1. Introduction
Cleaning works of art is a complicated task that requires an understanding of both the surface to be cleaned and the materials to be used for cleaning. Today, the use of wet cleaning methods with water, solvents, or, if needed, specific active ingredients, are established as a standard methodology in conservation [
1]. However, there are three main inconveniences arising from the use of wet cleaning methodologies: firstly, not all of the surfaces are water/solvent resistant; for example, in paints, the interaction with water or solvent might lead to swelling and/or leaching of organic components [
2]. Secondly, these types of methods often require a swab to place and remove the cleaning agent, thus inducing some mechanical damage on the surface. Finally, some solvents can present health- and environmental-related problems due to their toxicity. The former is an inconvenience from the health point of view, and also introduces complications regarding the logistics of the intervention.
To maintain further control of the cleaning agent release and perform gentler interventions, scientists and restorers have focused on the use of soft matter cleaning methodologies [
3,
4,
5,
6]. These methods allow for the safe transportation of organic solvents to be released only at the targeted surfaces. Selective release is a great advantage of soft materials compared to ordinary cleaning methods because (a) their selective action allows for a reduction in the amount of solvents and surfactant molecules used, and (b) the entrapment of solvents by reduction of the rate of evaporation has a big impact on the reduced toxicity and safety for the operators.
Gels, in particular, offer the main advantage of controlling water release and reducing the mechanical action to the surface. Given their rheological properties, gels can ensure good contact with the surface and the reduction of residues after the cleaning process [
7]. Moreover, gels can be designed to target specific substances to be removed from the surface [
4,
8,
9], for example, the removal of soiling particles [
6] and varnishes [
10]. Additionally, these soft materials allow specific functionalization to ensure the complete removal of the gel [
11].
Although lab-synthesized soft materials for cleaning offer many advantages, some of them (or their by-products) have one or a few inconveniences, namely: are hazardous, produce waste that is difficult to dispose, or require precursors that are harmful for the environment. Additionally, these materials might require higher user-safety considerations. Furthermore, some of these materials are complex to synthesize and/or expensive. Therefore, it is necessary to promote the usage of sustainable, safe, and economic cleaning methodologies that match the restoration’s objectives and needs.
Among the natural soft materials, agar
gel has been successfully implemented as a
green, cheap, and operator-safe methodology for the cleaning of diverse surfaces including historic buildings, sculptures, and paintings [
12,
13,
14,
15,
16,
17,
18]. Agar
gel is appreciated by many restorers due to its capacity to control the water release onto the surface of interest. Given its reduced price, agar is often used in cleaning campaigns that require large quantities of material. Furthermore, agar minimizes the remaining residues over the surface after its removal, and it can be combined with surfactants that act as hydrophobic and lipophilic to remove specific compounds (for example, it is quite common among conservators, to include the commercial product Tween20
®, a nonionic surfactant of the polysorbate family) [
19,
20]. Additionally, it can be used together with laser cleaning, improving color saturation of the surface, temperature control, and cleaning efficiency [
21]. Some of the limitations of agar
gel include its usage on extremely water sensitive materials, since they cannot tolerate the water released from the agar. Moreover, agar preferably should be prepared the day of the intervention to avoid the development of microorganisms.
The exact details of the cleaning mechanism of agar
gel are not completely known, and have mainly been studied on stone porous materials [
20,
22,
23]. However, it is known that an absorption–diffusion mechanism of the free water takes place through capillarity moving from the agar
gel to the substrate. The former is followed by the reabsorption of water to the agar
gel matrix [
24]. However, in this second step, the free water returns to the
gel containing the substances removed from the substrate. This process could be driven by counter diffusion of ions into the
gel matrix (slow rate) or by counter absorption due to the minor capillary radius (fast rate). The water release (and absorption) capacity of the agar
gel decreases with increasing agar concentration, since water mobility decreases with higher density of the polymeric network. In turn, agar
gel at 1% concentration behaves as a free water source, whereas at 5%, it offers the highest control on water transport [
20]. Concentration values above 5% are normally not used.
Finally, the agar
gel is a thermoreversible material; this property makes agar a versatile method for application into surfaces. Specifically, agar will maintain a random coil formation at high temperatures (above 35–40 °C), this is the
sol state. When the temperature of the agar is below the abovementioned range, the polysaccharide chains will reorganize into a double helix structure held together by hydrogen bonds and Van der Waals forces, forming a gel [
20,
25]. Furthermore, agar can be used on a variety of surfaces with diverse shapes and orientations. The three main forms of application are:
sol (its fluid form), on preformed
gel blocks, and
unstructured (obtained through milling the rigid gel blocks), each with its own advantages depending on the specifications of the object to be cleaned [
20,
26]. For example, the
sol form is mainly used in complex surface morphologies that can tolerate a slight adhesion of the
gel and temperatures around 40 °C. Moreover, it is evident from the literature [
20] that this form contains the highest amount of free water, thus allowing for a higher efficacy of cleaning. On the other hand, both
unstructured and rigid
gel blocks are suitable for delicate surfaces (a surface with intrinsic or induced matrix decohesion) thanks to their lower adhesion given by the reduced mobility of the macromolecules in the double helix conformation. Additionally, unstructured agar is sometimes used in the presence of nonpolar solvents, according to the specific needs of the restorers. The drawback of both (rigid gel and
unstructured agar) is related to the lower soiling removal.
In recent years, a new form of application of agar has been proposed and is rapidly becoming popular among conservators: agar foam generated by insufflating gasses in the sol phase (agar foam). The latter appears to have some advantages over the traditional agar applications, displaying a gentler action on very delicate surfaces and improving the ease of application. Moreover, the use of thermal isolating foaming chamber allows for the agar to be retained at stable temperature for longer. However, up to now, there have only been practical notes regarding the usage of agar foams, and no in-depth studies about their properties and behavior have been conducted. Consequently, the aim of the present work is to study the differences in the material properties and cleaning effectiveness amongst three agar systems: agar sol-gel, agar CO2 foam, and agar N2O foam. This was done through the study of the rheological properties and water release of these materials, together with the study of the cleaning effectiveness in laboratory conditions, and real case studies: a historical gypsum cast from the internal porch framing of the Abbey of Nonantola, and the 20th century gypsum cast of the Pietà Rondanini by Michelangelo, located in the Sforza Castle in Milan.
3. Results and Discussion
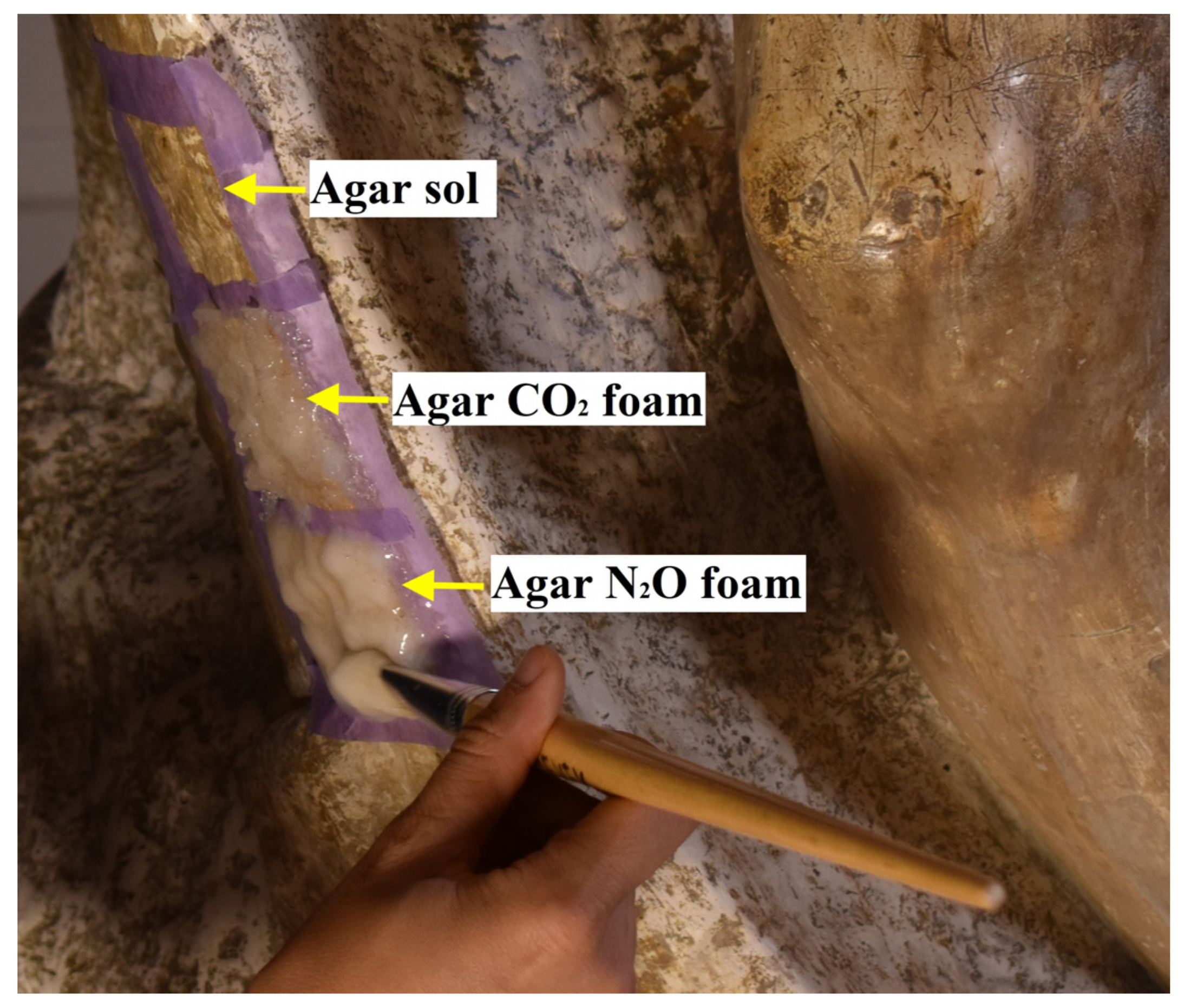
Agar foam is applied in the same manner as agar
sol, meaning while it is still fluid, allowing the operator to spread the foam along the surface to be cleaned. However, unlike agar
sol, which appears as a transparent film when applied, both agar foams have a whitish color due to the high light scattering of bubbles, as can be observed in
Figure 2. Over the gelification time, and due to the bubble coalescence, the amount of bubbles is reduced, and progressively, the agar foam turns transparent.
Even though both foams appear similar to the naked eye, when observed under the microscope (
Figure 3), it becomes clear that the bubble density inside each foam is different. Both foams are formed by bubbles that range between 70–420 µm in diameter; however, the amount of bubbles per unit volume is higher for CO
2 than for N
2O foam. This is consistent with the density measurements obtained for the agar
gel (1.067 ± 0.035 g/cm
3), agar CO
2 foam (0.483 ± 0.167 g/cm
3), and the agar N
2O foam (0.760 ± 0.062 g/cm
3), with CO
2 foam being the least dense material. Therefore, the amount of agar inside the CO
2 foam is the lowest for the same volume of material.
3.1. Flow Characterization
From the practical point of view, when the agar is applied to a surface in its fluid state, it is important that the material remains as much as possible in the area of interest without dripping or flowing. If the surfaces to be cleaned are oriented with a certain inclination with respect to the horizontal, naturally, the agar will tend to flow. Furthermore, the absorption–diffusion mechanism through which the cleaning takes place is favored by a good adhesion between the agar and the surface. On this basis, it is hypothesized that the reduction of flow will also have an influence on the cleaning efficacy, since the material remains in the same position of contact for longer effective time.
In order to characterize the flow behavior of the three fluid systems (agar
sol, agar CO
2, and N
2O foam), an inclined plane experiment was set. As can be observed in
Figure 4, the front velocity of the three agar systems without any inclination is practically null; actually, the only motion is the layer’s flattening under the material’s weight. For all the following degrees of inclination, as expected from the density values, a clear dominance of velocity is observed for the agar
sol. The faster flow is maintained until its matrix is gelified, hindering further movement.
In contrast with agar
sol, the agar foam systems show a lower flow velocity for all angles. In particular, it is observed that the movement is never totally impeded; however, the velocity decrease over time is faster, regardless of its onset (
Figure 4C,D). It is possible to observe that for the limit case (90°), after one-minute motion, the velocity of the systems was reduced by 42.3% for CO
2 and 22.5% for N
2O foams, and only 9.5% for the
sol.
One possible explanation of the flow reduction observed in the foams is that the gas bubbles inside the sol have a different behavior and flow differently than the sol. Bubbles apparently interfere with one another, slowing down the sol flow. Actually, these bubbles collide with each other, acting as a sort of entanglement to the free movement.
It is expected that for an increasing amount of bubbles, the velocity movement will be further impeded. This behavior is observed for both 45° and 90°, where the N2O foam, which is denser (that is, with a lower amount of bubbles), has also a lower capacity of flow-speed reduction than the CO2 foam. Slightly different is the case of an inclination of 15°; this is most probably due to the fact that at such shallow angles, the flowing effect is not so strong as to dominate over the initial velocity after pouring the samples onto the substrate.
The flowing behavior of the three systems confirms that both foams have the capacity to control the flowing movement of the agar in its fluid state. As stated before, this represents an advantage from the application point of view, since the reduction of the flow will also ease the application and reduce undesired dripping. The agar foam allows for a gentler approach towards the artworks: since the volumes of both foams are larger than that of the agar sol, when the foams are applied the layer formed by the foams is initially thicker than the one formed by the sol. Once applied, and while the material is still fluid, it is spread using a tool such as a painting brush. When using agar foams, the action of the spreading tool will naturally occur further away from the surface, acting as a sort of protective system toward the artwork surface.
3.2. Rheological Properties
The present rheological characterization of the agar systems was done through the analysis of both the storage (G′) and loss (G″) moduli. In general, the rheological behavior of a viscoelastic material can be characterized as
solid-like behavior when G′ > G″, since the material will store more elastic energy than that dissipated, whereas the dominance of G″ over G′ depicts a more viscous or
liquid-like behavior [
28].
In a polymeric solution, such as the agar one, a
gel will be formed when its constituents start to change from the random coil conformation to a double helix, which ultimately provides a network to form the
gel [
29]. This process is also referred to as
sol-gel transition [
20,
25]. In systems such as the agar, the onset of the conformation transformation (
Tsol-gel) is defined as the temperature at which G′ equals G″ during a cooling step [
30].
This change in molecular conformation continues to progress over time until a plateau is reached and no further change in G′ and G″ is observed (
Tgel). From this point on, there is a complete solidification of the material and this is no longer changing its structure. According to the datasheet of AgarArt, the gelation temperature of the
sol occurs between 38–42 °C. Moreover, in the literature, it is reported that
Tgel for agar
sols oscillates between 33 and 39 °C, depending on increasing agar concentration [
31]. However, these values also have an important dependency on the cooling rate, where a slower process will lead to a higher value of
Tgel. The origin of the raw material for the agar preparation also affects the gelification temperature (
Tgel) [
32]. Considering all the possible parameters, the general accepted range for
Tgel is 28–50 °C.
With the aim of understanding if foaming would have any effect on the
sol-gel transition temperature (
Tsol-gel) and the onset of the plateau region (
Tgel), a temperature sweep experiment (TS) was performed starting from 60 °C. In this test, the effect of temperature on both the conservative component (G′) and the loss component (G″) of the complex modulus behavior is observed for the agar
sol and the two foams (CO
2, and N
2O) (
Figure 5). It was found that for the agar
sol, the
sol-gel temperature can be observed at around 58 °C, whereas for the foams, the
sol-gel transition is never observed, because G′ > G″ at all tested temperatures, indicating that they already behave in a
solid-like manner at 60 °C.
The lack of a sol-gel transition point for both foams below 60 °C is an interesting result. It seems to indicate that even at a temperature where the agar molecules are non-structured, the material behaves as a solid, as would be expected for a material that has formed a three-dimensional network. It is hypothesized that the absence of Tsol-gel is a consequence of the formation of the agar network at a higher temperature, possibly due to the local increment in pressure inside the foaming thermal chamber.
On the other hand, Tgel was obtained by calculating the first derivative of the curves and using this criterion to determine the inflection point. Despite the differences in the sol-gel transition temperature between agar sol and the two foams, the gelification temperature (start of the plateau behavior) is observed in all three systems around 34 °C, in agreement with the datasheet (38–42 °C) and the value reported on the literature (ca. 30 °C). The present result confirms that foaming affects the conformation transformation process, but only in terms of the kinetics, and not in the temperature at which the gelification is completed. Moreover, a decrease of 20 kPa in the magnitude of the storage modulus is observed for the foams when all three systems are completely gelified.
The shear strain response was measured in order to determine the linear visco-elastic region (LVER) for the three systems. This is considered as the region in which both G′ and G″ are parallel and strain-independent. It was found that the response of the three agar systems to oscillatory strain is independent from shear strain amplitude up to a value of 10−1%, which is taken as the limit of the LVER.
Within the LVER, frequency sweep tests reported in
Figure 6 show a very limited frequency dependence of G′ for all investigated systems. This, and the dominance of G′ over G″, confirms the solid-like behavior expected for both the gel and the foams. At the mid frequency, the storage modulus, G′, is 14.27 ± 1.24 kPa in the case of
sol, 14.56 ± 2.75 kPa for CO
2 and 16.7 ± 8 kPa for N
2O foams, respectively. The values of all three systems are similar, confirming that foaming has little influence on the storage modulus of the material. It is worth observing that the value of the storage modulus (G′) of all three systems is a low value, typical of soft, easily deformable materials [
33,
34].
Unlike the behavior of G′, the loss modulus (G″) for the foams is higher than that of the gel in the investigated frequency range. This indicates a higher dissipation ability of the foams, which may originate from both a contribution of gas entrapped in bubbles and a higher mobility of the chains, and which might result in a better adhesion to the surface.
The time stability of the agar systems was studied by means of a time sweep test at constant amplitude and frequency over one hour. The results shown in
Figure 7 show a strong invariance of both G′ and G″ for the three systems over the tested period. The initial variations of the systems are related to the immediate relaxation response at the onset of the experiment. The time response of the agar
gel was as expected from the results obtained by Bertasa, et al. [
27]; however, it is important to highlight that foaming does not affect this performance.
Time stability of the systems is a desired characteristic for cleaning methodologies in the restoration framework. This becomes even more important for the mechanism of detachment of the cleaning agent. Agar is commonly used in periods of 30 min to 1 h, and its removal is performed by peeling of the layer with a movement that resembles the detachment of a thin membrane from a substrate. If the viscoelastic properties changed throughout the time of application, it could be possible that part of the material remained attached to the surface. Therefore, having a material that behaves in the same constant and predictable manner for the time of application ensures reliability of usage.
3.3. Water Release
Water release differences amongst agar systems can give insight into the cleaning process and efficacy. It is known that the amount of water released by the agar depends inversely on the concentration of the polysaccharide. The water contained by the agar gel is divided into free water (freezable) and bounded water (non-freezable). As the agar concentration increases, so does the amount of non-freezable water (which is bounded to the polymer network), and thus, the water release is reduced [
20]. In this sense, agar at low concentrations, such as 1%, acts as a free water source (c.a. 90% freezable water), whereas agar at 3% concentration has only around 80% of freezable water. However, in practice, there is a limitation: the consistency of the agar and speed of gelation increase with concentration. Therefore, a compromise between freezable water and the practical consistency of the material has to be made.
The behavior of the water release for each of the agar systems showed that at constant agar concentration, foaming slightly reduces the amount of water that is released to the surface. Nevertheless, agar
gel,
sol, and N
2O foam show very similar water release values (
Table 1). CO
2 agar foam releases a slightly lower amount of water (8.5%, as reported in
Table 1). Moreover, these results are consistent with what is reported in the literature for agar
sol and
gel, in which, the former releases the highest amount of water [
15,
24].
The observed reduction of water release can be explained through the reduction in density due to foaming, where agar CO
2 foam, being the least dense material, releases the lowest amount of water. A foam drainage effect might also be considered, where a material with lower density corresponds to a lower amount of freezable water close to the surface [
35].
As previously discussed in the present work, foaming has a relevant effect on the control of practical consistency of the agar, making it easier to handle. From the point of view of the application, the use of foam implies a better control when working on delicate surfaces. Moreover, CO2 foaming slightly influences the amount of water that is released to a surface.
3.4. Cleaning Effectiveness Evaluation
Three cleaning tests were analyzed: one reference laboratory test performed on artificially soiled gypsum specimens and two case studies with different surface conditions. All the cleaning tests were performed with the reference agar
sol/gel and the two foams in the same conditions (30-min cleaning). Cleaning effectiveness was evaluated through color measurements aimed to assess the total color difference achieved in the different tests (
Figure 8).
The laboratory cleaning experiments highlight that the three systems perform similarly in terms of their cleaning effectiveness. When observed more in depth, both agar sol/gel and N2O foam have the same behavior, in accordance with the water release testing. On the other hand, CO2 foam shows a slightly higher ∆E, despite the lower water release.
In the case of the cast of the Pietà Rondanini (
Figure 8), it is possible to observe that the agar
sol/gel has the highest effect on ∆
E, followed closely by the CO
2, and finally, by the N
2O foam. This was clearly evident upon visual observation on site (
Figure 9). On the other hand, the cleaning behavior on the historical gypsum cast of the porch framing of the Nonantola Abbey shows the complete reversed order of ∆
E, with the N
2O foam providing the highest cleaning effect. This difference between the results of the laboratory testing and the real case studies highlights the relevance of the nature and characteristics of the layers to be removed. The cast of the Pietà Rondanini and the historical gypsum cast from the porch framing of the Nonantola Abbey have very different surface layers. Micro-FTIR investigations (
Figure 10b) allowed for a characterization of the surface patination over the Pietà cast, showing organic residues of an intentional treatment with beeswax (red regions in the
Figure 10b) and silicon-based detaching agents (1260, 1020, 870, and 800 cm
−1). As regards the Nonantola Abbey historical gypsum cast (
Figure 10a), micro-FITR revealed that the surface deposit is composed of inorganic minerals coming from the environment: mainly feldspars, aluminosilicates (1355, 667 and 597 cm
−1), and quartz. Therefore, the adhesion and solubility of these two different surface mixtures is quite different.
The most coherent and adherent surface layer present on the Pietà cast is better removed by the agar
sol/gel. While the less coherent deposit present in the Nonantola Abbey gypsum cast is better cleaned by N
2O foam. This correlates with the rheological properties, and specifically with the difference in G″ values reported in
Figure 6, where it is demonstrated that the agar N
2O foam shows the highest
viscous behavior.
It is worth mentioning that in real-case applications, it is important to control the cleaning process as much as possible. The obtained results showed that thanks to the different properties of the three agar systems, it is possible to tune the cleaning effects according to the nature and level of soiling. Restorers have a great sensibility in selecting specific methodology according to the surface and the desired cleaning level. Despite the common chemical nature of the three systems, the rheological properties modified by the foaming process thus allow for a differentiation of the cleaning effectiveness.
4. Conclusions
The main objective of this work was to characterize and compare the material properties and cleaning effectiveness of three agar systems: sol/gel, CO2 foam, and N2O foam.
As expected, microscopy and density measurements showed that foaming reduces the density with respect to the conventional agar. In particular, CO2 foaming produces a material with a higher amount of bubbles and a lower density, whereas N2O provides a material with a moderate density reduction.
By means of the rheological test, it was possible to confirm that the gelification temperature remained unmodified for all three agar systems (33 °C), while the onset of sol-gel transition increased for the foams. Moreover, it is noteworthy to point out that the foams, when applied fresh, flow with a reduced velocity, thus allowing for better control and ease of application. Once gelified, the foams act as a soft solid-like material, as shown by rheological studies. Finally, agar foams, as the reference agar sol/gel, show a stable behavior for the typical time of application (30–60 min).
As concerns the water release, the laboratory test showed that the agar N2O foam and the sol/gel behave similarly, while CO2 foam releases a slightly lower amount of water.
Finally, the cleaning effectiveness was evaluated by colorimetric measurements on both artificially soiled gypsum samples and historical gypsum case studies. Results showed that in laboratory cleaning experiments, the three systems perform similarly. However, in real cases, the behavior of foams highly depends on the nature of the surface and soiling. For instance, the presence of organic materials, such as waxes, strongly affects the interaction with water, and therefore, the effectiveness of the cleaning. The availability of the three agar systems allows restorers to tune the desired cleaning effect.
To summarize, although the selection of a cleaning methodology depends on the specific case at hand, agar foam is a good cleaning system that is ideal for delicate surfaces with geometries and orientations that represent a challenge for conventional cleaning methods. Agar foam is more likely to remain in the application area and allow the water diffusion to remain active for longer. From the practical point of view, when the restorers apply the agar foam they use a lower amount of active material than with the conventional agar methodologies. The former has a direct impact in the cost of the intervention and the supply of material required. Additionally, the use of the thermally insulating chamber permits the maintainance of the precursor agar sol at an adequate temperature for longer periods of time. In this sense, the ability to control better the application temperature allows for the use of a larger spectrum of active ingredients.