Optimization of Electrical Properties of Nanocrystallized Na3M2(PO4)2F3 NASICON-like Glasses (M = V, Ti, Fe)
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
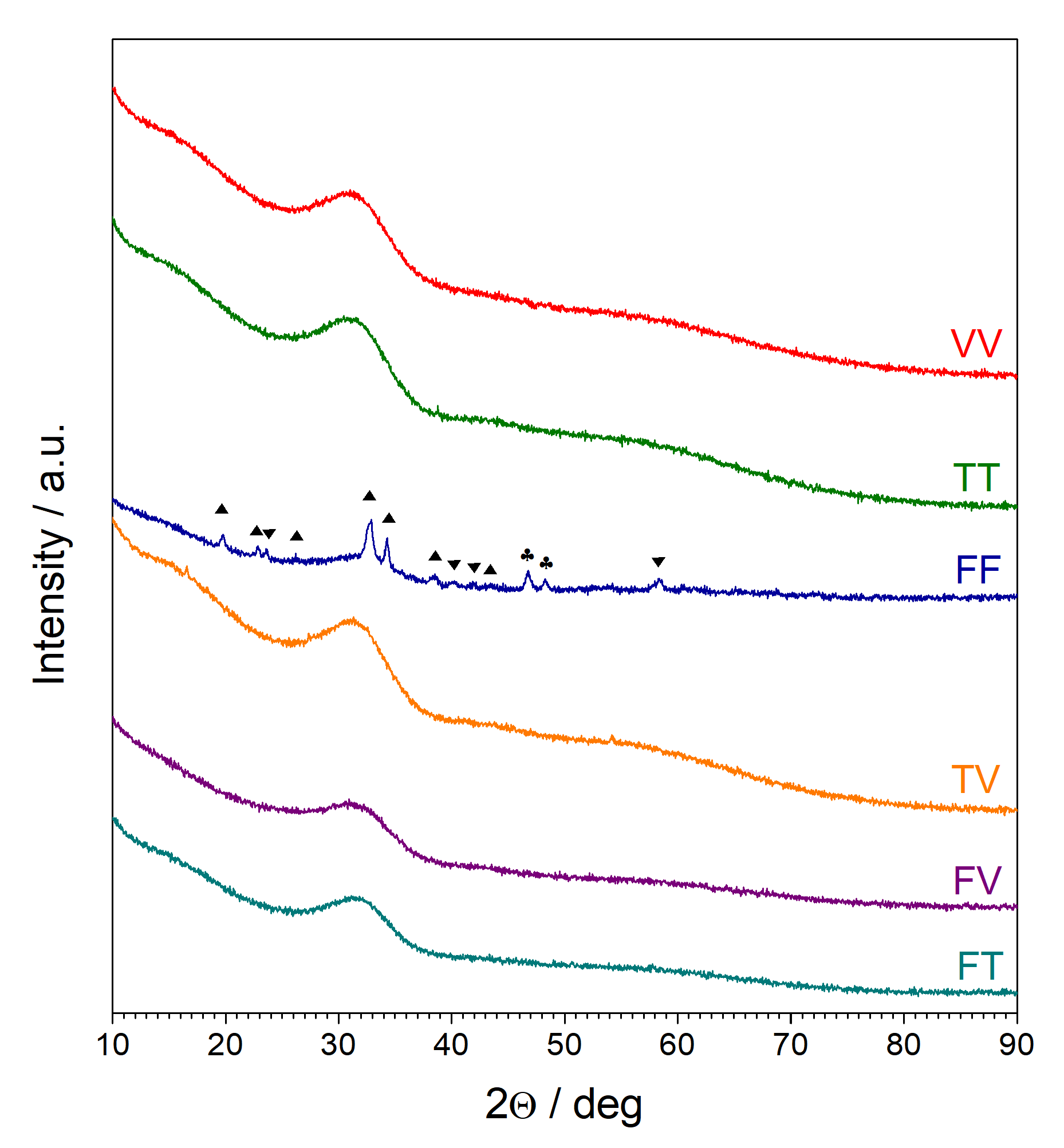
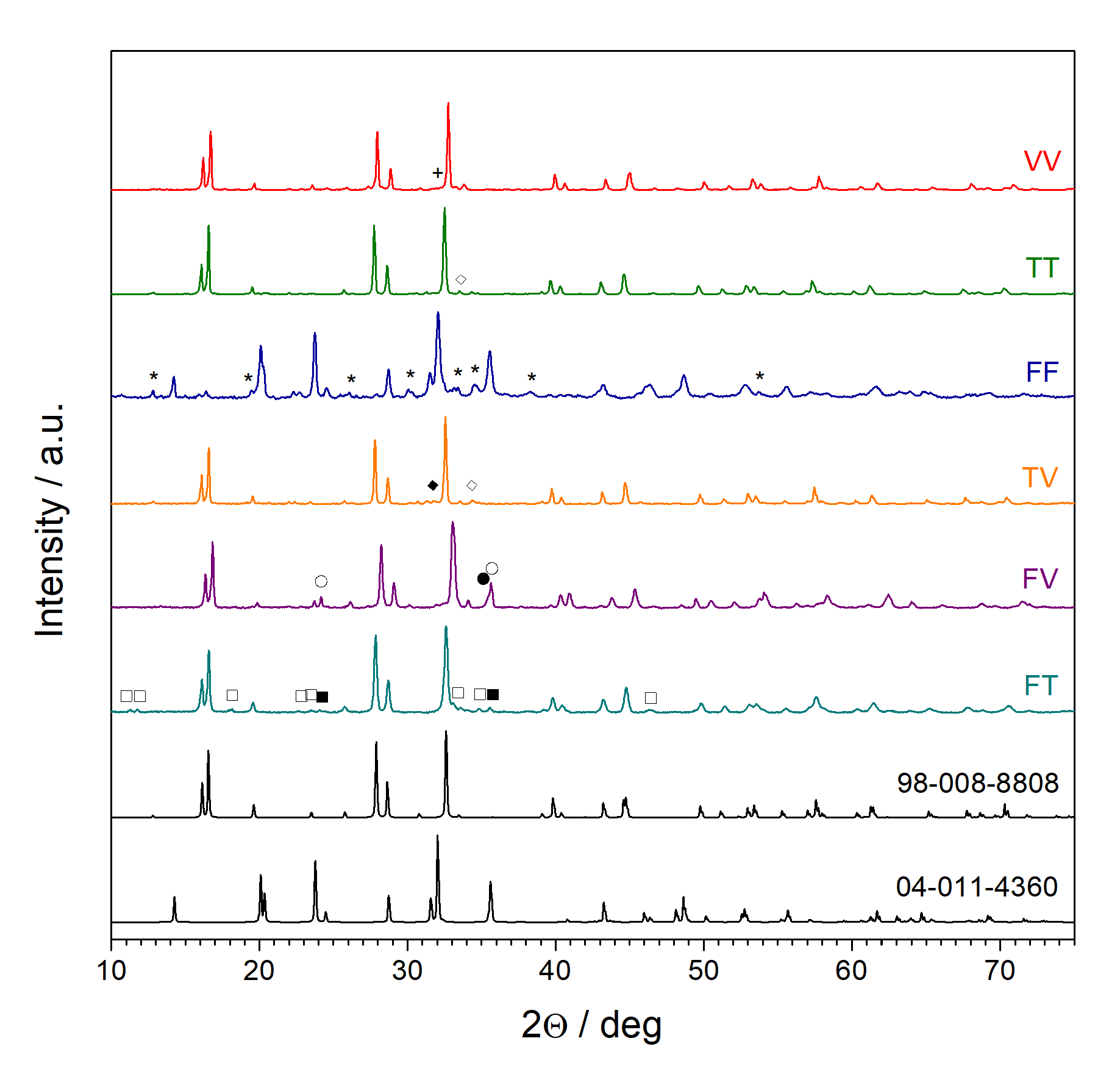
3.1. X-ray Diffractometry (XRD)
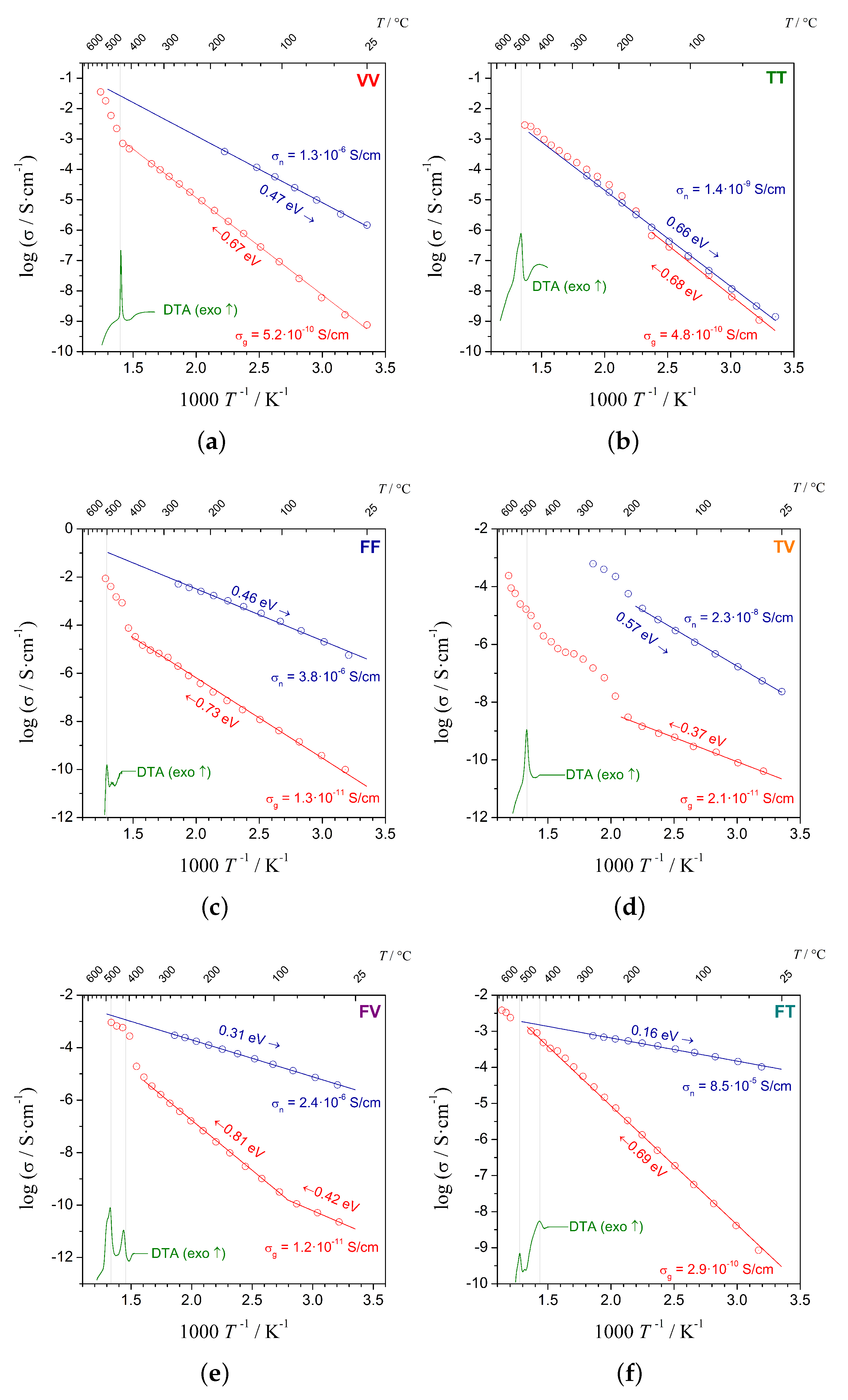
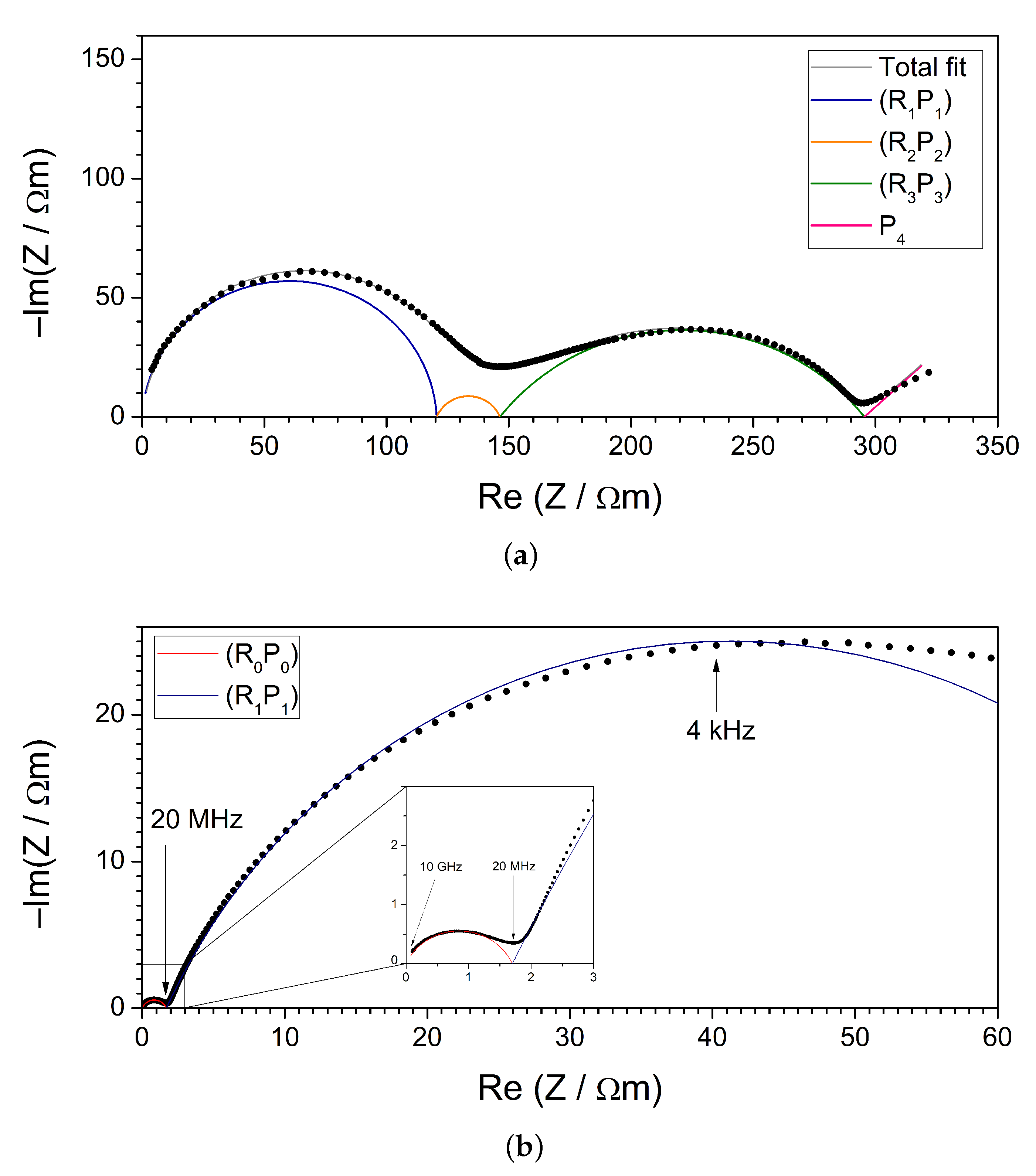
3.2. Impedance Spectroscopy (IS)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NASICON | NA-Super-Ionic-CONductor |
| XRD | X-ray Diffractometry |
| DTA | Differential Thermal analysis |
| IS | Impedance Spectroscopy |
| RT | Room Temperature |
References
- International Renewable Energy Agency. World Energy Transitions Outlook 2022: 1.5 °C Pathway; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2022. [Google Scholar]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Canepa, P.; Gautam, G.S.; Hannah, D.C.; Malik, R.; Liu, M.; Gallagher, K.G.; Persson, K.A.; Ceder, G. Odyssey of Multivalent Cathode Materials: Open Questions and Future Challenges. Chem. Rev. 2017, 117, 4287–4341. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Wu, C.; Shen, L.; Zhu, C.; Huang, Y.; Xi, K.; Maier, J.; Yu, Y. Challenges and Perspectives for NASICON-Type Electrode Materials for Advanced Sodium-Ion Batteries. Adv. Mater. 2017, 29, 1700431. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Vaddadi, V.S.C.S.; Panda, S.S.S.; Goona, N.K.; Parne, S.R.; Lakavat, M.; Bhaumik, A. Recent progress in the development of glass and glass-ceramic cathode/solid electrolyte materials for next-generation high capacity all-solid-state sodium-ion batteries: A review. J. Power Sources 2022, 521, 230930. [Google Scholar] [CrossRef]
- Ahsan, M.T.; Ali, Z.; Usman, M.; Hou, Y. Unfolding the structural features of NASICON materials for sodium-ion full cells. Carbon Energy 2022, 4, 776–819. [Google Scholar] [CrossRef]
- Muruganantham, R.; Chiu, Y.T.; Yang, C.C.; Wang, C.W.; Liu, W.R. An efficient evaluation of F-doped polyanion cathode materials with long cycle life for Na-ion batteries applications. Sci. Rep. 2017, 7, 14808. [Google Scholar] [CrossRef] [Green Version]
- Novikova, S.A.; Larkovich, R.V.; Chekannikov, A.A.; Kulova, T.L.; Skundin, A.M.; Yaroslavtsev, A.B. Electrical conductivity and electrochemical characteristics of Na3V2(PO4)3-based NASICON-type materials. Inorg Mater. 2018, 54, 794–804. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Hong, H.Y.-P.; Kafalas, J.A. Fast Na+—ion transport in skeleton structures. Mater. Res. Bull. 1976, 11, 203–220. [Google Scholar] [CrossRef]
- Anantharamulu, N.; Rao, K.K.; Rambabu, G.; Kumar, B.V.; Radha, V.; Vithal, M. A wide-ranging review on Nasicon type materials. J. Mater. Sci. 2011, 46, 2821–2837. [Google Scholar] [CrossRef]
- Meins, J.M.L.; Crosnier-Lopez, M.P.; Hemon-Ribaud, A.; Courbion, G. Phase Transitions in the Na3M2(PO4)2F3 Family (M = Al3+, V3+, Cr3+, Fe3+, Ga3+): Synthesis, Thermal, Structural, and Magnetic Studies. J. Solid State Chem. 1999, 148, 260–277. [Google Scholar] [CrossRef]
- Matts, I.L. Multi-Redox Active Polyanionic Cathodes for Alkali-ion Batteries. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2016. [Google Scholar]
- Chihara, K.; Kitajou, A.; Gocheva, I.D.; Okada, S.; Yamaki, J. Cathode properties of Na3M2(PO4)2F3 [M = Ti, Fe, V] for sodium-ion batteries. J. Power Sources 2013, 227, 80–85. [Google Scholar] [CrossRef]
- Matts, I.; Dacek, S.; Pietrzak, T.K.; Ceder, G. Explaining Performance-limiting Mechanisms in Fluorophosphate Na-ion Battery Cathodes Through Inactive Transition Metal Mixing and Firstprinciples Mobility Calculations. Chem. Mater. 2015, 27, 6008–6015. [Google Scholar] [CrossRef]
- Sharma, L.; Adiga, S.P.; Alshareef, H.N.; Barpanda, P. Fluorophosphates: Next Generation Cathode Materials for Rechargeable Batteries. Av. Energy Mater. 2020, 10, 2001449. [Google Scholar] [CrossRef]
- Pietrzak, T.K.; Wasiucionek, M.; Garbarczyk, J.E. Towards Higher Electric Conductivity and Wider Phase Stability Range via Nanostructured Glass-Ceramics Processing. Nanomaterials 2021, 11, 1321. [Google Scholar] [CrossRef]
- Pietrzak, T.K.; Kruk-Fura, P.E.; Mikołajczuk, P.J.; Garbarczyk, J.E. Syntheses and nanocrystallization of NaF-M2O3-P2O5 NASICON-like phosphate glasses (M = V, Ti, Fe). Int. J. Appl. Glass Sci. 2020, 11, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Nowagiel, M.; Samsel, M.J.; Pietrzak, T.K. Towards high phase purity of nanostructured alluaudite-type glass-ceramics cathode materials for sodium ion batteries. Materials 2021, 14, 4997. [Google Scholar] [CrossRef]
- Pietrzak, T.K. Multi-device software for impedance spectroscopy measurements with stabilization in low and high temperature ranges working under Linux environment. Ionics 2019, 25, 2445–2452. [Google Scholar] [CrossRef] [Green Version]
- Kežionis, A.; Kazlauskas, S. High temperature ultrabroadband impedance spectrometer. In Proceedings of the International Workshop on Impedance Spectroscopy 2013, Chemnitz, Germany, 25–27 September 2013; pp. 32–33. [Google Scholar]
- Kežionis, A.; Kazlauskas, S.; Petrulionis, D.; Orliukas, A.F. Broadband Method for the Determination of Small Sample’s Electrical and Dielectric Properties at High Temperatures. IEEE Trans. Microw. Theory Tech. 2014, 62, 2456–2461. [Google Scholar] [CrossRef]
- Michalski, P.P.; Pietrzak, T.K.; Nowiński, J.L.; Wasiucionek, M.; Garbarczyk, J.E. Dependence of a glass transition temperature on a heating rate in DTA experiments for glasses containing transition metal oxides. J. Non Cryst. Solids 2016, 443, 155–161. [Google Scholar] [CrossRef]
- Garbarczyk, J.E.; Pietrzak, T.K.; Wasiucionek, M.; Kaleta, A.; Dorau, A.; Nowiński, J.L. High electronic conductivity in nanostructured materials based on lithium-iron-vanadate-phosphate glasses. Solid State Ionics 2015, 272, 53–59. [Google Scholar] [CrossRef]

| ID | Compound | Reagents |
|---|---|---|
| VV | Na3V2(PO4)2F3 | NaF, V2O5, NH4H2PO4 |
| TT | Na3Ti2(PO4)2F3 | NaF, TiO2, NH4H2PO4 |
| FF | Na3Fe2(PO4)2F3 | NaF, FePO4 · 2H2O |
| TV | Na3TiV(PO4)2F3 | NaF, TiO2, V2O5, NH4H2PO4 |
| FV | Na3FeV(PO4)2F3 | NaF, FePO4 · 2H2O, V2O5, NH4H2PO4 |
| FT | Na3FeTi(PO4)2F3 | NaF, FePO4 · 2H2O, TiO2, NH4H2PO4 |
| Chemical | Purity | Manufacturer |
|---|---|---|
| NaF | ⩾99% | Sigma-Aldrich (Darmstad, Germany) |
| FePO4 · 2H2O | pure | Carl Roth (Karlsruhe, Germany) |
| V2O5 | ⩾99.6% | Sigma-Aldrich (Darmstadt, Germany) |
| TiO2 | ⩾99.8% | Sigma-Aldrich (Darmstadt, Germany) |
| NH4H2PO4 | ⩾99% | Polish Chemicals (Gliwice, Poland) |
| Sample | /C | (25 C)/Scm | (Glass)/eV | (25 C)/Scm | (Nano)/eV |
|---|---|---|---|---|---|
| VV | 532 | 5.2 | 0.67 | 1.3 | 0.47 |
| TT | 457 | 4.8 | 0.68 | 1.5 | 0.66 |
| FF | 556 | 1.3 | 0.73 | 3.2 | 0.46 |
| TV | 607 | 2.1 | 0.37 | 2.4 | 0.57 |
| FV | 506 | 1.2 | 0.42/0.81 | 4.0 | 0.31 |
| FT | 607 | 2.9 | 0.69 | 8.4 | 0.16 |
| This Work | Best Values from [19] | |||
|---|---|---|---|---|
| Sample | (25C)/Scm | (nano)/eV | (25C)/Scm | (nano)/eV |
| VV | 1.3 | 0.47 | 8.9 | 0.53 |
| TT | 1.5 | 0.66 | 1.3 | 0.65 |
| FF | 3.2 | 0.46 | 5.5 | 0.68 |
| TV | 2.4 | 0.57 | 4.8 | 0.69 |
| FV | 4.0 | 0.31 | 1.4 | 0.68 |
| FT | 8.4 | 0.16 | 1.8 | 0.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowagiel, M.; Hul, A.; Kazakevicius, E.; Kežionis, A.; Garbarczyk, J.E.; Pietrzak, T.K. Optimization of Electrical Properties of Nanocrystallized Na3M2(PO4)2F3 NASICON-like Glasses (M = V, Ti, Fe). Coatings 2023, 13, 482. https://doi.org/10.3390/coatings13030482
Nowagiel M, Hul A, Kazakevicius E, Kežionis A, Garbarczyk JE, Pietrzak TK. Optimization of Electrical Properties of Nanocrystallized Na3M2(PO4)2F3 NASICON-like Glasses (M = V, Ti, Fe). Coatings. 2023; 13(3):482. https://doi.org/10.3390/coatings13030482
Chicago/Turabian StyleNowagiel, Maciej, Anton Hul, Edvardas Kazakevicius, Algimantas Kežionis, Jerzy E. Garbarczyk, and Tomasz K. Pietrzak. 2023. "Optimization of Electrical Properties of Nanocrystallized Na3M2(PO4)2F3 NASICON-like Glasses (M = V, Ti, Fe)" Coatings 13, no. 3: 482. https://doi.org/10.3390/coatings13030482






