Abstract
This study aimed to improve the surface hardness and wear resistance of spindle hook teeth with special shapes to reduce the cost of replacing the spindle on cotton pickers. For this goal, a Ni-Co-β-SiC composite coating with different concentrations of β-SiC nanoparticles (0, 1, 2, 3, and 4 g/L) was electrodeposited on the surface of spindle hook teeth. The hardness, elemental composition, and micromorphology of the spindle hook teeth were characterized by microhardness tests, an energy spectrum analyzer, and a scanning electron microscope after cutting with the spindles. The actual wear process of the coating was determined by wear simulation and scratch wear tests, and the effect of the concentration of β-SiC nanoparticles on the properties of the coating was investigated. The results show that Ni-Co-β-SiC composite coating has a typical cellular structure. The hardness first increases and then decreases, and the wear resistance (including friction coefficient, scratch area, and shape of wear area) first decreases and then increases, mainly due to the pinning role and agglomeration of β-SiC nanoparticles. When the concentration of β-SiC was 1 g/L, the hardness reached a maximum of 506.2 HV0.1, the coefficient of friction reached a minimum of 0.13, and the wear area and wear micromorphology reached the most suitable values. Therefore, this Ni-Co-β-SiC composite coating had the best microhardness and wear resistance.
1. Introduction
With the development in agriculture and economics, agricultural equipment with good hardness and wear resistance has become crucial in reducing agricultural production costs. As the core part of the picking head of a cotton picker, the surface hardness and wear resistance of the spindle have received global attention [1]. Plating is commonly used to improve the surface performance and consequently the core performance of the spindle. The main failure form of picker spindles is wear: serious wear reduces the cotton pickers’ picking rate and increases the fallen cotton amount, thus greatly affecting performance [2].
A single nickel-based coating cannot satisfy the current requirements of agricultural and industrial appliances; hence, many researchers began to explore the properties of Ni-Co, Ni-W, Ni-P, and Ni-Cu composite coatings to improve their comprehensive performance [3,4,5,6]. Nickel-based composite coatings have good physical and chemical properties and are widely used in industrial production and agricultural tool production [7,8,9]. With the rapid development of science and technology, the optimal performance of different materials is required. Owing to their high purity, chemical property stability, and small size distribution, nanoparticles are widely used in material preparation. The comprehensive properties of a composite coating can be improved by co-depositing different insoluble second nanoparticles into the composite coating [10,11,12,13,14,15].
Cubic silicon carbide (β-SiC) nanoparticles are inorganic and nonmetallic materials that have a cubic crystalline structure and excellent properties, such as high hardness, high density, and chemical stability, and are widely used in mechanical manufacturing, industrial production, the ceramic industry, and other fields. Liu et al. [16] added β-SiC nanoparticles to a plating solution to improve the wear resistance of Ni-Mn alloy coatings and prepared Ni-Mn-SiC composite coatings by electrodeposition. The addition of SiC nanoparticles significantly improved the hardness, wear resistance, and adhesion of the composite. Du et al. [17] added β-SiC nanoparticles to prepare Ni-P-β-SiC composite coatings by pulse electrospray plating and applied COMSOL simulation to verify and analyze the effect of SiC nanoparticles on the hardness, wear resistance, and corrosion resistance of the composite coating.
Current research mainly focuses on the preparation of Cr-based coatings on the surface of the spindle. Based on this research status, most of the spindles used by cotton pickers at present have Cr coatings; however, the effect of preparing another layer of nickel-based composite coating on the surface of the spindle on the abrasion resistance of cotton stripping by the spindle itself is still unclear. In the present work, a Ni-Co-β-SiC composite coating was electrodeposited on the surface of a spindle, and the effect of SiC nanoparticles (0, 1, 2, 3, and 4 g/L) on the surface morphology of the coating was investigated. The hardness and wear resistance of the composite coating were analyzed, and a wear test was carried out. This study laid a foundation for preparing a Ni-Co-β-SiC composite coating with better wear resistance on spindles.
2. Materials and Methods
2.1. Materials
Thiourea (CH4N2S) and sodium dodecyl sulfate (C12H25SO4Na) were purchased from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). Nickel sulfate hexahydrate (NiSO4·6H2O), nickel chloride hexahydrate (NiCl4∙6H2O), cobalt sulfate heptahydrate (CoSO4·7H2O), and boric acid (H3BO3) were obtained from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). β-SiC was acquired from Shanghai New Materials Technology Co., Ltd. (Shanghai, China).
2.2. Experimental
The spindle of a PRO-16 picker was taken as the research object. The spindle material is 20CrMnTi steel, and the three rows of 12 hook teeth evenly distributed on the conical arc surface of the front end are the cotton-picking parts of the spindle. The structural parameters of the new spindle are shown in Table 1 [18], and the composition and content of the plating solution are listed in Table 2. For the chemical electrodeposition, the temperature was set as 60 °C and the current was 1 A. Prior to this process, the surface of the spindle was pretreated as follows: polishing → electrolytic deoiling → weak activation, including the ultrasonic cleaning of deionized water after each step. The size of the β-SiC nanoparticles was 30 nm. Ni-Co-β-SiC composite coatings with different concentrations of β-SiC nanoparticles were prepared on the surface of the spindles. The preparation time was 2 h. Finally, the processed spindles were cleaned ultrasonically and air-dried.

Table 1.
Structure parameters of the spindles.

Table 2.
Composition of plating solution.
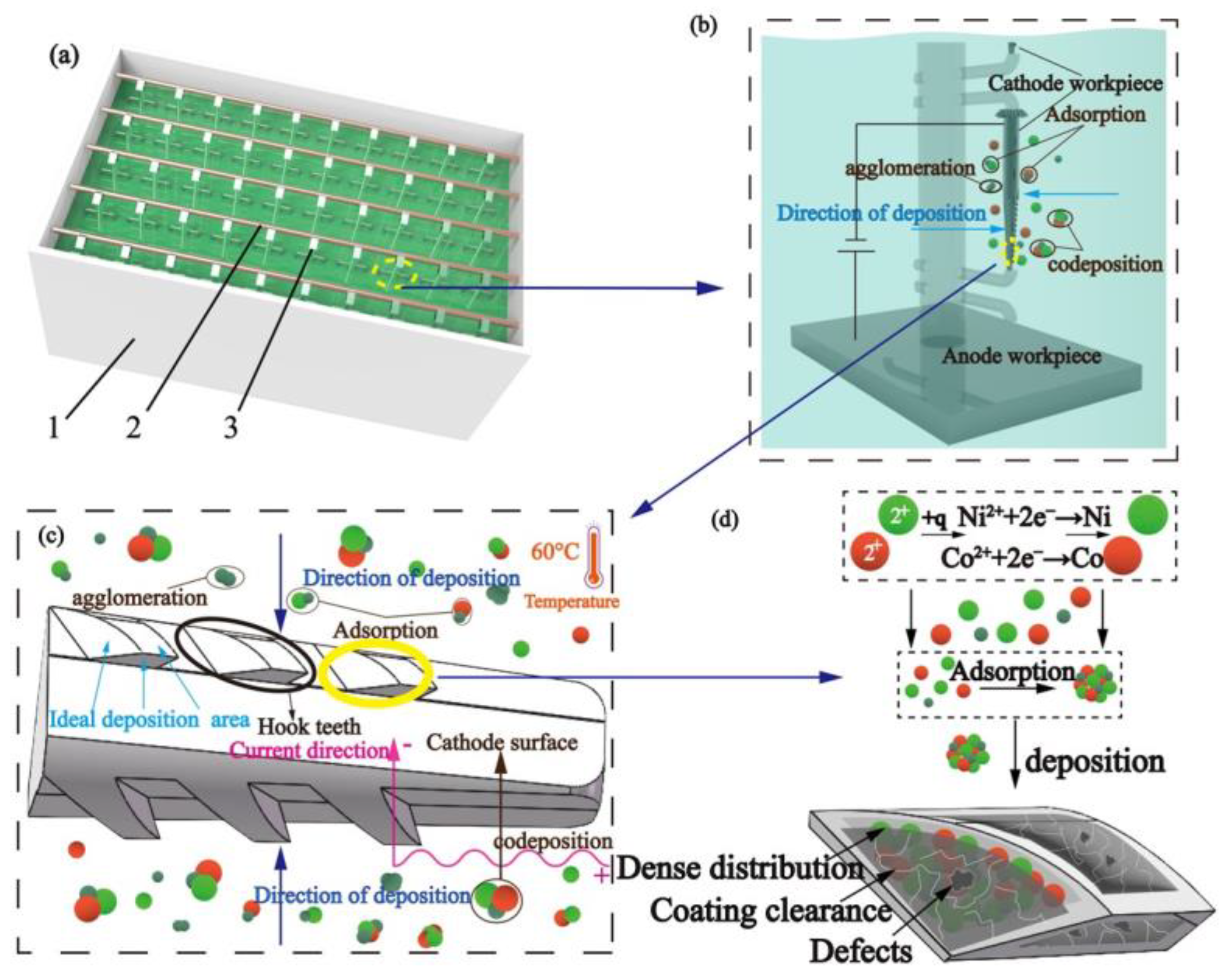
Figure 1 shows the experimental schematic of chemical electrodeposition. The spindles were fixed on the connection rod (2) and immersed in the electroplating bath (1) with the clamping device (3), which was connected to the cathode of the DC power source for reduction. The nickel sheet was placed at the bottom of the electroplating bath, which was connected to the anode of the DC power source for oxidation. Uniform coating on the surface of the pick-up hook tooth can be realized by controlling the flow and circulation of the plating solution in the bath (1). After the power supply was connected, the plating solution, spindle, and nickel sheet formed a closed-loop path to deposit the ions in the bath.
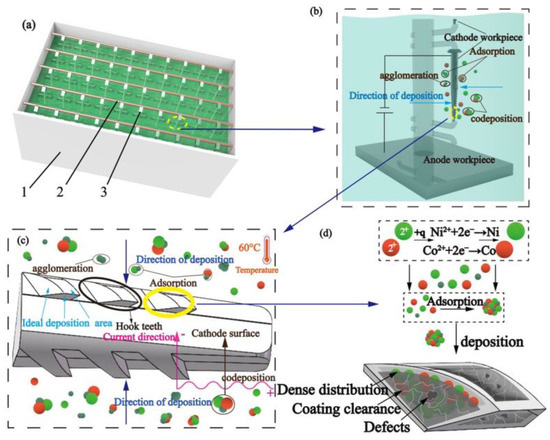
Figure 1.
Experimental schematic of chemical electrodeposition: (a) experimental device (1—electroplating bath, 2—connection rod, 3—clamping device), (b) single spindle hook tooth experiment diagram, (c) experimental response diagram of spindle hook teeth, and (d) experimental microcosmic principle.
The plating solution contained a significant amount of Ni and Co ions and β-SiC nanoparticles. When placed under an electric field, Ni2+ and Co2+ gained electrons and formed Ni and Co atoms, respectively, and were evenly deposited on the surface of the spindle. According to Guglielmi’s two-step adsorption model [19], β-SiC nanoparticles can adsorb ion layers, promote their strong adsorption with Ni2+ and Co2+, and codeposit on the surface of ingots to form a coating. Therefore, β-SiC nanoparticles can be distributed evenly in the gap of the Ni-Co coating and further form a composite coating with good surface quality.
2.3. Instruments
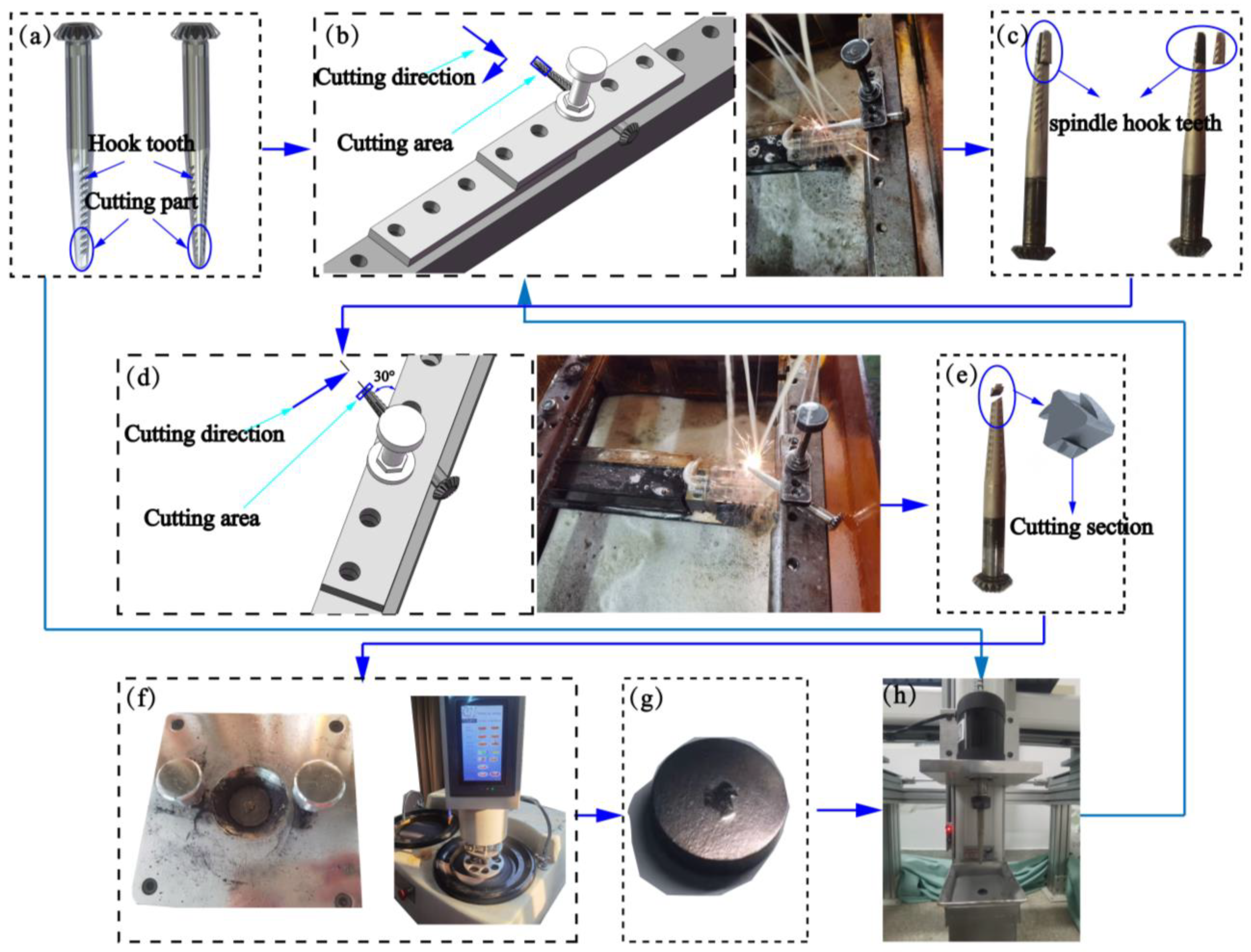
Figure 2 shows the sample preparation. For the morphological observation of the spindle after processing, the following instruments were used to cut and prepare samples of the spindle hook teeth: an EDM cutting machine, ultrasonic cleaning instrument, dryer, metallographic grinding machine, and metallographic inlay machine. The surface and cross-sectional morphology of the spindle hook teeth were observed using a Quanta FEG 250 Field Emission Scanning Electron Microscope from FEI Instruments, Inc. (Hillsboro, OR, USA) under the scanning speed of 30 μs (or time scanning one round) and magnifications of 50, 1000, and 5000 times. In addition, the content and distribution of elements in the composite coatings were analyzed by EDS from Bruker AXS, Inc. (Berlin, Germany). The surface of the coating was marked repeatedly for 20 min with a mark length of 3 mm under a load of 320 g using a CFT-I Material surface comprehensive performance tester from Zhongke Kaihua Instrument Equipment Co., Ltd. (Wuhan, China). For the analysis of the friction coefficient of the coating, the scratches were observed and analyzed using an Olympus LEXT 4100 Laser scanning confocal microscope from Olympus Tokyo, Inc. (Tokyo, Japan). The surface hardness of the coating and the substrate was comprehensively analyzed using a Duramin-40 microhardness tester from Struers, Inc. (Copenhagen, Denmark). The wear simulation of the spindle in an actual picking environment was carried out using a self-developed spindle hook tooth wear device [18]. At the same time, the mature brown corundum on the market is selected as the wear medium, with the specification of 100 meshes, the spindle motor speed set to 4000 r/min, and the wear time set to 6 h. Additionally, the worn samples were cut, prepared, and observed in the same way.
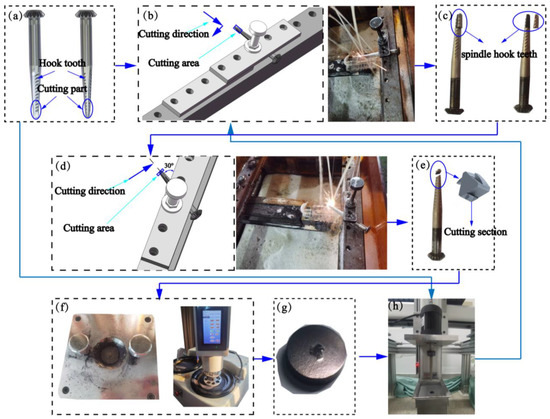
Figure 2.
Sample preparation: (a) sample of spindles, (b) first wire cutting, (c) sample after first wire cutting, (d) second wire cutting, (e) sample after second wire cutting, (f) inlay and polish, (g) sample after inlay and polish, and (h) wear process.
3. Results and Discussion
3.1. Analysis of the Micromorphology of the Composite Coating
3.1.1. Analysis of the Surface Micromorphology of the Composite Coating
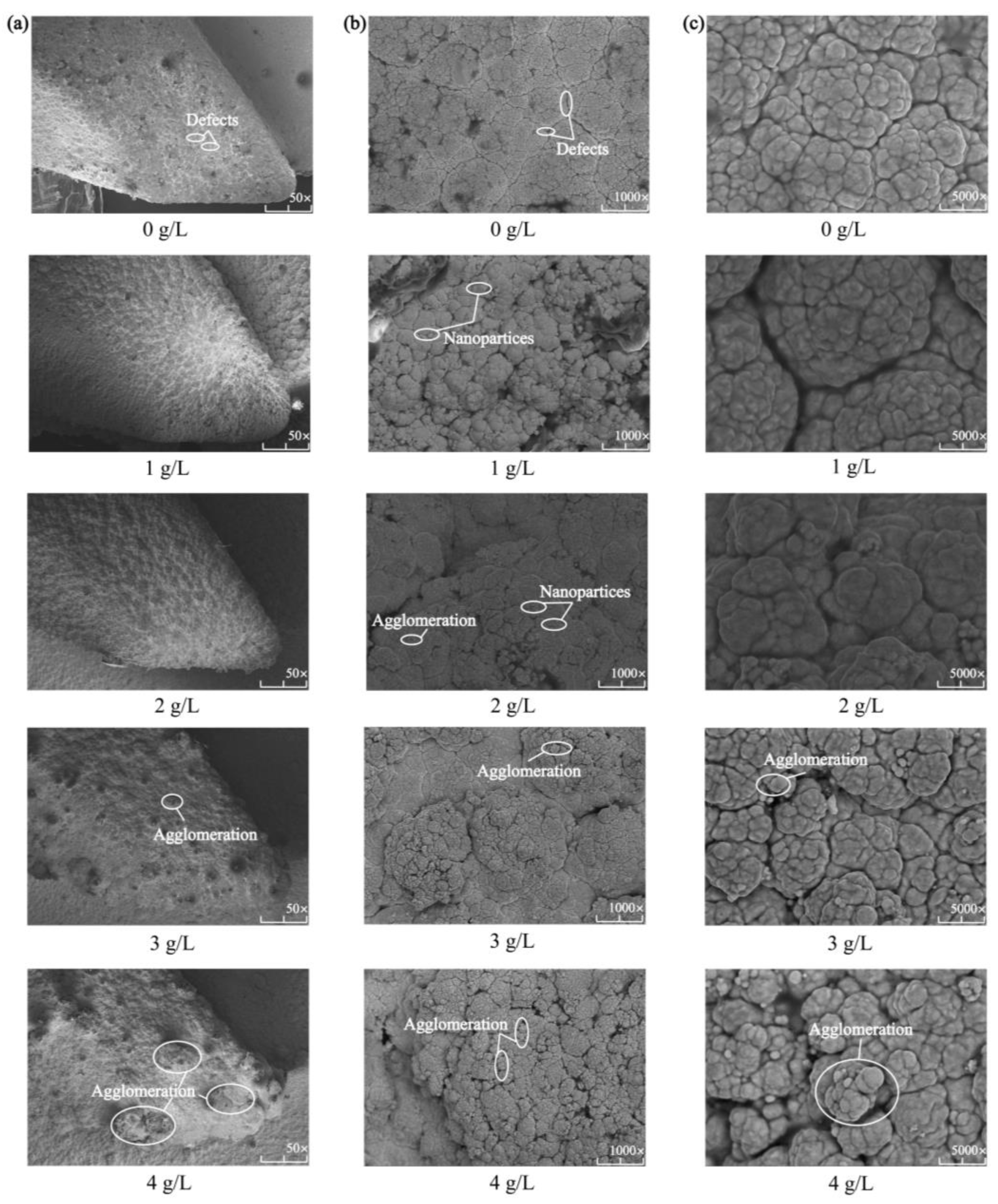
Figure 3 shows the surface morphology of the Ni-Co-β-SiC composite coating prepared with different β-SiC concentrations. As shown in Figure 3a, a compact composite coating was formed on the surface. Hence, the density of the coating is affected by the variation in SiC concentration. As shown in Figure 3b,c, the cell morphology in the composite coating exhibited a typical cellular structure. However, some defects were observed on the surface, and the cell size changed with the SiC concentration. The main reason is that the addition of SiC nanoparticles increases the cathode polarization and promotes the nucleation of Ni2+ [20]. Therefore, during the growth of the crystal nucleus in Ni-Co composite coatings, SiC disperses inside the Ni-Co crystal to form a dense Ni-Co-SiC displaced solid solution layer and codeposits on the spindle surface, which refines the surface structure of the composite coating. When the concentration of SiC nanoparticles changed from 0 g/L to 1 g/L and then to 2 g/L, the surface of the composite coating showed improved quality and was dense and smooth without defects. When the concentration of SiC nanoparticles changed from 2 g/L to 3 g/L and then to 4 g/L, the composite coating exhibited a rough surface, a large number of nanoparticles agglomerated, and the surface structure was no longer uniform and dense.
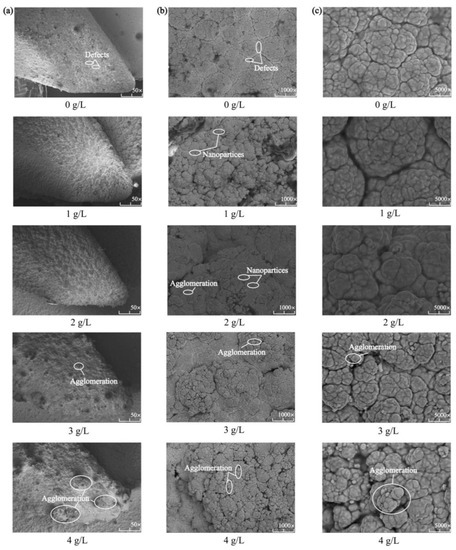
Figure 3.
Surface micromorphologies of Ni-Co-β-SiC composite coatings prepared with different β-SiC concentrations: (a) 50 magnification, (b) 1000 magnification, and (c) 5000 magnification.
The above results reveal that with the increase in the concentration of SiC nanoparticles, the deposition rate of SiC nanoparticles also increases. During processing, a large number of SiC nanoparticles suspended in the plating solution are surrounded by an ionic layer and are adsorbed onto the cathode surface, and cathode polarization is enhanced by the promotion of zeta potential [21], physical adsorption, and chemical adsorption. Thus, the rate of embedding SiC nanoparticles into the composite coating is accelerated, and the nucleation of the composite coating is promoted. Moreover, the dispersion strengthening and fine-grain strengthening of the composite coating surface were realized due to the synergistic effect of nanomaterials, so the quality of the composite coating is improved. However, when the concentration of SiC nanoparticles in the composite coating is too high, too many nucleation points are formed, leading to excessive grain refinement [22], uneven stress distribution in the coating, and grain precipitation. As a result, the efficiency of particle deposition is reduced and surface defects are formed. In addition, the excessive number of nanoparticles has a negative effect on the current, resulting in excessive surface energy during deposition [20]. The large number of inhomogeneous nanoparticles agglomerated on the surface of the coating reduces the ability of the elements to permeate and leads to a rough surface, uneven cell growth, and even pores, cracks, and other defects.
3.1.2. Analysis of the Cross-Sectional Micromorphology of the Composite Coating
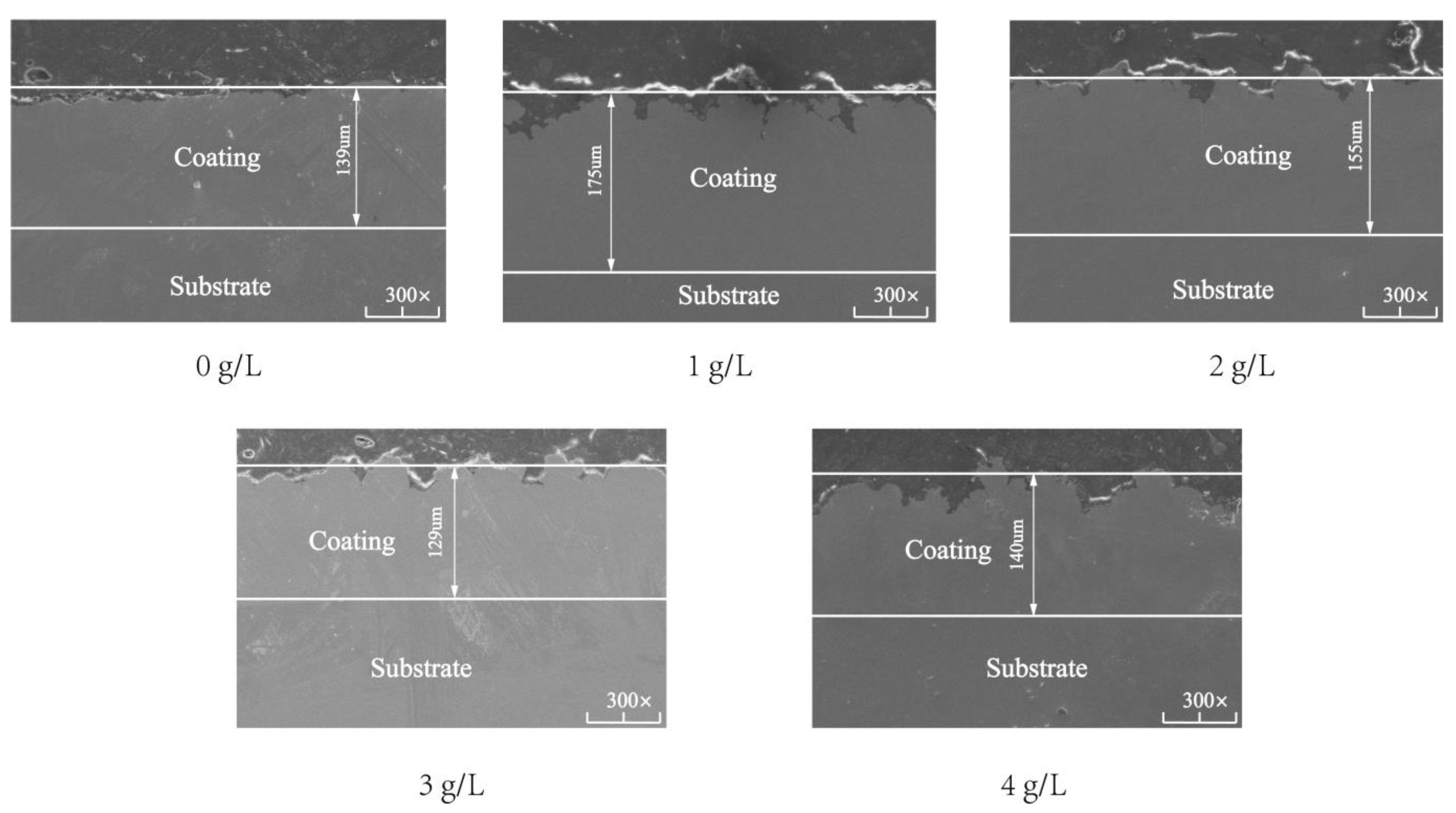
Figure 4 shows the SEM cross-sectional micromorphologies of the composite coating. When the SiC nanoparticle concentration was 0, 1, 2, 3, and 4 g/L, the composite coating thickness was 139, 175, 155, 129, and 140 μm, respectively. The overall thickness of the composite coating increased first and then decreased. When the concentration of SiC nanoparticles was 0 g/L, the cross-section of the composite coating was relatively compact. However, small pits were found on the contact surface between the substrate and the coating, and the internal smoothness of the coating was poor. These results show that Ni-Co composite coatings do not completely adhere to the surface of the spindle. When the concentration of SiC nanoparticles was 1 g/L, the composite coating was in close contact with the substrate and the thickness of the composite coating reached the maximum. The inner part of the coating was smooth, but the surface of the coating was less smooth. The main reason is that at 1 g/L, the nanoparticles can be distributed evenly in the coating, increasing the nucleation point of the Ni-Co composite coatings, the crystal nucleation growth, the crystal growth rate, and the coating thickness. However, the surface becomes rough because the sector hook teeth distributed on the conical structure cannot come into full contact with the nanoparticles during plating. At 2 g/L, the contact between the coating and substrate and the internal smoothness of the coating is almost unchanged. The thickness of the coating decreases, and the surface of the coating becomes smooth; the reason is that when the SiC concentration continues to increase, the nucleation point is too much, and a reduction is observed in the potential difference between the nanoparticles and cathode, the deposition efficiency, and the thickness. However, the contact between the coating and substrate becomes full during plating and the surface becomes flat. At 3 g/L, the cross-section quality of the coating decreases and the inside of the coating becomes rough. With further increase in the nanoparticles, they start to agglomerate and become adsorbed in the form of agglomerates on the interior and surface of the coating, thus reducing the current efficiency during adsorption and changing the stress distribution inside the coating. Hence, the nucleation point distribution is not uniform, and the overall quality of the coating is reduced. At 4 g/L, the thickness of the coating increases abnormally, and the surface quality decreases because of the excessive agglomeration of nanoparticles. This phenomenon decreases the conductivity of the plating bath, thus affecting the efficiency of electroplating and reducing the formation of effective nucleation points and deteriorating the surface quality.
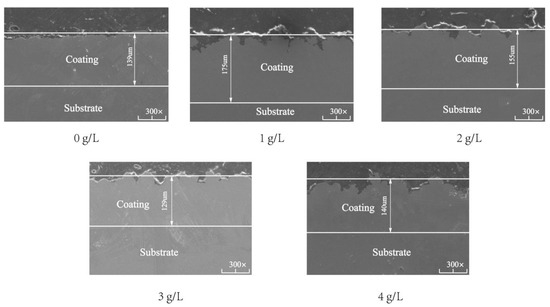
Figure 4.
Cross-sectional micromorphologies of Ni-Co-β-SiC composite coatings prepared with different β-SiC concentrations.
3.2. Effect of β-SiC Concentrations on Coating Composition
EDS and Mass Fraction Analysis of the Composite Coating
Table 3 shows the elemental content of Ni-Co-β-SiC composite coating with different β-SiC nanoparticle concentrations. Different concentrations of SiC nanoparticles exhibited different effects on the mass fraction of the main elements in the composite coating. The mass fraction of Ni increased first and then decreased. When the concentration of SiC nanoparticles was 1 g/L, the mass fraction of Ni was 67.03%, that of Co was 9.60%, and that of Si was the largest. These results show that increasing the concentration of SiC nanoparticles can effectively increase the nucleation point and current efficiency, which is beneficial to the codeposition of Ni and SiC nanoparticles. The relative contents of Ni and Si in the composite coating increase, and the relative content of Co decreases. With the increase in the concentration of SiC nanoparticles (2 g/L, 3 g/L, and 4 g/L), agglomeration decreases the number of SiC nanoparticles and the relative masses of Ni and Si. Owing to the SiC nanoparticles refining the cell and promoting the deposition, the large number of available deposition points allow the Ni, Co, and SiC nanoparticles in the plating solution to be deposited quickly and uniformly on the substrate. Thus, the mass fraction of the elements deposited in the composite coating is higher than that of other elements indicated by the matrix.

Table 3.
Mass fraction of the elements in the coating with different nanoparticle concentrations.
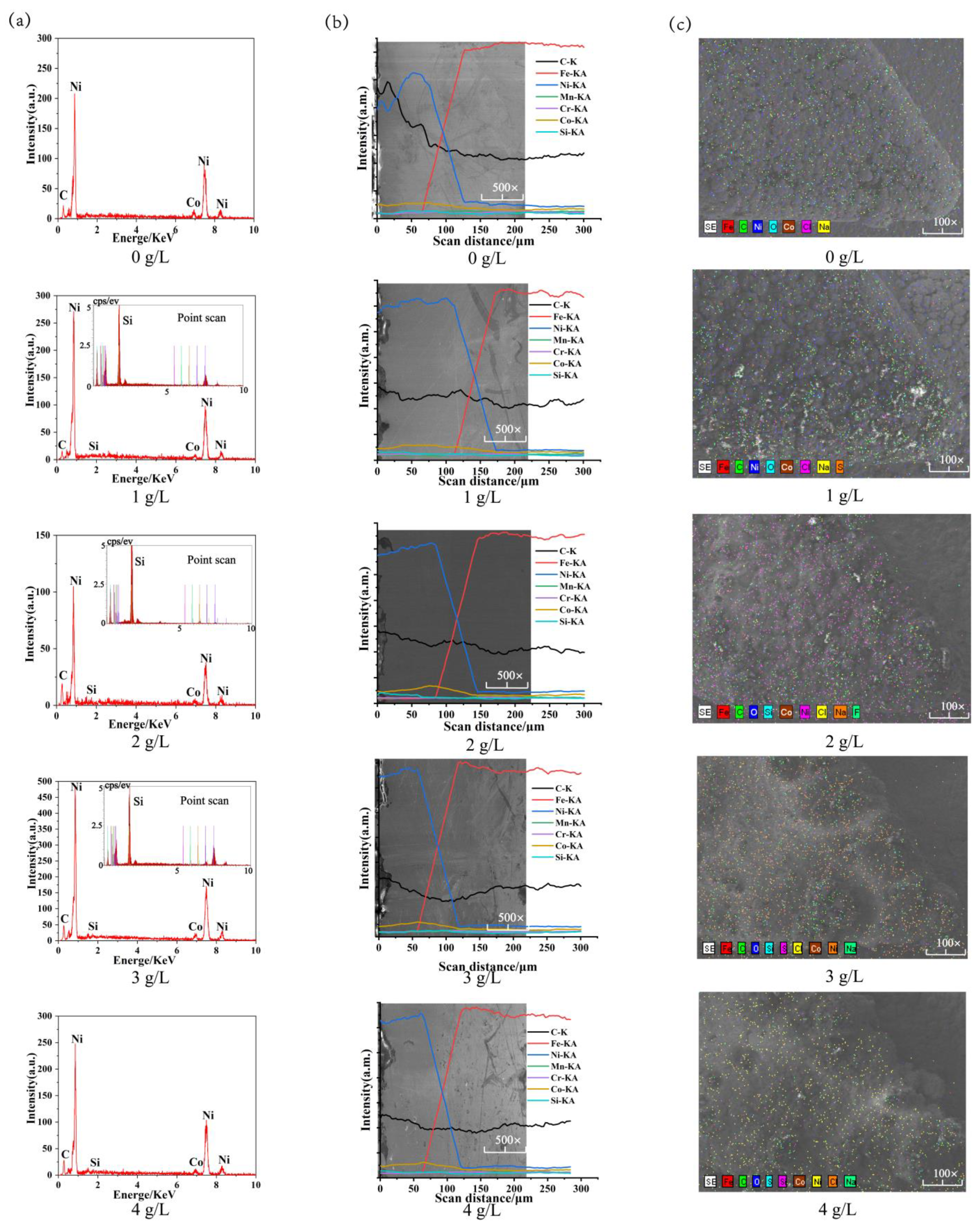
Figure 5 shows the EDS spectroscopic analysis of Ni-Co-β-SiC composite coatings prepared at different SiC concentrations. Figure 5a shows that Ni, Co, Si, and C are the main elements in the coating, and Si is the weak peak value. When the concentration of SiC nanoparticles is 1, 2, or 3 g/L, the particles in the coating are spot-scanned, and SiC nanoparticles were found in the coating. Figure 5b shows that Ni, Co, Si, and C tend to decrease and Fe tends to increase in the process of cross-sectional line scanning from coating to substrate, with the element permeation region as the boundary. The results show that the main components of the bath are deposited on the surface of the substrate. When the concentration of SiC nanoparticles is 1 g/L, the element permeation zone between Ni-Co coating and substrate by electrodeposition is 45.50 μm, which indicates that the element permeation phenomenon is weak when the coating and substrate are adsorbed onto each other without SiC nanoparticles. When the concentration of SiC nanoparticles changes from 0 g/L to 1 g/L, the element permeation zone between the coating and the substrate becomes 60.66 μm, which indicates that the addition of SiC nanoparticles contributes to the mutual diffusion of the elements between the coating and the substrate, the main reason is that with the addition of SiC nanoparticles, the nucleation points on the surface of the composite coating increase and the adhesion forms tend to be diversified; the nucleation rate is increased, the cell growth is facilitated, and the element permeation region is enlarged. When the concentration of SiC nanoparticles changes from 1 to 4 g/L (1, 2, 3, 4 g/L), the length of the element permeation zone between the coating and the substrate decreases to 54.49 μm, which shows that with the increase in the concentration of SiC nanoparticles, the agglomeration phenomenon increases and the nucleation point on the substrate surface increases, which reduces the current efficiency. At the same time, the internal stress is too large, which causes the defects of the coating in different forms, affects the growth rate of the cell, reduces the thickness of the coating, and reduces the length of the element penetration zone. Figure 5c shows that there are large amounts of Ni and Co in the coating, and the distribution of Ni and Co on the surface of the coating tends to become uniform first and then disperse with the increase in the concentration of SiC nanoparticles. At the same time, the distribution of the Si element exists on the surface of the coating, which indicates that the coating contains a Si element. The main reason is that with the addition of SiC nanoparticles concentration, the nucleation point on the surface of the coating increases, which is beneficial to the homogeneous codeposition of SiC, Ni, and Co on the surface of the substrate, accelerating the growth of crystal cells and improving the distribution of elements on the surface of the coating. However, with the addition of too much SiC, agglomerations begin to occur, which reduces the current efficiency and prevents the coating from being deposited on the substrate, resulting in the uneven distribution of elements.
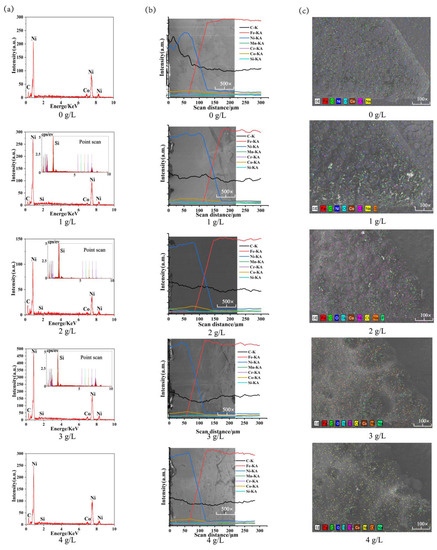
Figure 5.
EDS spectroscopic analysis of Ni-Co-β-SiC coatings prepared with different SiC concentrations: (a) selected area scanning on the surface of the spindle hook tooth, (b) cross-sectional line scanning, and (c) scanning on the surface of the spindle hook tooth.
3.3. Effect of β-SiC Concentrations on Coating Hardness and Wear Resistance
3.3.1. Analysis of the Microhardness of the Composite Coating
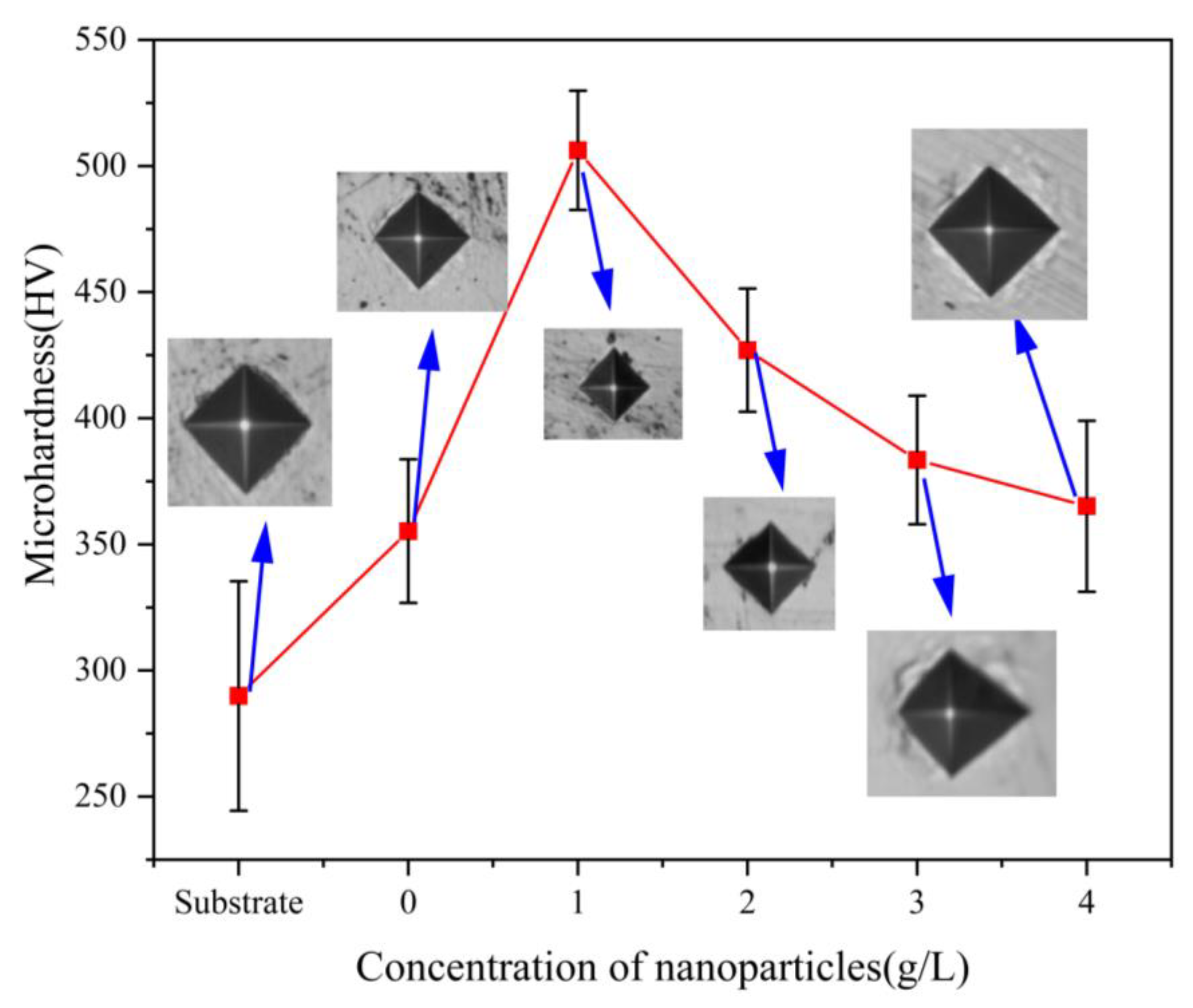
Figure 6 shows the relationship between the hardness of the composite coating and the nanoparticle concentration. The microhardness of the Ni-Co-β-SiC composite coating increased at first and then decreased, and the hardness of the Ni-Co-β-SiC composite coating was higher than that of the substrate. When the concentration of β-SiC nanoparticles was changed from 0 to 1 g/L, the composite microhardness showed a gradually increasing trend. The main reason is that β-SiC is a ceramic material with high hardness; adding a certain concentration of nanoparticles can improve the overall hardness of the coating and refine its microstructure due to Hall–Petch strengthening [23] and Orowan strengthening. The incorporated particles [24] fill in the defects between Ni-Co grains, thus improving the dislocation resistance of the grains and the microhardness of the composite coating. When the concentration of β-SiC nanoparticles was gradually changed from 1 g/L to 4 g/L (1 g/L, 2 g/L, 3 g/L, 4 g/L), the composite microhardness showed a decreasing trend. The main reason for this is that when the concentration of nanoparticles increases, the substrate has a limited ability to encapsulate and cannot bind to all the nanoparticles. Hence, the nanoparticles begin to agglomerate, reducing their surface charge density. At the same time, the uniformity of nanoparticles in the plating solution decreases, thus affecting the degree of the surface flatness and reducing the hardness. When the concentration of β-SiC nanoparticles was 1 g/L, the composite microhardness was 502.6 Hv0.1. This microhardness value is the highest among the coatings and is 40% higher than that of the composite coating without β-SiC nanoparticles. In addition, the surface of the composite coating is the densest.
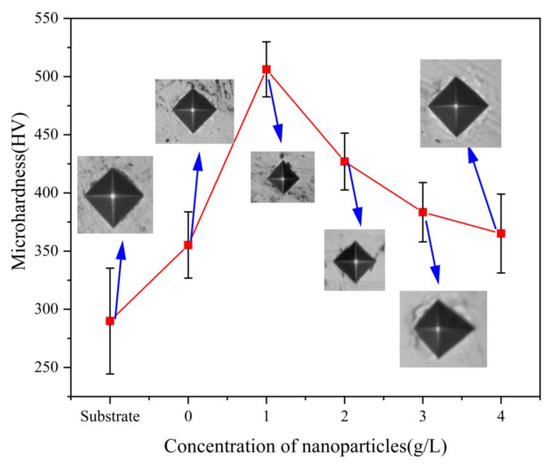
Figure 6.
Relationship between the hardness of the composite coating and nanoparticle concentration.
3.3.2. Friction Coefficient Analysis of the Composite Coating
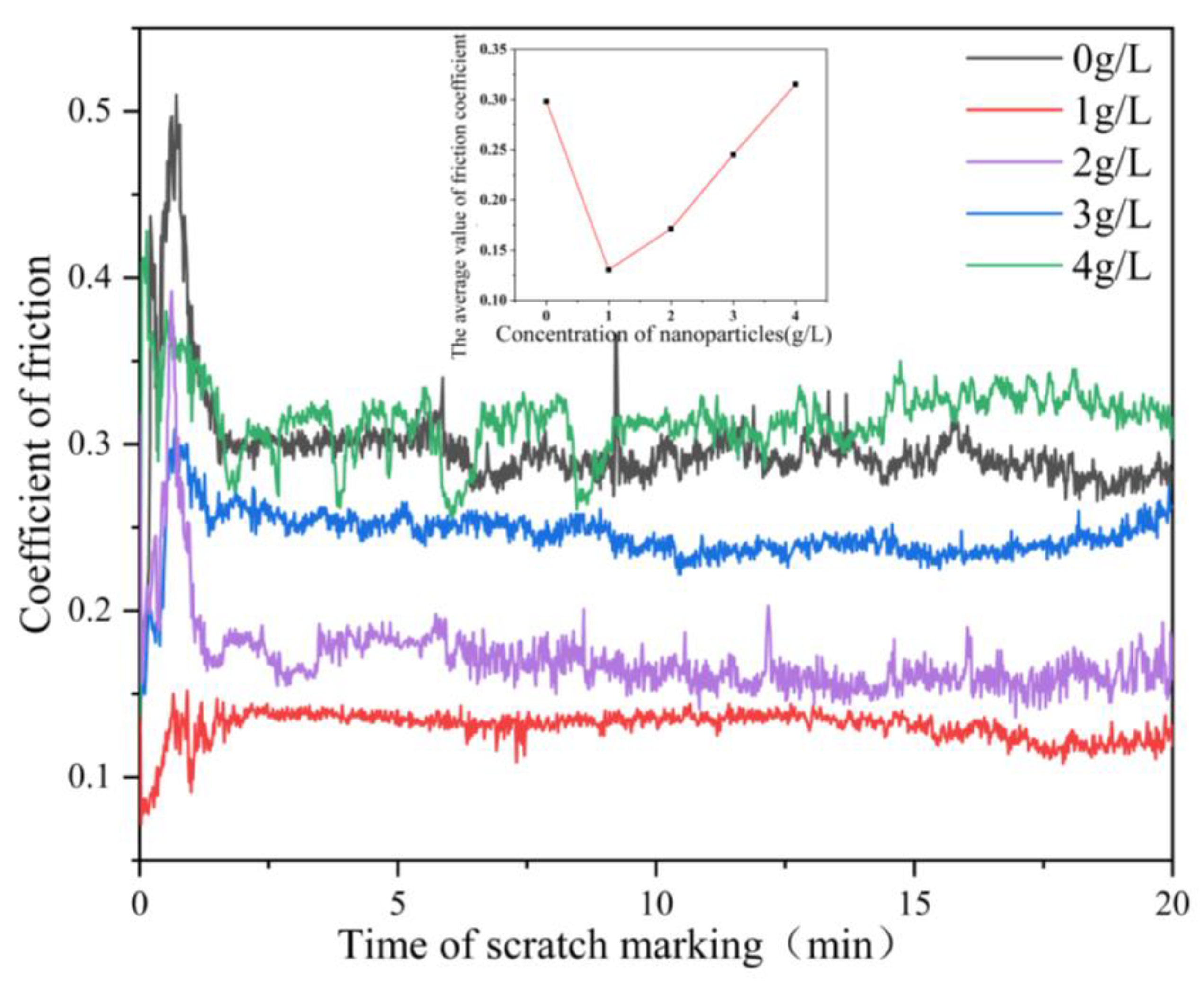
Figure 7 shows the relationship between the friction coefficient of the composite coating and the nanoparticle concentration. The friction coefficient of Ni-Co-β-SiC composite coating decreased first and then increased with the increase in SiC nanoparticle concentration. The friction coefficient of the coating increased sharply in a short time from the beginning of friction because the coating has a rough surface and the friction is not sufficient. When the friction progressed, the friction head came into full contact with the coating and stabilized quickly [25]. When the concentration of β-SiC nanoparticles was changed from 0 to 1 g/l, the friction coefficient also changed from 0.3 to 0.13. The main reason is that, through the adsorption and deposition of Ni2+ on the surface of the substrate, the addition of a few SiC nanoparticles to the plating bath plays a pinning role in the coating. This phenomenon limits the movement of dislocations, improves the mechanical properties between grains, and effectively reduces the occurrence of tiny displacement during friction and the coefficient of friction. When the concentration of β-SiC nanoparticles was changed from 1 g/L to 4 g/L (1 g/L, 2 g/L, 3 g/L, 4 g/L), the friction coefficient also changed from 0.13 to 0.28. The main reason is that, with the increase in the concentration of SiC nanoparticles, the concentration and uniformity of SiC nanoparticles in the coating decrease due to agglomeration. Some SiC nanoparticles fall off and take part in friction, which will produce slight groove characteristics and increase the depth of wear marks. Therefore, the coating will be damaged to a certain extent, resulting in wear debris, and the friction coefficient consequently increases. When the concentration of SiC nanoparticles was 1 g/L, the friction coefficient was at the minimum of 0.13, which is 2.3 times lower than that of the surface without nanoparticles. The distribution of nanoparticles on the surface is the most uniform and the surface compactness is the best.
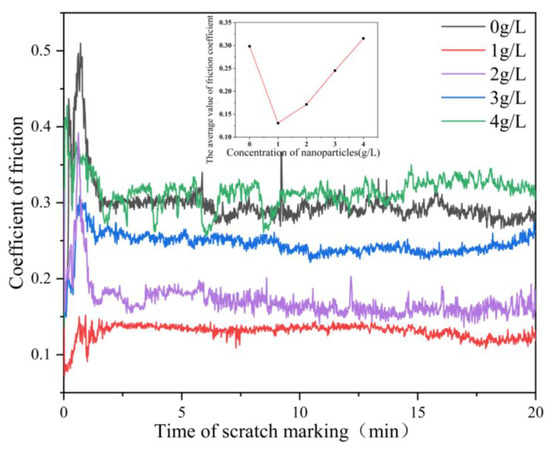
Figure 7.
Relationship between the friction coefficient of the composite coating and the nanoparticle concentration.
3.3.3. Wear Resistance Analysis of the Composite Coating
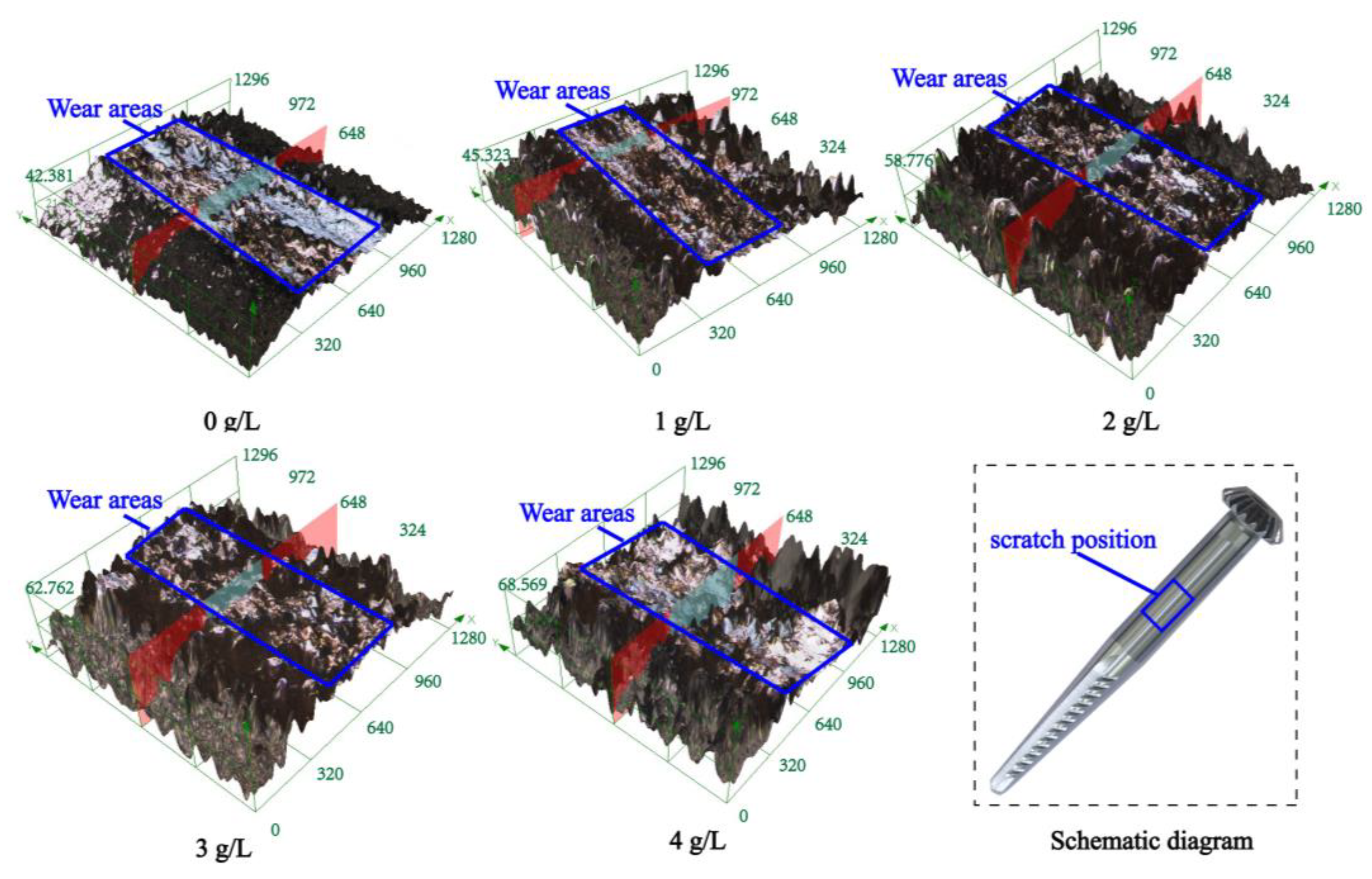
Figure 8 and Table 4 show the micromorphology and size parameters of the scratch wear of the composite coating with different concentrations of β-SiC nanoparticles observed by confocal microscopy. With the increase in SiC nanoparticle concentration, the width, height, and cross-sectional area of wear scratches decreased first and then increased. When the concentration of β-SiC nanoparticles was changed from 0 to 1 g/L, the scratch dent degree and wear scratch on the surface decreased and the scratch surface uniformity increased slightly. When the concentration of β-SiC nanoparticles was changed from 1 g/L to 4 g/L (1 g/L, 2 g/L, 3 g/L, 4 g/L), the degree of scratch depression gradually increased. However, the turning point of the trend of the change of surface area lagged when the scratch surface uniformity began to decrease. When the concentration of β-SiC nanoparticles was 1 g/L, the scratch surface uniformity was the best, and the scratch width, scratch height, and scratch surface area were the smallest at 395.266 µm, 34.496 µm, and 0.995 mm2.
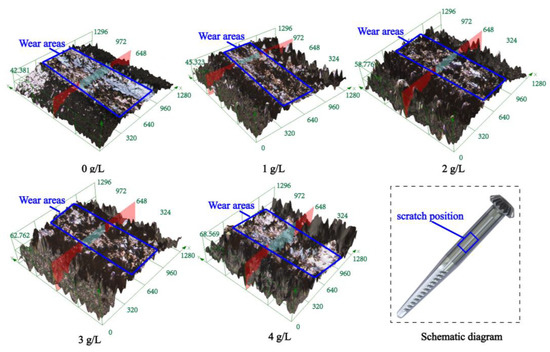
Figure 8.
Wear and scratch surface morphology of the composite coating with different β-SiC concentrations prepared on the spindle.

Table 4.
Section parameters of wear scars on the composite coating with different β-SiC concentrations.
The above results show that, on the one hand, the wear resistance of the cylinder surface has the same change trend as the hardness of the hook tooth [26]. The higher the hardness of the hook tooth, the better the wear resistance of the cylinder surface. When a few SiC nanoparticles are added to the plating solution, the nanoparticles are embedded in the composite coating and strengthen the composite coating and improve its mechanical properties. The hardness of the coating is increased, its antiplastic deformation ability is improved, and its wear resistance is enhanced. However, when the concentration of nanoparticles increases, agglomeration occurs and increases the surface roughness and the friction force on the surface during scratch wear, leading to the poor wear resistance of the nanoparticles. On the other hand, the uniform and fine composite coating based on the second phase particle SiC has good mechanical properties and wear resistance, which helps improve the wear resistance of the coating, limits the dislocation during wear, and enhances the stress effect of the composite coating. On the basis of the above two aspects, the wear resistance of the composite coating has been improved.
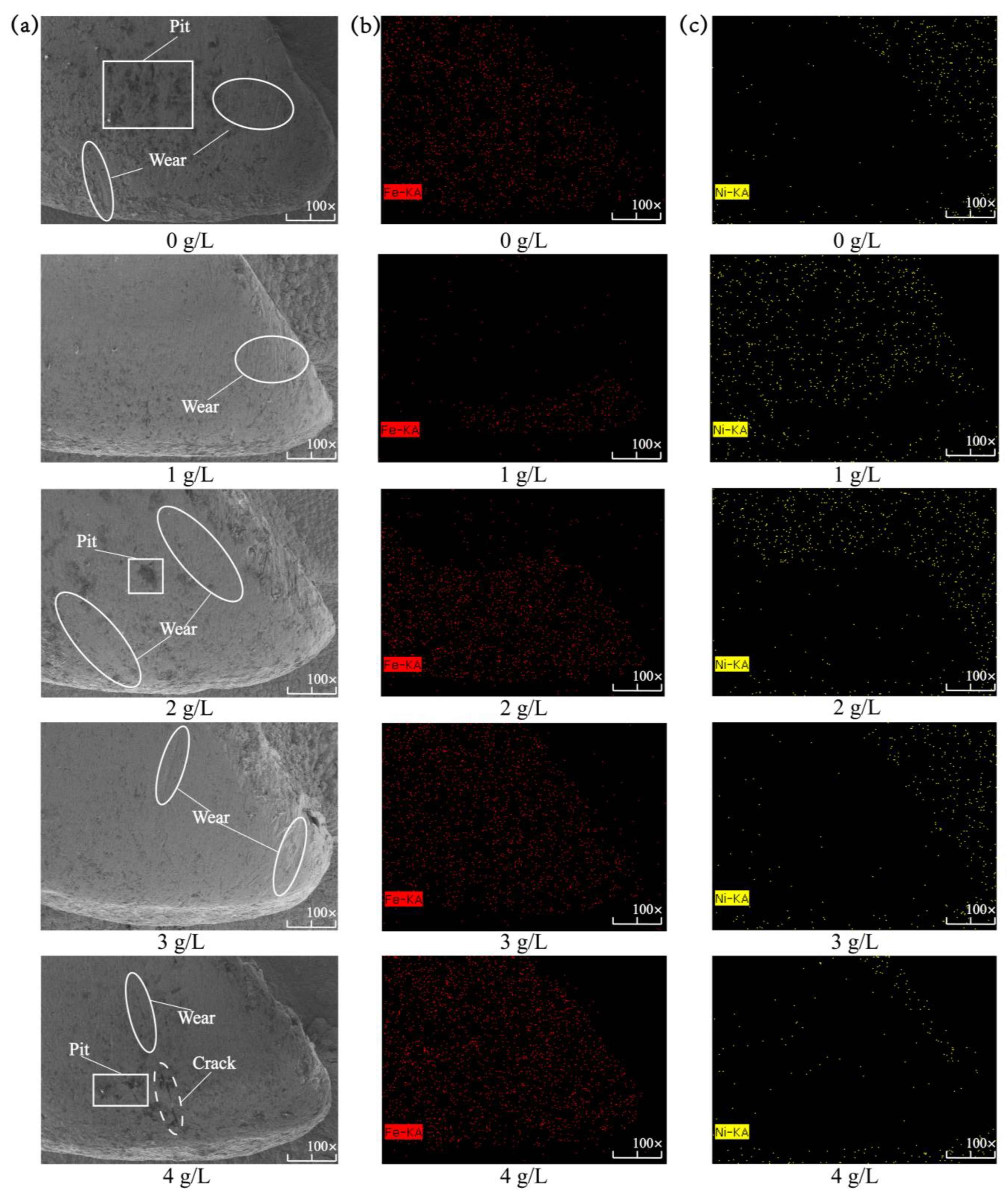
Figure 9 shows the micromorphology and EDS spectroscopic analysis of the composite coating on the surface of the spindle hook tooth after the simulation of wear in the field. Table 5 shows the elemental content of the Ni-Co-β-SiC composite coating with different β-SiC nanoparticle concentrations after wear. When the concentration of β-SiC nanoparticles was 0 g/L, the mass fraction of Fe is slightly higher than that of Ni. A large number of slight wear marks, a small number of deep wear marks, and some voids were found on the surface after wear in simulated fieldwork. Additionally, the wear area extends to the entire hook tooth. The wear mark on the surface of the composite coating is typical abrasive wear. During wear simulation, the hard particles slide on the surface of the composite coating, leading to plastic deformation [27]. The formation of voids is due to the uneven stress distribution in the wear coating that results in uneven stress. Their joint action caused a large area of wear. When the concentration of β-SiC nanoparticles was 1 g/L, the mass fraction of Ni was much higher than that of Fe, and there was a small amount of wear on the tooth tip. In addition, only a small amount of slight abrasion was observed on the surface of the hook teeth because the SiC nanoparticles are uniformly adsorbed on the composite coating after deposition. Owing to the pinprick action, the comprehensive mechanical properties are strengthened, and the wear resistance of the surface of the hook teeth is improved, causing the surface to inevitably present slight abrasion. Therefore, wear only occurs at a small portion of the tooth tip. When the concentration of β-SiC nanoparticles was changed from 1 g/L to 4 g/L (1 g/L, 2 g/L, 3 g/L, 4 g/L), the mass fraction of Fe was gradually higher than that of Ni, and the wear surface gradually extended from the tooth tip to the tooth side and finally to the entire hook tooth. In addition, the surface of the β-SiC nanoparticles became hollow, and deep wear marks and cracks appeared again. The nanoparticles have agglomerated on the surface of the coating, thus reducing the efficiency of the plating process and destroying the surface uniformity. Hence, the internal stress produced during wear becomes extensive and leads to additional wear. At the same time, the superfluous nanoparticles are worn off, so the surface of the composite coating develops many voids. As a result, the mechanical properties of the composite coating gradually decline, and cracks are eventually formed. Therefore, the best simulation fieldwork effect and wear resistance are obtained at a 1 g/L concentration of β-SiC nanoparticles and the minimum mass fraction of Fe.
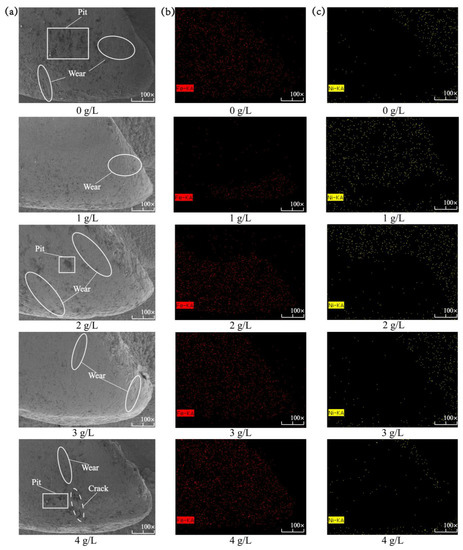
Figure 9.
Microstructure and EDS spectroscopic analysis of the composite coating with different concentrations of β-SiC nanoparticles prepared on the surface of the spindle hook teeth after simulated wear: (a) microstructure, (b) scanning on the surface of the spindle hook tooth of Fe, and (c) scanning on the surface of the spindle hook tooth of Ni.

Table 5.
Mass fraction of the elements in the coating with different nanoparticle concentrations after wear.
4. Conclusions
On the basis of the wear failure of spindle hook teeth, the preparation and properties of spindle hook teeth composite coatings were studied. Experiments were carried out with different concentrations of β-SiC nanoparticles. The surface morphology, section morphology, and element distribution of the spindle hook teeth were characterized, and the variation regularity of hardness, friction coefficient, and wear resistance were investigated. The following conclusions were drawn:
(1) Ni-Co-β-SiC composite coating had a typical cellular structure. When the concentration of β-SiC nanoparticles was 2 g/L, the surface distribution of the composite coating was uniform, and the surface quality was the best;
(2) When the concentration of β-SiC nanoparticles was 1 g/L, the intercellular structure of the composite coating was relatively compact, and the hardness was the highest. The nanoparticles were distributed uniformly in the composite coating, and the friction coefficient was the smallest;
(3) The wear resistance of Ni-Co-β-SiC composite coating was the same in the scratch test and simulated field test. Other surface phenomena were observed in the simulated field operation. When the concentration of β-SiC nanoparticles was 1 g/L, the nanoparticles in the composite coating played the role of “lubrication” in the wear process and reduced the occurrence of wear marks, holes, and cracks by forming a protective coating. As a result, the wear resistance was improved.
In order to better measure the extent of wear, in future research, we can pick the area of more detailed analysis, such as the use of phenotypic methods to calculate more accurate wear area; the concentration of SiC nanoparticles can be even more finely divided. Alternatively, the effect of roughness on the coating can be further explored.
Author Contributions
Conceptualization, H.N., H.F. and X.F.; methodology, H.N.; software, H.N., H.F.; validation, X.F., H.F. and H.N.; formal analysis, H.N.; investigation, H.N., H.F. and H.Z.; resources, H.N.; data curation, H.N.; writing—original draft preparation, H.N.; writing—review and editing, H.W. and Y.Z.; visualization, H.N. and X.F.; supervision, H.F., H.N. and X.F.; project administration, H.W., H.Z., Y.Z. and X.F.; funding acquisition, H.N. and X.F.; All authors have read and agreed to the published version of the manuscript.
Funding
Financial support for this work was provided by the Fundamental Research Funds for the Central Universities (Grant No. KYLH2022002), Tarim University–Nanjing Agricultural University Joint Fund (Grant No. NNLH202202), Student Innovation Research and Entrepreneurship Training (Grant No. 202210307222Y).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fan, J.R. Present situation research and development trend of cotton harvest machine. Mech. Res. Appl. 2011, 24, 1–4. (In Chinese) [Google Scholar]
- Sun, K. Wear mechanism analysis of cotton picker spindle. Agric. Dev. Equip. 2021, 11, 46–47. (In Chinese) [Google Scholar]
- Lin, L.B.; Dai, P.Q.; Lin, L.F.; Liao, Y.X. Corrosion characteristics of nanocrystalline Co-Ni-Fe deposits in 3.5% NaCl solution. Rare Metal Mater. Eng. 2012, 41, 851–856. (In Chinese) [Google Scholar]
- Zhang, H.Z.; Li, J.; Ou, X.M.; Chen, H. Electroless Deposition and Corrosion Resistance of Ni-Co-Cu-P Amorphous Coatings. Rare Metal Mater. Eng. 2016, 45, 2965–2969. (In Chinese) [Google Scholar]
- Hu, J.; Wang, B.; Hu, J.T.; Xu, Y.C.; Pu, D.L.; Zhou, C. Effect of nSiO2 on Corrosion Resistance of Ni-W-P Coating in High Temperature and High Pressure Environment. Surf. Technol. 2018, 47, 68–74. (In Chinese) [Google Scholar]
- Wang, J.Y.; Zhang, X.; Zhang, X.G.; Liu, J.T.; Wang, X.C. Performance Characterization of Ni-P-xZrO2 Nano-Composite Coating by Electrodeposition on Cylinder Liner Surface of Robot Arm. Mater. Prot. 2021, 54, 97–101. (In Chinese) [Google Scholar]
- Zhang, K.; Li, Y.F.; Zhao, H.Y.; Han, H.J.; Yang, Z.J. Preparation and Properties of Sol-Enhanced Ni-P-Al2O3 Nanocomposite Coatings. Mater. Prot. 2020, 53, 77–83. (In Chinese) [Google Scholar]
- Safavi, M.S.; Rasooli, A. Ni-P-TiO2 nanocomposite coatings with uniformly dispersed Ni3Ti intermetallics: Effects of TiO2 nanoparticles concentration. Surf. Eng. 2019, 35, 1070–1080. [Google Scholar] [CrossRef]
- Shen, M.Q.; Fu, X.Q.; Kang, M.; Zhang, Y. Process of Jet-Electrodeposited Ni-P-ZrO2 Coating on Rotary Body Parts. Mater. Prot. 2019, 52, 96–101. (In Chinese) [Google Scholar]
- Jiang, W.; Shen, L.D.; Xu, M.Y.; Wang, Z.W.; Tian, Z.J. Mechanical properties and corrosion resistance of Ni-Co-SiC composite coatings by magnetic field-induced jet electrodeposition. J. Alloys Compd. 2019, 791, 847–855. [Google Scholar] [CrossRef]
- Ahmadkhaniha, D.; Zanella, C. The Effects of Additives, Particles Load and Current Density on Codeposition of SiC Particles in NiP Nanocomposite Coatings. Coatings 2019, 9, 554. [Google Scholar] [CrossRef]
- Jiang, W.; Shen, L.D.; Wang, K.; Wang, Z.W.; Tian, Z.J. Wear resistance of Ni-Co/SiC composite coating by jet electrodeposition in the presence of magnetic field. Proc. Inst. Mech. Eng. Part B 2019, 243, 431–438. [Google Scholar] [CrossRef]
- Kazimierczak, H.; Szymkiewicz, K.; Bobrowski, P.; Swiatek, Z.; Rogal, L.; Gileadi, E.; Eliaz, N. The Effect of SiC Nanoparticle Size on the Electrodeposition of Zn–SiC Nanocomposite Coatings from Citrate Bath. J. Electrochem. Soc. 2018, 165, 774–782. [Google Scholar] [CrossRef]
- Yang, Z.G.; Fu, X.Y.; Yi, S.J.; Wang, S.; Li, Q.; Guo, S.J. Effects of different deposition methods on microstructure and friction properties of Ni-W/SiC composite coatings. J. Funct. Mater. 2022, 53, 6205–6211. (In Chinese) [Google Scholar]
- Su, P.; Liu, H.; Wang, X.H.; Long, W.; Jiang, Y.G.; Li, Z.S.; Wang, Q. Effect of heat treatment temperature on microstructure and properties of Ni-W-P/TiO2composite coating. Trans. Mater. Heat Treat. 2022, 43, 166–174. (In Chinese) [Google Scholar]
- Liu, Y.T. Research on the Process and Properties of Ni-Mn-SiC Composite Coatings by Jet-Electrodeposition; Master of Engineering; Nanjing Agricultural University: Nanjing, China, 2020. (In Chinese) [Google Scholar]
- Du, X.X. Research on the Process and Properties of Ni-P-β-SiC Composite Coating by Pulse jet Electrodeposition; Master of Engineering; Nanjing Agricultural University: Nanjing, China, 2017. (In Chinese) [Google Scholar]
- Li, J. Research on Wear Law and Wear Device of Spindle Hook Teeth Coating; Professional Master of Mechanical Engineering; Nanjing Agricultural University: Nanjing, China, 2022. (In Chinese) [Google Scholar]
- Guglielmi, N. Kinetics of the deposition of inert particles from electrolytic baths. J. Electrochem. Soc. 1972, 119, 1009–1012. [Google Scholar] [CrossRef]
- Shen, Z.Y. Study on Preparation of Ni-P-SiC Composite Coatings by Scanning Electrodeposition on 45 Steel Substrates with Sandblasting Pretreatment; Professional Master of Mechanical Engineering; Nanjing Agricultural University: Nanjing, China, 2021. (In Chinese) [Google Scholar]
- Garcia-Lecina, E.; Garcia-Urrutia, I.; Diez, J.A.; Salvo, M.; Smeacetto, F.; Gautier, G.; Seddon, R.; Martin, R. Electrochemical preparation and characterization of Ni/SiC compositionally graded multilayered coatings. Electrochim. Acta 2009, 54, 2556–2562. [Google Scholar] [CrossRef]
- Pavlatou, E.A.; Stroumbouli, M.; Gyftou, P.; Spyrellis, N. Hardening effect induced by incorporation of SiC particles in nickel electrodeposits. J. Appl. Electrochem. 2005, 36, 385–394. [Google Scholar] [CrossRef]
- Chavoshi, S.Z.; Branicio, P.S.; An, Q. Transition between Hall-Petch and inverse Hall-Petch behavior in nanocrystalline silicon carbide. Phys. Rev. Mater. 2021, 5, 073606. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, D.L. Contribution of Orowan strengthening effect in particulate-reinforced metal matrix nanocomposites. Mater. Sci. Eng. A 2008, 483, 148–152. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, M.; Fu, X.Q.; Li, H.Z.; Liu, Y.T. Surface structure and wear resistance of Ni-Co-P-BN (h)- Al2O3binary nano composite coatings. Mater. Sci. Technol. 2019, 27, 55–66. (In Chinese) [Google Scholar]
- Jin, P.; Sun, C.F.; Zhou, C.Y.; Shi, L.; Liu, C. Effect of SiC particle size on structures and properties of Ni–SiC nanocomposites deposited by magnetic pulse electrodeposition technology. Ceram. Int. 2019, 45, 20155–20164. [Google Scholar] [CrossRef]
- Gu, Y.Q.; Zhang, H.W.; Fu, X.Q.; Wang, L.; Wang, J.; Cai, Y.X.; Li, H.Y. Comparative Analysis of the Wear Performance of Spindle Hook Teeth During Fieldwork. J. Tribol. 2022, 144, 011706. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).