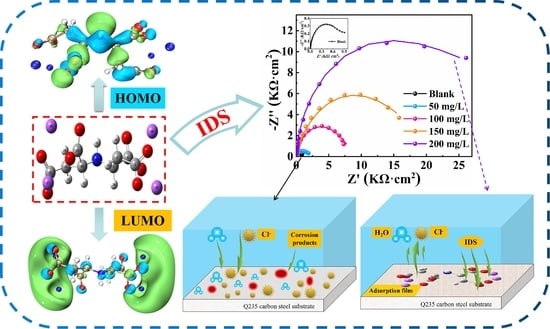
Corrosion Behaviors of Tetrasodium Iminodisuccinate (IDS) as an Environmentally Friendly Inhibitor: Experimental and Theoretical Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Test Solutions
2.2. Electrochemical Testing
2.3. Surface Morphology Testing
2.4. Quantum Chemical Calculations
2.5. MD Simulations
3. Results and Discussion
3.1. Open Circuit Potential
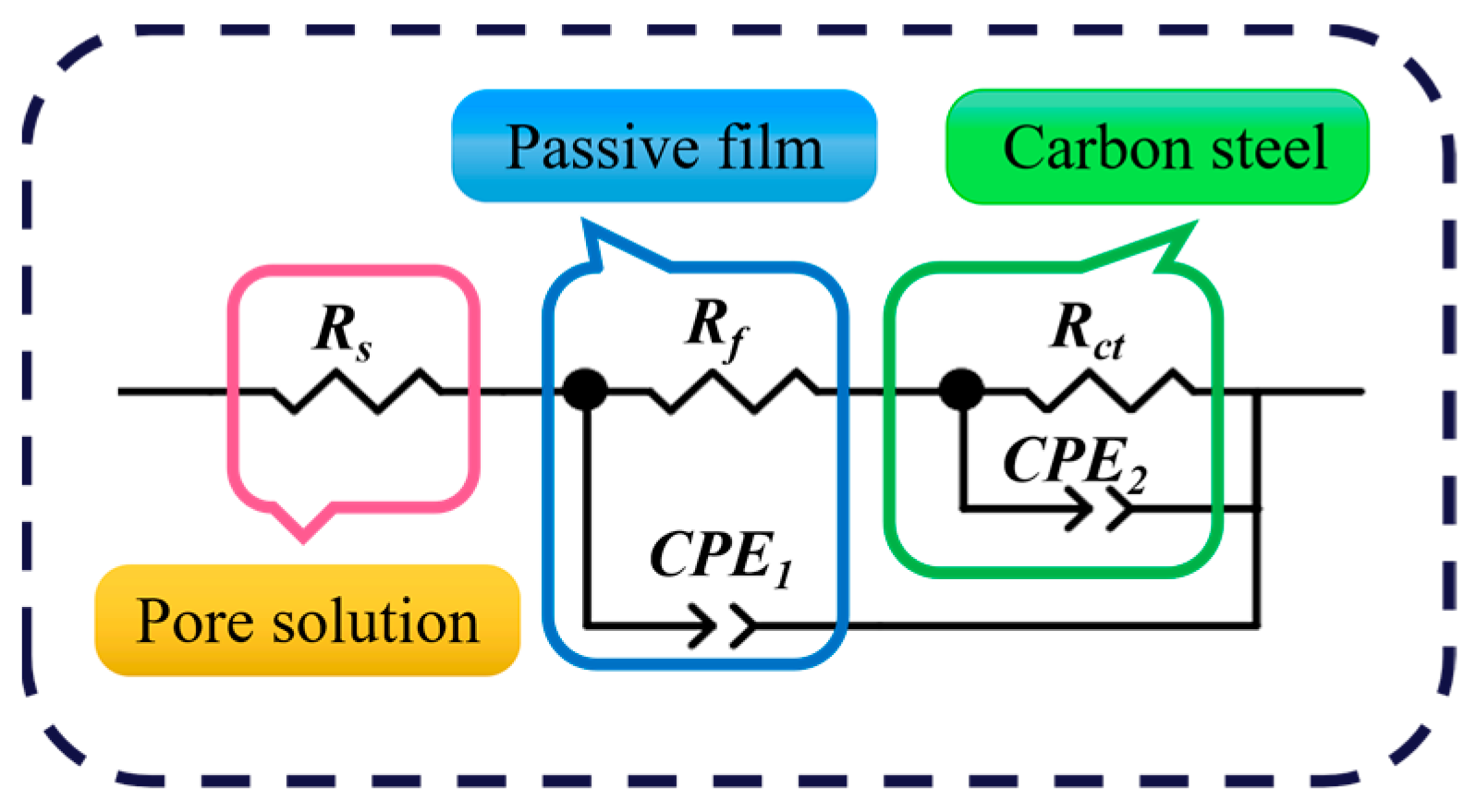
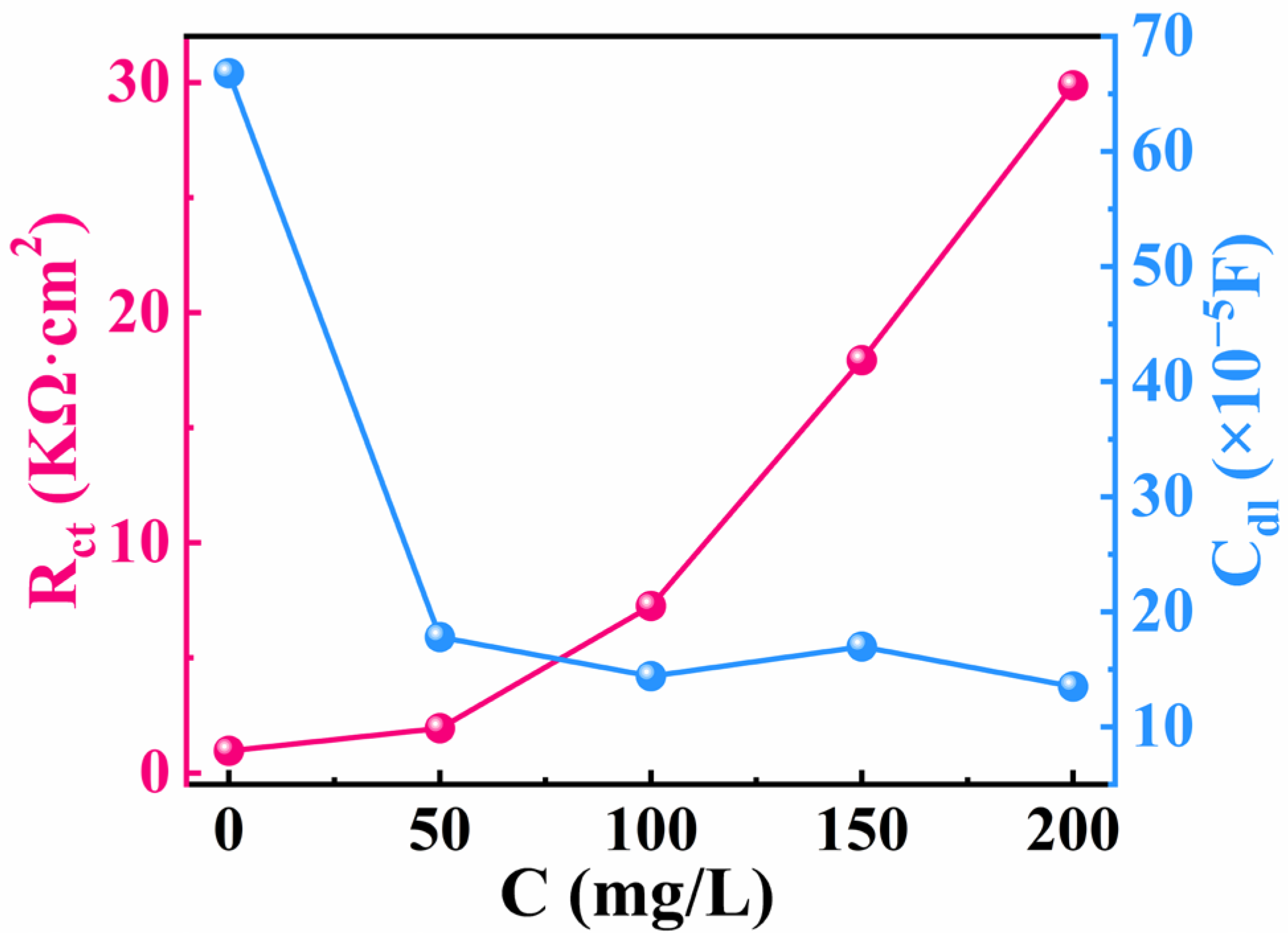
3.2. Electrochemical Impedance Spectroscopy
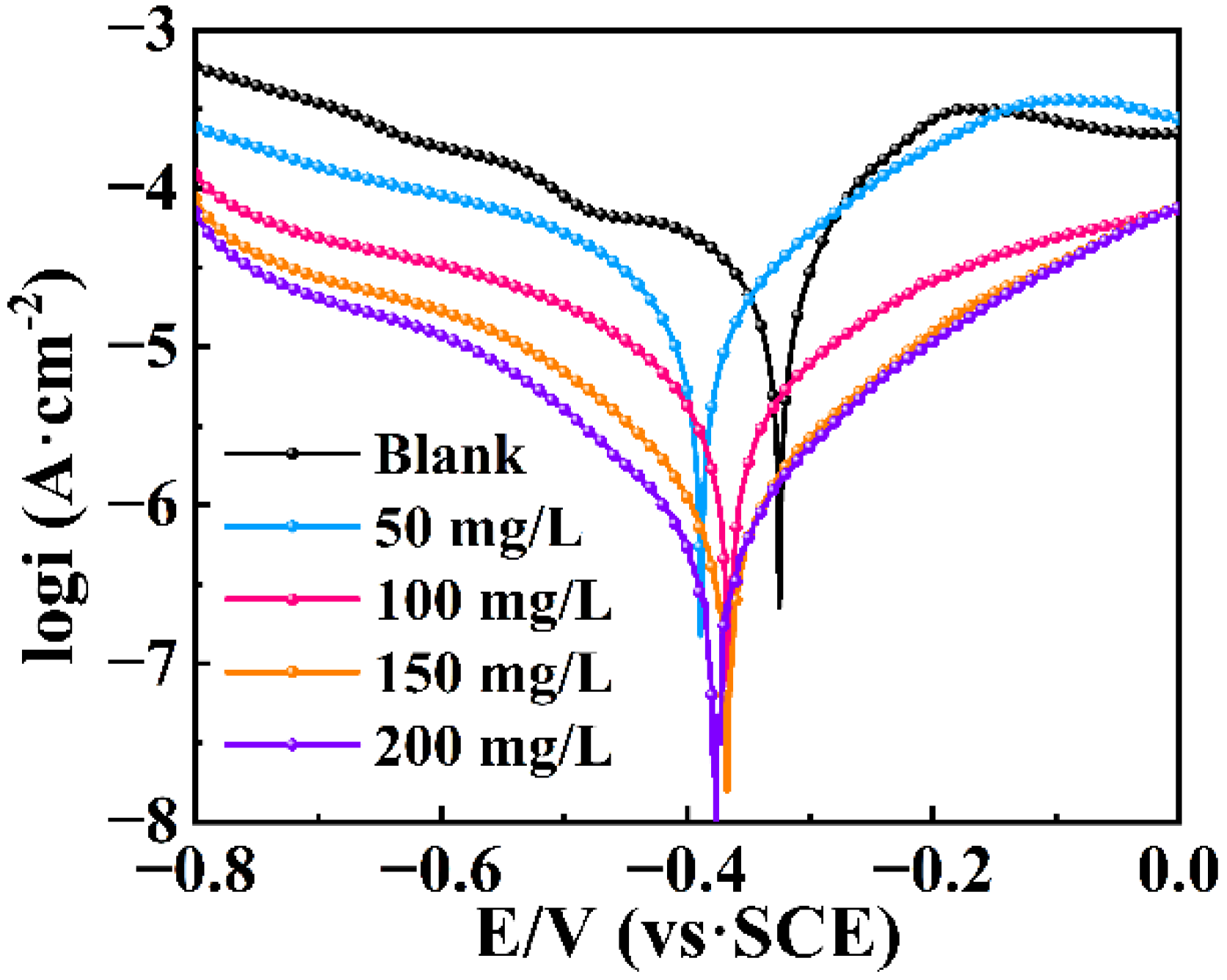
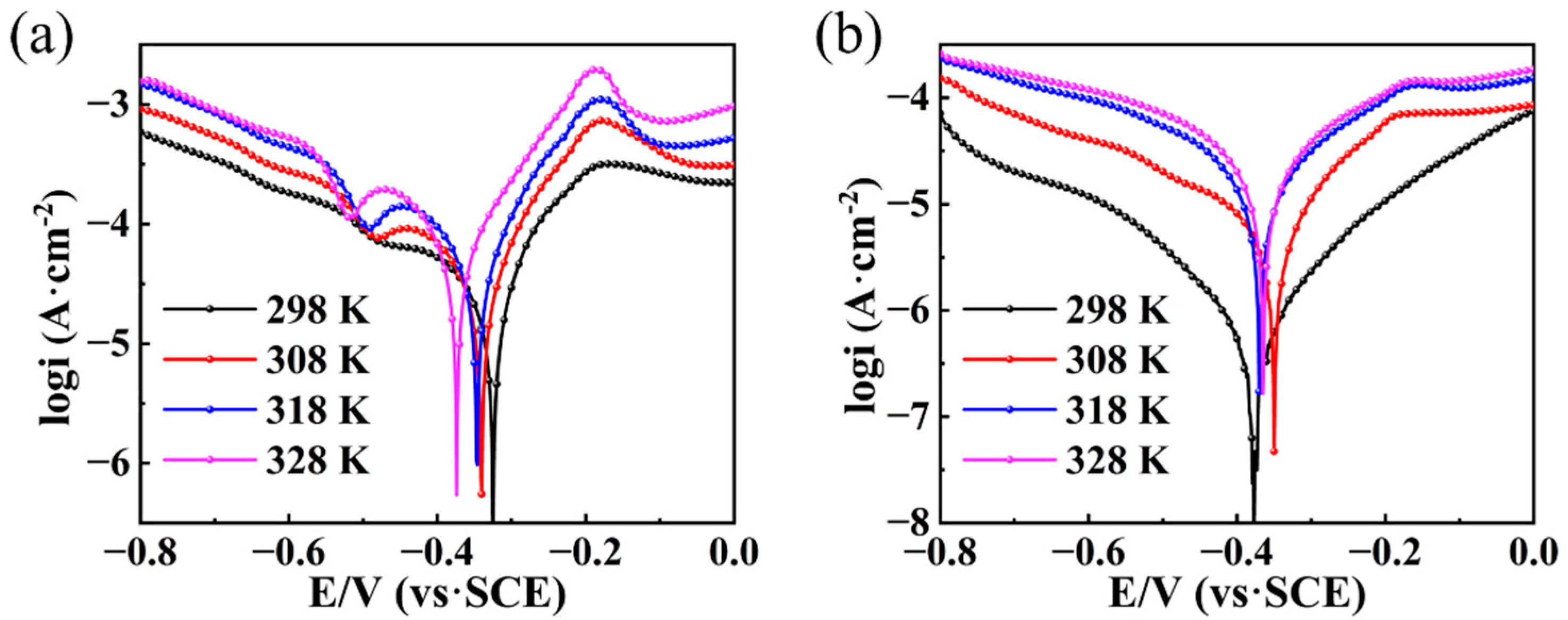
3.3. Tafel Polarization Curves
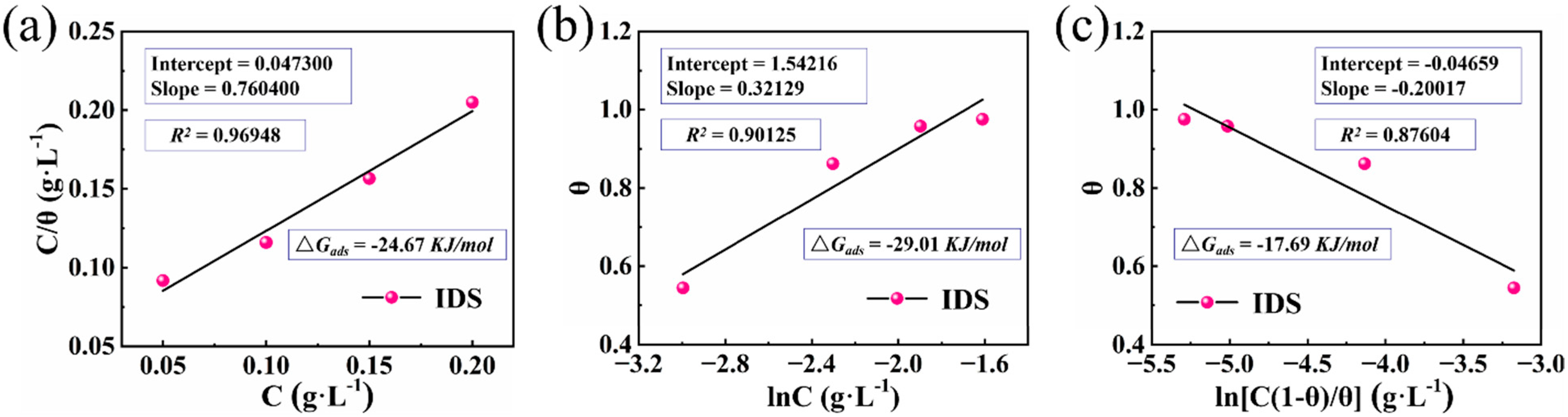
3.4. Adsorption Isotherm Behavior
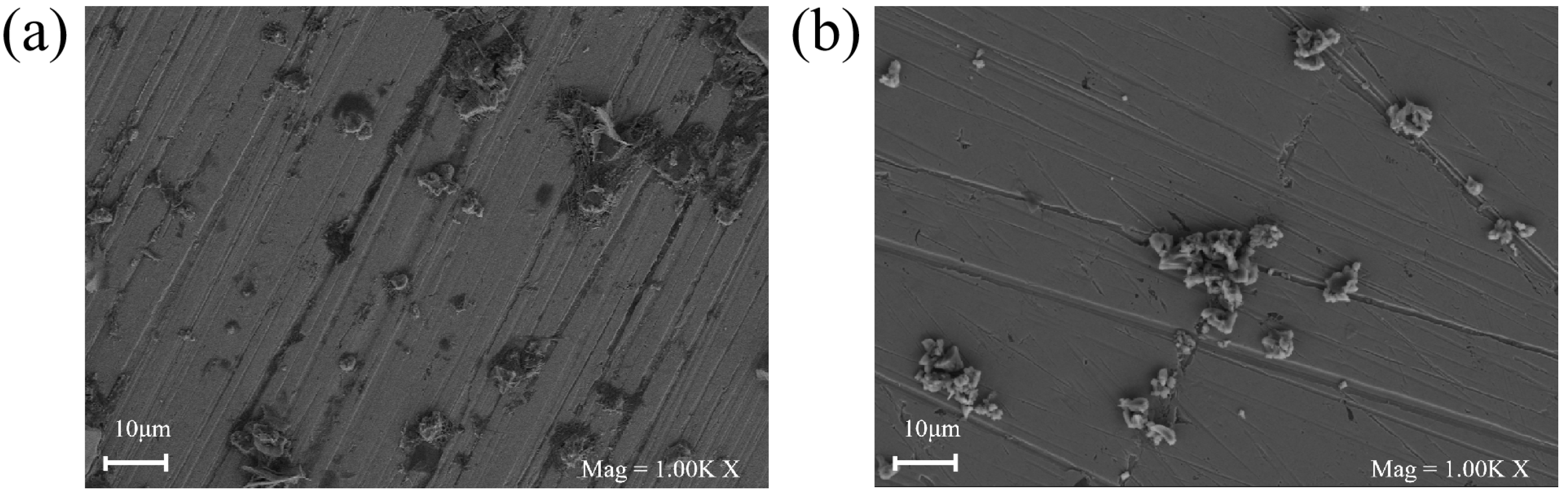
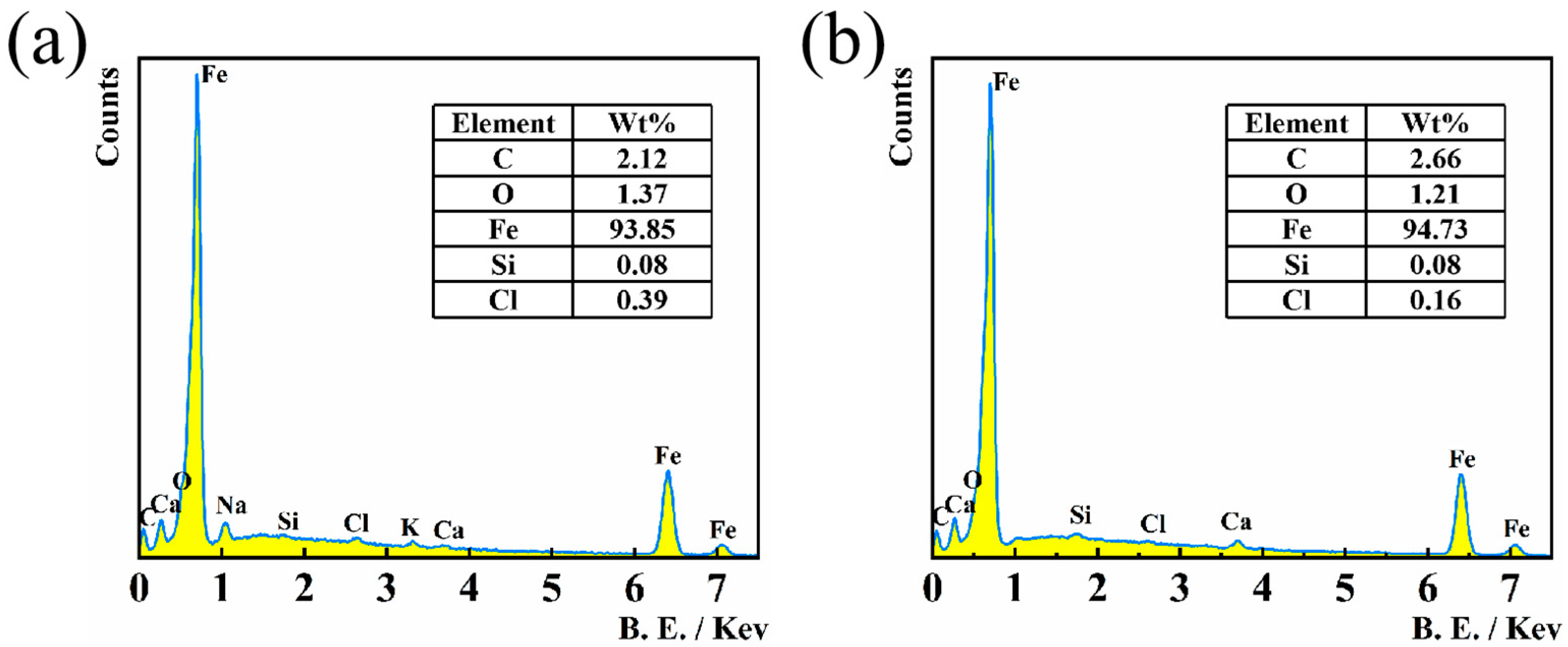
3.5. Surface Studies
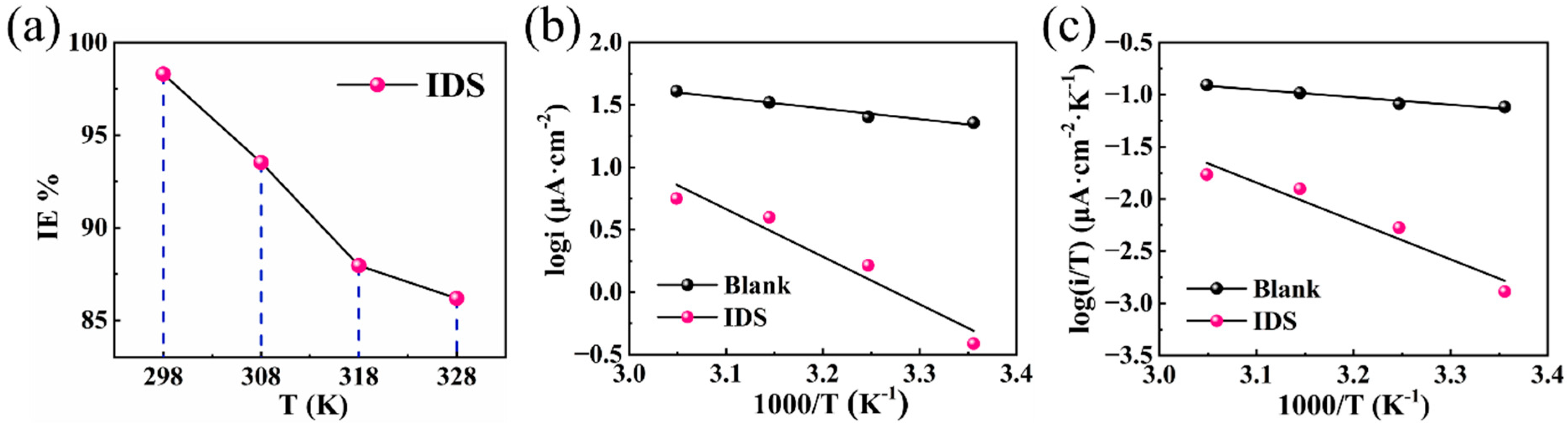
3.6. Effect of Temperature
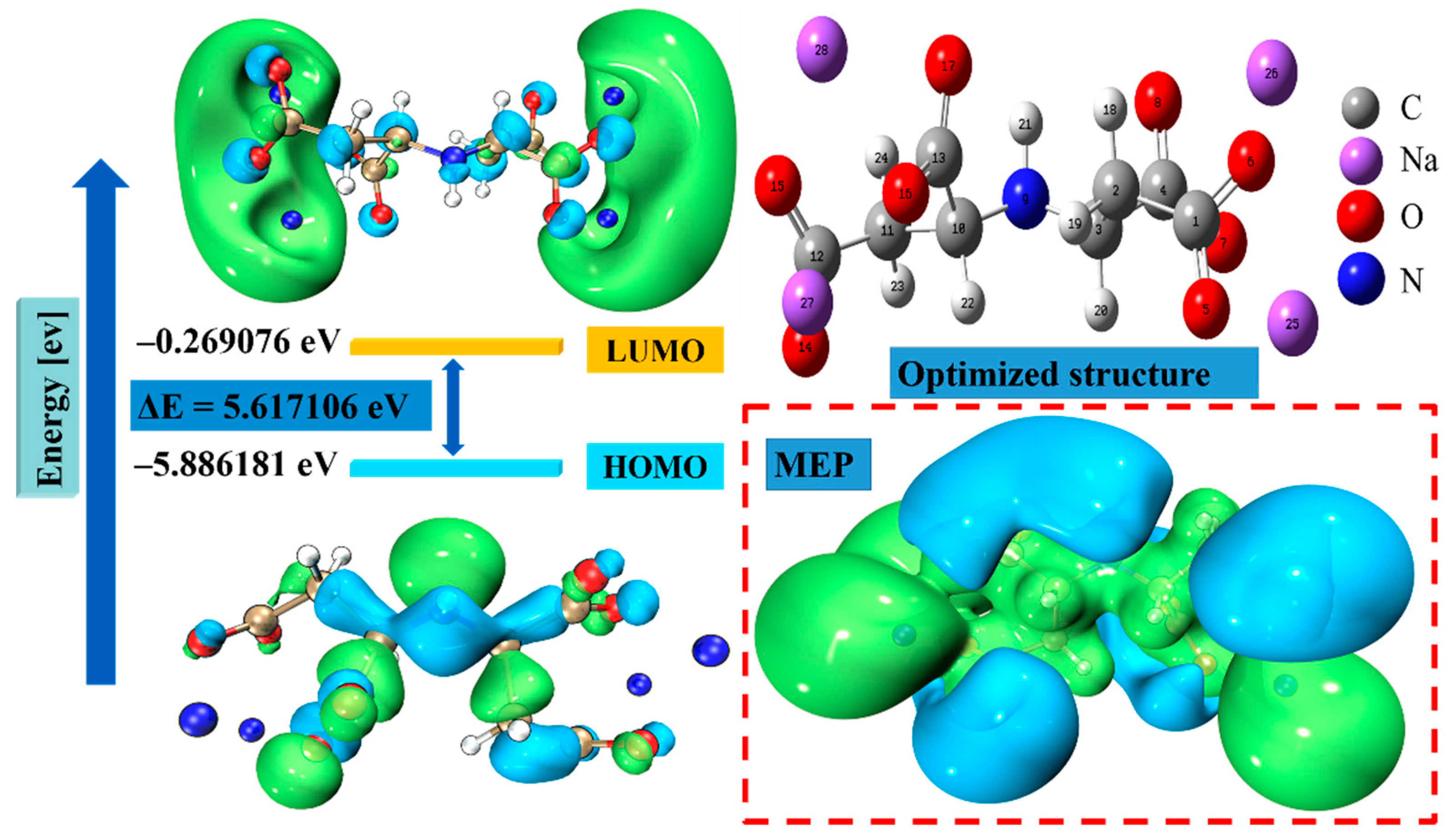
3.7. DFT Calculations
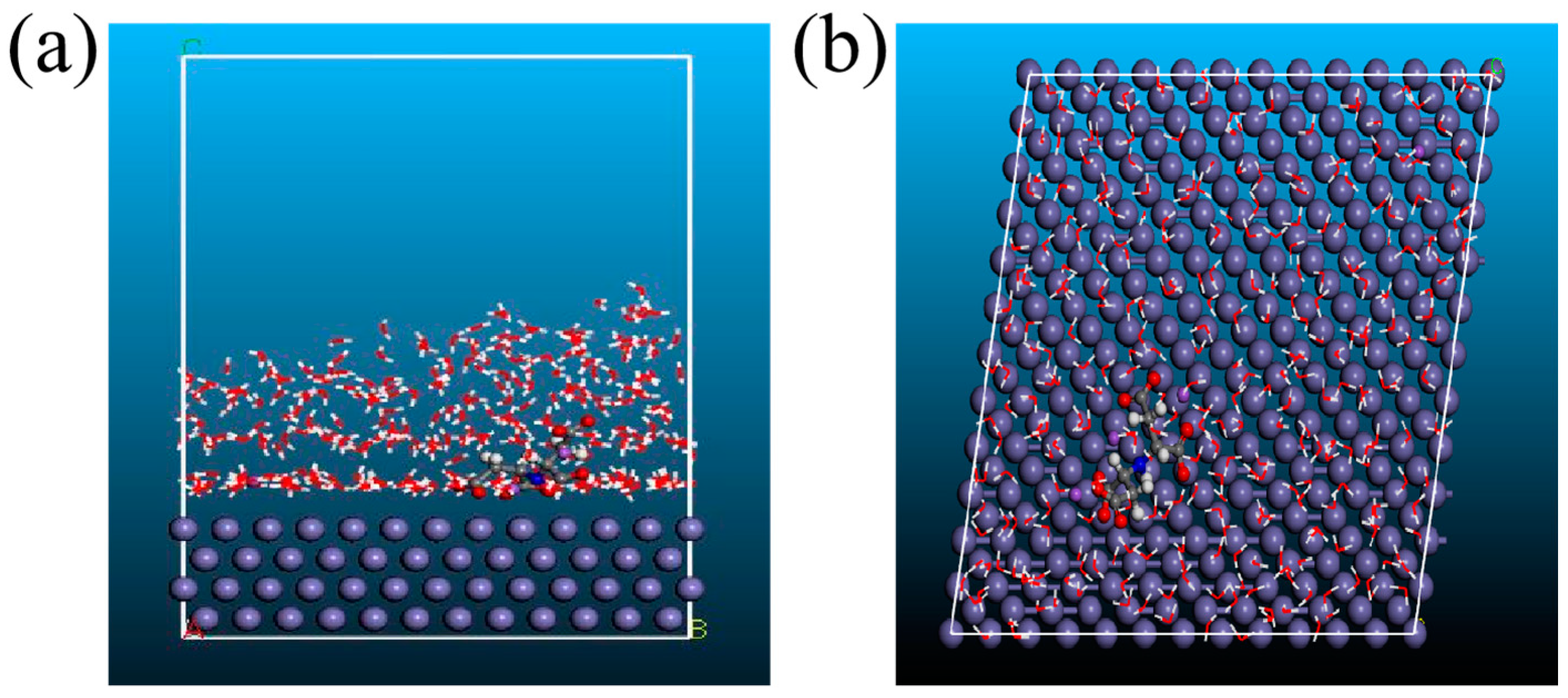
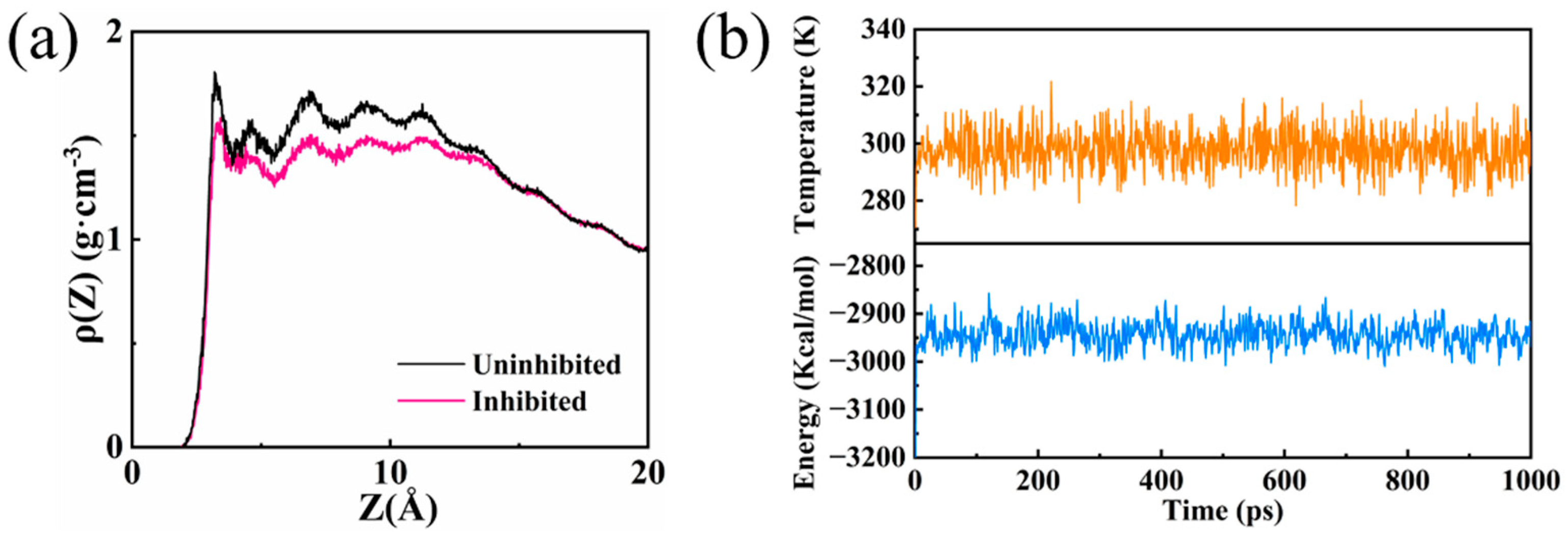
3.8. MD Simulations
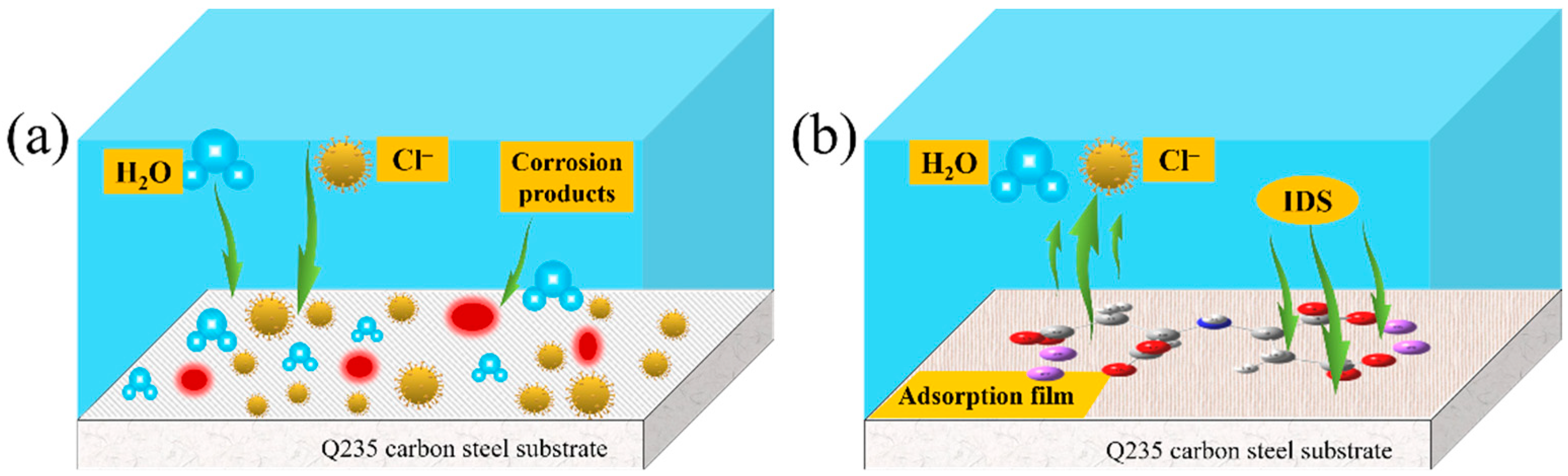
3.9. Mechanism of Inhibition
3.10. Comparison of Similar Corrosion Inhibitors
4. Conclusions
- The ddouble-layer capacitance (Cdl) was significantly reduced, and the charge transfer resistance (Rct) was significantly increased after adding IDS. This demonstrates that corrosion of Q235 carbon steel can be effectively retarded when adding IDS to the simulated concrete solution.
- IDS acts on both the cathode and anode of Q235 carbon steel, the IDS efficiency reached 97.54% at 200 mg/L.
- IDS on carbon steel surface is spontaneous, mainly physical adsorption, which matches the Langmuir adsorption model well.
- The DFT and MD simulations support the results of the tests and further indicate the sodium carboxylate groups in the IDS molecule play a major role in the corrosion inhibition process. It can be used as a new type of highly efficient inhibitor.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, Y.; Miao, Y.; Zuo, Y.; Zhang, G.; Wang, C. Corrosion behavior of steel in simulated concrete pore solutions treated with calcium silicate hydrates. Constr. Build. Mater. 2011, 30, 252–256. [Google Scholar] [CrossRef]
- Ben Mansour, H.; Dhouibi, L.; Idrissi, H. Effect of Phosphate-based inhibitor on prestressing tendons corrosion in simulated concrete pore solution contaminated by chloride ions. Constr. Build. Mater. 2018, 171, 250–260. [Google Scholar] [CrossRef]
- Rathod, M.R.; Rajappa, S.; Minagalavar, R.L.; Praveen, B.; Devendra, B.K.; Kittur, A. Investigation of African mangosteen leaves extract as an environment-friendly inhibitor for low carbon steel in 0.5 M H2SO4. Inorg. Chem. Commun. 2022, 140, 109488. [Google Scholar] [CrossRef]
- Matad, P.B.; Mokshanatha, P.B.; Hebbar, N.; Venkatesha, V.T.; Tandon, H.C. Ketosulfone Drug as a Green Corrosion Inhibitor for Mild Steel in Acidic Medium. Ind. Eng. Chem. Res. 2014, 53, 8436–8444. [Google Scholar] [CrossRef]
- Hebbar, N.; Praveen, B.; Prasanna, B.; Vishwanath, P. Electrochemical and Adsorption Studies of 4-Chloro, 8-(Trifluoromethyl) Quinoline (CTQ) for Mild Steel in Acidic Medium. J. Fail. Anal. Prev. 2020, 20, 1516–1523. [Google Scholar] [CrossRef]
- Bryan, N.S.; Alexander, D.D.; Coughlin, J.R.; Milkowski, A.L.; Boffetta, P. Ingested nitrate and nitrite and stomach cancer risk: An updated review. Food Chem. Toxicol. 2012, 50, 3646–3665. [Google Scholar] [CrossRef]
- Praveen, B.M.; Alhadhrami, A.; Prasanna, B.M.; Hebbar, N.; Prabhu, R. Anti-Corrosion Behavior of Olmesartan for Soft-Cast Steel in 1 mol dm−3 HCl. Coatings 2021, 11, 965. [Google Scholar] [CrossRef]
- Lin, B.; Zuo, Y. Corrosion inhibition of carboxylate inhibitors with different alkylene chain lengths on carbon steel in an alkaline solution. RSC Adv. 2019, 9, 7065–7077. [Google Scholar] [CrossRef] [Green Version]
- Wysocka, J.; Cieslik, M.; Krakowiak, S.; Ryl, J. Carboxylic acids as efficient corrosion inhibitors of aluminium alloys in alkaline media. Electrochim. Acta 2018, 289, 175–192. [Google Scholar] [CrossRef]
- Praveen, B.; Prasanna, B.; Mallikarjuna, N.; Jagadeesh, M.; Hebbar, N.; Rashmi, D. Investigation of anticorrosive behaviour of novel tert-butyl 4-[(4-methyl phenyl) carbonyl] piperazine-1-carboxylate for carbon steel in 1M HCl. Heliyon 2021, 7, e06090. [Google Scholar] [CrossRef]
- Faisal, M.; Saeed, A.; Shahzad, D.; Abbas, N.; Larik, F.A.; Channar, P.A.; Fattah, T.A.; Khan, D.M.; Shehzadi, S.A. General properties and comparison of the corrosion inhibition efficiencies of the triazole derivatives for mild steel. Corros. Rev. 2018, 36, 507–545. [Google Scholar] [CrossRef] [Green Version]
- Marzorati, S.; Verotta, L.; Trasatti, S.P. Green Corrosion Inhibitors from Natural Sources and Biomass Wastes. Molecules 2018, 24, 48. [Google Scholar] [CrossRef] [Green Version]
- Zeino, A.; Abdulazeez, I.; Khaled, M.; Jawish, M.W.; Obot, I.; Alhooshani, K. Mechanistic study of polyepoxy succinic acid (PESA) as green corrosion inhibitor on carbon steel in aerated NaCl Solution. Mater. Today Commun. 2021, 29, 102848. [Google Scholar] [CrossRef]
- Azamian, I.; Allahkaram, S.; Johari, M.; Teymouri, F. Interfacial Interaction Study of EDTA with the Defect Structure of Fe 3-δ O 4 Passive Film in an Aggressive Alkaline Medium Based on the Lattice Theory of Point Defects. RSC Adv. 2022, 12, 3524–3541. [Google Scholar] [CrossRef]
- Teymouri, F.; Allahkaram, S.R.; Shekarchi, M.; Azamian, I.; Johari, M. A comprehensive study on the inhibition behaviour of four carboxylate-based corrosion inhibitors focusing on efficiency drop after the optimum concentration for carbon steel in the simulated concrete pore solution. Constr. Build. Mater. 2021, 296, 123702. [Google Scholar] [CrossRef]
- Lashgari, S.M.; Bahlakeh, G.; Ramezanzadeh, B. Detailed theoretical DFT computation/molecular simulation and electrochemical explorations of Thymus vulgaris leave extract for effective mild-steel corrosion retardation in HCl solution. J. Mol. Liq. 2021, 335, 115897. [Google Scholar] [CrossRef]
- Zhang, Z.; Ba, H.; Wu, Z.; Zhu, Y. The inhibition mechanism of maize gluten meal extract as green inhibitor for steel in concrete via experimental and theoretical elucidation. Constr. Build. Mater. 2018, 198, 288–298. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2016.
- Zhang, Q.; Jiang, Z.; Li, Y.; Wang, X.; Xiong, W.; Liu, H.; Zhang, G. In-Depth Insight into the Inhibition Mechanism of the Modified and Combined Amino Acids Corrosion Inhibitors: “Intramolecular Synergism” vs. “Intermolecular Synergism”. Chem. Eng. J. 2022, 437, 135439. [Google Scholar] [CrossRef]
- Al Zoubi, W.; Ko, Y.G. Self-assembly of hierarchical N-heterocycles-inorganic materials into three-dimensional structure for superior corrosion protection. Chem. Eng. J. 2018, 356, 850–856. [Google Scholar] [CrossRef]
- Ouakki, M.; Galai, M.; Rbaa, M.; Abousalem, A.; Lakhrissi, B.; Rifi, E.; Cherkaoui, M. Quantum chemical and experimental evaluation of the inhibitory action of two imidazole derivatives on mild steel corrosion in sulphuric acid medium. Heliyon 2019, 5, e02759. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Mortier, W.J. The use of global and local molecular parameters for the analysis of the gas-phase basicity of amines. J. Am. Chem. Soc. 1986, 108, 5708–5711. [Google Scholar] [CrossRef]
- Hsissou, R. Review on epoxy polymers and its composites as a potential anticorrosive coatings for carbon steel in 3.5% NaCl solution: Computational approaches. J. Mol. Liq. 2021, 336, 116307. [Google Scholar] [CrossRef]
- Hsissou, R.; Abbout, S.; Benhiba, F.; Seghiri, R.; Safi, Z.; Kaya, S.; Briche, S.; Serdaroğlu, G.; Erramli, H.; Elbachiri, A.; et al. Insight into the Corrosion Inhibition of Novel Macromolecular Epoxy Resin as Highly Efficient Inhibitor for Carbon Steel in Acidic Mediums: Synthesis, Characterization, Electrochemical Techniques, AFM/UV–Visible and Computational Investigations. J. Mol. Liq. 2021, 337, 116492. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, J.; Zhao, W.; Wang, C.; Wu, B.; Lu, G. Investigating the anti-corrosion behaviors of the waterborne epoxy composite coatings with barrier and inhibition roles on mild steel. Prog. Org. Coat. 2019, 133, 8–18. [Google Scholar] [CrossRef]
- Javidparvar, A.A.; Naderi, R.; Ramezanzadeh, B. Epoxy-polyamide nanocomposite coating with graphene oxide as cerium nanocontainer generating effective dual active/barrier corrosion protection. Compos. Part B Eng. 2019, 172, 363–375. [Google Scholar] [CrossRef]
- Kornblum, N.; Blackwood, R.K.; Mooberry, D.D. The Reaction of Aliphatic Nitro Compounds with Nitrite Esters1,2. J. Am. Chem. Soc. 1956, 78, 1501–1504. [Google Scholar] [CrossRef]
- Wang, Q.; Fu, S.; Yang, X.; Sun, Q.; Zhang, J.; Peng, Y.; Zhang, R.; Liang, Z.; Li, J. An imide-based organic polymer as an inhibitor for HRB400 steel in simulated concrete pore solution: Experimental and theoretical calculations. J. Mol. Struct. 2022, 1265, 133426. [Google Scholar] [CrossRef]
- Qiu, S.; Li, W.; Zheng, W.; Zhao, H.; Wang, L. Synergistic Effect of Polypyrrole-Intercalated Graphene for Enhanced Corrosion Protection of Aqueous Coating in 3.5% NaCl Solution. ACS Appl. Mater. Interfaces 2017, 9, 34294–34304. [Google Scholar] [CrossRef]
- Obot, I.; Macdonald, D.; Gasem, Z. Density functional theory (DFT) as a powerful tool for designing new organic corrosion inhibitors. Part 1: An overview. Corros. Sci. 2015, 99, 1–30. [Google Scholar] [CrossRef]
- Peme, T.; Olasunkanmi, L.O.; Bahadur, I.; Adekunle, A.S.; Kabanda, M.M.; Ebenso, E.E. Adsorption and Corrosion Inhibition Studies of Some Selected Dyes as Corrosion Inhibitors for Mild Steel in Acidic Medium: Gravimetric, Electrochemical, Quantum Chemical Studies and Synergistic Effect with Iodide Ions. Molecules 2015, 20, 16004–16029. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Xu, L.; Wang, W.; Lin, Z.; Li, X. Synthesis and characterisation of composite nanoparticles of mesoporous silica loaded with inhibitor for corrosion protection of Cu-Zn alloy. Corros. Sci. 2017, 120, 139–147. [Google Scholar] [CrossRef]
- Ansari, K.R.; Quraishi, M.A.; Singh, A.; Ramkumar, S.; Obote, I.B. Corrosion inhibition of N80 steel in 15% HCl by pyrazolone derivatives: Electrochemical, surface and quantum chemical studies. RSC Adv. 2016, 6, 24130–24141. [Google Scholar] [CrossRef]
- Umoren, S.A.; Gasem, Z.M.; Obot, I.B. Natural Products for Material Protection: Inhibition of Mild Steel Corrosion by Date Palm Seed Extracts in Acidic Media. Ind. Eng. Chem. Res. 2013, 52, 14855–14865. [Google Scholar] [CrossRef]
- Peimani, A.; Nasr-Esfahani, M. Application of Anise Extract for Corrosion Inhibition of Carbon Steel in CO2 Saturated 3.0% NaCl Solution. Prot. Met. Phys. Chem. Surfaces 2018, 54, 122–134. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.; Quraishi, M.; Lgaz, H.; Lin, Y. Synthesis and investigation of pyran derivatives as acidizing corrosion inhibitors for N80 steel in hydrochloric acid: Theoretical and experimental approaches. J. Alloys Compd. 2018, 762, 347–362. [Google Scholar] [CrossRef]
- Ituen, E.B.; Akaranta, O.; Umoren, S.A. N-acetyl cysteine based corrosion inhibitor formulations for steel protection in 15% HCl solution. J. Mol. Liq. 2017, 246, 112–118. [Google Scholar] [CrossRef]
- Singh, P.; Chauhan, D.; Chauhan, S.; Singh, G.; Quraishi, M. Chemically modified expired Dapsone drug as environmentally benign corrosion inhibitor for mild steel in sulphuric acid useful for industrial pickling process. J. Mol. Liq. 2019, 286, 110903. [Google Scholar] [CrossRef]
- El Aatiaoui, A.; Koudad, M.; Chelfi, T.; Erkan, S.; Azzouzi, M.; Aouniti, A.; Savaş, K.; Kaddouri, M.; Benchat, N.; Oussaid, A. Experimental and Theoretical Study of New Schiff Bases Based on Imidazo (1, 2-a) Pyridine as Corrosion Inhibitor of Mild Steel in 1M HCl. J. Mol. Struct. 2021, 1226, 129372. [Google Scholar] [CrossRef]
- Domínguez-Crespo, M.; García-Murillo, A.; Torres-Huerta, A.; Carrillo-Romo, F.; Onofre-Bustamante, E.; Yáñez-Zamora, C. Characterization of ceramic sol–gel coatings as an alternative chemical conversion treatment on commercial carbon steel. Electrochim. Acta 2009, 54, 2932–2940. [Google Scholar] [CrossRef]
- Myung, N.V.; Park, D.-Y.; Yoo, B.-Y.; Sumodjo, P.T. Development of electroplated magnetic materials for MEMS. J. Magn. Magn. Mater. 2003, 265, 189–198. [Google Scholar] [CrossRef]
- Zhang, Z.; Ba, H.; Wu, Z. Sustainable corrosion inhibitor for steel in simulated concrete pore solution by maize gluten meal extract: Electrochemical and adsorption behavior studies. Constr. Build. Mater. 2019, 227, 117080. [Google Scholar] [CrossRef]
- Fernandes, C.M.; Alvarez, L.X.; dos Santos, N.E.; Barrios, A.C.M.; Ponzio, E.A. Green synthesis of 1-benzyl-4-phenyl-1H-1,2,3-triazole, its application as corrosion inhibitor for mild steel in acidic medium and new approach of classical electrochemical analyses. Corros. Sci. 2019, 149, 185–194. [Google Scholar] [CrossRef]
- Madkour, L.H.; Kaya, S.; Obot, I.B. Computational, Monte Carlo simulation and experimental studies of some arylazotriazoles (AATR) and their copper complexes in corrosion inhibition process. J. Mol. Liq. 2018, 260, 351–374. [Google Scholar] [CrossRef]
- Bellal, Y.; Benghanem, F.; Keraghel, S. A new corrosion inhibitor for steel rebar in concrete: Synthesis, electrochemical and theoretical studies. J. Mol. Struct. 2020, 1225, 129257. [Google Scholar] [CrossRef]
- Badiea, A.M.; Mohana, K.N. Effect of fluid velocity and temperature on the corrosion mechanism of low carbon steel in industrial water in the absence and presence of 2-hydrazino benzothiazole. Korean J. Chem. Eng. 2008, 25, 1292–1299. [Google Scholar] [CrossRef]
- Tawfik, S.M. Ionic liquids based gemini cationic surfactants as corrosion inhibitors for carbon steel in hydrochloric acid solution. J. Mol. Liq. 2016, 216, 624–635. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B. Green Eucalyptus leaf extract: A potent source of bio-active corrosion inhibitors for mild steel. Bioelectrochemistry 2019, 130, 107339. [Google Scholar] [CrossRef]
- Belghiti, M.; Echihi, S.; Dafali, A.; Karzazi, Y.; Bakasse, M.; Elalaoui-Elabdallaoui, H.; Olasunkanmi, L.; Ebenso, E.; Tabyaoui, M. Computational simulation and statistical analysis on the relationship between corrosion inhibition efficiency and molecular structure of some hydrazine derivatives in phosphoric acid on mild steel surface. Appl. Surf. Sci. 2019, 491, 707–722. [Google Scholar] [CrossRef]
- Madkour, L.H.; Kaya, S.; Guo, L.; Kaya, C. Quantum chemical calculations, molecular dynamic (MD) simulations and experimental studies of using some azo dyes as corrosion inhibitors for iron. Part 2: Bis–azo dye derivatives. J. Mol. Struct. 2018, 1163, 397–417. [Google Scholar] [CrossRef]
- Lukovits, I.; Kálmán, E.; Zucchi, F. Corrosion Inhibitors—Correlation between Electronic Structure and Efficiency. Corrosion 2001, 57, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Kokalj, A. Is the analysis of molecular electronic structure of corrosion inhibitors sufficient to predict the trend of their inhibition performance. Electrochim. Acta 2010, 56, 745–755. [Google Scholar] [CrossRef]
- Ghailane, T.; Balkhmima, R.; Ghailane, R.; Souizi, A.; Touir, R.; Touhami, M.E.; Marakchi, K.; Komiha, N. Experimental and theoretical studies for mild steel corrosion inhibition in 1M HCl by two new benzothiazine derivatives. Corros. Sci. 2013, 76, 317–324. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, G.; Niu, F.; Shi, Y.; Du, Z.; Lu, G.; Liu, Z. Understanding the inhibition performance of novel dibenzimidazole derivatives on Fe (110) surface: DFT and MD simulation insights. J. Mater. Res. Technol. 2022, 17, 211–222. [Google Scholar] [CrossRef]




| Parameter | Description |
|---|---|
| Molecular Structure |  |
| Appearance | White powder |
| pH (1% water solution) | 11 |
| Molecular formula | C8H7NNa4O8 |
| Molecular weight | 337.10200 |
| Inhibitor | C (mg/L) | Rs (Ω cm2) | Rf (Ω cm2) | Y1 (×10−5 sn·ohm−1·cm−2) | n1 | Rct (Ω cm2) | Y2 (×10−5sn·ohm−1· cm−2) | n2 | Cdl (×10−5 F) | IEEIS% |
|---|---|---|---|---|---|---|---|---|---|---|
| Blank | 0 | 1.49 | 697.90 | 8.99 | 0.89 | 957.90 | 231.19 | 0.56 | 66.81 | — |
| IDS | 50 | 1.91 | 900.30 | 7.74 | 0.79 | 1948.00 | 65.93 | 0.36 | 17.80 | 50.83 |
| 100 | 1.53 | 3024.00 | 11.62 | 0.88 | 7254.00 | 14.37 | 0.24 | 14.41 | 86.79 | |
| 150 | 2.80 | 6388.00 | 6.25 | 0.86 | 17,933.00 | 9.99 | 0.43 | 16.99 | 94.66 | |
| 200 | 3.78 | 7193.00 | 7.95 | 0.97 | 29,866.00 | 6.78 | 0.50 | 13.51 | 96.79 |
| Inhibitor | C (mg/L) | Ecorr (V) | βa (mV/Dec) | βc (mV/Dec) | Icorr (μA/cm−2) | CR (mm/year) | IETP % |
|---|---|---|---|---|---|---|---|
| Blank | 0 | −0.32441 | 92.79 | 204.88 | 22.71 | 0.263 | — |
| IDS | 50 | −0.38642 | 108.25 | 128.44 | 9.51 | 0.110 | 58.10 |
| 100 | −0.35376 | 141.31 | 183.74 | 3.27 | 0.038 | 85.62 | |
| 150 | −0.37065 | 123.69 | 117.29 | 0.71 | 0.008 | 96.86 | |
| 200 | −0.38989 | 117.36 | 98.00 | 0.39 | 0.004 | 98.30 |
| Inhibitor | C (mg/L) | IEEIS (%) | IETP (%) | Average of IE (%) |
|---|---|---|---|---|
| IDS | 50 | 50.83 | 58.10 | 54.46 |
| 100 | 86.79 | 85.62 | 86.21 | |
| 150 | 94.66 | 96.86 | 95.76 | |
| 200 | 96.79 | 98.30 | 97.54 |
| T | Inhibitor | Ecorr (V) | βa (mV/Dec) | βc (mV/Dec) | Icorr (μA/cm−2) | IETP (%) |
|---|---|---|---|---|---|---|
| 298 K | Blank | −0.32441 | 92.79 | 204.88 | 22.71 | - |
| IDS | −0.38989 | 117.36 | 98.00 | 0.39 | 98.30 | |
| 308 K | Blank | −0.32636 | 59.43 | 166.72 | 25.29 | - |
| IDS | −0.34479 | 41.39 | 72.15 | 1.64 | 93.53 | |
| 318 K | Blank | −0.34050 | 74.73 | 129.54 | 33.08 | - |
| IDS | −0.36863 | 54.93 | 57.47 | 3.98 | 87.96 | |
| 328 K | Blank | −0.36870 | 82.32 | 112.23 | 40.70 | - |
| IDS | −0.36087 | 65.61 | 56.74 | 5.62 | 86.19 |
| Inhibitor | Ea (KJ mol−1) | ΔHa (KJ mol−1) | ΔSa (J mol−1 K−1) |
|---|---|---|---|
| Blank | 16.34 | 13.75 | −173.19 |
| IDS | 73.02 | 70.42 | −14.58 |
| Descriptor | IDS |
|---|---|
| EHOMO (ev) | −5.88618 |
| ELUMO (ev) | −0.26908 |
| I.P (ev) | 5.88618 |
| E.A (ev) | 0.26908 |
| ∆E (ev) | 5.61711 |
| χ (ev) | 3.07763 |
| η (ev) | 2.80855 |
| σ (ev−1) | 0.35606 |
| ω | 1.68624 |
| ∆N | 0.69829 |
| μ (Debye) | 6.74497 |
| Atoms | fk+ | fk− | fk2 | σk+ | σk− | ∆σk | ω+ | ω− | ∆ω |
|---|---|---|---|---|---|---|---|---|---|
| 1(C) | 0.0028 | 0.0186 | −0.158 | 0.0125 | 0.0817 | −0.0692 | 0.0029 | 0.02571 | −0.02281 |
| 2(C) | 0.0014 | 0.0319 | −0.0305 | 0.006 | 0.1399 | −0.1339 | 0.00139 | 0.04410 | −0.04271 |
| 3(C) | 0.0015 | 0.02 | −0.0186 | 0.0065 | 0.088 | −0.0815 | 0.00152 | 0.02765 | −0.02613 |
| 4(C) | 0.0032 | 0.0052 | −0.002 | 0.0142 | 0.0228 | −0.0086 | 0.0033 | 0.00719 | −0.00389 |
| 5(O) | 0.0065 | 0.0609 | −0.0544 | 0.0286 | 0.2675 | −0.2389 | 0.00665 | 0.08419 | −0.07754 |
| 6(O) | −0.0026 | 0.0345 | −0.0371 | −0.0113 | 0.1516 | −0.1629 | −0.00263 | 0.04769 | −0.05032 |
| 7(O) | −0.0021 | 0.0432 | −0.0452 | −0.009 | 0.1896 | −0.1986 | −0.0021 | 0.05972 | −0.06182 |
| 8(O) | 0.0056 | 0.028 | −0.0224 | 0.0246 | 0.123 | −0.0984 | 0.00573 | 0.03871 | −0.03298 |
| 9(N) | 0.0042 | 0.1933 | −0.1891 | 0.0183 | 0.8485 | −0.8302 | 0.00426 | 0.26722 | −0.26296 |
| 10(C) | 0.0015 | 0.0216 | −0.0201 | 0.0064 | 0.0947 | −0.0883 | 0.00149 | 0.02986 | −0.02837 |
| 11(C) | 0.0034 | 0.0157 | −0.0124 | 0.0149 | 0.0691 | −0.0542 | 0.00347 | 0.02170 | −0.01823 |
| 12(C) | 0.0041 | 0.0084 | −0.0042 | 0.0182 | 0.0367 | −0.0185 | 0.00423 | 0.01161 | −0.00738 |
| 13(C) | 0.0016 | 0.0228 | −0.0213 | 0.007 | 0.1003 | −0.0933 | 0.00162 | 0.03152 | −0.02990 |
| 14(O) | 0.0085 | 0.0326 | −0.0242 | 0.0372 | 0.1433 | −0.1061 | 0.00865 | 0.04507 | −0.03642 |
| 15(O) | −0.0038 | 0.0257 | −0.0295 | −0.0165 | 0.1128 | −0.1293 | −0.00385 | 0.03553 | −0.03938 |
| 16(O) | −0.0032 | 0.0791 | −0.0823 | −0.0139 | 0.3472 | −0.3611 | −0.00325 | 0.10935 | −0.11260 |
| 17(O) | 0.003 | 0.0395 | −0.0364 | 0.0133 | 0.1732 | −0.1599 | 0.00311 | 0.05460 | −0.05149 |
| 25(Na) | 0.2344 | 0.0435 | 0.1908 | 1.029 | 0.1911 | 0.8379 | 0.23956 | 0.06013 | 0.17943 |
| 26(Na) | 0.2276 | 0.0405 | 0.1871 | 0.9992 | 0.1778 | 0.8214 | 0.23264 | 0.05599 | 0.17665 |
| 27(Na) | 0.2197 | 0.0381 | 0.1817 | 0.9647 | 0.1672 | 0.7975 | 0.2246 | 0.05267 | 0.17193 |
| 28(Na) | 0.2611 | 0.0427 | 0.2184 | 1.1463 | 0.1874 | 0.9589 | 0.26689 | 0.05903 | 0.20786 |
| Etotal (kcal/mol) | Esurface (kcal/mol) | Einhibitor (kcal/mol) | Eads (kcal/mol) |
|---|---|---|---|
| −55,044.77 | −54,499.59 | −472.04 | −73.14 |
| Inhibitor | 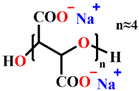 | 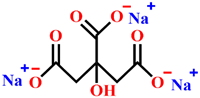 | 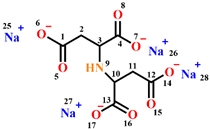 |
| Metal | mild steel | Q235 carbon steel | Q235 carbon steel |
| Corrosive media | Aerated 3% NaCl solution | 0.5 M NaCl simulated concrete pore solution | 3.5% NaCl simulated concrete pore solution |
| Optimum concentration | 2 mg/L Zn+ + 2 mg/L PESA | 0.1 M | 200 mg/L |
| Protection efficiency | 92% | 91% | 97.54% |
| References | [13] | [15] | In this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, S.; Yang, X.; Peng, Y.; Wang, Q.; Sun, Q.; Zhang, J.; Wang, X.; Liang, Z.; Li, J. Corrosion Behaviors of Tetrasodium Iminodisuccinate (IDS) as an Environmentally Friendly Inhibitor: Experimental and Theoretical Studies. Coatings 2023, 13, 613. https://doi.org/10.3390/coatings13030613
Fu S, Yang X, Peng Y, Wang Q, Sun Q, Zhang J, Wang X, Liang Z, Li J. Corrosion Behaviors of Tetrasodium Iminodisuccinate (IDS) as an Environmentally Friendly Inhibitor: Experimental and Theoretical Studies. Coatings. 2023; 13(3):613. https://doi.org/10.3390/coatings13030613
Chicago/Turabian StyleFu, Shaopeng, Xingyao Yang, Yichun Peng, Qi Wang, Qinghao Sun, Junwei Zhang, Xinping Wang, Zezhou Liang, and Jianfeng Li. 2023. "Corrosion Behaviors of Tetrasodium Iminodisuccinate (IDS) as an Environmentally Friendly Inhibitor: Experimental and Theoretical Studies" Coatings 13, no. 3: 613. https://doi.org/10.3390/coatings13030613
APA StyleFu, S., Yang, X., Peng, Y., Wang, Q., Sun, Q., Zhang, J., Wang, X., Liang, Z., & Li, J. (2023). Corrosion Behaviors of Tetrasodium Iminodisuccinate (IDS) as an Environmentally Friendly Inhibitor: Experimental and Theoretical Studies. Coatings, 13(3), 613. https://doi.org/10.3390/coatings13030613