Transparent Superhydrophobic Coatings with Mechanical and Chemical Stability Prepared by Modified Polyhedral Oligosilsesquioxanes via UV-Curable Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Coating Preparation
2.3. Characterization
3. Results and Discussion
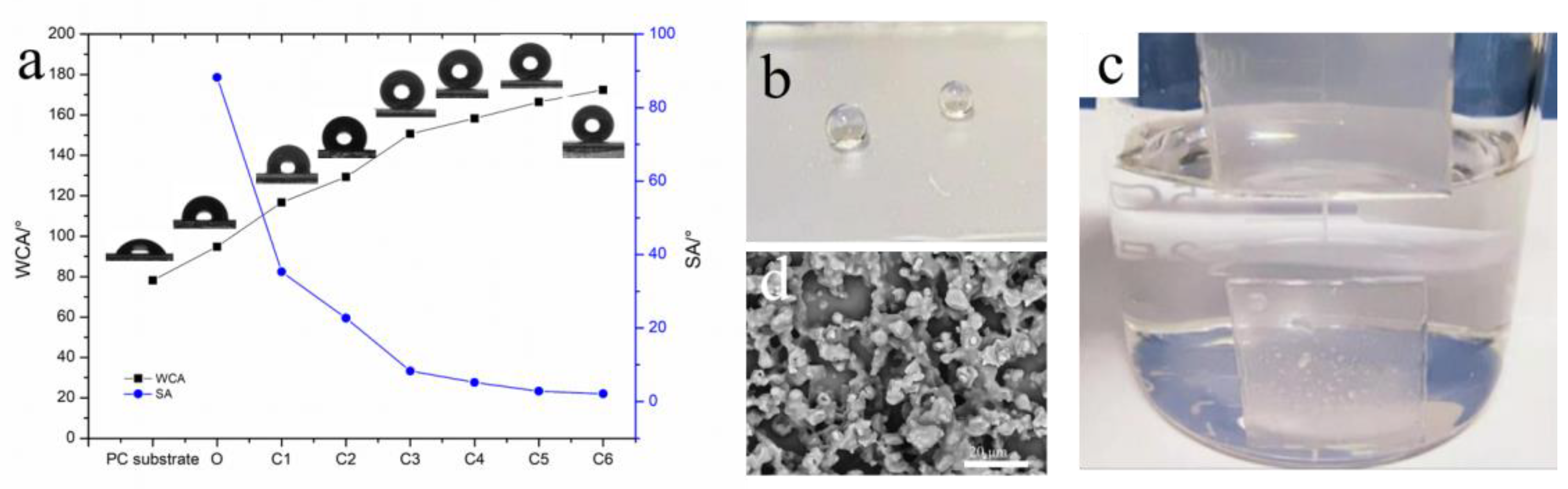
3.1. Surface Wettability
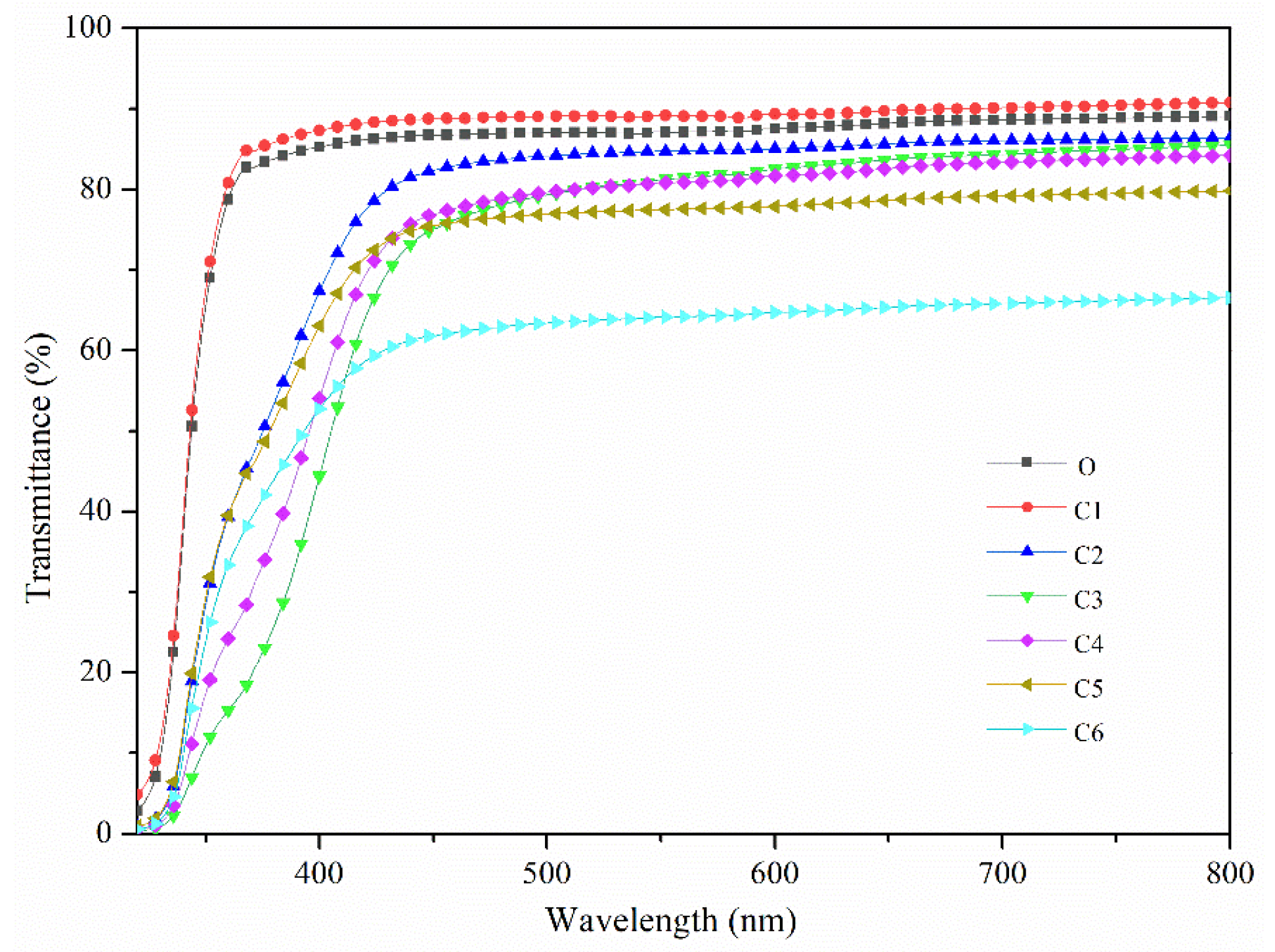
3.2. Transmittance
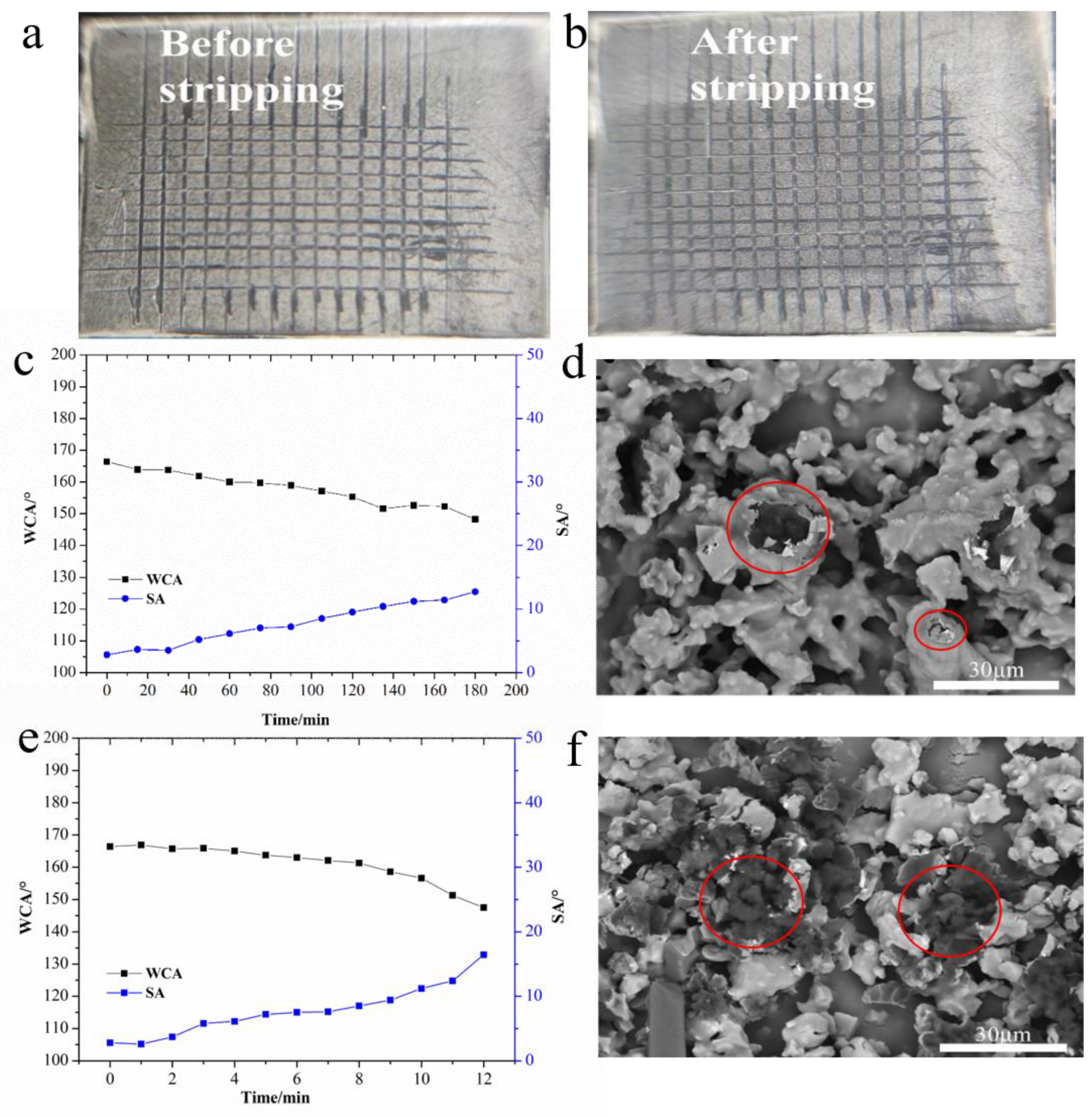
3.3. Mechanical Stability
3.3.1. Strip Tape Peel Test
3.3.2. Water Droplet Impact Test
3.3.3. Sand Impact Test
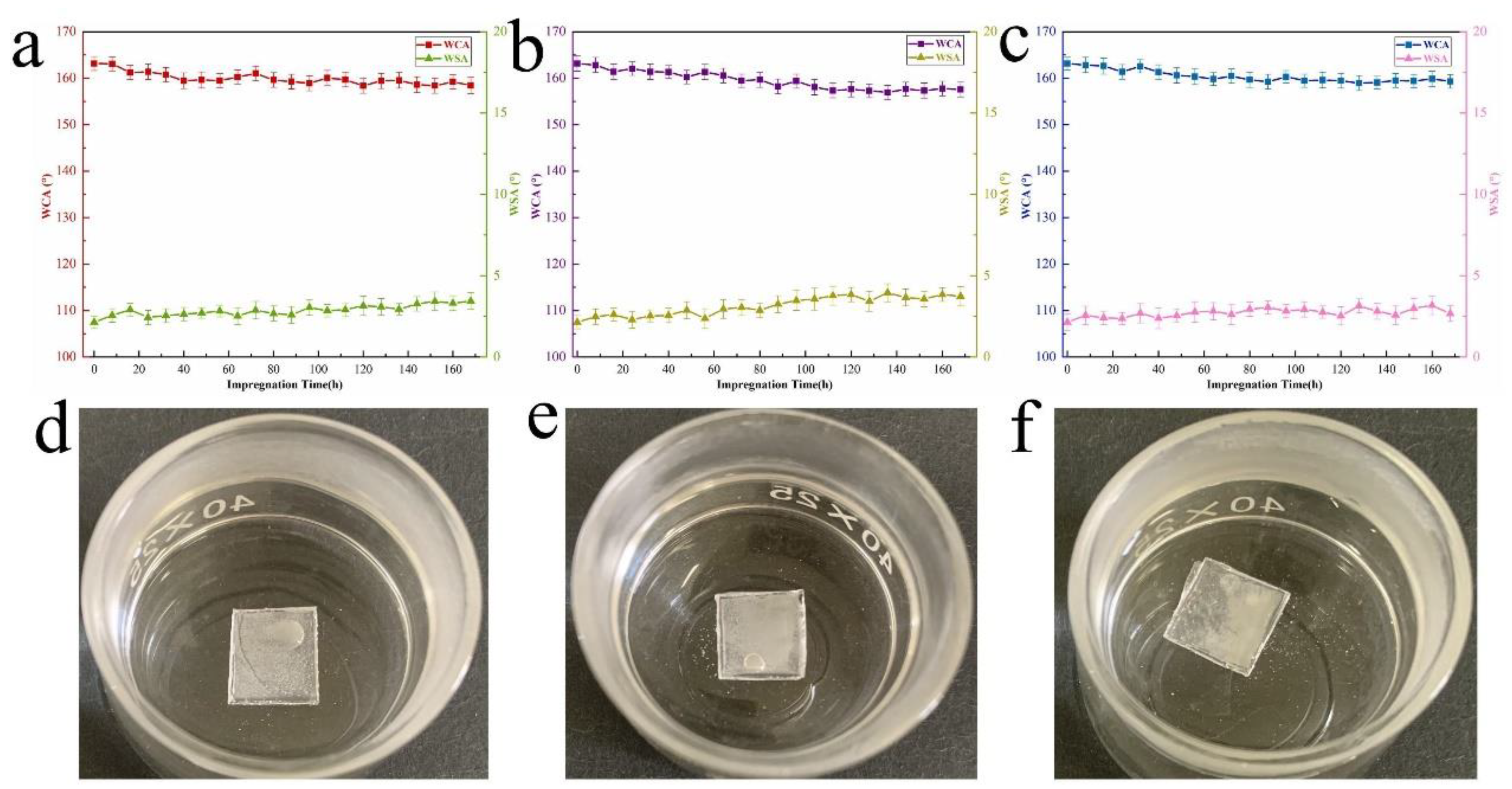
3.4. Chemical Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neinhuis, C.; Barthlott, W. Characterization and distribution of water-repellent, self-cleaning plant surfaces. Ann. Bot. 1997, 79, 667–677. [Google Scholar] [CrossRef]
- Gao, L.C.; McCarthy, T.J. The “lotus effect” explained: Two reasons why two length scales of topography are important. Langmuir 2006, 22, 2966–2967. [Google Scholar] [CrossRef]
- Gao, X.F.; Jiang, L. Water-repellent legs of water striders. Nature 2004, 432, 36. [Google Scholar] [CrossRef] [PubMed]
- Lum, K.; Chandler, D.; Weeks, J.D. Hydrophobicity at small and large length scales. J. Phys. Chem. B 1999, 103, 4570–4577. [Google Scholar] [CrossRef]
- Chen, J.; Yuan, L.; Shi, C.; Wu, C.; Long, Z.; Qiao, H.; Wang, K.; Fan, Q.H. Nature-Inspired Hierarchical Protrusion Structure Construction for Washable and Wear-Resistant Superhydrophobic Textiles with Self-Cleaning Ability. ACS Appl. Mater. Interfaces 2021, 13, 18142–18151. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Tian, L.; Bing, W.; Zhao, J.; Ren, L. Bioinspired marine antifouling coatings: Status, prospects, and future. Prog. Mater. Sci. 2022, 124, 100889. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, K.; Liu, J.; Tian, Y.; Zhang, H.; Wang, R.; Zhang, B.; Zhang, H.; Zhou, F.; Zhang, Q. Design and preparation of a multi-fluorination organic superhydrophobic coating with high mechanical robustness and icing delay ability. Appl. Surf. Sci. 2019, 497, 143663. [Google Scholar] [CrossRef]
- Jia, L.; Sun, J.; Li, X.; Zhang, X.; Chen, L.; Tian, X. Preparation and Anti-frost Performance of PDMS-SiO2/SS Superhydrophobic Coating. Coatings 2020, 10, 1051. [Google Scholar] [CrossRef]
- Nie, Y.; Ma, S.; Tian, M.; Zhang, Q.; Huang, J.; Cao, M.; Li, Y.; Sun, L.; Pan, J.; Wang, Y.; et al. Superhydrophobic silane-based surface coatings on metal surface with nanoparticles hybridization to enhance anticorrosion efficiency, wearing resistance and antimicrobial ability. Surf. Coat. Technol. 2021, 410, 126966. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Gong, X. Design and fabrication of polydopamine based superhydrophobic fabrics for efficient oil-water separation. Soft Matter 2021, 17, 6542–6551. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Sheng, W.; Wang, C.; Ye, Y. Solvent-free fabrication of tough self-crosslinkable short-fluorinated copolymer nanocoatings for ultradurable superhydrophobic fabrics. Chem. Eng. J. 2021, 416, 128043. [Google Scholar] [CrossRef]
- Jang, G.G.; Smith, D.B.; List, F.A.; Lee, D.F.; Ievlev, A.V.; Collins, L.; Park, J.; Polizos, G. The anti-soiling performance of highly reflective superhydrophobic nanoparticle-textured mirrors. Nanoscale 2018, 10, 14600–14612. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Ham, D.S.; Lee, D.Y.; Bong, H.; Che, K. Transparent Superhydrophobic/Translucent Superamphiphobic Coatings Based on Silica-Fluoropolymer Hybrid Nanoparticles. Langmuir 2013, 29, 15051–15057. [Google Scholar] [CrossRef] [PubMed]
- Sutha, S.; Suresh, S.; Raj, B.; Ravi, K.R. Transparent alumina based superhydrophobic self-cleaning coatings for solar cell cover glass applications. Sol. Energy Mater. Sol. Cells 2017, 165, 128–137. [Google Scholar] [CrossRef]
- Torun, I.; Celik, N.; Hancer, M.; Es, F.; Emir, C.; Turan, R.; Onses, M.S. Water Impact Resistant and Antireflective Superhydrophobic Surfaces Fabricated by Spray Coating of Nanoparticles: Interface Engineering via End-Grafted Polymers. Macromolecules 2018, 51, 10011–10020. [Google Scholar] [CrossRef]
- Xi, Y.; Yang, Z.; Zhang, J. Fabrication of superhydrophobic bilayer composite coating for roof cooling and cleaning. Constr. Build. Mater. 2021, 291, 123283. [Google Scholar] [CrossRef]
- Zhan, W.; Wang, W.; Xiao, Z.; Yu, X.; Zhang, Y. Water-free dedusting on antireflective glass with durable superhydrophobicity. Surf. Coat. Technol. 2018, 356, 123–131. [Google Scholar] [CrossRef]
- Allione, M.; Limongi, T.; Marini, M.; Torre, B.; Zhang, P.; Moretti, M.; Perozziello, G.; Candeloro, P.; Napione, L.; Pirri, C.F.; et al. Micro/Nanopatterned Superhydrophobic Surfaces Fabrication for Biomolecules and Biomaterials Manipulation and Analysis. Micromachines 2021, 12, 1501. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H. Fabrication of a superhydrophobic surface by a template-assisted chemical deposition method. Mater. Express 2020, 10, 1346–1351. [Google Scholar] [CrossRef]
- Wei, D.; Wang, J.; Liu, Y.; Wang, D.; Li, S.; Wang, H. Controllable superhydrophobic surfaces with tunable adhesion on Mg alloys by a simple etching method and its corrosion inhibition performance. Chem. Eng. J. 2021, 404, 126444. [Google Scholar] [CrossRef]
- Chen, F.; Xiang, W.; Yin, S.; Huang, S. Magnetically Responsive Superhydrophobic Surface with Switchable Adhesivity Based on Electrostatic Air Spray Deposition. ACS Appl. Mater. Interfaces 2021, 13, 20885–20896. [Google Scholar] [CrossRef]
- Pratiwi, N.; Zulhadjri; Arief, S.; Admi; Wellia, D.V. Self-cleaning material based on superhydrophobic coatings through an environmentally friendly sol-gel method. J. Sol-Gel Sci. Technol. 2020, 96, 669–678. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, L.; Zhang, Z.; Wang, Y.; Yang, Z.; Liu, C.; Li, Z.; Zhao, Y. Bioinspired superhydrophobic surface by hierarchically colloidal assembling of microparticles and colloidal nanoparticles. Chem. Eng. J. 2020, 394, 125008. [Google Scholar] [CrossRef]
- Biria, S.; Hosein, I.D. Superhydrophobic Microporous Substrates via Photocuring: Coupling Optical Pattern Formation to Phase Separation for Process Tunable Pore Architectures. ACS Appl. Mater. Interfaces 2018, 10, 3094–3105. [Google Scholar] [CrossRef]
- Jiang, S.; Meng, X.; Chen, B.; Wang, N.; Chen, G. Electrospinning superhydrophobic-superoleophilic PVDF-SiO2 nanofibers membrane for oil-water separation. J. Appl. Polym. Sci. 2020, 137, 49546. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhou, H.; Huang, J.; Guo, Z. Review on the recent development of durable superhydrophobic materials for practical applications. Nanoscale 2021, 13, 11734–11764. [Google Scholar] [CrossRef]
- Zhou, H.; Ye, Q.; Xu, J. Polyhedral oligomeric silsesquioxane-based hybrid materials and their applications. Mater. Chem. Front. 2017, 1, 212–230. [Google Scholar] [CrossRef]
- Chrusciel, J.J.; Lesniak, E. Modification of epoxy resins with functional silanes, polysiloxanes, silsesquioxanes, silica and silicates. Prog. Polym. Sci. 2015, 41, 67–121. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, C.; Zheng, S. Organic-inorganic poly(hydroxyether of bisphenol A) copolymers with double-decker silsesquioxane in the main chains. J. Mater. Chem. 2011, 21, 19344–19352. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, W.; Zhang, S.; Huang, H.; Ouyang, L.; Peng, W.; Ye, J.; Chen, C. A Comparative Study of Adhesion Evaluation Methods on Ophthalmic AR Coating Lens. Coatings 2020, 10, 979. [Google Scholar] [CrossRef]
- Zhang, P.; Qin, B.; Xia, J. UV Curable Robust Durable Hydrophobic Coating Based on Epoxy Polyhedral Oligomeric Silsesquioxanes (EP-POSS) and Their Derivatives. ACS Omega 2022, 7, 17108–17118. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.L.; Song, F.; Wang, X.L.; Wang, Y.Z. Surface modification with hierarchical CuO arrays toward a flexible, durable superhydrophobic and self-cleaning material. Chem. Eng. J. 2017, 313, 1328–1334. [Google Scholar] [CrossRef]
| System NO. | Composition (wt. %) | |||
|---|---|---|---|---|
| Acrylate Oligomer(8110) | HDDA | POSS Component | 2-M-4-MT-2-MP(I907) | |
| O | 64 | 32 | - | 4 |
| C1 | 57 | 29 | 10 | 4 |
| C2 | 51 | 25 | 20 | 4 |
| C3 | 38 | 18 | 40 | 4 |
| C4 | 31 | 15 | 50 | 4 |
| C5 | 21 | 10 | 65 | 4 |
| C6 | 14 | 7 | 75 | 4 |
| PC Substrate | O | C1 | C2 | C3 | C4 | C5 | C6 | |
|---|---|---|---|---|---|---|---|---|
| WCA (°) | 78.2 | 94.8 | 116.7 | 129.3 | 150.6 | 158.2 | 166.4 | 172.4 |
| SA (°) | - | 88.2 | 35.3 | 22.7 | 8.3 | 5.2 | 2.8 | 2.1 |
| Transmittance (%) | 89.1 | 90.8 | 86.4 | 85.5 | 84.2 | 79.8 | 76.8 | 66.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; Xu, Y.; He, J.; Dong, X. Transparent Superhydrophobic Coatings with Mechanical and Chemical Stability Prepared by Modified Polyhedral Oligosilsesquioxanes via UV-Curable Method. Coatings 2023, 13, 498. https://doi.org/10.3390/coatings13030498
Zhu W, Xu Y, He J, Dong X. Transparent Superhydrophobic Coatings with Mechanical and Chemical Stability Prepared by Modified Polyhedral Oligosilsesquioxanes via UV-Curable Method. Coatings. 2023; 13(3):498. https://doi.org/10.3390/coatings13030498
Chicago/Turabian StyleZhu, Weibiao, Yazhou Xu, Jinxin He, and Xia Dong. 2023. "Transparent Superhydrophobic Coatings with Mechanical and Chemical Stability Prepared by Modified Polyhedral Oligosilsesquioxanes via UV-Curable Method" Coatings 13, no. 3: 498. https://doi.org/10.3390/coatings13030498
APA StyleZhu, W., Xu, Y., He, J., & Dong, X. (2023). Transparent Superhydrophobic Coatings with Mechanical and Chemical Stability Prepared by Modified Polyhedral Oligosilsesquioxanes via UV-Curable Method. Coatings, 13(3), 498. https://doi.org/10.3390/coatings13030498





