Abstract
High-efficient heat dissipation materials are urgently required in advanced electronic packaging technology because effectively releasing the internal heat flow density of electronic devices is a key factor during their operation. In this work, a novel vertical graphene nanosheets/carbon fibers (VGNs/CF) composite film, with a vertically oriented structure and excellent heat dissipation properties, is fabricated on the stainless steel substrate by a facile thermochemical growth method. The preparation of composite film is green, safe, and highly efficient. CF is used as a thermally conductive filler to provide thermal conductivity channels for VGNs, and both of them construct a continuous thermally conductive network. The through-plane thermal conductivity of the VGNs/CF composite film could reach 17.7 W/(m·K), and the addition of CF significantly improved the heat dissipation performance of the composite film compared with the pure VGNs film (13.9 W/(m·K)). Conclusively, the simple preparation method and outstanding thermal conductivity capacity of the VGNs/CF composite film are expected to meet the application requirements of the electronics industry.
1. Introduction
With the development of electronic devices trending toward miniaturization and high integration, heat flow density is gradually increasing, and the failure of heat dissipation becomes the primary problem hindering the performance and service life of electronic equipment [1,2,3]. It has been reported that with every 10 °C increase in the temperature of individual electronic components, within 70–80 °C, the reliability of the system decreases by 50%, and more than 55% of electronic devices cannot function properly because of excessive heat [4,5,6]. Thus, how to efficiently enhance the heat dissipation of electronic components is an important topic. Efficient heat dissipation materials with high thermal conductivity play a vital role in improving heat dissipation efficiency, which can transfer the excessive heat away from the electronic device to reduce its operating temperature [7].
The bulk heat dissipation materials typically include carbon nanotubes, graphite, copper, aluminum, etc. [8]. However, there are various limitations in their applications. For example, although the thermal conductivity of carbon nanotubes is very high (3000~3500 W/(m·K)), many problems still exist, such as poor coupling, high boundary thermal resistance, orientation misalignment, and catalyst impurities in the preparation process [9]. The metal materials such as copper and aluminum exhibit ideal thermal conductivity, but they possess high smelting costs and complex machining process. Conventional graphite heat dissipation film is a mainly artificial film formed by the carbonization and high-temperature graphitization of polyimide (PI), and its thermal conductivity can reach 1750 W/(m·K) [10,11]. However, the strict requirements for the original PI molecular structure and the tedious time-consuming production processes (including polymerization, film formation, long carbonization, graphitization, etc.) have seriously reduced the productivity of the graphite film [12].
In contrast, graphene nanosheet composites, widely used as ideal candidates for heat dissipation films, exhibit an ultra-high in-plane thermal conductivity (5300 W/(m∙K)) which can mitigate local hot spots by transferring heat along the surface of the electronic device [13,14]. However, due to the two-dimensional (2D) structure of graphene nanosheets, with weak van der Waals interactions between their layers, the thermal conductivity of graphene is anisotropic. They show strong heat transfer capacity along the in-plane direction and unsatisfactory thermal conductivity along its thickness direction, which limits its application for the heat dissipation of electronic devices [15,16]. Compared with ordinary horizontally oriented graphene nanosheets, vertical graphene nanosheets (VGNs) have great potential for the through-plane direction heat dissipation of electronic components due to their unique three-dimensional (3D) structure and vertical orientation. Ci et al. reported that the use of vertically oriented graphene nanowalls as a buffer layer between the sapphire substrate and the AlN film can effectively enhance the through-plane heat dissipation between them [15], providing a new strategy to effectively spread the excess heat vertically from the heat sources. Zhang et al. prepared vertical graphene film/epoxy (VGF/EP) composites by using continuous vertical-aligned graphene film as a heat dissipation channel [17]. Compared with pure EP resin, the vertical composite reduced the temperature of light emitting diode (LED) lamps by about 20.7 °C, apparently enhancing the cooling performance of LED lamps. In addition, Zhang et al. fabricated VGNs/PDMS composites by rolling graphite films and infiltrating polydimethylsiloxane (PDMS) precursors, and the composites exhibited a super vertical thermal conductivity of 5.349 W/(m·K) [18]. These works demonstrate that VGNs films can provide a fast and effective heat transfer pathway and improve the thermal performance of the composite materials.
Generally, the thermal energy transfer of VGNs film mainly relies on phonon conduction and lattice vibration [19]. However, most of graphene nanosheets are difficult to fully crystallize and show strong phonon scattering, which results in high thermal resistance. It has been reported that the interfacial thermal resistance among VGNs can be reduced by adding nanofillers with fewer defects to form a thermally conductive network. For example, CF has a one-dimensional (1D) structure, a large length/diameter ratio and high thermal conductivity [20,21,22,23]. Zhang et al. used CF as a filler to optimize the properties of expanded graphite/paraffin gypsum composites (EGPG). Moreover, it was experimentally discovered that the thermal conductivity (1.350 W/(m·K)) increased by 36.0%, and the flexural strength (12.4 MPa) increased by 51.6%, respectively, when 1 wt% CF was added into EGPG [24]. Cui et al. found that the flexural strength of cement slurry and alkali activated slag mixed with 1 wt% CF increased by 130.8% and 302.6%, respectively, after 28 days. It was also demonstrated that the addition of a suitable amount of CF could improve the mechanical strength of the composites [25]. As a thermal conductive filler, CF can be added to the VGNs films, and the “channel” will be formed. The continuous overlap between fillers provides a channel of rapid heat conduction, which plays a role in uniform heat dissipation, eventually leading to long service life and the improved stability of electronic devices [26].
So far, the methods for preparing VGNs film mainly include plasma-enhanced chemical vapor deposition [27,28,29,30,31,32,33,34,35,36], arc discharge [37,38], sputtering [39], hot filament chemical vapor deposition [40,41], etc. Among these, plasma-enhanced chemical vapor deposition is mainly used for the preparation of VGNs. Zhou et al. prepared VGNs on Cu and Ni foil substrates by plasma-enhanced chemical vapor deposition. They used CH4 as the carbon source, H2/Ar as the protecting gas, and a 120 w radio-frequency (RF) to decompose CH4 [42]. Wei et al. successfully synthesized vertical graphene films on flexible soda lime glass substrates, with CH4 and pyridine as carbon sources and Ar/H2 as carrier gases, with the assistance of copper foam [43]. Hong et al. prepared VGNs by feeding a mixture of H2 and CH4 into the ICPCVD chamber using Cu foil as a substrate and adjusting the appropriate RF power and bias voltage [44]. During the preparation process, the density and size of the VGNs arrays in the films can be controlled by adjusting the experimental parameters. However, all of these methods exhibit problems. For example, all of them require excessive energy consumption, high equipment maintenance cost, complex control systems, storage safety, and large-scale preparation. Meanwhile, the growth precursors are mostly dependent on dangerous carbon sources (such as CH4, C2F6, C2H2, CF4, etc.), and impurities are easily introduced during the growth process, which will contaminate the VGNs film and seriously hinder its performance and industrial application. Similarly, the traditional chemical vapor precipitation method of VGNs film provides less energy to decompose the carbon source, which makes it very difficult to grow. Thus, researchers introduced catalysts (such as plating nickel on the substrate surface [45,46]) to promote the growth of VGNs, but it is still a complicated operation process. It is worth noting that current research has revealed that VGNs can be directly grown on the surface of the stainless steel (SS) substrate at high temperatures because of the metal elements (such as Ni, Fe, Co, etc.) contained in stainless steel as catalytic agents [47,48,49], making it very suitable to serve as a substrate with catalytic activity for preparing the VGNs film.
In recent years, with the development of the miniaturization of electronic devices, the thermal management capability of chip materials has been required to gradually increase, and the problem of uneven local overheating inside the chip is still one of the urgent difficulties to be solved [50]. The unique structure of VGNs stands out among heat dissipation materials. However, the methods for VGNs preparation are more complicated, and the heat dissipation aspect is still affected by anisotropy. Therefore, the development of simple and green preparation methods for VGNs, along with the improvement of its thermal performance, are urgent challenges to be solved. In addition, the CF with high thermal conductivity solves the problem of uneven VGNs inside the film and builds a good thermal conductivity network structure, which is expected to be better developed in the heat sink, electronic chip, and other heat dissipation components.
Here, an ideal thermal-transportation structure was designed to enhance the through-plane thermal conductivity of a VGNs/CF composite film. Using glucose solution and CF as the carbon precursor and thermal conductive filler, respectively, the VGNs/CF composite film was successfully achieved on the 304 SS substrate using the thermochemical growth method in a tube furnace. As a result, the prepared VGNs/CF composite film exhibits a higher through-plane thermal conductivity of 17.7 W/(m·K) compared with the pure VGNs films (13.9 W/(m·K)). The simple preparation method and outstanding through-plane heat dissipation capacity of VGNs/CF composite films can facilitate its practical application for the heat dissipation of electronic components.
2. Experimental
2.1. Materials
Concentrated sulfuric acid (H2SO4, 98.0%) was purchased from Sichuan Xilong Science Co. Ltd., concentrated nitric acid (HNO3, 68.0%) was purchased from Baiyin Liangyou Chemical Reagent Co. Ltd., and carbon fibers (CF, maximum length/diameter ratio 7.1:1) were purchased from Toray, Japan. Glucose (C6H12O6, CAS 14431-43-7) was purchased from Chengdu Colon Chemical Co., LTD. Other chemicals were of analytical grade and were used without further purification. Ultrapure water (18.25 MΩ·cm) was employed for preparation and rinsing.
2.2. Synthesis of Materials
The SS substrate was polished with SiC sandpaper (120 mesh) to obtain the roughened SS (RSS) substrate, which was cleaned sequentially with acetone, ethanol, and deionized water, and then dried with flowing nitrogen gas. Subsequently, it was treated with oxygen plasma for the following experiments.
To improve the dispersion of CF in the carbon precursor solution, 0.5 g CF was ultrasonically dispersed in a mixture of HNO3 and H2SO4 (3:1, v/v) for 30 min, followed by refluxing at 100 °C for 5 h. After cooling to room temperature, the obtained solution was washed with deionized water for neutralization, dried at 60 °C for 48 h, and then collected for future use.
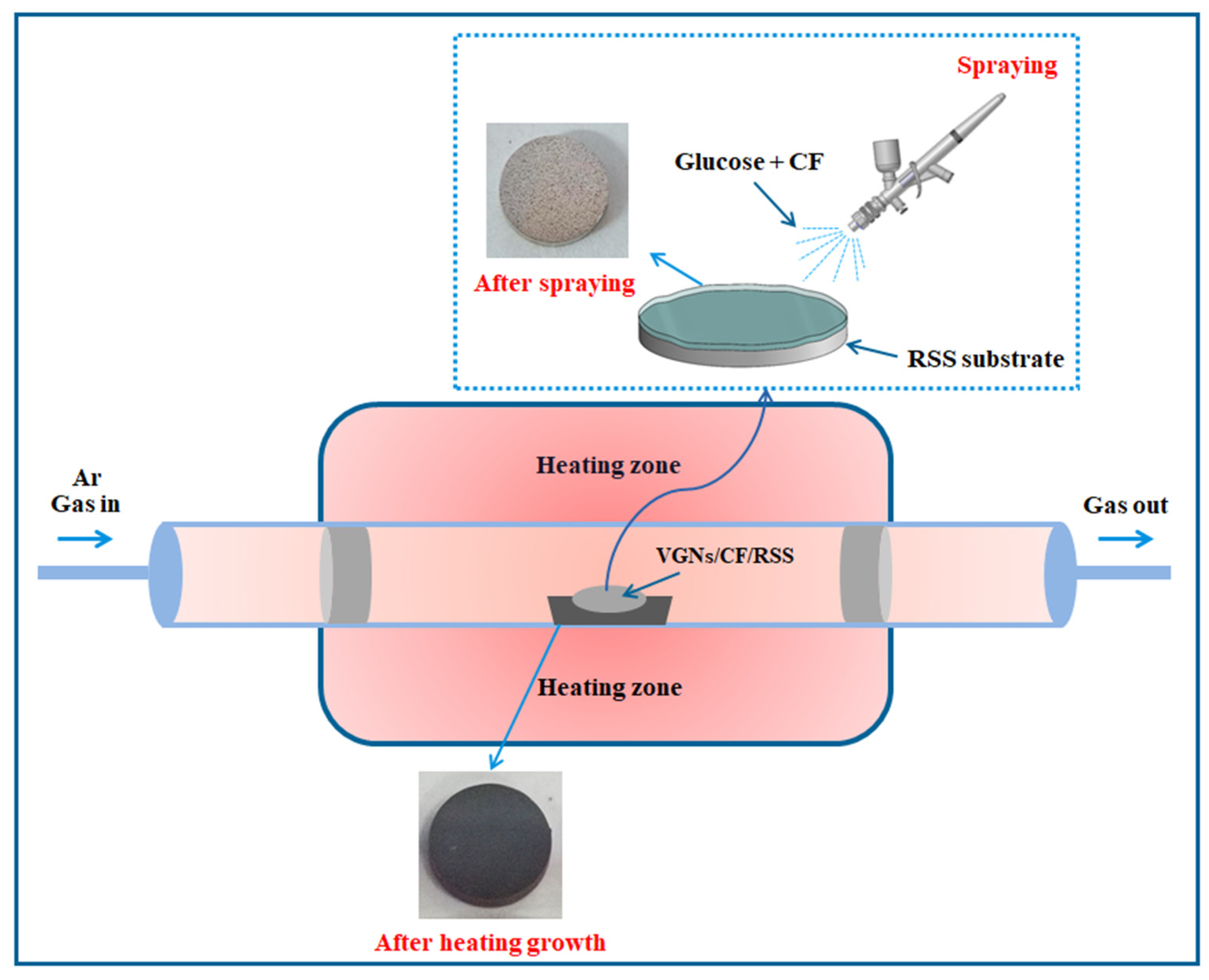
A schematic view of the preparation of VGNs/CF by a facile thermochemical growth method is shown in Figure 1. The VGNs/CF composite films were obtained as follows: 1 mg CF powder was homogeneously mixed with glucose solution at a set concentration to form the precursor solution that was subsequently applied onto the surface of the RSS substrate. The obtained sample was dried at 60 °C for 30 min and then transferred to the central heating zone of a quartz tube furnace, where it was heated at 5 °C/min to 850 °C for 3 h, after which it was naturally cooled to room temperature to obtain the VGNs/CF composite films.
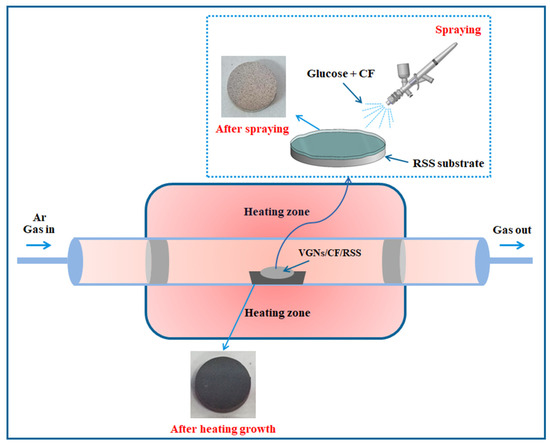
Figure 1.
Schematic diagram of VGNs/CF composite films preparation process.
2.3. Characterizations
The surface morphology and roughness (Ra) of the RSS substrate were measured by an atomic force microscope (AFM, Multimode8). The micromorphology of VGNs/CF composite films was also observed by a scanning electron microscope (SEM, Quanta 650 FEG) and a transmission electron microscope (TEM, TF20, USA). The crystal structure of the samples was determined by an X-ray diffractometer (XRD-6100). The Raman spectrum was measured by a micro confocal Raman spectrometer (LabRAM HR Evolution with excitation wavelength of 532 nm). The composition of the sample surface was analyzed using a Fourier transform infrared spectrometer (FT-IR, V70). The internal composition of the samples was analyzed using an X-ray photoelectron spectrometer (XPS, ESCALAB XI+).
The heat dissipation performance of the VGNs/CF composite films was tested on an infrared thermal imager (Yoseen X640D) and a laser thermal conductivity meter (LFA457). The through-plane thermal conductivity value of the composites films was measured using the laser flash technique in a closed system under a vacuum of 10−2 mbar. In the test of through-plane thermal conductivity, argon gas was introduced at a flow rate of 80 mL/min, and a laser thermal conductivity meter was used to heat the lower surface of the sample by emitting a short laser pulse at room temperature, causing an instantaneous increase in the temperature of the surface layer after absorbing the light energy and propagating the energy to the upper surface by 1D heat conduction as the hot end. Then, the heating process at the center of the upper surface was continuously measured using an infrared detector to obtain the corresponding thermal diffusion coefficient, and the thermal conductivity (K) of the sample was calculated by Equation (1) [14,51].
where α, ρ, and Cp represent the thermal diffusivity (mm2/s), density (g/cm3), and specific heat capacity (J/(g·K)), respectively, of the composites.
K = α × ρ × Cp
3. Results and Discussion
3.1. Structural Characterization of VGNs/CF Composite Films
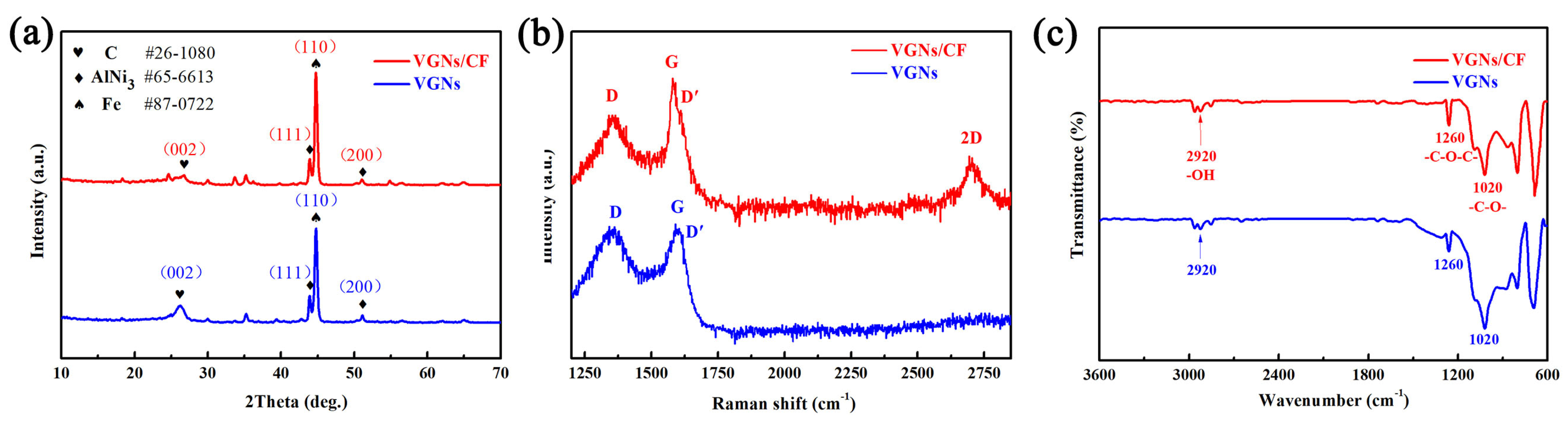
To investigate the component and phrase structure of the RSS surface, it was evaluated by XRD measurement and the results are shown in Figure 2a. A diffraction peak at approximately 2θ = 26.10° is indexed as the (002) plane of graphite, indicating that the sample surface possessed the graphite phase [52]. Two diffraction peaks at 2θ = 43.91°and 51.14° are attributable to the (111) and (200) planes of AlNi3 alloy formed by Al and Ni elements inside the RSS substrate after high temperature treatment. However, a well-resolved diffraction peak can be clearly seen at 2θ = 44.82°, which is the (110) plane of the Fe element exposed inside the SS substrate after roughening treatment. To confirm the successful growth of VGNs/CF on the RSS substrate, a Raman spectra test was carried out, and the results are shown in Figure 2b. The Raman spectrum of the graphene film is displayed as a visible D band at 1350 cm−1 and a pronounced G band at 1580 cm−1. The D peak is related to the edge and structural disorder of graphene, while the G peak is a peak characteristic of the carbon sp2 structure caused by the in-plane vibration of carbon atoms [53,54,55]. These findings suggested that the graphene material was obtained. A weak shoulder peak (D′) can be seen in the VGNs/CF Raman spectrum near 1620 cm−1, which is assigned to graphene edges and finite size graphene crystals, suggesting the signal peak appears at the edge site of VGNs [56,57]. Meanwhile, the typical feature of the 2D band of graphene at 2700 cm−1 is caused by the double phonon resonance of graphene nanosheets and indicates their stacking degree, but it is not seen in the Raman spectrum of VGNs film. On the other hand, the intensity ratio (ID/IG) of the D band to the G band of the samples can be used to reflect the degree of disorder and the density of defects inside the sample. A larger intensity ratio of ID/IG represents a higher degree of defects and disorder inside the sample, i.e., a lower graphitization degree of the sample [47]. The intensity ratios of ID/IG for VGNs/CF and VGNs films are 0.85 and 0.98, respectively, while the intensity ratio of I2D/IG for VGNs/CF is 0.65 < 1, which indicates that the graphene sheets are multilayered [58].
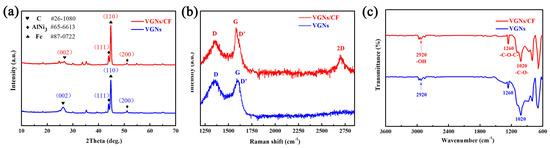
Figure 2.
(a) XRD patterns, (b) Raman spectra, and (c) FT-IR spectra of the VGNs/CF composite films and the pure VGNs films prepared by different precursor solutions.
The prepared VGNs/CF composite film and pure VGNs film were further characterized by the FT-IR spectra, and the results are depicted in Figure 2c. In detail, for both samples, the peaks at 2920 cm−1 are attributed to the stretching mode of the hydroxyl groups (-OH). The peak located at 1020 cm−1 is assigned to the stretching vibration of the -C-O- groups, while the peak at 1260 cm−1 is attributed to the stretching vibration of the -C-O-C- groups [51]. These findings suggested that at least three functional groups of -OH, -C-O-, and -C-O-C- were successfully introduced in the films prepared by the two precursors under the experimental conditions. The presence of -OH, -C-O-, and -C-O-C- in the FT-IR spectra proves the successful generation of graphene from another point of view. In addition, the presence of oxygen-containing functional groups proves that the resulting graphene has been oxidized, but theoretically, completely oxygen-free graphene does not exist.
3.2. Study on the Preparation Process of VGNs/CF Composite Films
3.2.1. Effect of Coating Methods on VGNs/CF Composite Films
The effects of the preparation method on VGNs/CF composite films was studied by using the spin-coating and spraying techniques. In the spin-coating process, the precursor solution is applied to the substrate surface using a spinner at 1500 r/pm for 10 min. In the spraying process, the precursor solution is applied to the substrate surface in a mist form using a spray gun under nitrogen pressure. The results are shown in Figure S1.
It is obvious from Figure S1a that the uniformity of the films prepared by the spin-coating method is poor, and a serious agglomeration of CF occurs, while the film prepared using the spraying method (Figure S1b) shows better overall surface uniformity. After being held and grown at 850 °C, the morphology of the film prepared by the two techniques is shown in Figure S2. It is found that the surface of the film prepared by the spin-coating technique is not uniform, exhibiting a peeling phenomenon and poor integrity. The uniformity and growth area of the films on the substrate surface usually require the experimental conditions to reach a certain value, and this is difficult to control (as shown in Figure S3). These undesired results are attributed to the decreased amount of the precursor solution retained on the substrate surface during the spin-coating process, and the obvious thickness difference between the middle and edge of the substrate can be observed. In contrast, the film prepared by the spraying technique (Figure S2b) exhibits higher surface integrity and better bonding with the substrate. Compared with the spin-coating technique, the growth of VGNs/CF composite films prepared by spraying technology is more uniform. Therefore, the films prepared by the spraying technique were employed for the following experimental procedures.
3.2.2. Effect of Pretreatment of Stainless Steel Substrate Surface on VGNs/CF Composite Films
The 304 SS contains a variety of metal elements. By roughing the SS substrate, more metal elements will be exposed to the surface, producing more nucleation sites for the growth of VGNs [47,59]. To present the clearer chemical composition and the element information of the SS substrate before and after roughing, XPS measurement was carried out on the SS samples, and the results are shown in Figure S4. The roughness of SS only provides a surface parameter of physical polishing and has no effect on the species and chemical state of elements. To spread the carbon precursor solution uniformly on the substrate surface, the wettability of the RSS surface was improved by oxygen plasma treatment. The results are reflected by the change in the surface contact angle of the water droplet in Figure S5. The surface contact angle of RSS becomes significantly smaller after oxygen plasma treatment. The improved wettability facilitates the spreading of the precursor solution on the SS substrate surface, and the obtained films exhibited a more uniform appearance.
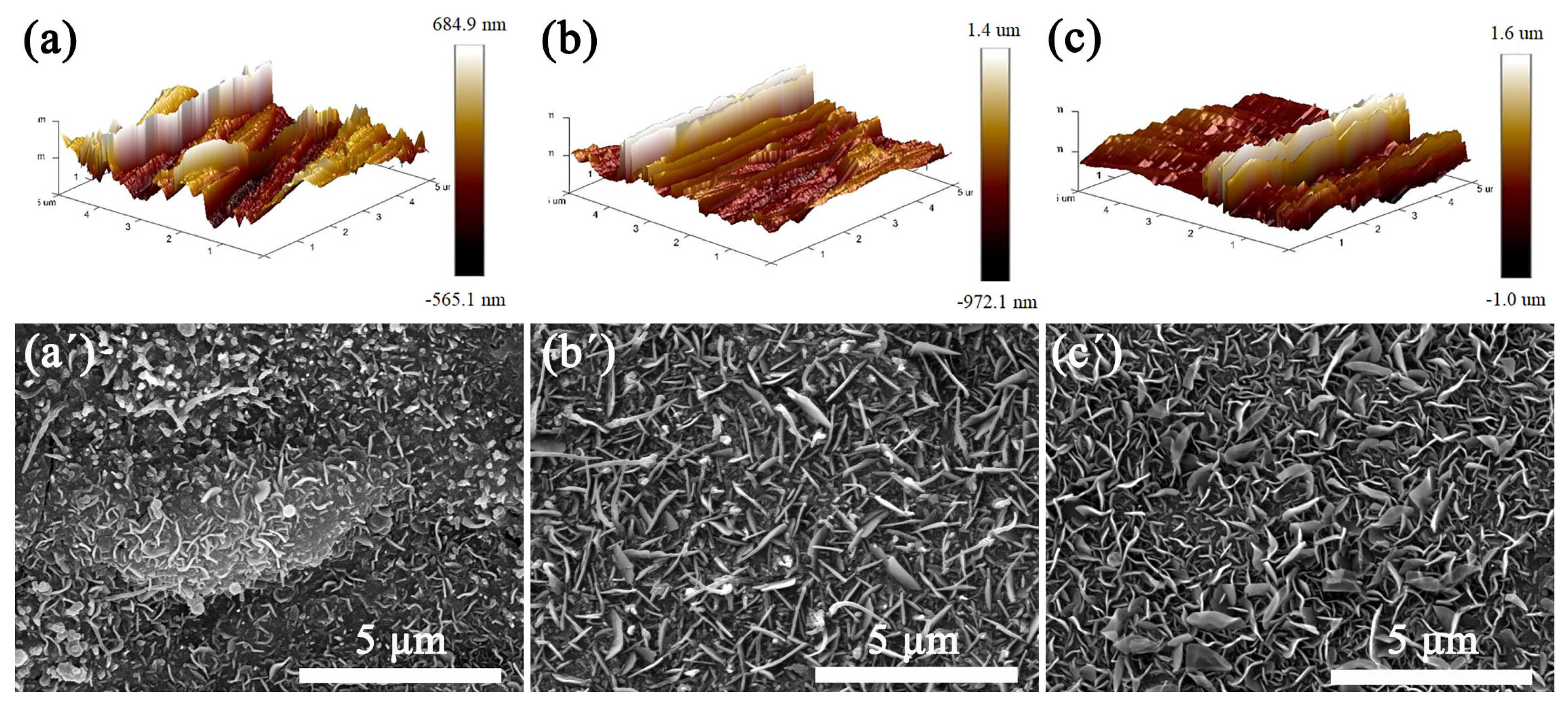
The SS substrates were polished for different times to obtain surfaces with different roughness values. The morphology of the RSS was characterized using AFM. Figure 3a–c shows the surface morphology of SS substrates polished for 10 min, 20 min, and 30 min, whose surface roughness values (Ra) are 149 nm, 199 nm, and 224 nm, respectively. Figure 3a′–c′ show the SEM images of the VGNs/CF composite films grown on the surface of the corresponding roughness substrates by holding them at 850 °C for 3 h, respectively. With increasing Ra values of the substrate surface from 149 nm to 224 nm, the quality of VGNs/CF composite films gradually tends to become uniform, dense, and homogeneous. The main reason for this is that the rougher surface of the polished SS substrate contains more catalytic metal elements, such as Ni, Fe, and Co, which are used as active sites for the more favorable growth of VGNs/CF composite films.
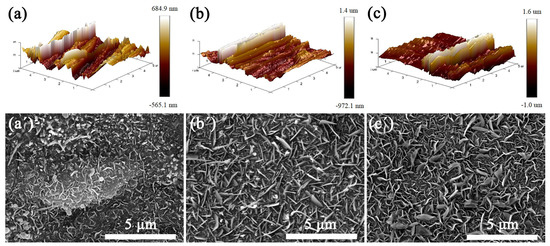
Figure 3.
AFM images of SS with different Ra values of (a) 149 nm, (b) 199 nm, (c) 224 nm; (a′–c′) SEM images of VGNs/CF composite films obtained by spraying a 0.6 g/mL glucose and CF mixture onto the SS surface of the corresponding roughness.
3.2.3. Effect of Carbon Precursor Solution Concentration and Dosage on VGNs/CF Composite Films
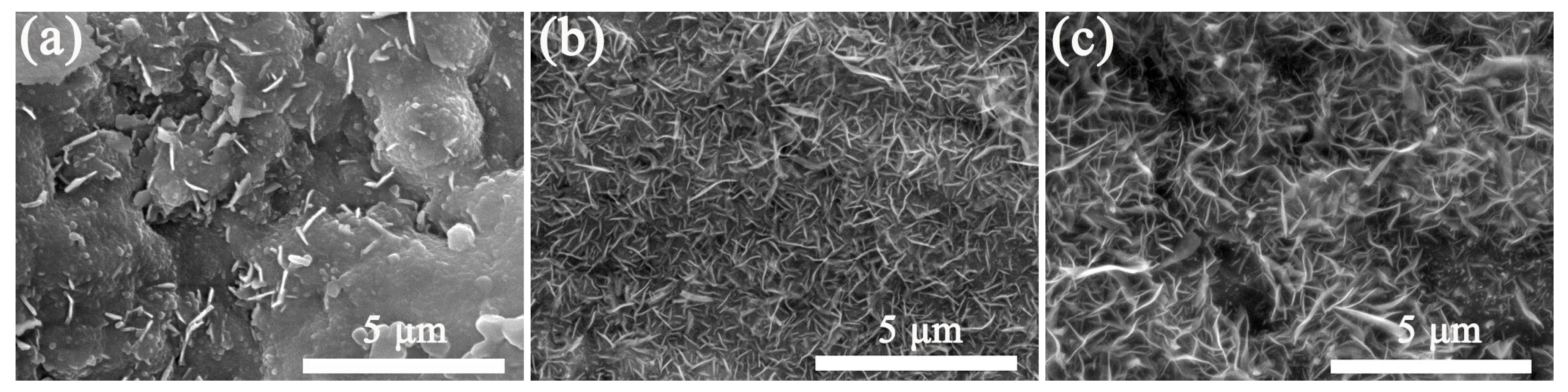
When the surface roughness of the SS substrate is fixed, the concentration of the precursor solution has an important impact on the quality of VGNs/CF composite films. Figure 4 shows the relationship between the precursor concentration and the structure of the VGNs/CF composite films when the RSS surface was polished for 30 min and the amount of CF was fixed. When the precursor concentration was 0.4 g/mL, the VGNs in the composite film were the most homogeneous in size, without mutual entanglement and agglomeration (Figure 4b). In contrast, the VGNs/CF composite films prepared by 0.2 g/mL precursor solution showed large, whole agglomerated particles (Figure 4a). At 0.6 g/mL of precursor solution concentration, the VGNs/CF composite film was incomplete, and the VGNs presented mutual entanglement and agglomeration, as clearly shown in Figure 4c.

Figure 4.
SEM images of VGNs/CF composite films prepared at 850 °C using the glucose solution with different concentrations of (a) 0.2 g/mL, (b) 0.4 g/mL, (c) 0.6 g/mL, respectively.
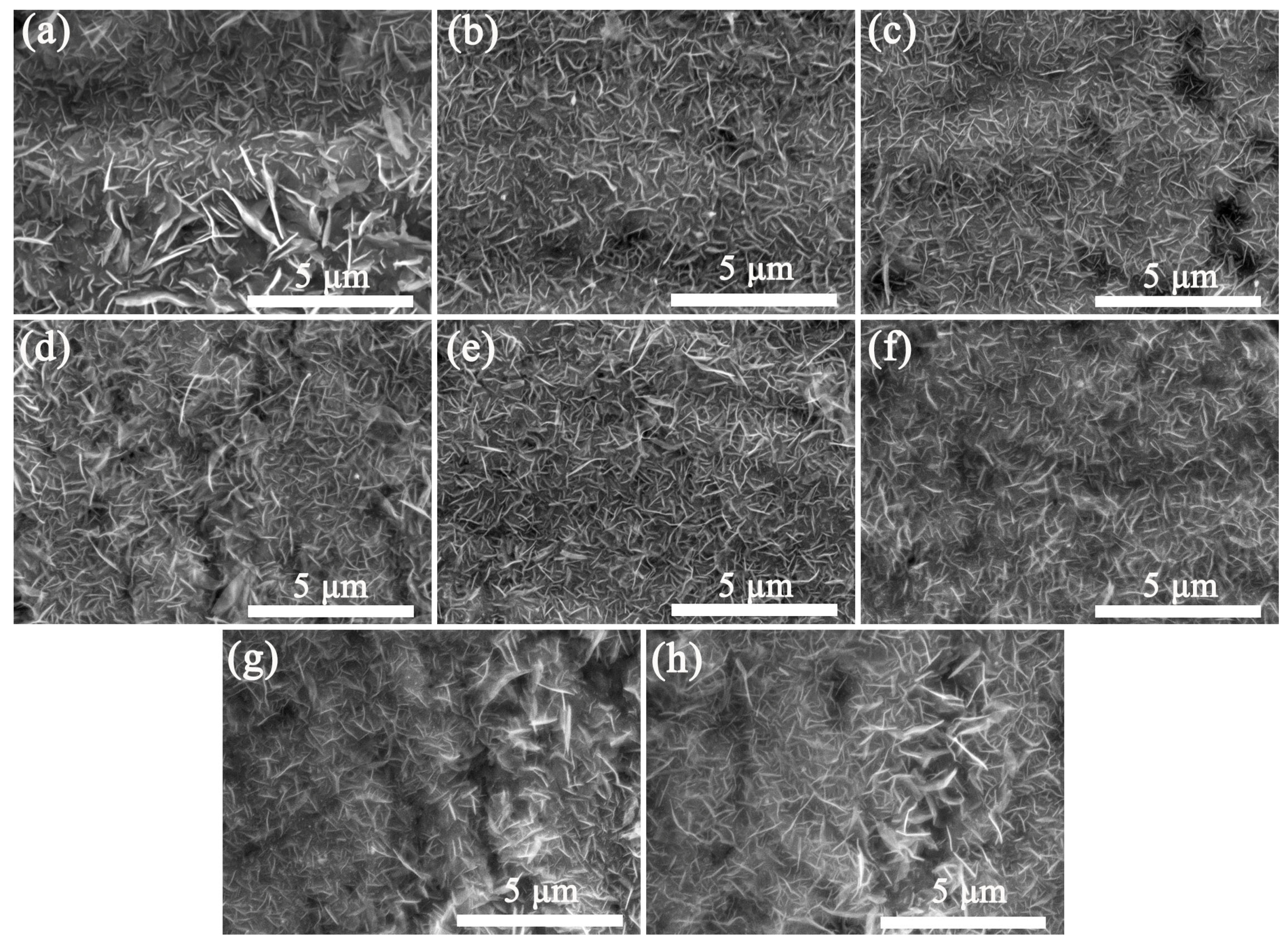
The effect of the spraying times of 0.4 g/mL precursor solution on the morphology and uniformity of VGNs/CF composite films were also explored. As indicated in Figure 5, by increasing the spraying time, the uniformity and integrity of VGNs/CF composite films gradually improve. When the spraying number was increased to 30 and 40 times (Figure 5g,h), the VGNs in the composite films presented an obvious agglomeration phenomenon, and the uniformity and integrity of the composite films became poor. Therefore, when the concentration of the precursor solution was set as 0.4 g/mL, the morphology and uniformity of the VGNs/CF composite films prepared by 15–20 times of spraying reached the optimal state (as shown in Figure 5e,f).
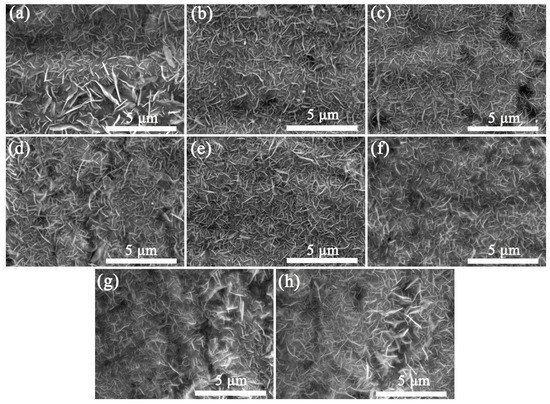
Figure 5.
Effect of the spraying time of 0.4 g/mL precursor solution on the morphology and uniformity of VGNs/CF composite films at 850 °C: (a) 1, (b) 2, (c) 5, (d) 10, (e) 15, (f) 20, (g) 30, and (h) 40 times, respectively.
3.2.4. Effect of Growth Time on the VGNs/CF Composite Films
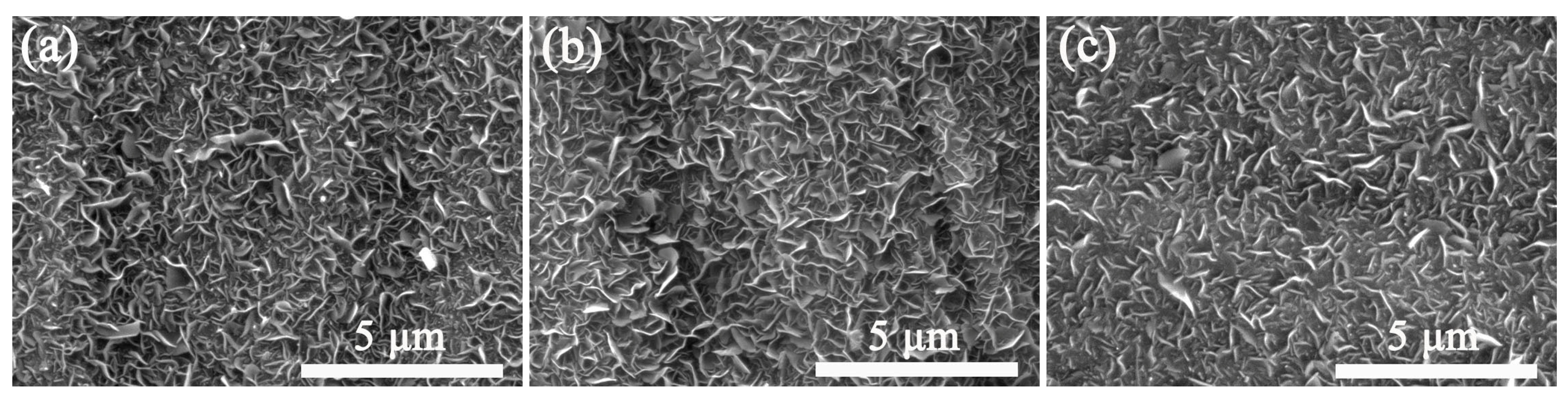
Effect of growth time on the surface morphology of the VGNs/CF composite films obtained by heating 0.4 g/mL glucose precursor solution at 850 °C for different times was further investigated. After growing for 3 h (Figure 6b), the VGNs in the composite films grew more densely and uniformly, with a clearer vertical morphology. In contrast, the morphologies of the VGNs/CF composite films showed relatively poor growth after 2 h (Figure 6a) and 4 h (Figure 6c), and the vertical structure of the VGNs on the surface of the composite films could not be presented clearly. According to the results, 3 h is the optimal growth time for the VGNs/CF composite films at 850 °C in the tube furnace.

Figure 6.
SEM images of the VGNs/CF composite films obtained by heating the 0.4 g/mL precursor solution at 850 °C for different growth times of (a) 2 h, (b) 3 h, and (c) 4 h, respectively.
3.3. The Growth Mechanism of VGNs/CF Composite Films
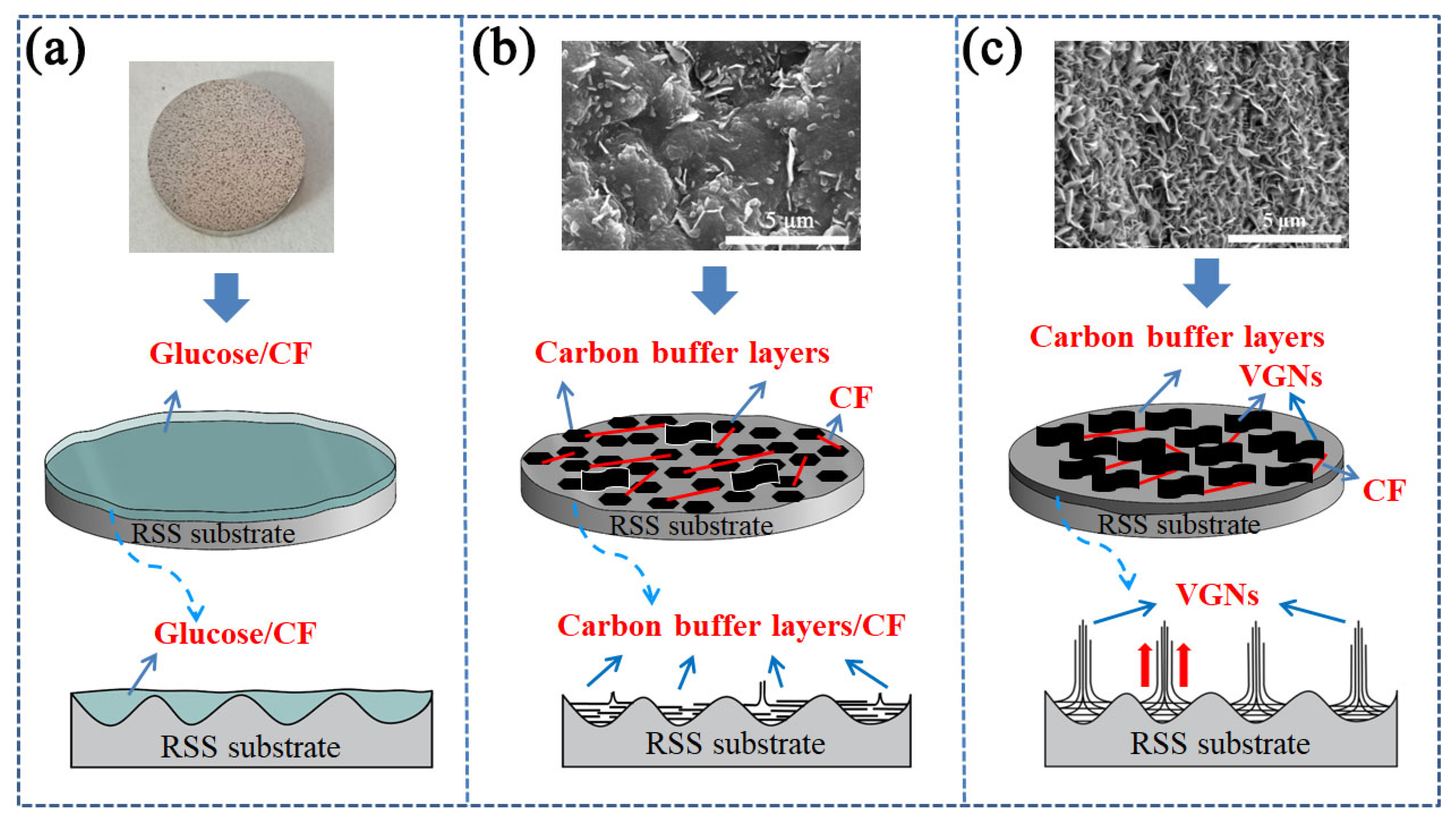
The growth mechanism of the VGNs/CF composite film on the surface of the RSS substrate is depicted in Figure 7, where the growth of the VGNs in the composite films can be divided into the following three stages. (1) Early-growth stage: the rough surface of the SS substrate (Figure 7a) first forms an intricate and mutually cross-linked 3D structure. The metal atoms, such as Fe, Ni, and Co, in these large 3D structures of the surface form some small island-like active sites to provide the prerequisite for the growth of VGNs [60]. (2) Growth stage: with the increase in the temperature in the furnace, the stress between the amorphous carbon structures was formed, with increasing carbon precursors. Then, the carbon precursor films were cleaved under the action of catalytic active sites on the RSS substrate surface. As a buffer layer, a large agglomerated granular amorphous carbon structure was generated on the surface until a small amount of VGNs was grown by mutual extrusion [61], as shown in Figure 7b. (3) Stabilization stage: when the temperature reaches a certain value and remains stable, the number and size of vertical graphene nanosheets driven by the carbon concentration of the carbon precursor is further increased until all the available active sites on the substrate surface are occupied, finally forming a high-density and homogeneous VGNs film, as shown in Figure 7c. In the above process, the substrate provides active sites as the basis for the growth of VGNs, while the decisive factor is the concentration of carbon precursor solution, which provides a sufficient carbon source for the growth of VGNs, rendering the growth of VGNs more complete and uniform [53].
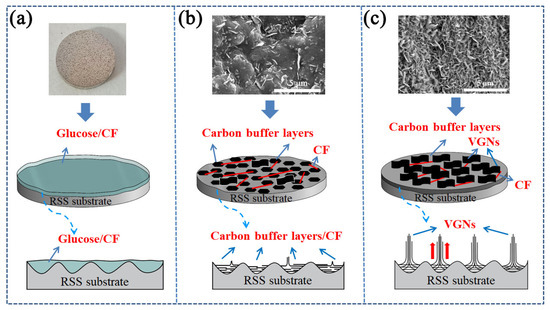
Figure 7.
Schematic diagram of the growth mechanism of VGNs/CF composite film on the surface of the RSS substrate: (a) early-growth stage, (b) growth stage, (c) stabilization stage.
3.4. The Heat Dissipation Performance of VGNs/CF Composite Films
There are some inherent defects, including the presence of microgasbags, the lack of surface crystal structure, and the introduction of impurities in the preparation process of graphene [62]. These adverse factors lead to the decrease in the thermal conductivity of graphene nanosheets, which leads to phonon scattering. However, as a 1D thermal conductivity filler, CF possesses a large cross-sectional area and can form good heat transfer channels. On the one hand, CF can fill in the microgasbags in graphene films and expel the air, improving the thermal conductivity. On the other hand, effective thermal conductivity channels formed between the CF and the graphene nanosheets, promoting connectivity between the graphene nanosheets and contributing to the effective export of heat. The TEM images of the CF and the CF in VGNs/CF composite film are shown in Figure S6. The morphology of CF is not affected by high temperature treatment, and it is distributed between the graphene nanosheets to act as a bridge.
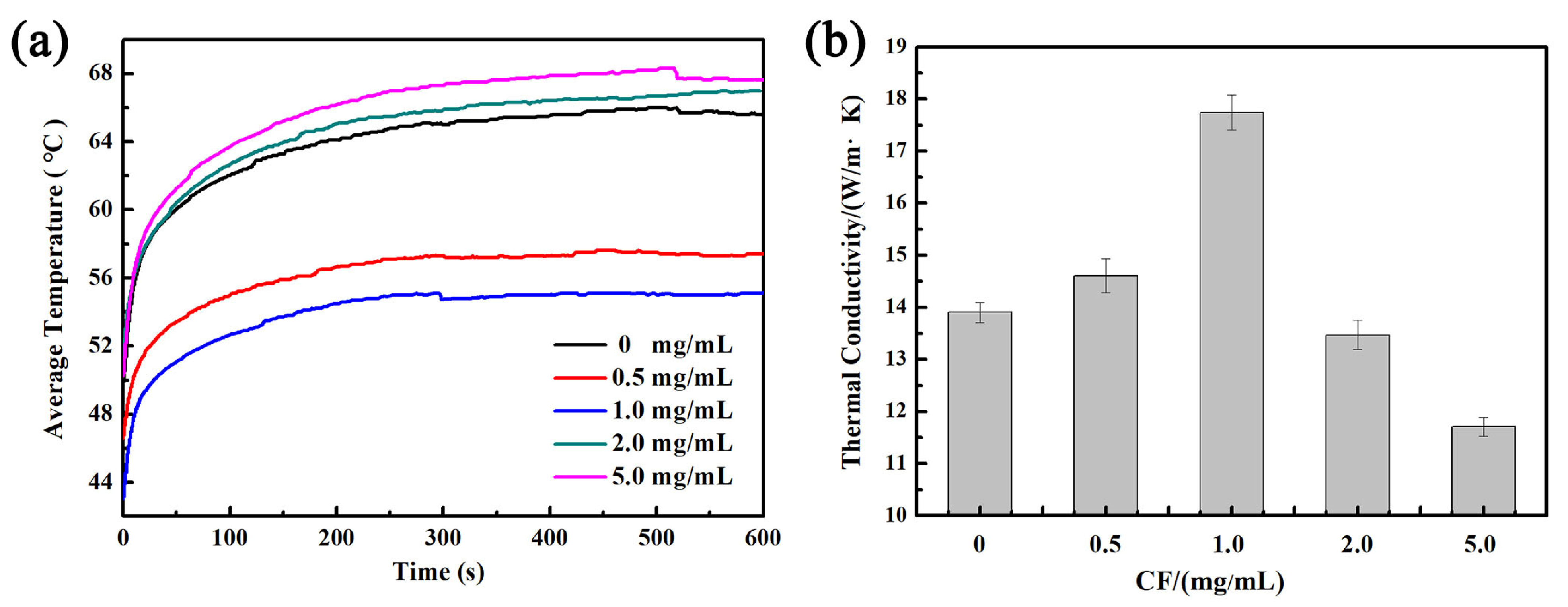
To demonstrate the surface heat dissipation of the VGNs/CF composite film, the VGNs/CF composite films were placed on a heating plate preheated to 80 °C and heated uniformly for 10 min. The changes in the average surface temperature of the VGNs/CF composite film with time were recorded using a thermal infrared imager at 25 °C, as shown in Figure 8a. The results showed that the average surface temperature of the composite film increased with time to 54.3 °C and remained stable when the CF concentration was 1.0 mg/mL. At the same time, the temperature of the VGNs/CF composite film with 1.0 mg/mL CF was the lowest compared to other composite films, showing the best heat dissipation and stable performance. With the concentration increase in CF from 0.5 to 5.0 mg/mL, the through-plane thermal conductivity of the VGNs/CF composite film shows a trend of first increase and then decrease (Figure 8b). When the CF concentration is less than 1.0 mg/mL, CF is dispersed in the film, and the laps of graphene nanosheets are not completely connected by CF. Thus, there is still a part of the laps where heat transfer is blocked, thus not effectively contributing to thermal conductivity. With increasing the CF concentration, a heat conduction network is formed between the CF and VGNs, which forces heat to pass through the CF quickly, avoiding the influence of the microgasbags. When the CF concentration was 1.0 mg/mL, the through-plane thermal conductivity was as high as 17.7 W/(m·K). However, the excess CF (>1.0 mg/mL) is easily entangled and agglomerated due to van der Waals forces, leading to the heterogeneous distribution of CF in the composite films, which results in the decrease in thermal conductivity [26]. The above results reveal that 1.0 mg/mL CF significantly guarantees the formation of the continuous thermally conductive network, which is an appropriate concentration for the ideal heat dissipation performance of the VGNs/CF composite films.
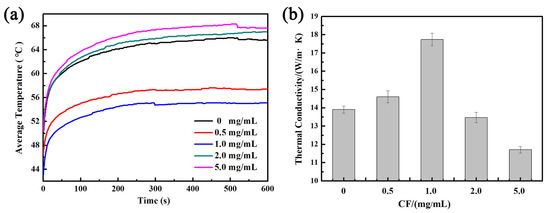
Figure 8.
Effect of CF concentration on the through-plane (a) time-average temperature change curve and (b) thermal conductivity of VGNs/CF composite films.
4. Conclusions
In this work, a new type of VGNs/CF composite film was prepared, and its heat dissipation behaviors were investigated. The Ni, Fe, and Co elements of stainless steel as catalytic components promoted the growth of VGNs. The appropriate amount of CF filler was introduced into the VGNs film, providing efficient thermal conduction channels and further enhancing the heat dissipation capacity of the VGNs/CF composite film. Some main effects, including the impact of the roughness of the SS substrate surface, the concentration and dosage of the carbon precursor solution, and the growth time and content of CF on the growth of the VGNs/CF composite film were studied in detail. It is determined that these factors could greatly affect the morphology of the composite film. The results of through-plane thermal conductivity testing showed that the VGNs/CF composite film could reach 17.7 W/(m·K), which is 27.6% higher than that of the pure VGNs film (13.9 W/(m·K)). Therefore, the prepared VGNs/CF composite film can find its practical application in the heat dissipation field of electronic devices.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/coatings13020407/s1, Figure S1: Optical photographs of VGNs/CF films on RSS surfaces prepared by (a) spin-coating (b) spraying techniques; Figure S2: Comparison of SEM images after heating films prepared by (a) spin-coating and (b) spraying techniques; Figure S3: SEM images of VGNs/CF composite films on RSS surfaces grown at 850°C for 3 h using different concentrations and dosages of precursor solutions: (a) 0.4 g/mL, 0.6 mL; (b) 0.4 g/ml, 0.8 mL; (c) 0.4 g/mL, 1.0 mL; (d) 0.6 g/mL, 0.6 mL; (e) 0.6 g/mL, 0.8 mL; (f) 0.6 g/mL, 1.0 mL; (g) 0.8 g/mL, 0.6 mL; (h) 0.8 g/mL, 0.8 mL; (i) 0.8 g/mL, 1.0 mL; Figure S4: XPS images of stainless steel (SS) substrate before and after roughening treatment; Figure S5: Wettability comparison of water droplet on RSS substrate surface (a) before and (b) after oxygen plasma pretreatment; Figure S6: TEM images of (a) the CF and (b) the CF in VGNs/CF composite film.
Author Contributions
Conceptualization, investigation, writing—original draft preparation, and writing, M.Y.; data curation, methodology, resources, and supervision, W.J.; resources and supervision, Y.Y.; writing—review and editing, and supervision, Q.Z.; resources and supervision, L.M.; conceptualization, methodology, resources, supervision, writing—review and editing, and project administration, J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science and Technology Project of Gansu Province (Grant No. 21JR7RA085) and the National Natural Science Foundation of China (Grant No. 52175203).
Data Availability Statement
The data that support the findings of this study are available within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Balandin, A.A. Chill out. IEEE Spectr. 2009, 46, 34–39. [Google Scholar] [CrossRef]
- Majumdar, A. Helping chips to keep their cool. Nat. Nanotechnol. 2009, 4, 214–215. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.; Omar, M.N.; Ishak, M.S.A.; Rahman, R.; Yahaya, N.Z.; Razab, M.K.A.A.; Thirmizir, M.Z.A. Comparison between CNT thermal interface materials with graphene thermal interface material in term of thermal conductivity. Mater. Sci. Forum 2020, 1010, 160–165. [Google Scholar] [CrossRef]
- Dai, W.; Ma, T.; Yan, Q.; Gao, J.; Tan, X.; Lv, L.; Hou, H.; Wei, Q.; Yu, J.; Wu, J.; et al. Metal-level thermally conductive yet soft graphene thermal interface materials. ACS Nano 2019, 13, 11561–11571. [Google Scholar] [CrossRef] [PubMed]
- Due, J.; Robinson, A.J. Reliability of thermal interface materials: A review. Appl. Therm. Eng. 2013, 50, 455–463. [Google Scholar] [CrossRef]
- Sudhindra, S.; Ramesh, L.; Balandin, A.A. Graphene thermal interface materials–state-of-the-art and application prospects. IEEE Open J. Nanotechnol. 2022, 3, 169–181. [Google Scholar] [CrossRef]
- Anandan, S.; Ramalingam, V. Thermal management of electronics: A review of literature. Therm. Sci. 2008, 12, 5–26. [Google Scholar] [CrossRef]
- Prasher, R. Graphene spreads the heat. Science 2010, 328, 185–186. [Google Scholar] [CrossRef]
- Liu, Y.; Li, P.; Wang, F.; Fang, W.; Xu, Z.; Gao, W.; Gao, C. Rapid roll-to-roll production of graphene films using intensive Joule heating. Carbon 2019, 155, 462–468. [Google Scholar] [CrossRef]
- Inagaki, M.; Harada, S.; Sato, T.; Nakajima, T.; Horino, Y.; Morita, K. Carbonization of polyimide film “Kapton”. Carbon 1989, 27, 253–257. [Google Scholar] [CrossRef]
- Inagaki, M.; Meng, L.-J.; Ibuki, T.; Sakai, M.; Hishiyama, Y. Carbonization and graphitization of polyimide film “Novax”. Carbon 1991, 29, 1239–1243. [Google Scholar] [CrossRef]
- Suhng, Y.; Hashizume, K.; Kaneko, T.; Otani, S.; Yoshimura, S. The study of the graphitization behavior for polyimide and polyamide films. Synth. Met. 1995, 71, 1751–1752. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Yang, G.; Yi, H.; Yao, Y.; Li, C.; Li, Z. Thermally conductive graphene films for heat dissipation. ACS Appl. Nano Mater. 2020, 3, 2149–2155. [Google Scholar] [CrossRef]
- Ci, H.; Chang, H.; Wang, R.; Wei, T.; Wang, Y.; Chen, Z.; Sun, Y.; Dou, Z.; Liu, Z.; Li, J.; et al. Enhancement of heat dissipation in ultraviolet light-emitting diodes by a vertically oriented graphene nanowall buffer layer. Adv. Mater. 2019, 31, e1901624. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.S.; Perrier, T.; Barani, Z.; Kargar, F.; Balandin, A.A. Thermal interface materials with graphene fillers: Review of the state of the art and outlook for future applications. Nanotechnology 2021, 32, 142003. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Ren, Y.-J.; Bai, S.-L. Vertically aligned graphene film/epoxy composites as heat dissipating materials. Int. J. Heat Mass Transf. 2018, 118, 510–517. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Han, D.; Fang, H.M.; Bai, S.L. A facile method to transfer vertical aligned graphene film/polydimethylsiloxane composite to thermal interface materials. In Proceedings of the 2016 China Semiconductor Technology International Conference (CSTIC), Shanghai, China, 13–14 March 2016. [Google Scholar]
- Balandin, A.A. Phononics of graphene and related materials. ACS Nano 2020, 14, 5170–5178. [Google Scholar] [CrossRef]
- Barrau, S.; Demont, P.; Perez, E.; Peigney, A.; Laurent, C.; Lacabanne, C. Effect of palmitic acid on the electrical conductivity of carbon nanotubes−epoxy resin composites. Macromolecules 2003, 36, 9678–9680. [Google Scholar] [CrossRef]
- Choi, Y.K.; Sugimoto, K.I.; Song, S.M.; Endo, M. Mechanical and thermal properties of vapor-grown carbon nanofiber and polycarbonate composite sheets. Mater. Lett. 2005, 59, 3514–3520. [Google Scholar] [CrossRef]
- Yeh, M.-K.; Tai, N.-H.; Liu, J.-H. Mechanical behavior of phenolic-based composites reinforced with multi-walled carbon nanotubes. Carbon 2006, 44, 1–9. [Google Scholar] [CrossRef]
- Zhu, H.; Li, X.; Ci, L.; Xu, C.; Wu, D.; Mao, Z. Hydrogen storage in heat-treated carbon nanofibers prepared by the vertical floating catalyst method. Mater. Chem. Phys. 2003, 78, 670–675. [Google Scholar] [CrossRef]
- Zhang, B.; Tian, Y.; Jin, X.; Lo, T.Y.; Cui, H. Thermal and Mechanical Properties of Expanded Graphite/Paraffin Gypsum-Based Composite Material Reinforced by Carbon Fiber. Materials 2018, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Feng, T.; Yang, H.; Bao, X.; Tang, W.; Fu, J. Experimental study of carbon fiber reinforced alkali-activated slag composites with micro-encapsulated PCM for energy storage. Constr. Build. Mater. 2018, 161, 442–451. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Fan, C.; Yao, H.; Chen, X.; Liu, Y. Study on the preparation of a high-efficiency carbon fiber dissipating coating. Coatings 2017, 7, 94. [Google Scholar] [CrossRef]
- Cho, H.J.; Kondo, H.; Ishikawa, K.; Sekine, M.; Hiramatsu, M.; Hori, M. Density control of carbon nanowalls grown by CH4/H2 plasma and their electrical properties. Carbon 2014, 68, 380–388. [Google Scholar] [CrossRef]
- Fu, W.; Zhao, X.; Zheng, W. Growth of vertical graphene materials by an inductively coupled plasma with solid-state carbon sources. Carbon 2021, 173, 91–96. [Google Scholar] [CrossRef]
- Hussain, S.; Amade, R.; Boyd, A.; Musheghyan-Avetisyan, A.; Alshaikh, I.; Martí-Gonzalez, J.; Pascual, E.; Meenan, B.J.; Bertran-Serra, E. Three-dimensional Si/vertically oriented graphene nanowalls composite for supercapacitor applications. Ceram. Int. 2021, 47, 21751–21758. [Google Scholar] [CrossRef]
- Kim, S.Y.; Choi, W.S.; Lee, J.-H.; Hong, B. Substrate temperature effect on the growth of carbon nanowalls synthesized via microwave PECVD. Mater. Res. Bull. 2014, 58, 112–116. [Google Scholar] [CrossRef]
- Sahoo, G.; Polaki, S.R.; Ghosh, S.; Krishna, N.G.; Kamruddin, M. Temporal-stability of plasma functionalized vertical graphene electrodes for charge storage. J. Power Sources 2018, 401, 37–48. [Google Scholar] [CrossRef]
- Sha, Z.; Zhou, Y.; Huang, F.; Yang, W.; Yu, Y.; Zhang, J.; Wu, S.; Brown, S.A.; Peng, S.; Han, Z.; et al. Carbon fibre electrodes for ultra long cycle life pseudocapacitors by engineering the nano-structure of vertical graphene and manganese dioxides. Carbon 2021, 177, 260–270. [Google Scholar] [CrossRef]
- Tanaka, K.; Yoshimura, M.; Okamoto, A.; Ueda, K. Growth of carbon nanowalls on a SiO2 substrate by microwave plasma-enhanced chemical vapor deposition. Jpn. J. Appl. Phys. 2005, 44, 2074–2076. [Google Scholar] [CrossRef]
- Wang, J.; Ito, T. CVD growth and field emission characteristics of nano-structured films composed of vertically standing and mutually intersecting nano-carbon sheets. Diam. Relat. Mater. 2007, 16, 589–593. [Google Scholar] [CrossRef]
- Yu, K.; Bo, Z.; Lu, G.; Mao, S.; Cui, S.; Zhu, Y.; Chen, X.; Ruoff, R.S.; Chen, J. Growth of carbon nanowalls at atmospheric pressure for one-step gas sensor fabrication. Nanoscale Res. Lett. 2011, 6, 202. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, Z.; Wang, Y.; Yang, R.; Shi, D.; Zhang, G. Catalyst-free growth of nanographene films on various substrates. Nano Res. 2010, 4, 315–321. [Google Scholar] [CrossRef]
- Ando, Y.; Zhao, X.; Ohkohchi, M. Production of petal-like graphite sheets by hydrogen arc discharge. Carbon 1997, 35, 153–158. [Google Scholar] [CrossRef]
- Sun, D.; Li, H.; Li, M.; Li, C.; Qian, L.; Yang, B. Electrochemical immunosensors with AuPt-vertical graphene/glassy carbon electrode for alpha-fetoprotein detection based on label-free and sandwich-type strategies. Biosens. Bioelectron. 2019, 132, 68–75. [Google Scholar] [CrossRef]
- Wang, Z.; Xue, L.; Li, M.; Li, C.; Li, P.; Li, H. Au@SnO2-vertical graphene-based microneedle sensor for in-situ determination of abscisic acid in plants. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 127, 112237. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, Z.; Chen, C.; Ma, W.; Han, S.; Li, X.; Lu, S.; Hu, X. High efficient oxygen reduced reaction electrodes by constructing vertical graphene sheets on separated papillary granules formed nanocrystalline diamond films. Carbon 2020, 168, 536–545. [Google Scholar] [CrossRef]
- Wang, B.B.; Zheng, K.; Cheng, Q.J.; Ostrikov, K. Plasma effects in aligned carbon nanoflake growth by plasma-enhanced hot filament chemical vapor deposition. Appl. Surf. Sci. 2015, 325, 251–257. [Google Scholar] [CrossRef]
- Zhou, H.-T.; Yu, N.; Zou, F.; Yao, Z.-H.; Gao, G.; Shen, C.-M. Controllable preparation of vertically standing graphene sheets and their wettability and supercapacitive properties. Chin. Phys. B 2016, 25, 096106. [Google Scholar] [CrossRef]
- Wei, N.; Li, Q.; Cong, S.; Ci, H.; Song, Y.; Yang, Q.; Lu, C.; Li, C.; Zou, G.; Sun, J.; et al. Direct synthesis of flexible graphene glass with macroscopic uniformity enabled by copper-foam-assisted PECVD. J. Mater. Chem. A 2019, 7, 4813–4822. [Google Scholar] [CrossRef]
- Hong, T.; Zhan, R.; Zhang, Y.; Deng, S. High crystallinity vertical few-layer graphene grown using template method assisted ICPCVD approach. Nanomaterials 2022, 12, 3746. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.K.; Shukla, A.K.; Vankar, V.D.; Kumar, V. Growth, structure and field emission characteristics of petal like carbon nano-structured thin films. Thin Solid Film. 2005, 492, 124–130. [Google Scholar] [CrossRef]
- Wu, Y.; Qiao, P.; Chong, T.; Shen, Z. Carbon nanowalls grown by microwave plasma enhanced chemical vapor deposition. Adv. Mater. 2002, 14, 64–67. [Google Scholar] [CrossRef]
- Guo, X.; Li, Y.; Ding, Y.; Chen, Q.; Li, J. Direct patterned growth of intrinsic/doped vertical graphene nanosheets on stainless steel via heating solid precursor films for field emission application. Mater. Des. 2019, 162, 293–299. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, A.; Kaya, S.; Marzouki, R.; Zhang, F.; Guo, L. Recent advancements in surface modification, characterization and functionalization for enhancing the biocompatibility and corrosion resistance of biomedical implants. Coatings 2022, 12, 1459. [Google Scholar] [CrossRef]
- Thakur, A.; Kaya, S.; Abousalem, A.S.; Sharma, S.; Ganjoo, R.; Assad, H.; Kumar, A. Computational and experimental studies on the corrosion inhibition performance of an aerial extract of Cnicus Benedictus weed on the acidic corrosion of mild steel. Process Saf. Environ. Prot. 2022, 161, 801–818. [Google Scholar] [CrossRef]
- Fu, Y.; Hansson, J.; Liu, Y.; Chen, S.; Zehri, A.; Samani, M.K.; Wang, N.; Ni, Y.; Zhang, Y.; Zhang, Z.-B.; et al. Graphene related materials for thermal management. 2d Mater. 2020, 7, 012001. [Google Scholar] [CrossRef]
- Du, W.; Zhang, Z.; Su, H.; Lin, H.; Li, Z. Urethane-functionalized graphene oxide for improving compatibility and thermal conductivity of waterborne polyurethane composites. Ind. Eng. Chem. Res. 2018, 57, 7146–7155. [Google Scholar] [CrossRef]
- Nong, J.; Wei, W.; Song, X.; Tang, L.; Yang, J.; Sun, T.; Yu, L.; Luo, W.; Li, C.; Wei, D. Direct growth of graphene nanowalls on silica for high-performance photo-electrochemical anode. Surf. Coat. Technol. 2017, 320, 579–583. [Google Scholar] [CrossRef]
- Prasad, K.; Bandara, C.D.; Kumar, S.; Singh, G.P.; Brockhoff, B.; Bazaka, K.; Ostrikov, K.K. Effect of precursor on antifouling efficacy of vertically-oriented graphene nanosheets. Nanomaterials 2017, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.-H.; Chen, W.; Fang, H.-C.; Tzeng, Y.; Liu, C.-P. Heteroepitaxial nucleation and growth of graphene nanowalls on silicon. Carbon 2013, 54, 234–240. [Google Scholar] [CrossRef]
- Zhao, R.; Ahktar, M.; Alruqi, A.; Dharmasena, R.; Jasinski, J.B.; Thantirige, R.M.; Sumanasekera, G.U. Electrical transport properties of graphene nanowalls grown at low temperature using plasma enhanced chemical vapor deposition. Mater. Res. Express 2017, 4, 055007. [Google Scholar] [CrossRef]
- Hiramatsu, M.; Shiji, K.; Amano, H.; Hori, M. Fabrication of vertically aligned carbon nanowalls using capacitively coupled plasma-enhanced chemical vapor deposition assisted by hydrogen radical injection. Appl. Phys. Lett. 2004, 84, 4708–4710. [Google Scholar] [CrossRef]
- Zhang, Z.; Lee, C.-S.; Zhang, W. Vertically aligned graphene nanosheet arrays: Synthesis, properties and applications in electrochemical energy conversion and storage. Adv. Energy Mater. 2017, 7, 1700678. [Google Scholar] [CrossRef]
- Liu, J.; Sun, W.; Wei, D.; Song, X.; Jiao, T.; He, S.; Zhang, W.; Du, C. Direct growth of graphene nanowalls on the crystalline silicon for solar cells. Appl. Phys. Lett. 2015, 106, 043904. [Google Scholar] [CrossRef]
- Thirumal, V.; Yuvakkumar, R.; Kumar, P.S.; Ravi, G.; Velauthapillai, D. Direct growth of multilayered graphene nanofibers by chemical vapour deposition and their binder-free electrodes for symmetric supercapacitor devices. Prog. Org. Coat. Int. Rev. J. 2021, 161, 106511. [Google Scholar] [CrossRef]
- Sui, Y.; Chen, Z.; Zhang, Y.; Hu, S.; Liang, Y.; Ge, X.; Li, J.; Yu, G.; Peng, S.; Jin, Z.; et al. Growth promotion of vertical graphene on SiO2/Si by Ar plasma process in plasma-enhanced chemical vapor deposition. RSC Adv. 2018, 8, 18757–18761. [Google Scholar] [CrossRef]
- Deng, J.H.; Zheng, R.T.; Zhao, Y.; Cheng, G.A. Vapor–solid growth of few-layer graphene using radio frequency sputtering deposition and its application on field emission. ACS Nano 2012, 6, 3727–3733. [Google Scholar] [CrossRef]
- Peng, L.; Xu, Z.; Liu, Z.; Guo, Y.; Li, P.; Gao, C. Ultrahigh thermal conductive yet superflexible graphene films. Adv. Mater. 2017, 29, 1700589. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).