Direct Liquid Reactor-Injector of Nanoparticles: A Safer-by-Design Aerosol Injection for Nanocomposite Thin-Film Deposition Adapted to Various Plasma-Assisted Processes
Abstract
:1. Introduction
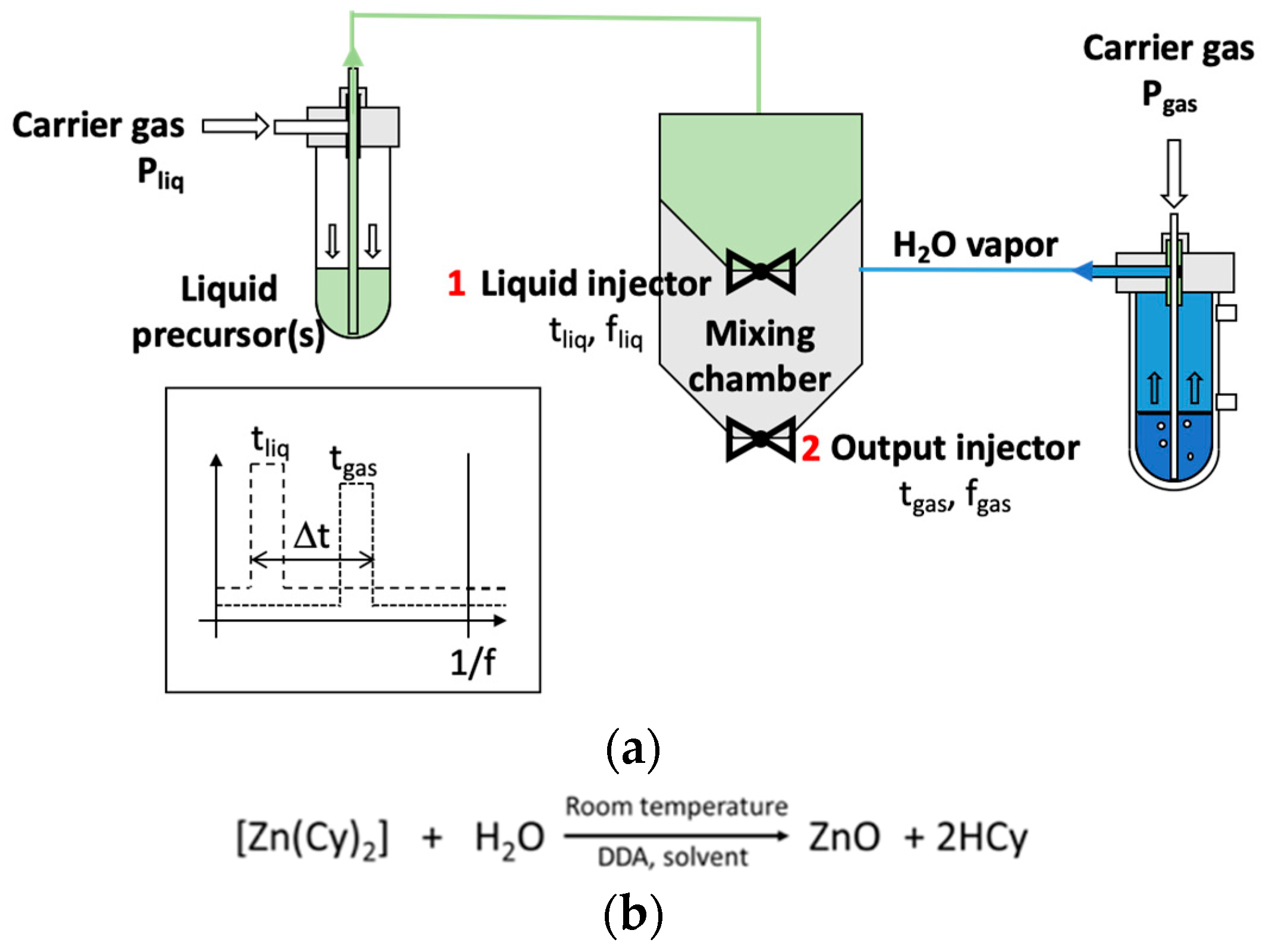
2. Materials and Methods
3. Results
3.1. DLRI in a Low-Pressure Plasma Process
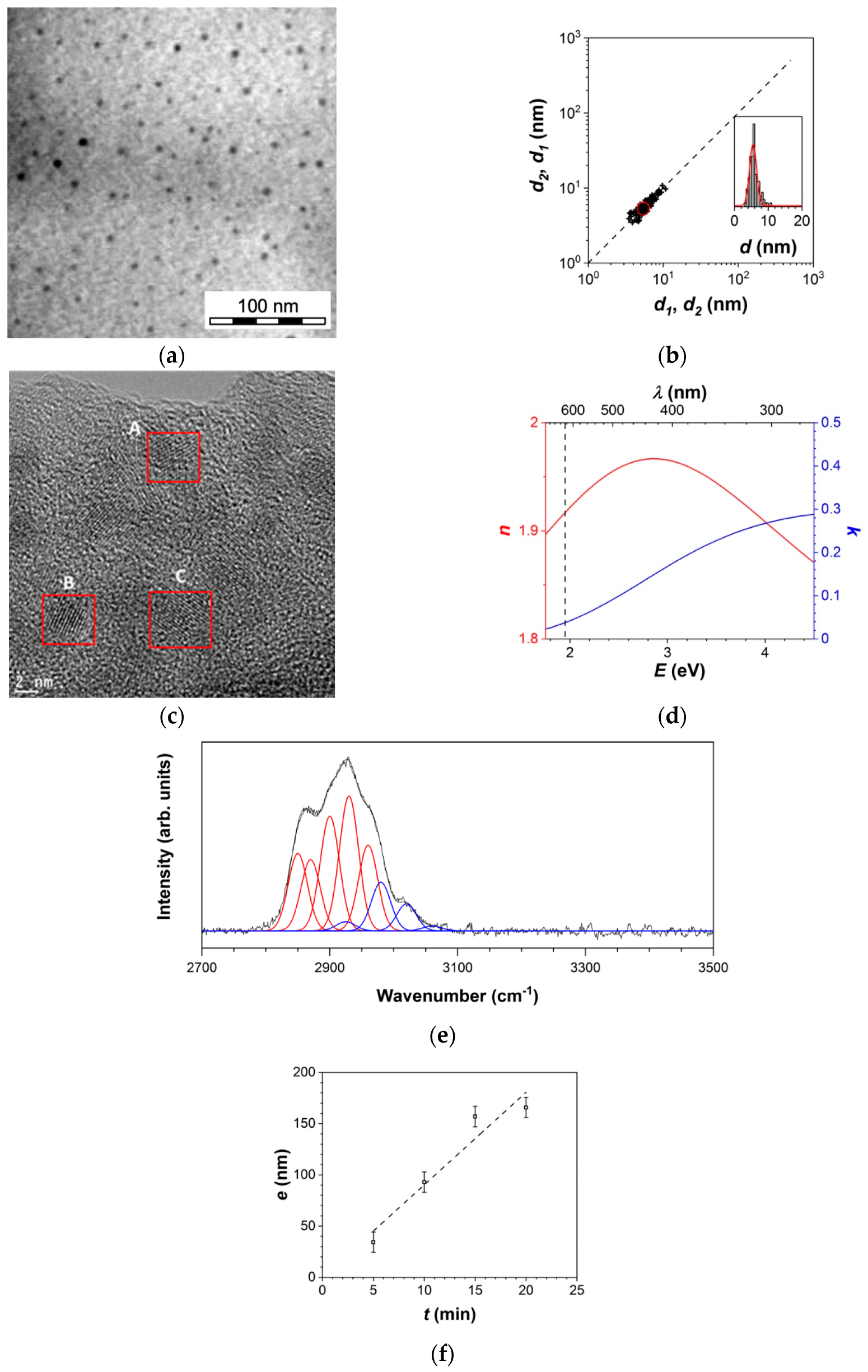
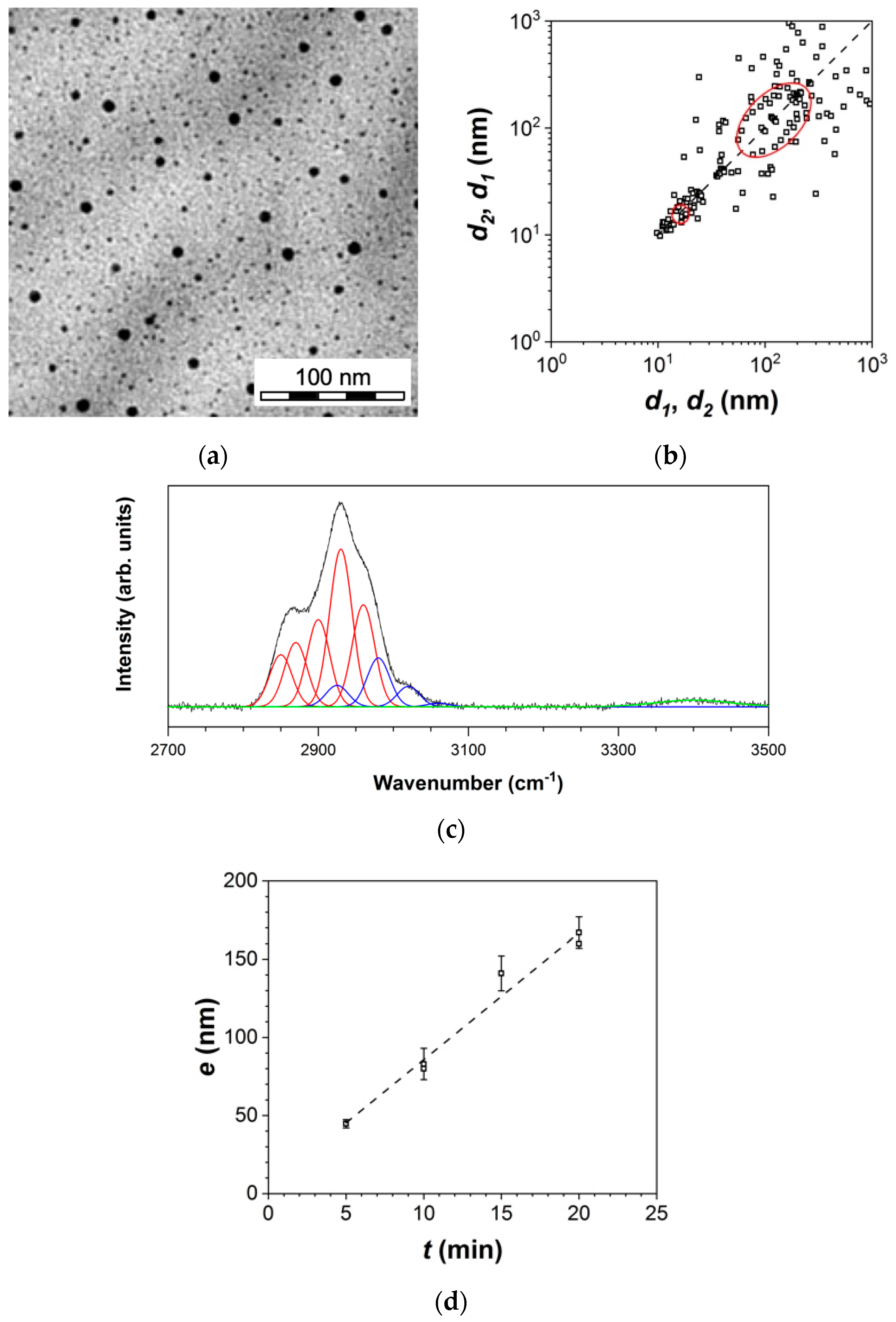
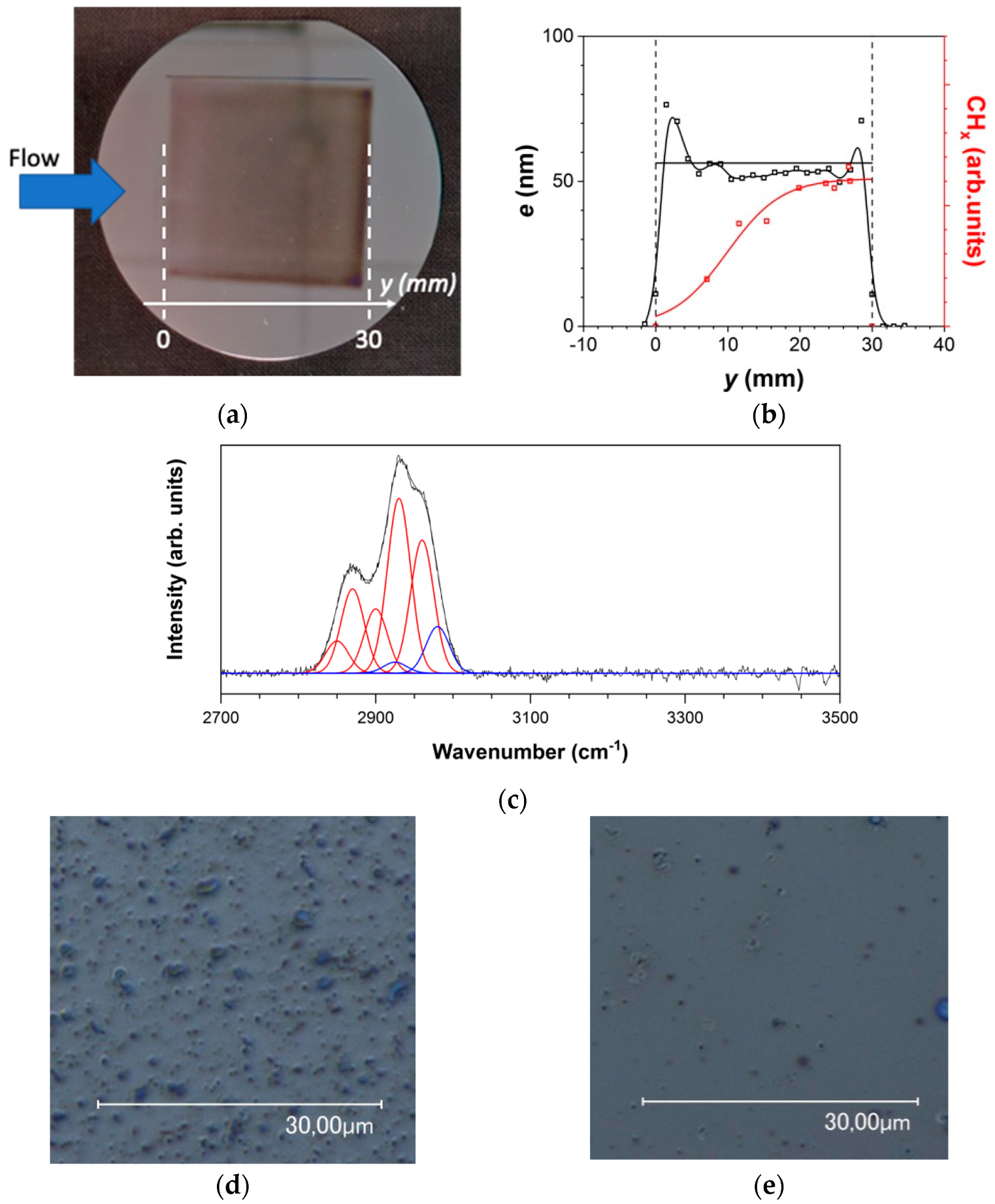
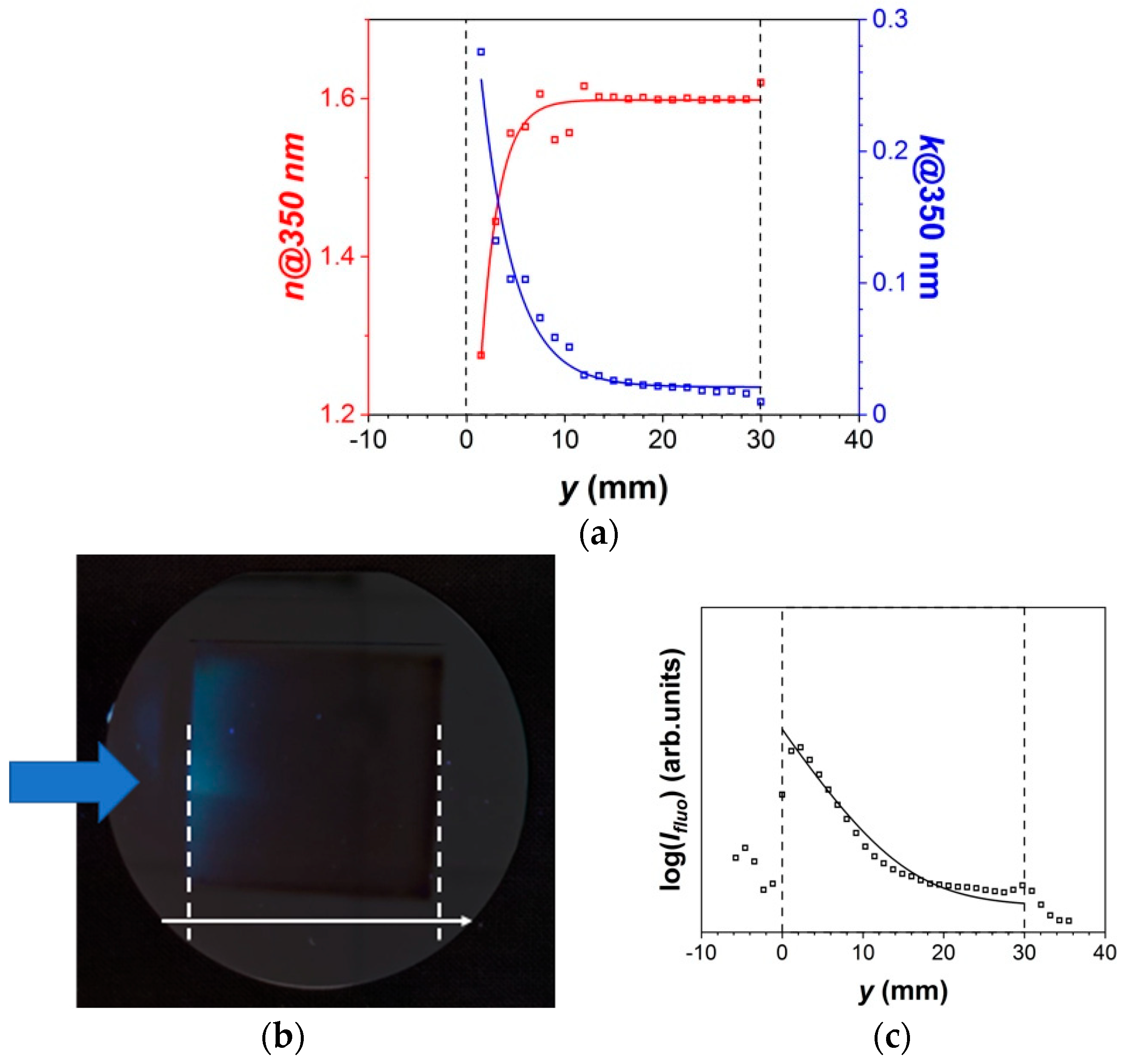
3.1.1. Thin Film Characteristics
3.1.2. Mass Balance
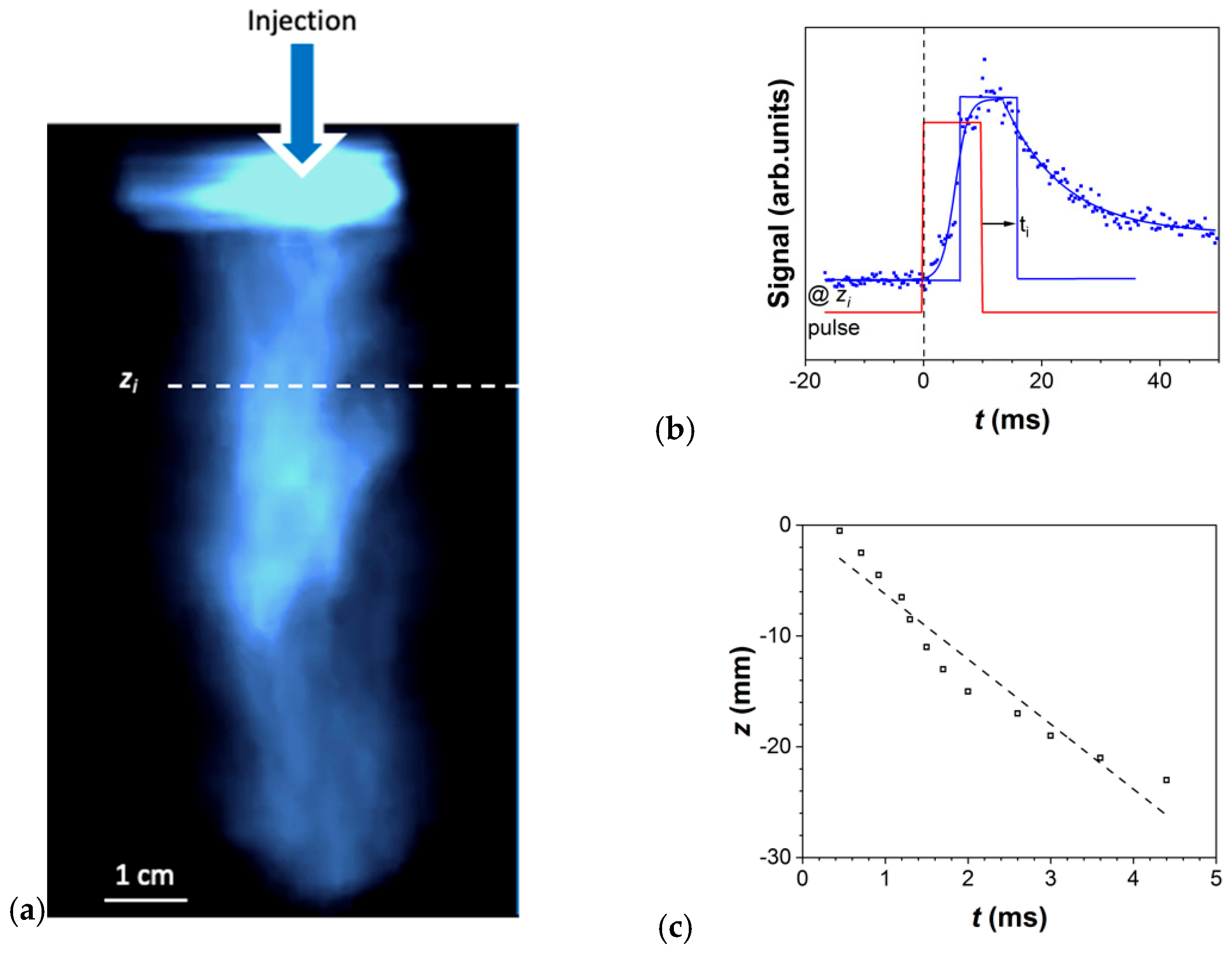
3.1.3. Plasma Behavior
3.1.4. DLRI vs. DLI of Colloidal Solutions
3.2. DLRI in an Atmospheric-Pressure Plasma Process
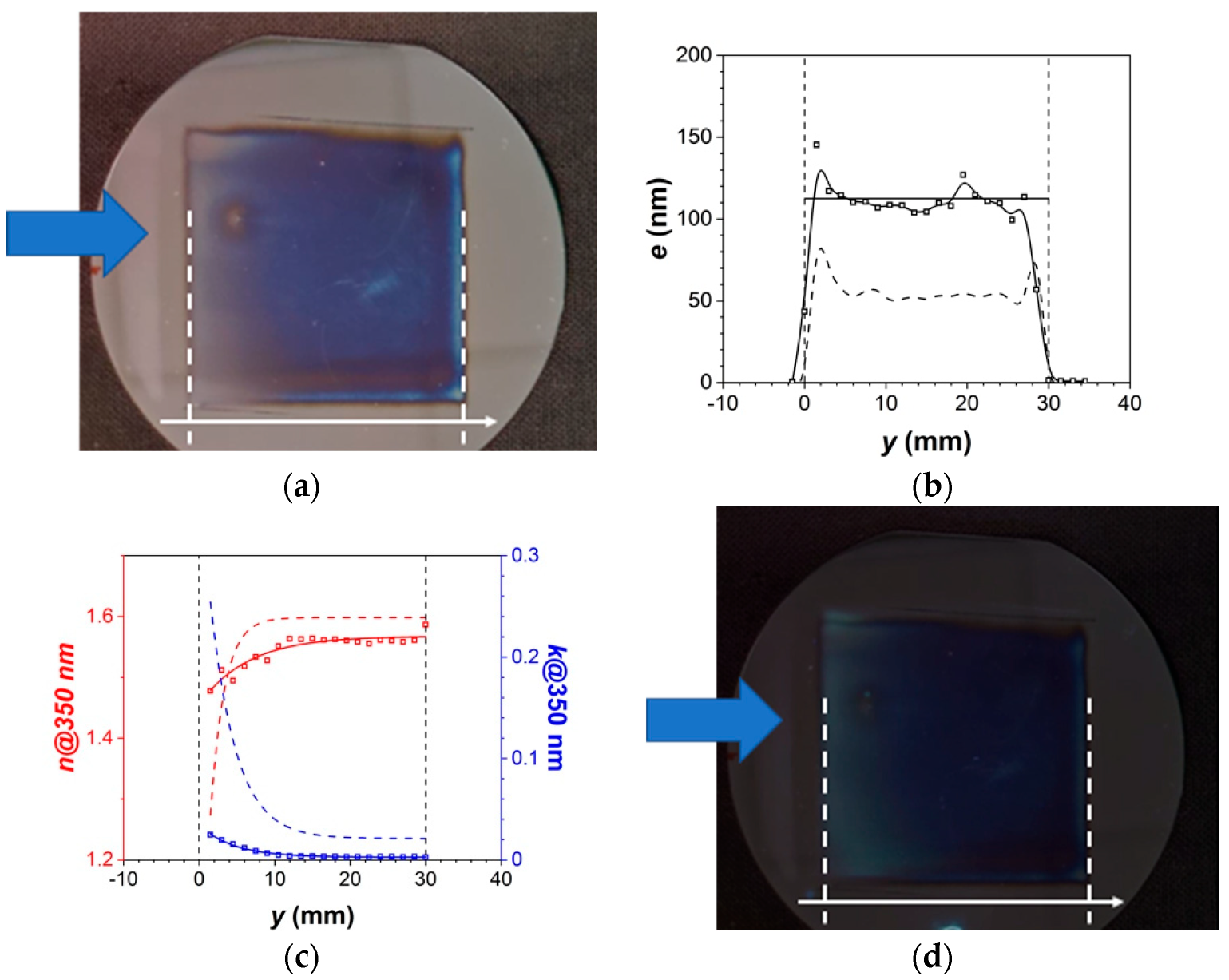
3.2.1. Thin Film Characteristics
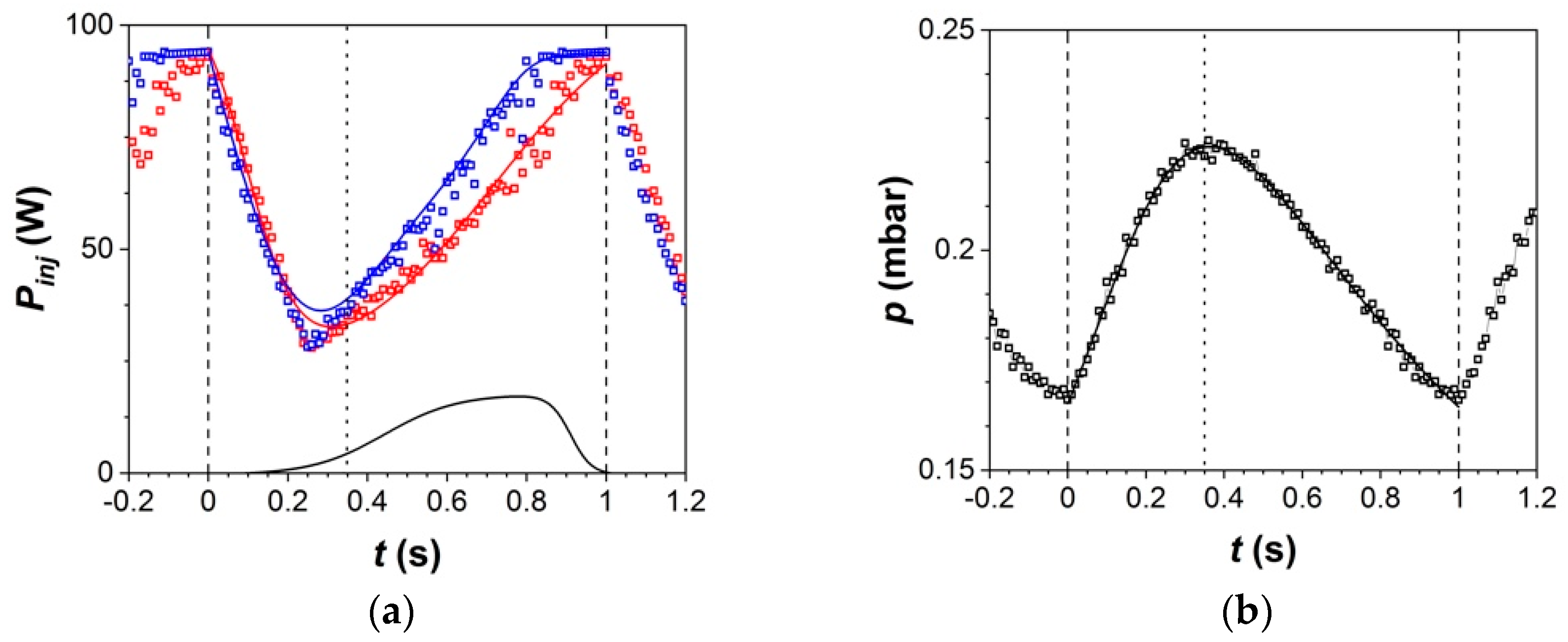
3.2.2. Mass Balance
3.2.3. Plasma Behavior
3.2.4. DLRI vs. DLI of Colloidal Solution
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanemann, T.; Szabó, D.V. Polymer-nanoparticle composites: From synthesis to modern applications. Materials 2010, 3, 3468–3517. [Google Scholar] [CrossRef]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of hybrid organic–inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Jeong, Y.-M.; Lee, J.-K.; Ha, S.-C.; Kim, S.H. Fabrication of cobalt-organic composite thin film via plasma-enhanced chemical vapor deposition for antibacterial applications. Thin Solid Film. 2009, 517, 2855–2858. [Google Scholar] [CrossRef]
- Jiang, X.; Klages, C.-P. Synthesis of diamond/β-SiC composite films by microwave plasma assisted chemical vapor deposition. Appl. Phys. Lett. 1992, 61, 1629–1631. [Google Scholar] [CrossRef]
- Perraud, S.; Quesnel, E.; Parola, S.; Barbé, J.; Muffato, V.; Faucherand, P.; Morin, C.; Jarolimek, K.; Van Swaaij, R.A.C.M.M.; Zeman, M.; et al. Silicon nanocrystals: Novel synthesis routes for photovoltaic applications. Phys. Status Solidi A 2013, 210, 649–657. [Google Scholar] [CrossRef]
- Sattler, K.; Mühlbach, J.; Recknagel, E. Generation of metal clusters containing from 2 to 500 atoms. Phys. Rev. Lett. 1980, 45, 821–824. [Google Scholar] [CrossRef]
- Millière, L.; Makasheva, K.; Laurent, C.; Despax, B.; Boudou, L.; Teyssedre, G. Silver nanoparticles as a key feature of a plasma polymer composite layer in mitigation of charge injection into polyethylene under dc stress. J. Phys. D Appl. Phys. 2015, 49, 015304. [Google Scholar] [CrossRef]
- Drabik, M.; Hanus, J.; Kousal, J.; Choukourov, A.; Biederman, H.; Slavinska, D.; Mackova, A.; Pesicka, J. Composite TiOx/hydrocarbon plasma polymer films prepared by magnetron sputtering of TiO2 and poly (propylene). Plasma Process. Polym. 2007, 4, 654–663. [Google Scholar] [CrossRef]
- Drewes, J.; Vahl, A.; Carstens, N.; Strunskus, T.; Polonskyi, O.; Faupel, F. Enhancing composition control of alloy nanoparticles from gas aggregation source by in operando optical emission spectroscopy. Plasma Process. Polym. 2021, 18, 2000208. [Google Scholar] [CrossRef]
- Calafat, M.; Yuryev, P.; Drenik, A.; Slim, A.; Clergereaux, R. Carbon Nanoparticle/Hydrogenated Amorphous Carbon Composite Thin Films Formed in ECR Plasma. Plasma Process. Polym. 2011, 8, 401–408. [Google Scholar] [CrossRef]
- Palumbo, F.; Porto, C.L.; Fracassi, F.; Favia, P. Recent advancements in the use of aerosol-assisted atmospheric pressure plasma deposition. Coatings 2020, 10, 440. [Google Scholar] [CrossRef]
- Mitronika, M.; Granier, A.; Goullet, A.; Richard-Plouet, M. Hybrid approaches coupling sol–gel and plasma for the deposition of oxide-based nanocomposite thin films: A review. SN Appl. Sci. 2021, 3, 1–23. [Google Scholar] [CrossRef]
- Collette, S.; Hubert, J.; Batan, A.; Baert, K.; Raes, M.; Vandendael, I.; Daniel, A.; Archambeau, C.; Terryn, H.; Reniers, F. Photocatalytic TiO2 thin films synthesized by the post-discharge of an RF atmospheric plasma torch. Surf. Coat. Technol. 2016, 289, 172–178. [Google Scholar] [CrossRef]
- Borella, M.; Plissonnier, M.; Belmonte, T. PECVD Synthesis of Polysiloxane-Like Thin Films with Very Low Contact Angle Hysteresis. Plasma Process. Polym. 2007, 4, S771–S775. [Google Scholar] [CrossRef]
- Brunet, P.; Rincón, R.; Margot, J.; Massines, F.; Chaker, M. Deposition of homogeneous carbon-TiO2 composites by atmospheric pressure DBD. Plasma Process. Polym. 2017, 14, 1600075. [Google Scholar] [CrossRef]
- Profili, J.; Levasseur, O.; Blaisot, J.-B.; Koronai, A.; Stafford, L.; Gherardi, N. Nebulization of nanocolloidal suspensions for the growth of nanocomposite coatings in dielectric barrier discharges. Plasma Process. Polym. 2016, 13, 981–989. [Google Scholar] [CrossRef]
- Magnan, R.; Clergereaux, R.; Villeneuve-Faure, C.; Lantin, B.; Carnide, G.; Raynaud, P.; Naudé, N. Aerosol assisted atmospheric pressure plasma jet for a high deposition rate of silica-like thin films. Eur. Phys. J. Appl. Phys. 2022, 97, 37. [Google Scholar] [CrossRef]
- Cacot, L.; Carnide, G.; Kahn, M.; Clergereaux, R.; Naudé, N.; Stafford, L. Kinetics driving thin-film deposition in dielectric barrier discharges using a direct liquid injector operated in a pulsed regime. J. Phys. D Appl. Phys. 2022, 55, 475202. [Google Scholar] [CrossRef]
- Palgrave, R.G.; Parkin, I.P. Aerosol assisted chemical vapor deposition using nanoparticle precursors: A route to nanocomposite thin films. J. Am. Chem. Soc. 2006, 128, 1587–1597. [Google Scholar] [CrossRef]
- Rubio-Garcia, J.; Coppel, Y.; Lecante, P.; Mingotaud, C.; Chaudret, B.; Gauffre, F.; Kahn, M. One-step synthesis of metallic and metal oxide nanoparticles using amino-PEG oligomers as multi-purpose ligands: Size and shape control, and quasi-universal solvent dispersibility. Chem. Commun. 2011, 47, 988–990. [Google Scholar] [CrossRef]
- Ramirez, E.; Jansat, S.; Philippot, K.; Lecante, P.; Gomez, M.; Masdeu-Bulto, A.; Chaudret, B. Influence of organic ligands on the stabilization of palladium nanoparticles. J. Organomet. Chem. 2004, 689, 4601–4610. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M. Atom economy—A challenge for organic synthesis: Homogeneous catalysis leads the way. Angew. Chem. Int. Ed. Engl. 1995, 34, 259–281. [Google Scholar] [CrossRef]
- Archer, J.; Walker, J.; Gregson, F.; Hardy, D.; Reid, J.P. Drying kinetics and particle formation from dilute colloidal suspensions in aerosol droplets. Langmuir 2020, 36, 12481–12493. [Google Scholar] [CrossRef]
- Ogawa, D.; Saraf, I.; Sra, A.; Timmons, R.; Goeckner, M.; Overzet, L. The direct injection of liquid droplets into low pressure plasmas. J. Vac. Sci. Technol. A Vac. Surf. Film. 2009, 27, 342–351. [Google Scholar] [CrossRef]
- Borra, J.-P. Review on water electro-sprays and applications of charged drops with focus on the corona-assisted cone-jet mode for High Efficiency Air Filtration by wet electro-scrubbing of aerosols. J. Aerosol Sci. 2018, 125, 208–236. [Google Scholar] [CrossRef]
- Potočňáková, L.; Synek, P.; Hoder, T. Viscous droplet in nonthermal plasma: Instability, fingering process, and droplet fragmentation. Phys. Rev. E 2020, 101, 063201. [Google Scholar] [CrossRef]
- Coppins, M. Electrostatic breakup in a misty plasma. Phys. Rev. Lett. 2010, 104, 065003. [Google Scholar] [CrossRef]
- Rojo, M.; Glad, X.; Margot, J.; Dap, S.; Clergereaux, R. Charging and heating processes of dust particles in an electron cyclotron resonance plasma. Plasma Sources Sci. Technol. 2019, 28, 085004. [Google Scholar] [CrossRef]
- Tardiveau, P.; Marode, E. Point-to-plane discharge dynamics in the presence of dielectric droplets. J. Phys. D Appl. Phys. 2003, 36, 1204–1211. [Google Scholar] [CrossRef]
- Garofano, V.; Berard, R.; Boivin, S.; Joblin, C.; Makasheva, K.; Stafford, L. Multi-scale investigation in the frequency domain of Ar/HMDSO dusty plasma with pulsed injection of HMDSO. Plasma Sources Sci. Technol. 2019, 28, 055019. [Google Scholar] [CrossRef]
- Chouteau, S.; Mitronika, M.; Goullet, A.; Richard-Plouet, M.; Granier, A.; Stafford, L. Kinetics driving nanocomposite thin film deposition in misty plasma processes. J. Phys. D Appl. Phys. 2022, 55, 505303. [Google Scholar] [CrossRef]
- Stancampiano, A.; Gallingani, T.; Gherardi, M.; Machala, Z.; Maguire, P.; Colombo, V.; Pouvesle, J.; Robert, E. Plasma and aerosols: Challenges, opportunities and perspectives. Appl. Sci. 2019, 9, 3861. [Google Scholar] [CrossRef] [Green Version]
- Garofano, V.; Berard, R.; Glad, X.; Joblin, C.; Makasheva, K.; Stafford, L. Time-resolved analysis of the precursor fragmentation kinetics in an hybrid PVD/PECVD dusty plasma with pulsed injection of HMDSO. Plasma Process. Polym. 2019, 16, 1900044. [Google Scholar] [CrossRef] [Green Version]
- Clergereaux, R.; Escaich, D.; de Larclause, I.; Bernecker, B.; Raynaud, P. Plasma deposition of carbon layer: Correlations between plasma parameters, film structure and properties. Diam. Relat. Mater. 2006, 15, 888–892. [Google Scholar] [CrossRef]
- Cacot, L.; Carnide, G.; Kahn, M.; Naudé, N.; Stafford, L.; Clergereaux, R. Soft polymerization of hexamethyldisiloxane by coupling pulsed direct-liquid injections with dielectric barrier discharge. Plasma Process. Polym. 2023, 20, e2200165. [Google Scholar] [CrossRef]
- Hegemann, D.; Nisol, B.; Gaiser, S.; Watson, S.; Wertheimer, M.R. Energy conversion efficiency in low-and atmospheric-pressure plasma polymerization processes with hydrocarbons. Phys. Chem. Chem. Phys. 2019, 21, 8698–8708. [Google Scholar] [CrossRef]
- Kraegeloh, A.; Suarez-Merino, B.; Sluijters, T.; Micheletti, C. Implementation of safe-by-design for nanomaterial development and safe innovation: Why we need a comprehensive approach. Nanomaterials 2018, 8, 239. [Google Scholar] [CrossRef] [Green Version]
- Carnide, G.; Champouret, Y.; Vallapil, D.; Vahlas, C.; Mingotaud, A.; Clergereaux, R.; Kahn, M. Secured Nanosynthesis–Deposition Aerosol Process for Composite Thin Films Incorporating Highly Dispersed Nanoparticles. Adv. Sci. 2023, 10, 2204929. [Google Scholar] [CrossRef]
- Levasseur, O.; Stafford, L.; Gherardi, N.; Naudé, N.; Beche, E.; Esvan, J.; Blanchet, P.; Riedl, B.; Sarkissian, A. Role of substrate outgassing on the formation dynamics of either hydrophilic or hydrophobic wood surfaces in atmospheric-pressure, organosilicon plasmas. Surf. Coat. Technol. 2013, 234, 42–47. [Google Scholar] [CrossRef] [Green Version]
- Naudé, N.; Cambronne, J.; Gherardi, N.; Massines, F. Electrical model and analysis of the transition from an atmospheric pressure Townsend discharge to a filamentary discharge. J. Phys. D Appl. Phys. 2005, 38, 530. [Google Scholar] [CrossRef]
- Hong, J.; Goullet, A.; Turban, G. Ellipsometry and Raman study on hydrogenated amorphous carbon (a-C:H) films deposited in a dual ECR-rf plasma. Thin Solid Film. 1999, 352, 41–48. [Google Scholar] [CrossRef]
- Dowling, D.; Donnelly, K.; Monclus, M.; McGuinness, M. The use of refractive index as a measure of diamond-like carbon film quality. Diam. Relat. Mater. 1998, 7, 432–434. [Google Scholar] [CrossRef]
- Lazar, G.; Zellama, K.; Vascan, I.; Stamate, M.; Lazar, I.; Rusu, I. Infrared absorption properties of amorphous carbon films. J. Optoelectron. Adv. Mater. 2005, 7, 647–652. [Google Scholar]
- Simonnet, C.; Parmar, D.; Zavvou, Z.; Aussonne, A.; Girardot, A.; Pozsgay, V.; Verdier, T.; Carnide, G.; Kahn, M.; Stafford, L.; et al. Pulsed-aerosol-assisted low-pressure plasma deposition of diamond-like carbon coatings. J. Vac. Sci. Technol. A Vac. Surf. Film. 2023. submitted. [Google Scholar]
- Pastol, A.; Catherine, Y. Optical emission spectroscopy for diagnostic and monitoring of CH4 plasmas used for a-C:H deposition. J. Phys. D Appl. Phys. 1990, 23, 799. [Google Scholar] [CrossRef]
- Peter, S.; Graupner, K.; Grambole, D.; Richter, F. Comparative experimental analysis of the a-C:H deposition processes using CH4 and C2H2 as precursors. J. Appl. Phys. 2007, 102, 053304. [Google Scholar] [CrossRef]
- Berndt, J.; Kovačević, E.; Stefanović, I.; Stepanović, O.; Hong, S.; Boufendi, L.; Winter, J. Some Aspects of Reactive Complex Plasmas. Contrib. Plasma Phys. 2009, 49, 107–133. [Google Scholar] [CrossRef]
- Laguardia, L.; Cremona, A.; De Angeli, M.; Lazzaro, E.; Ratynskaia, S.; Passoni, M.; Dellasega, D.; Gervasini, G.; Grosso, G.; Shiavone, R. Formation of dust in low-pressure magnetized hydrocarbon plasmas. New J. Phys. 2011, 13, 063006. [Google Scholar] [CrossRef]
- Sadek, T.; Vinchon, P.; Durocher-Jean, A.; Carnide, G.; Kahn, M.; Clergereaux, R.; Stafford, L. Time-resolved analysis of the electron temperature in RF magnetron discharges with a pulsed gas injection. Atoms 2022, 10, 147. [Google Scholar] [CrossRef]
- Monge, M.; Kahn, M.; Maisonnat, A.; Chaudret, B. Room-Temperature Organometallic Synthesis of Soluble and Crystalline ZnO Nanoparticles of Controlled Size and Shape. Angew. Chem. Int. Ed. 2003, 42, 5321–5324. [Google Scholar] [CrossRef]
- Martin, S.; Massines, F.; Gherardi, N.; Jimenez, C. Atmospheric pressure PE-CVD of silicon based coatings using a glow dielectric barrier discharge. Surf. Coat. Technol. 2004, 177, 693–698. [Google Scholar] [CrossRef]
- Kahn, M.L.; Cardinal, T.; Bousquet, B.; Monge, M.; Jubera, V.; Chaudret, B. Optical properties of zinc oxide nanoparticles and nanorods synthesized using an organometallic method. ChemPhysChem 2006, 7, 2392–2397. [Google Scholar] [CrossRef]
- Sarra-Bournet, C.; Gherardi, N.; Glénat, H.; Laroche, G.; Massines, F. Effect of C2H4/N2 ratio in an atmospheric pressure dielectric barrier discharge on the plasma deposition of hydrogenated amorphous carbon-nitride films (a-C:N:H). Plasma Chem. Plasma Process. 2010, 30, 213–239. [Google Scholar] [CrossRef]
- Massines, F.; Sarra-Bournet, C.; Fanelli, F.; Naudé, N.; Gherardi, N. Atmospheric pressure low temperature direct plasma technology: Status and challenges for thin film deposition. Plasma Process. Polym. 2012, 9, 1041–1073. [Google Scholar] [CrossRef]
- de Mejanes, N.; Profili, J.; Babaei, S.; Naudé, N.; Stafford, L. Refined analysis of current–voltage characteristics in Townsend dielectric barrier discharges in nitrogen at atmospheric pressure. J. Phys. D Appl. Phys. 2020, 54, 095204. [Google Scholar] [CrossRef]
- Naudé, N.; Cambronne, J.-P.; Gherardi, N.; Massines, F. Electrical model of an atmospheric pressure Townsend-like discharge (APTD). Eur. Phys. J. Appl. Phys. 2005, 29, 173–180. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carnide, G.; Cacot, L.; Champouret, Y.; Pozsgay, V.; Verdier, T.; Girardeau, A.; Cavarroc, M.; Sarkissian, A.; Mingotaud, A.-F.; Vahlas, C.; et al. Direct Liquid Reactor-Injector of Nanoparticles: A Safer-by-Design Aerosol Injection for Nanocomposite Thin-Film Deposition Adapted to Various Plasma-Assisted Processes. Coatings 2023, 13, 630. https://doi.org/10.3390/coatings13030630
Carnide G, Cacot L, Champouret Y, Pozsgay V, Verdier T, Girardeau A, Cavarroc M, Sarkissian A, Mingotaud A-F, Vahlas C, et al. Direct Liquid Reactor-Injector of Nanoparticles: A Safer-by-Design Aerosol Injection for Nanocomposite Thin-Film Deposition Adapted to Various Plasma-Assisted Processes. Coatings. 2023; 13(3):630. https://doi.org/10.3390/coatings13030630
Chicago/Turabian StyleCarnide, Guillaume, Laura Cacot, Yohan Champouret, Vincent Pozsgay, Thomas Verdier, Adèle Girardeau, Marjorie Cavarroc, Andranik Sarkissian, Anne-Françoise Mingotaud, Constantin Vahlas, and et al. 2023. "Direct Liquid Reactor-Injector of Nanoparticles: A Safer-by-Design Aerosol Injection for Nanocomposite Thin-Film Deposition Adapted to Various Plasma-Assisted Processes" Coatings 13, no. 3: 630. https://doi.org/10.3390/coatings13030630




