Enhanced Photocatalytic Activity of the Cu2SnS3 + GO Composite for the Degradation of Navy Blue ME2RL Industrial Dye
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
2.2. Preparation of Photocatalyst
2.3. Characterization Techniques
2.4. Photocatalytic Activity of CTS Nanoparticles
3. Results and Discussion
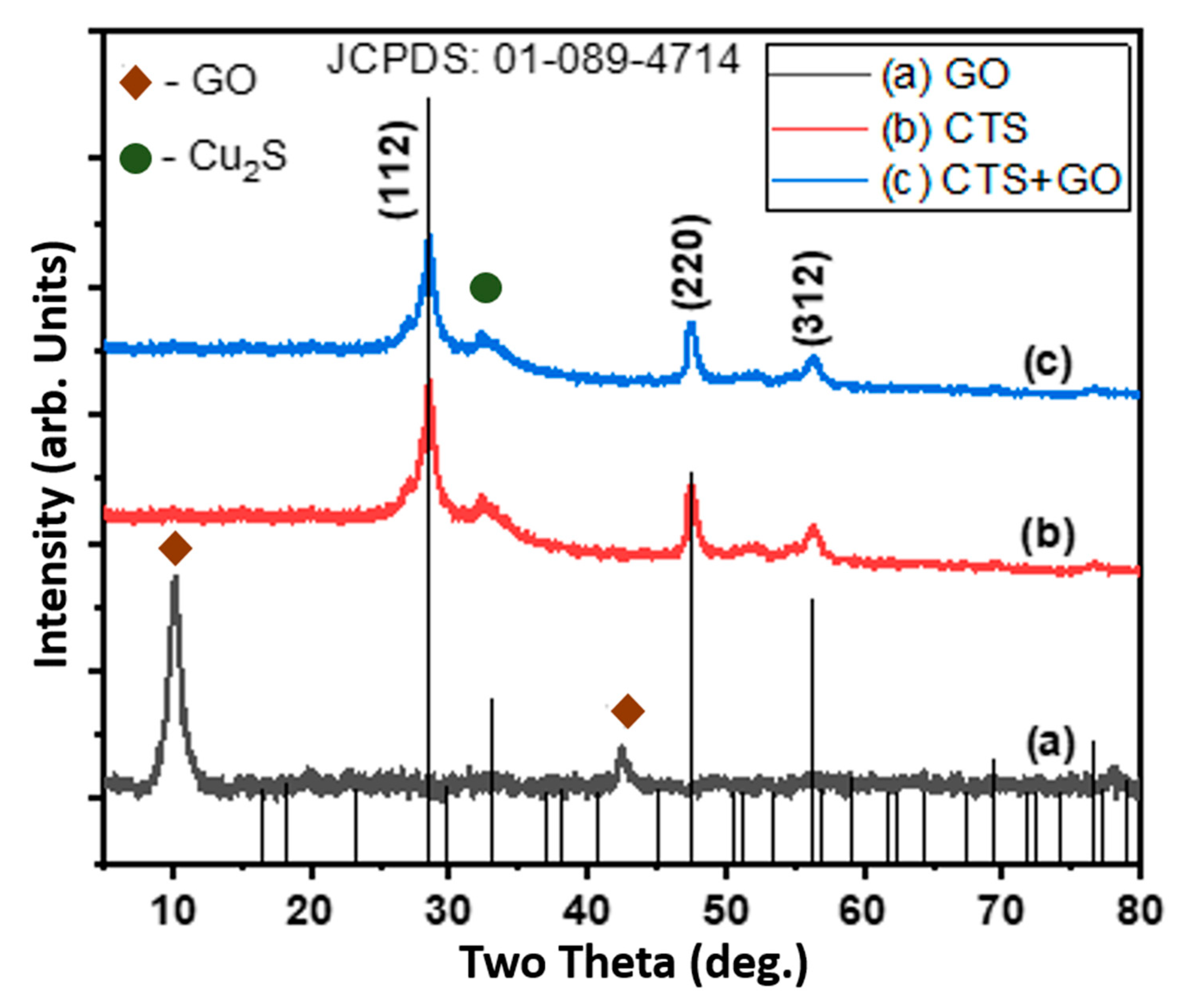
3.1. Structural Analysis
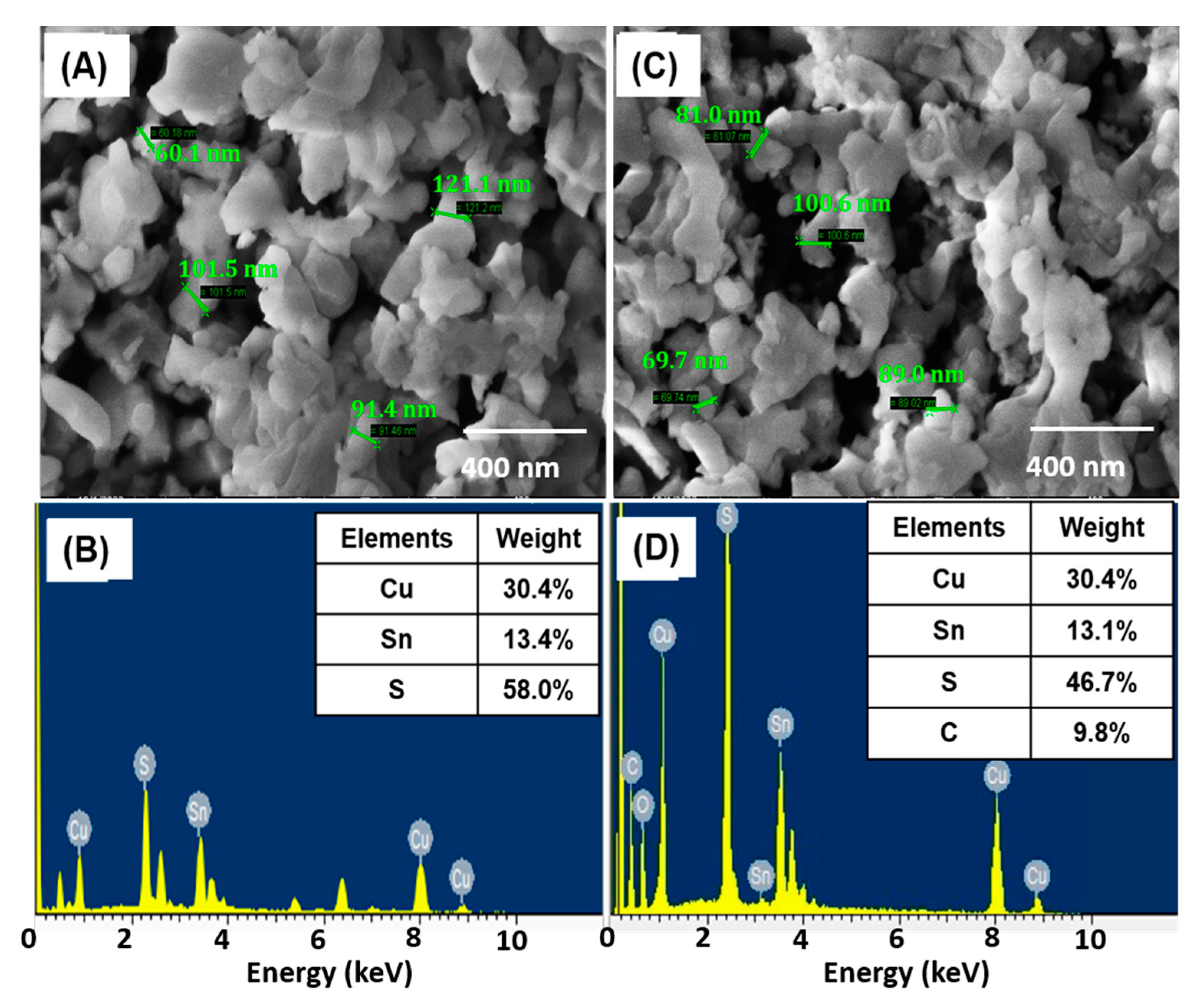
3.2. Morphological Analysis
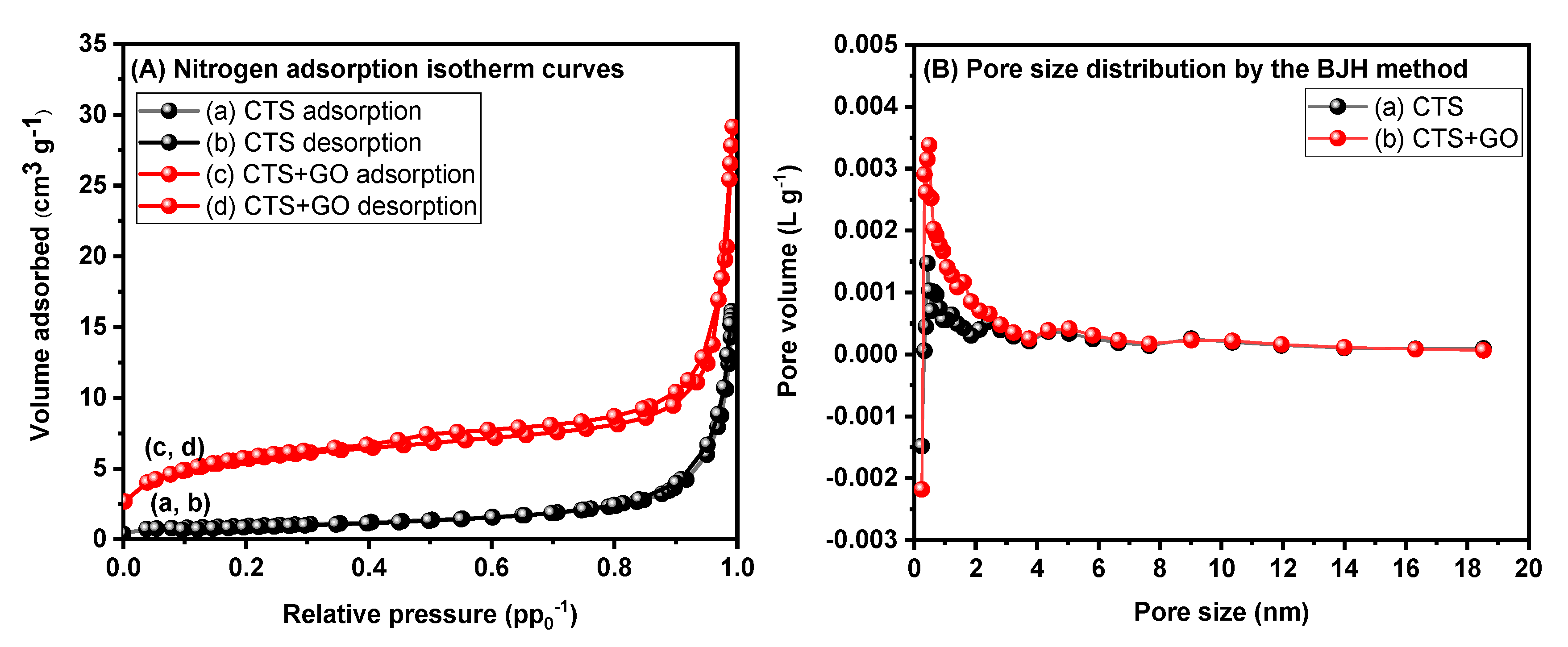
3.3. Brunauer-Emmett-Teller (BET) Analysis
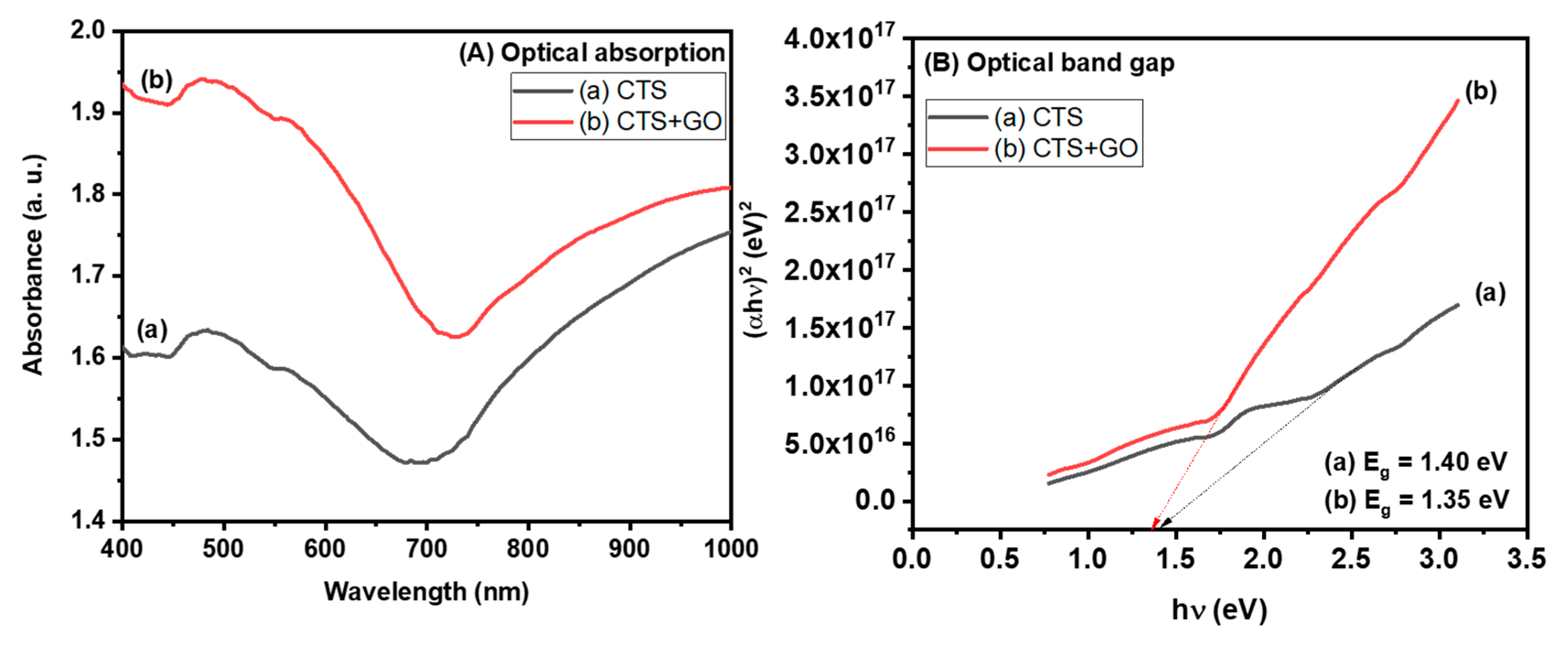
3.4. Optical Analysis
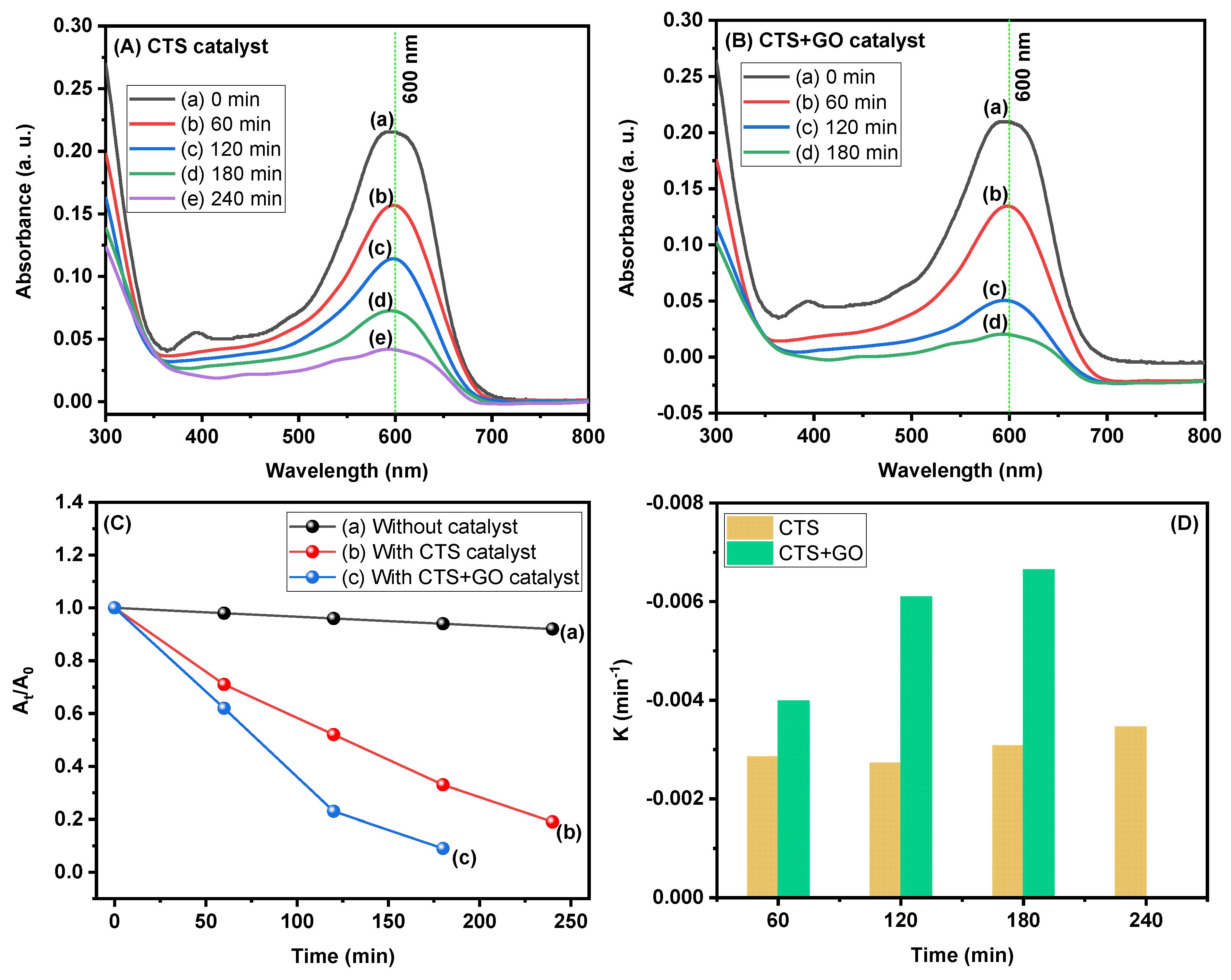
3.5. Photocatalytic Activity
3.6. Photocatalytic Process Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.; Li, J.; Wang, H.; Li, Y.; Duan, X. Impact of co-contamination by PAHs and heavy metals on micro-ecosystem in bioretention systems with soil, sand, and water treatment residuals. J. Clean. Prod. 2023, 383, 135417. [Google Scholar] [CrossRef]
- Mandeep; Gupta, G.K.; Liu, H.; Shukla, P. Pulp and paper industry-based pollutants, their health hazards and environmental risks. Curr. Opin. Environ. Sci. Health 2019, 12, 48–56. [Google Scholar] [CrossRef]
- Lin, C.; Liu, H.; Guo, M.; Zhao, Y.; Su, X.; Zhang, P.; Zhang, Y. Plasmon-induced broad spectrum photocatalytic overall water splitting: Through non-noble bimetal nanoparticles hybrid with reduced graphene oxide. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 646, 128962. [Google Scholar] [CrossRef]
- Lops, C.; Ancona, A.; Di Cesare, K.; Dumontel, B.; Garino, N.; Canavese, G.; Hérnandez, S.; Cauda, V. Sonophotocatalytic degradation mechanisms of rhodamine B dye via radicals generation by micro- and nano-particles of ZnO. Appl. Catal. B Environ. 2019, 243, 629–640. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Guo, C.; Wang, Y. Molybdenum carbide-based photocatalysts: Synthesis, functionalization, and applications. Langmuir 2022, 38, 12739–12756. [Google Scholar] [CrossRef] [PubMed]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Wagh, S.S.; Jagtap, C.V.; Kadam, V.S.; Shaikh, S.F.; Ubaidullah, M.; Pandit, B.; Salunkhe, D.B.; Patil, R.S. Silver doped ZnO nanoparticles synthesized for photocatalysis application. ES Energy Environ. 2022, 17, 94–105. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Wang, G. Photocatalytic advanced oxidation processes for water treatment: Recent advances and perspective. Chem.—Asian J. 2020, 15, 3239–3253. [Google Scholar] [CrossRef]
- Xia, C.; Wu, W.; Yu, T.; Xie, X.; van Oversteeg, C.; Gerritsen, H.C.; de Mello Donega, C. Size-dependent band-gap and molar absorption coefficients of colloidal CuInS2 quantum dots. ACS Nano 2018, 12, 8350–8361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shelke, H.D.; Machale, A.R.; Survase, A.A.; Pathan, H.M.; Lokhande, C.D.; Lokhande, A.C.; Shaikh, S.F.; Rana, A.u.H.S.; Palaniswami, M. Multifunctional Cu2SnS3 nanoparticles with enhanced photocatalytic dye degradation and antibacterial activity. Materials 2022, 15, 3126. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Li, G.; Shang, C.; Mao, E.; Huang, L.; Wang, X.; Ma, G.; Wang, X.; Zhou, G. Reduced graphene oxide boosted ultrafine Cu2SnS3 nanoparticles for high-performance sodium storage. ChemElectroChem 2019, 6, 2949–2955. [Google Scholar] [CrossRef]
- Hu, P.; Liu, X.; Liu, B.; Li, L.; Qin, W.; Yu, H.; Zhong, S.; Li, Y.; Ren, Z.; Wang, M. Hierarchical layered Ni3S2-graphene hybrid composites for efficient photocatalytic reduction of Cr(VI). J. Colloid Interface Sci. 2017, 496, 254–260. [Google Scholar] [CrossRef]
- Zou, L.; Wang, X.; Xu, X.; Wang, H. Reduced graphene oxide wrapped CdS composites with enhanced photocatalytic performance and high stability. Ceram. Int. 2016, 42, 372–378. [Google Scholar] [CrossRef]
- Borthakur, P.; Das, M.R. Hydrothermal assisted decoration of NiS2 and CoS nanoparticles on the reduced graphene oxide nanosheets for sunlight driven photocatalytic degradation of azo dye: Effect of background electrolyte and surface charge. J. Colloid Interface Sci. 2018, 516, 342–354. [Google Scholar] [CrossRef]
- Borthakur, P.; Boruah, P.K.; Darabdhara, G.; Sengupta, P.; Das, M.R.; Boronin, A.I.; Kibis, L.S.; Kozlova, M.N.; Fedorov, V.E. Microwave assisted synthesis of CuS-reduced graphene oxide nanocomposite with efficient photocatalytic activity towards azo dye degradation. J. Environ. Chem. Eng. 2016, 4, 4600–4611. [Google Scholar] [CrossRef]
- Nakate, Y.T.; Sangale, S.S.; Shaikh, S.F.; Shinde, N.M.; Shirale, D.J.; Mane, R.S. Human urine-derived naturally heteroatom doped highly porous carbonaceous material for gas sensing and supercapacitor applications. Ceram. Int. 2022, 48, 28942–28950. [Google Scholar] [CrossRef]
- Sebastian, A.; Maheskumar, V.; Bhuvanesh, N.; Vidhya, B.; Nandhakumar, R.; Jiang, Z. Photocatalytic performance of Cu3SnS4 (CTS)/reduced graphene oxide (RGO) composite prepared via ball milling and solvothermal approach. J. Mater. Sci. Mater. Electron. 2020, 31, 21408–21418. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Z.; Jin, Z.; Su, Y.; Wang, Y. A reduced graphene oxide supported Cu3SnS4 composite as an efficient visible-light photocatalyst. Dalt. Trans. 2014, 43, 7491–7498. [Google Scholar] [CrossRef]
- Vadivel, S.; Maruthamani, D.; Paul, B.; Dhar, S.S.; Habibi-Yangjeh, A.; Balachandran, S.; Saravanakumar, B.; Selvakumar, A.; Selvam, K. Biomolecule-assisted solvothermal synthesis of Cu2SnS3 flowers/RGO nanocomposites and their visible-light-driven photocatalytic activities. RSC Adv. 2016, 6, 74177–74185. [Google Scholar] [CrossRef]
- Olatunde, O.C.; Onwudiwe, D.C. Synthesis of reduced graphene oxide/copper tin sulfide (Cu2SnS3) composite for the photocatalytic degradation of tetracycline. J. Inorg. Organomet. Polym. Mater. 2022, 32, 2578–2590. [Google Scholar] [CrossRef]
- Tao, H.-C.; Zhu, S.-C.; Yang, X.-L.; Zhang, L.-L.; Ni, S.-B. Reduced graphene oxide decorated ternary Cu2SnS3 as anode materials for lithium ion batteries. J. Electroanal. Chem. 2016, 760, 127–134. [Google Scholar] [CrossRef]
- Maheskumar, V.; Sheebha, I.; Vidhya, B.; Deebasree, J.P.; Selvaraju, T.; Akash, S. Enhanced electrocatalytic and photocatalytic activity of ball milled copper tin sulphide by incorporating GO and RGO. Appl. Surf. Sci. 2019, 484, 265–275. [Google Scholar] [CrossRef]
- Yao, S.; Xu, L.; Gao, Q.; Wang, X.; Kong, N.; Li, W.; Wang, J.; Li, G.; Pu, X. Enhanced photocatalytic degradation of rhodamine b by reduced graphene oxides wrapped-Cu2SnS3 flower-like architectures. J. Alloys Compd. 2017, 704, 469–477. [Google Scholar] [CrossRef]
- Machale, A.R.; Phaltane, S.A.; Shelke, H.D.; Kadam, L.D. Facile hydrothermal synthesis of Cu2SnS3 nanoparticles for photocatalytic dye degredation of mythelene blue. Mater. Today Proc. 2021, 43, 2768–2773. [Google Scholar] [CrossRef]
- Machale, A.R.; Shelke, H.D.; Phaltane, S.A.; Kadam, L.D. Influence of hydrothermal temperature on the structural, morphological, optical and photocatalytic properties of ternary Cu2SnS3 nanoparticles. Chem. Phys. Lett. 2022, 808, 140097. [Google Scholar] [CrossRef]
- Zhang, J.; Xiong, Z.; Zhao, X.S. Graphene-metal-oxide composites for the degradation of dyes under visible light irradiation. J. Mater. Chem. 2011, 21, 3634–3640. [Google Scholar] [CrossRef]
- Gao, P.; Liu, J.; Sun, D.D.; Ng, W. Graphene oxide-CdS composite with high photocatalytic degradation and disinfection activities under visible light irradiation. J. Hazard. Mater. 2013, 250–251, 412–420. [Google Scholar] [CrossRef]
- Padmanabhan, N.T.; Thomas, N.; Louis, J.; Mathew, D.T.; Ganguly, P.; John, H.; Pillai, S.C. Graphene coupled TiO2 photocatalysts for environmental applications: A review. Chemosphere 2021, 271, 129506. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shelke, H.D.; Machale, A.R.; Surwase, A.A.; Shaikh, S.F.; Rana, A.u.H.S.; Pathan, H.M. Enhanced Photocatalytic Activity of the Cu2SnS3 + GO Composite for the Degradation of Navy Blue ME2RL Industrial Dye. Coatings 2023, 13, 522. https://doi.org/10.3390/coatings13030522
Shelke HD, Machale AR, Surwase AA, Shaikh SF, Rana AuHS, Pathan HM. Enhanced Photocatalytic Activity of the Cu2SnS3 + GO Composite for the Degradation of Navy Blue ME2RL Industrial Dye. Coatings. 2023; 13(3):522. https://doi.org/10.3390/coatings13030522
Chicago/Turabian StyleShelke, Harshad D., Archana R. Machale, Avinash A. Surwase, Shoyebmohamad F. Shaikh, Abu ul Hassan S. Rana, and Habib M. Pathan. 2023. "Enhanced Photocatalytic Activity of the Cu2SnS3 + GO Composite for the Degradation of Navy Blue ME2RL Industrial Dye" Coatings 13, no. 3: 522. https://doi.org/10.3390/coatings13030522





