rev. B 2003, 68, 245414. [Google Scholar] [CrossRef]
- Pai, W.; Hsu, C.L. Ordering of an Incommensurate Molecular Layer with Adsorbate-Induced Reconstruction: C60/Ag(100). Phys. Rev. B-Condens. Matter Mater. Phys. 2003, 68, 121403. [Google Scholar] [CrossRef]
- Hinterstein, M.; Torrelles, X.; Felici, R.; Rius, J.; Huang, M.; Fabris, S.; Fuess, H.; Pedio, M. Looking underneath Fullerenes on Au(110): Formation of Dimples in the Substrate. Phys. Rev. B 2008, 77, 153412. [Google Scholar] [CrossRef]
- Rosei, F.; Schunack, M.; Jiang, P.; Gourdon, A.; Lægsgaard, E.; Stensgaard, I.; Joachim, C.; Besenbacher, F. Organic Molecules Acting as Templates on Metal Surfaces. Science 2002, 296, 328–331. [Google Scholar] [CrossRef] [Green Version]
- Otero, R.; Rosei, F.; Naitoh, Y.; Jiang, P.; Thostrup, P.; Gourdon, A.; Laegsgaard, E.; Stensgaard, I.; Joachim, C.; Besenbacher, F. Nanostructuring Cu Surfaces Using Custom-Designed Molecular Molds. Nano Lett. 2004, 4, 75–78. [Google Scholar] [CrossRef]
- Schunack, M.; Rosei, F.; Naitoh, Y.; Jiang, P.; Gourdon, A.; Lægsgaard, E.; Stensgaard, I.; Joachim, C.; Besenbacher, F. Adsorption Behavior of Lander Molecules on Cu(110) Studied by Scanning Tunneling Microscopy. J. Chem. Phys. 2002, 117, 6259–6265. [Google Scholar] [CrossRef]
- Schunack, M.; Petersen, L.; Kuhnle, A.; Laegsgaard, E.; Stensgaard, I.; Johannsen, I.; Besenbacher, F. Anchoring of Organic Molecules to a Metal Surface: HtBDC on Cu(110). Phys. Rev. Lett. 2001, 86, 456–459. [Google Scholar] [CrossRef]
- Witte, G.; Hanel, K.; Busse, C.; Birkner, A.; Woell, C. Molecules Coining Patterns into a Metal: The Hard Core of Soft Matter. Chem. Mater. 2007, 19, 4228–4233. [Google Scholar] [CrossRef]
- Tseng, T.C.; Urban, C.; Wang, Y.; Otero, R.; Tait, S.L.; Alcamí, M.; Écija, D.; Trelka, M.; Gallego, J.M.; Lin, N.; et al. Charge-Transfer-Induced Structural Rearrangements at Both Sides of Organic/Metal Interfaces. Nat. Chem. 2010, 2, 374–379. [Google Scholar] [CrossRef]
- Böhringer, M.; Berndt, R.; Schneider, W.D. Transition from Three-Dimensional to Two-Dimensional Faceting of Ag(110) Induced by Cu-Phthalocyanine. Phys. Rev. B-Condens. Matter Mater. Phys. 1997, 55, 1384–1387. [Google Scholar] [CrossRef]
- Gross, L.; Moresco, F.; Alemani, M.; Tang, H.; Gourdon, A.; Joachim, C.; Rieder, K.H. Lander on Cu(211)—Selective Adsorption and Surface Restructuring by a Molecular Wire. Chem. Phys. Lett. 2003, 371, 750–756. [Google Scholar] [CrossRef]
- Kuhnle, A.; Molina, L.M.; Linderoth, T.R.; Hammer, B.; Besenbacher, F. Growth of Unidirectional Molecular Rows of Cysteine on Au(110)-(1x2) Driven by Adsorbate-Induced Surface Rearrangements. Phys. Rev. Lett. 2004, 93, 086101. [Google Scholar] [CrossRef]
- Gross, L.; Rieder, K.H.; Moresco, F.; Stojkovic, S.M.; Gourdon, A.; Joachim, C. Trapping and Moving Metal Atoms with a Six-Leg Molecule. Nat. Mater. 2005, 4, 892–895. [Google Scholar] [CrossRef]
- Trevethan, T.; Such, B.; Glatzel, T.; Kawai, S.; Shluger, A.L.; Meyer, E.; de Mendoza, P.; Echavarren, A.M. Organic Molecules Reconstruct Nanostructures on Ionic Surfaces. Small 2011, 7, 1264–1270. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, S.X.; Yuan, B.K.; Deng, K.; Sun, B.Y.; Qiu, X.H. Monolayered Adatom Aggregation Induced by Metallofullerene Molecules on Cu(100). Surf. Sci. 2012, 606, 78–82. [Google Scholar] [CrossRef]
- Dong, L.; Sun, Q.; Zhang, C.; Li, Z.W.; Sheng, K.; Kong, H.H.; Tan, Q.G.; Pan, Y.X.; Hu, A.G.; Xu, W. A Self-Assembled Molecular Nanostructure for Trapping the Native Adatoms on Cu(110). Chem. Commun. 2013, 49, 1735–1737. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Choi, B.Y.; Salmeron, M. Water Chains Guide the Growth of Monoatomic Copper Wires on Cu(110). J. Phys. Chem. C 2013, 117, 17119–17122. [Google Scholar] [CrossRef]
- Abadía, M.; González-Moreno, R.; Sarasola, A.; Otero-Irurueta, G.; Verdini, A.; Floreano, L.; Garcia-Lekue, A.; Rogero, C. Massive Surface Reshaping Mediated by Metal–Organic Complexes. J. Phys. Chem. C 2014, 118, 29704–29712. [Google Scholar] [CrossRef]
- Kalashnyk, N.; Rochford, L.A.; Li, D.Z.; Smogunov, A.; Dappe, Y.J.; Jones, T.S.; Guillemot, L. Unraveling Giant Cu(110) Surface Restructuring Induced by a Non-Planar Phthalocyanine. Nano Res. 2018, 11, 2605–2611. [Google Scholar] [CrossRef]
- Paßens, M.; Karthäuser, S. Interfacial and Intermolecular Interactions Determining the Rotational Orientation of C60 Adsorbed on Au(111). Surf. Sci. 2015, 642, 11–15. [Google Scholar] [CrossRef]
- Pai, W.W.; Hsu, C.L.; Lin, M.C.; Lin, K.C.; Tang, T.B. Structural Relaxation of Adlayers in the Presence of Adsorbate-Induced Reconstruction: C60/Cu(111). Phys. Rev. B-Condens. Matter Mater. Phys. 2004, 69, 125405. [Google Scholar] [CrossRef]
- Li, H.I.; Pussi, K.; Hanna, K.J.; Wang, L.L.; Johnson, D.D.; Cheng, H.P.; Shin, H.; Curtarolo, S.; Moritz, W.; Smerdon, J.A.; et al. Surface Geometry of C60 on Ag(111). Phys. Rev. Lett. 2009, 103, 056101. [Google Scholar] [CrossRef]
- Stöhr, M.; Gabriel, M.; Möller, R. Investigation of the Growth of PTCDA on Cu(1 1 0): An STM Study. Surf. Sci. 2002, 507–510, 330–334. [Google Scholar] [CrossRef]
- Murray, P.; Pedersen, M.; Lægsgaard, E.; Stensgaard, I.; Besenbacher, F. Growth of on Cu(110) and Ni(110) Surfaces:-Induced Interfacial Roughening. Phys. Rev. B-Condens. Matter Mater. Phys. 1997, 55, 9360–9363. [Google Scholar] [CrossRef]
- Jones, P.G.; Blaschette, A.; Henschel, D.; Weitze, A. Redetermination of the Crystal Structure of triphenylbismuth, (C6H5)3Bi. Zeitschrift Fur Krist. 1995, 210, 377–378. [Google Scholar] [CrossRef]
- Wang, Z.F.; Liu, Z.; Liu, F. Organic Topological Insulators in Organometallic Lattices. Nat. Commun. 2013, 4, 1–5. [Google Scholar] [CrossRef]
- Wang, R.S.; Cheng, J.; Wu, X.L.; Yang, H.; Chen, X.J.; Gao, Y.; Huang, Z.B. Superconductivity at 3.5 K and/or 7.2 K in Potassium-Doped Triphenylbismuth. J. Chem. Phys. 2018, 149, 144502. [Google Scholar] [CrossRef]
- Wang, R.S.; Yang, H.; Cheng, J.; Wu, X.L.; Fu, M.A.; Chen, X.J.; Gao, Y.; Huang, Z.B. Discovery of Superconductivity in Potassium-Doped Tri-p-Tolylbismuthine. J. Phys. Chem. C 2019, 123, 19105–19111. [Google Scholar] [CrossRef]
- Vande, V.J.; Krack, M.; Mohamed, F.; Parrinello, M.; Chassaing, T.; Hutter, J. QUICKSTEP: Fast and accurate density functional calculations using a mixed Gaussian and plane waves approach. Comput. Phys. Commun. 2005, 167, 103–128. [Google Scholar] [CrossRef]
- Perdew, J.; Burke, P.K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special points for Brilloofn-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Bucko, T.; Hafner, J.; Lebegue, S.; Angy’an, J.G. Improved Description of the Structure of Molecular and Layered Crystals: Ab Initio DFT Calculations with van der Waals Corrections. J. Phys. Chem. A 2010, 114, 11814–11824. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, M.; Li, Z.; Shi, M.; Tao, M.; Sun, K.; Yang, X.; Zhang, Y.; Wang, J. Reconstructed Cd(0001) Surface Induced by Adsorption of Triphenyl Bismuth. Coatings 2023, 13, 394. https://doi.org/10.3390/coatings13020394
Bai M, Li Z, Shi M, Tao M, Sun K, Yang X, Zhang Y, Wang J. Reconstructed Cd(0001) Surface Induced by Adsorption of Triphenyl Bismuth. Coatings. 2023; 13(2):394. https://doi.org/10.3390/coatings13020394
Chicago/Turabian StyleBai, Mengmeng, Zuo Li, Mingxia Shi, Minlong Tao, Kai Sun, Xiaotian Yang, Yufeng Zhang, and Junzhong Wang. 2023. "Reconstructed Cd(0001) Surface Induced by Adsorption of Triphenyl Bismuth" Coatings 13, no. 2: 394. https://doi.org/10.3390/coatings13020394





