Characterization of Nitrogen-Doped TiO2 Films Prepared by Arc Ion Plating without Substrate Heating in Various N2/O2 Gas Mixture Ratios
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Sample Characterization
3. Results and Discussion
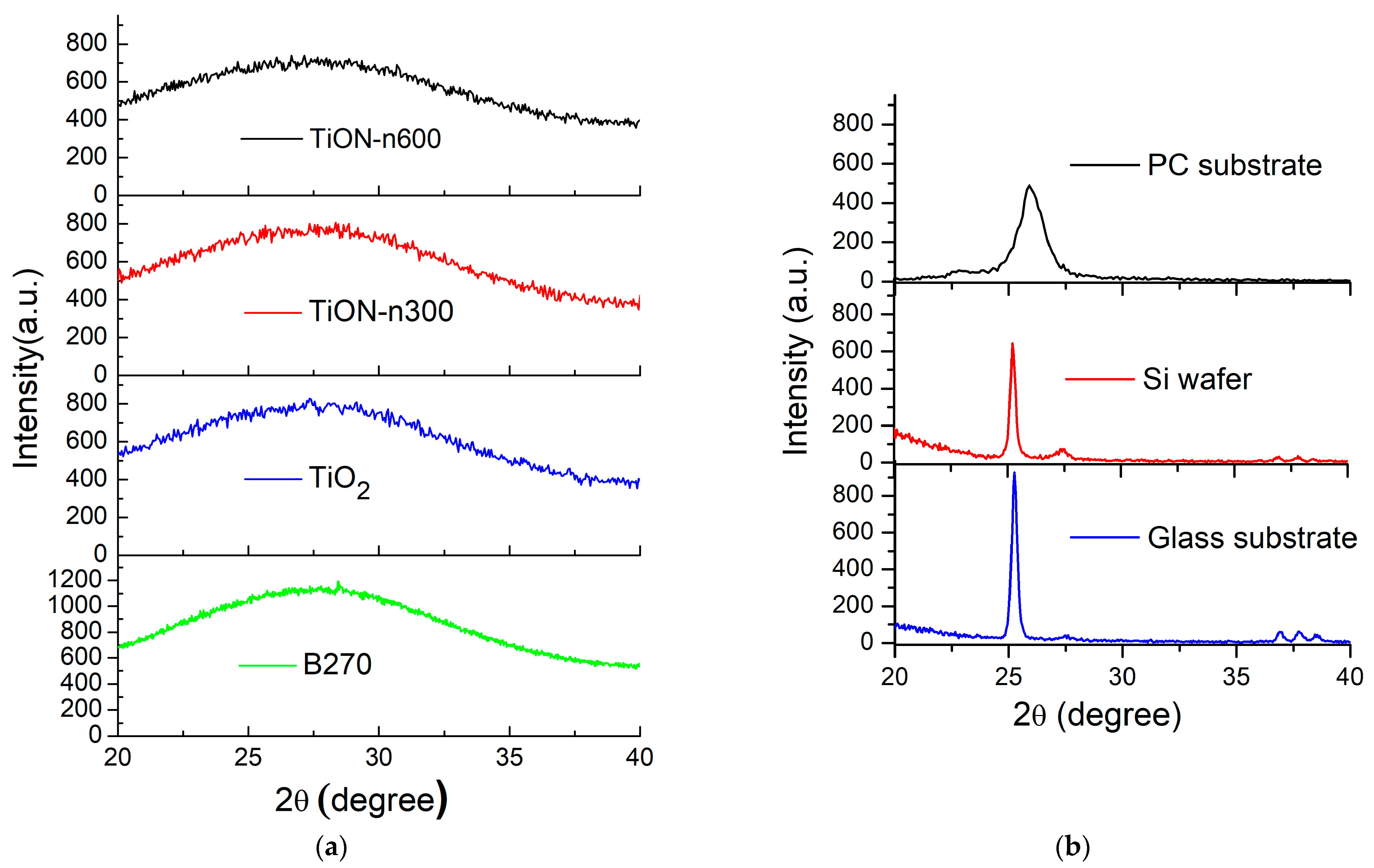
3.1. XRD and Surface Morphologies of N-Doped TiO2 Films
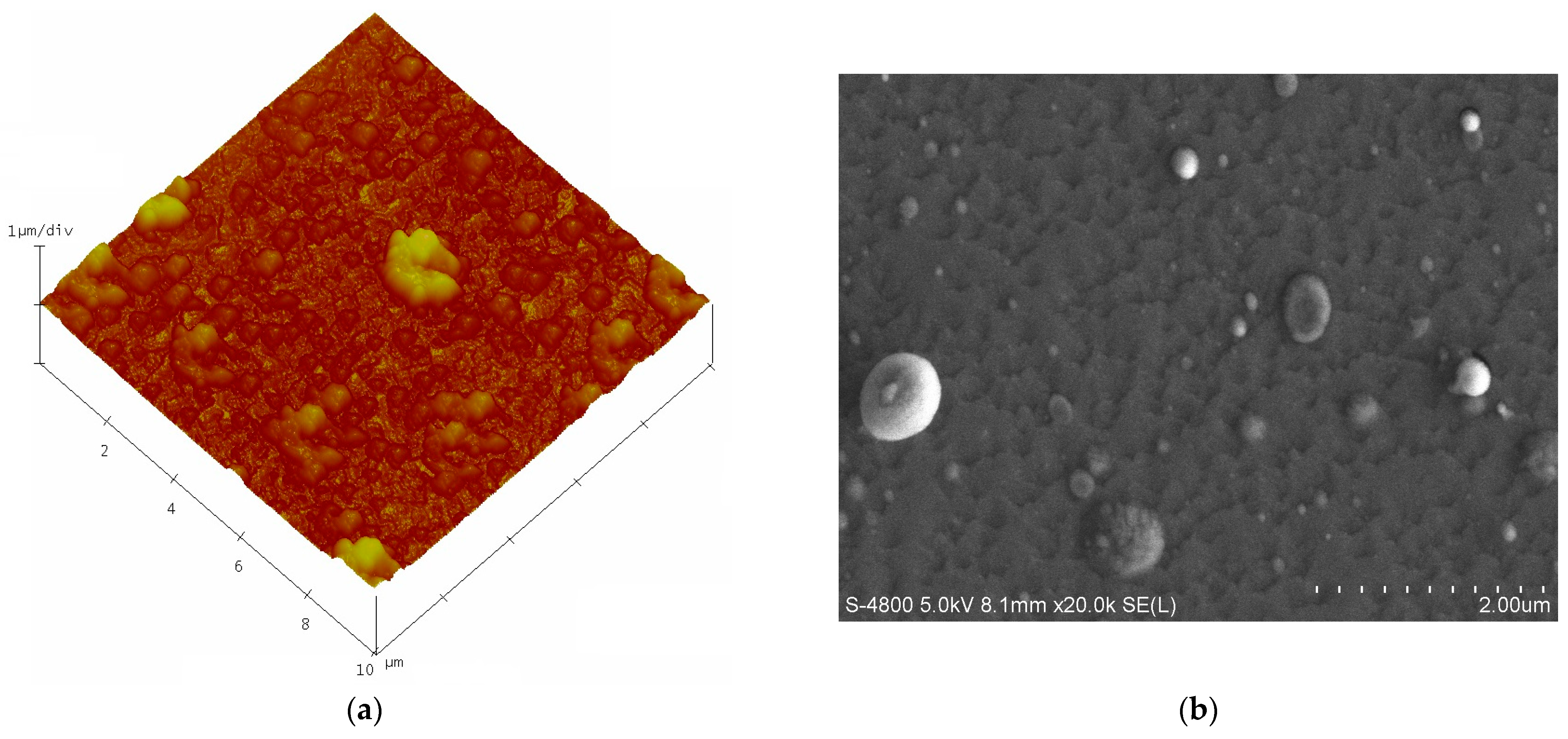
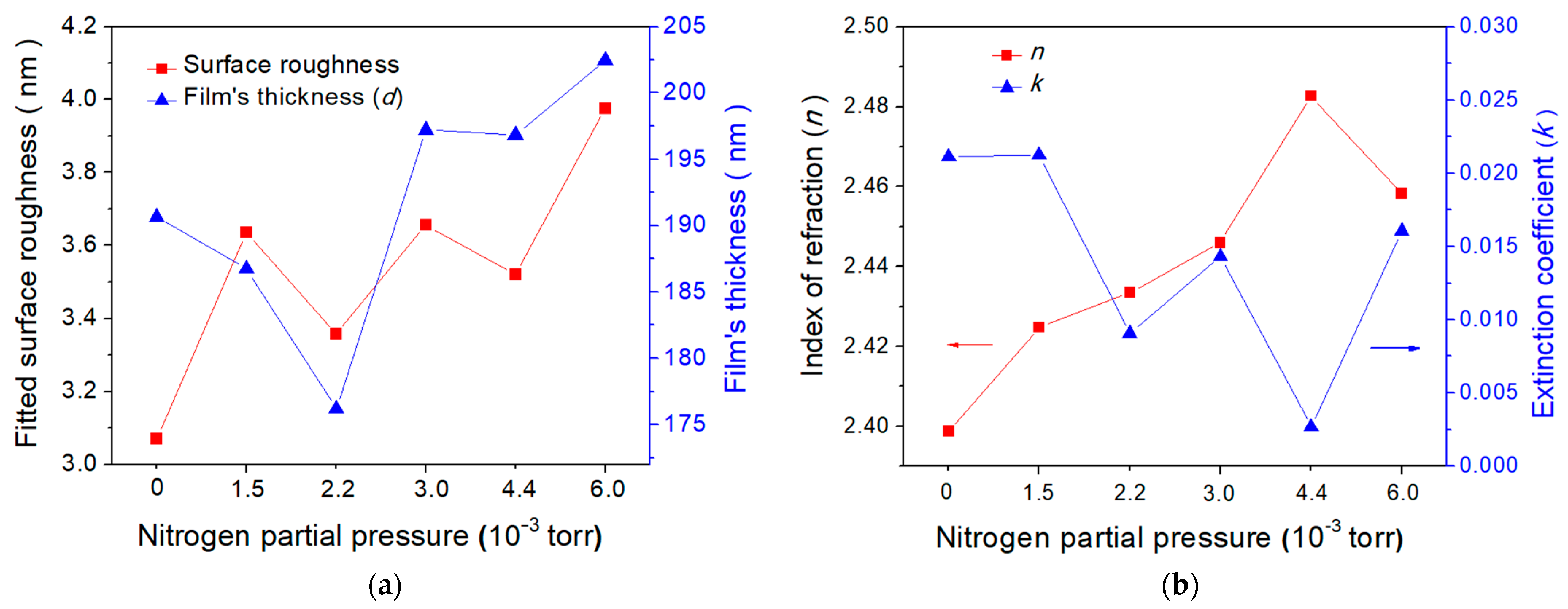
3.2. Surface Morphology of N-Doped TiO2 Films
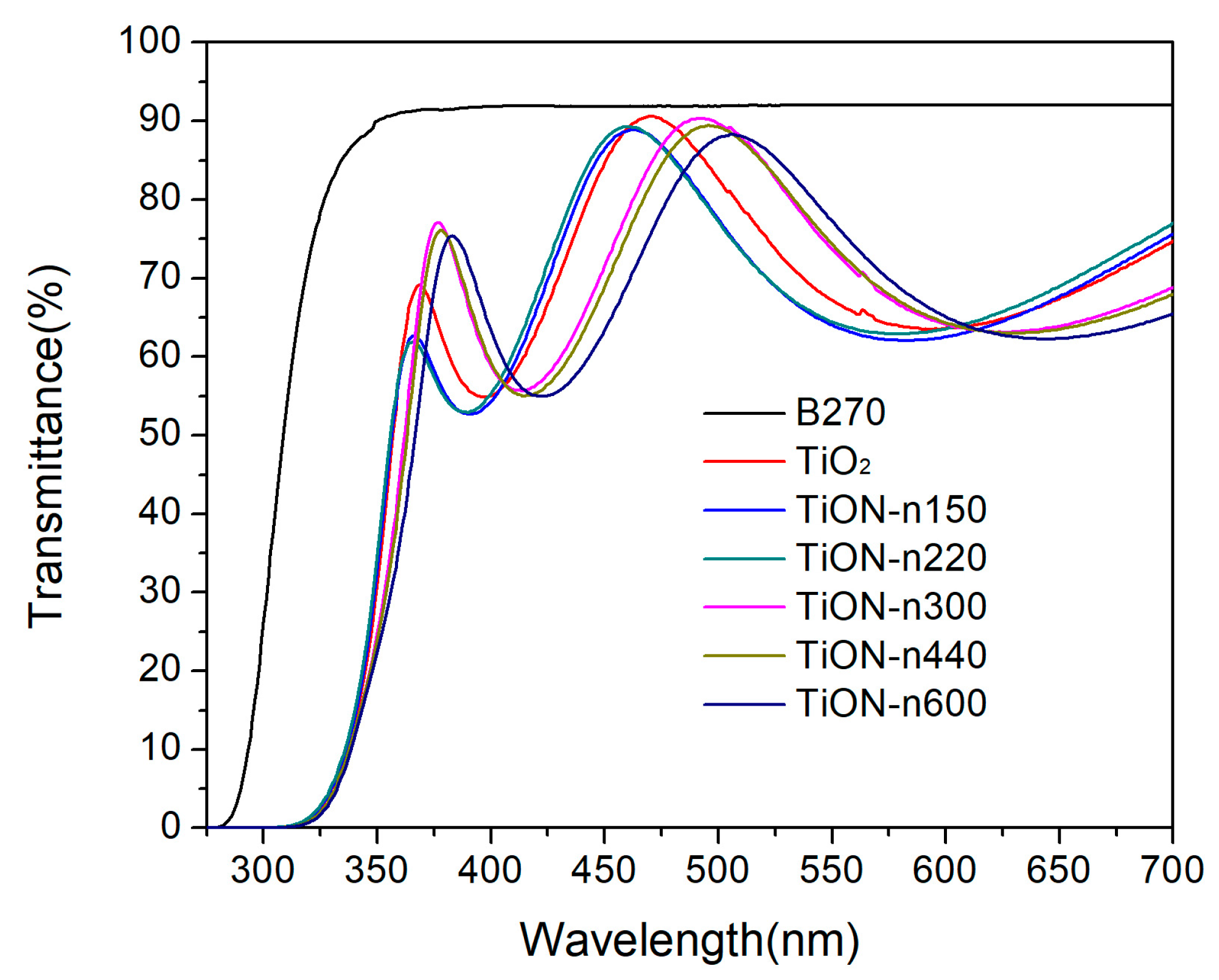
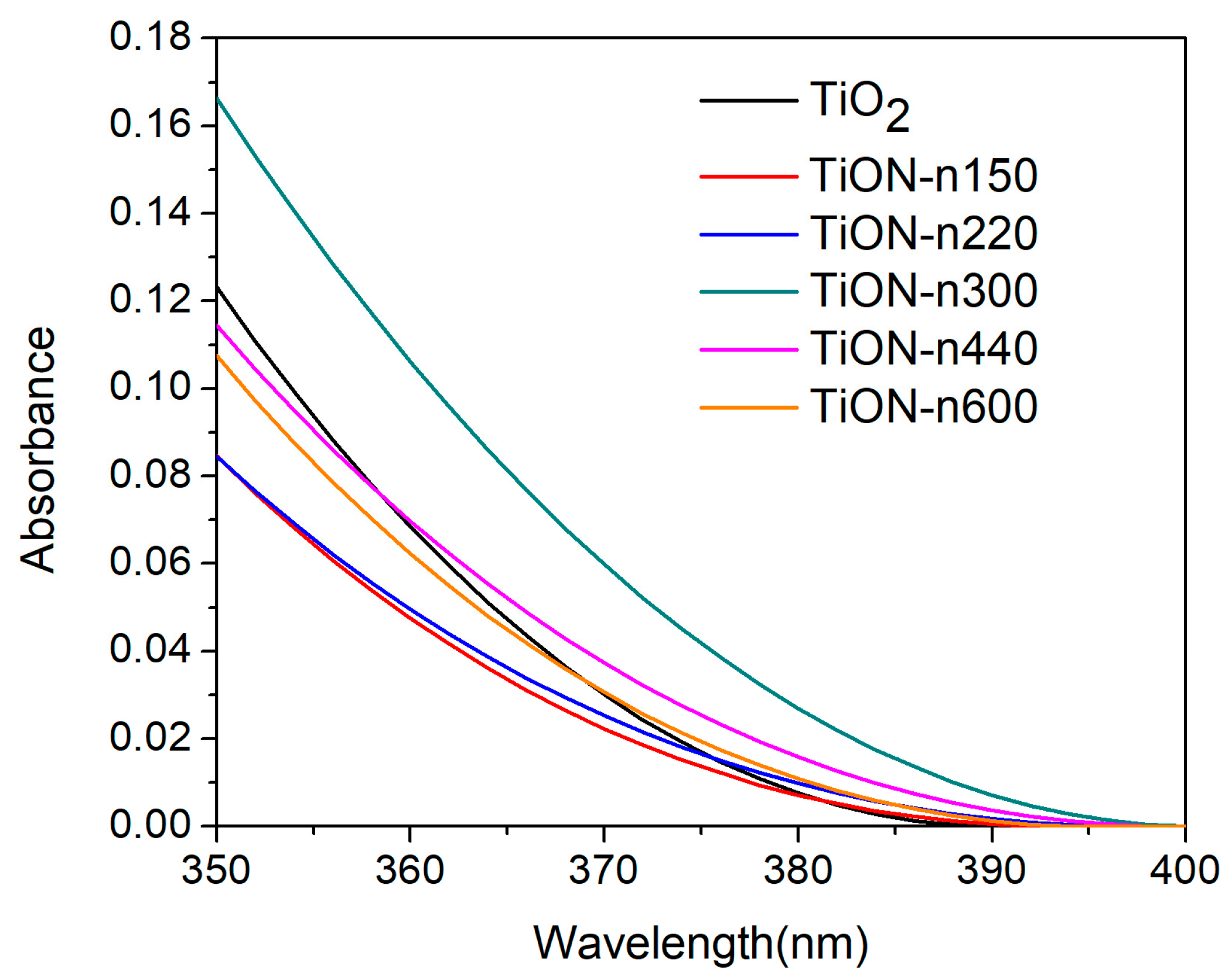
3.3. Transmittance of N-Doped TiO2 Films
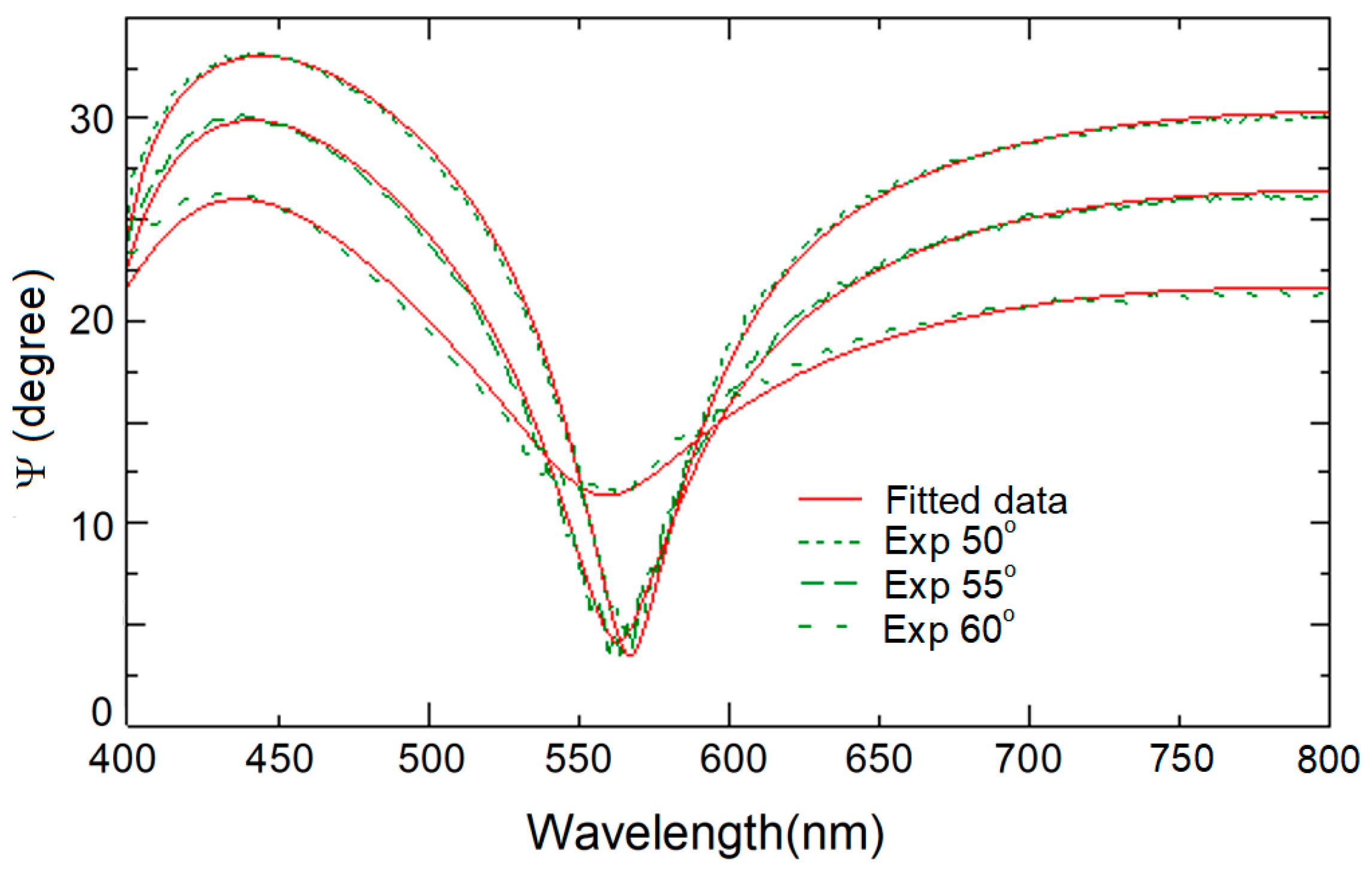
3.4. Optical Properties of N-Doped TiO2 Films
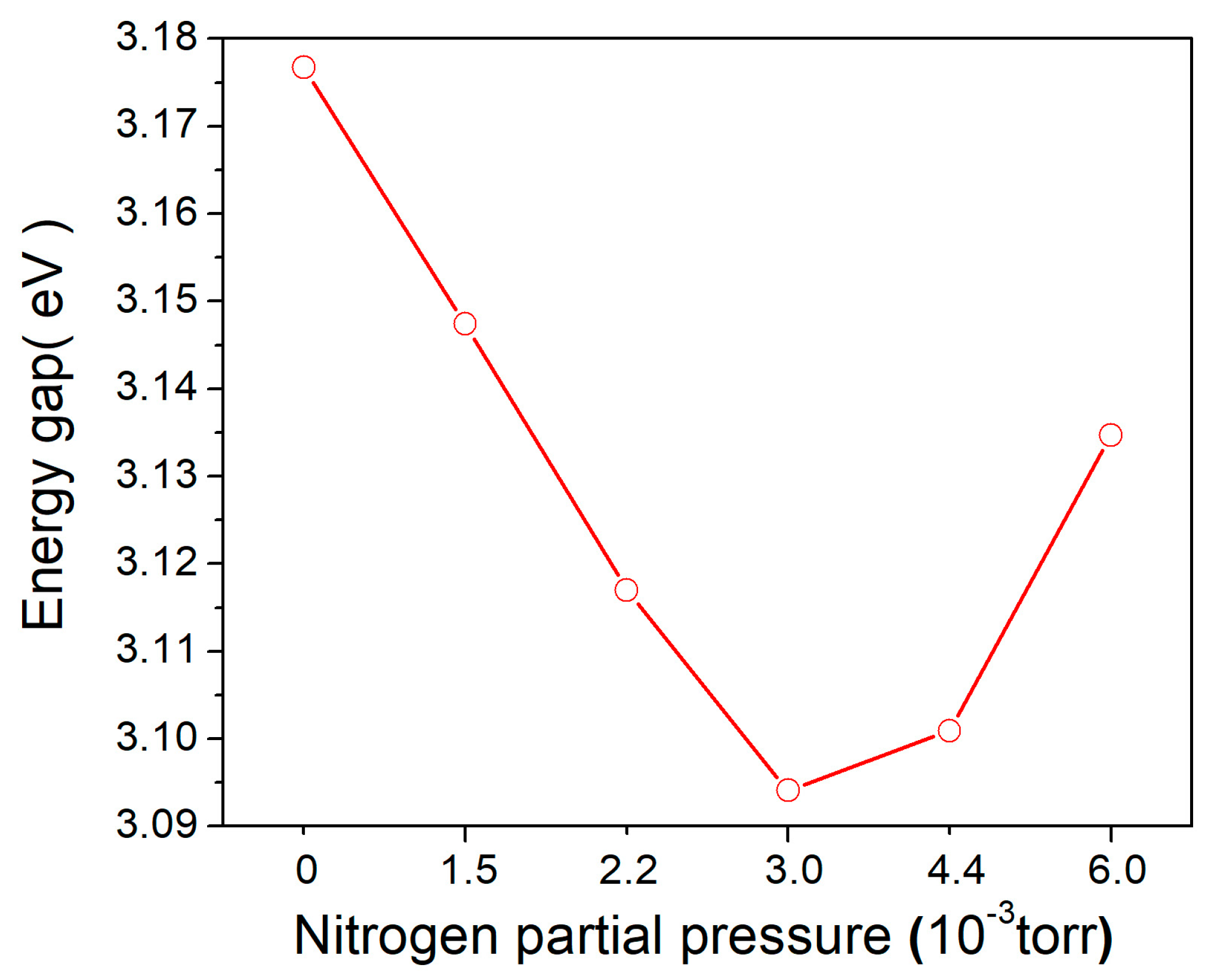
3.5. Bandgap Energy of N-Doped TiO2 Films
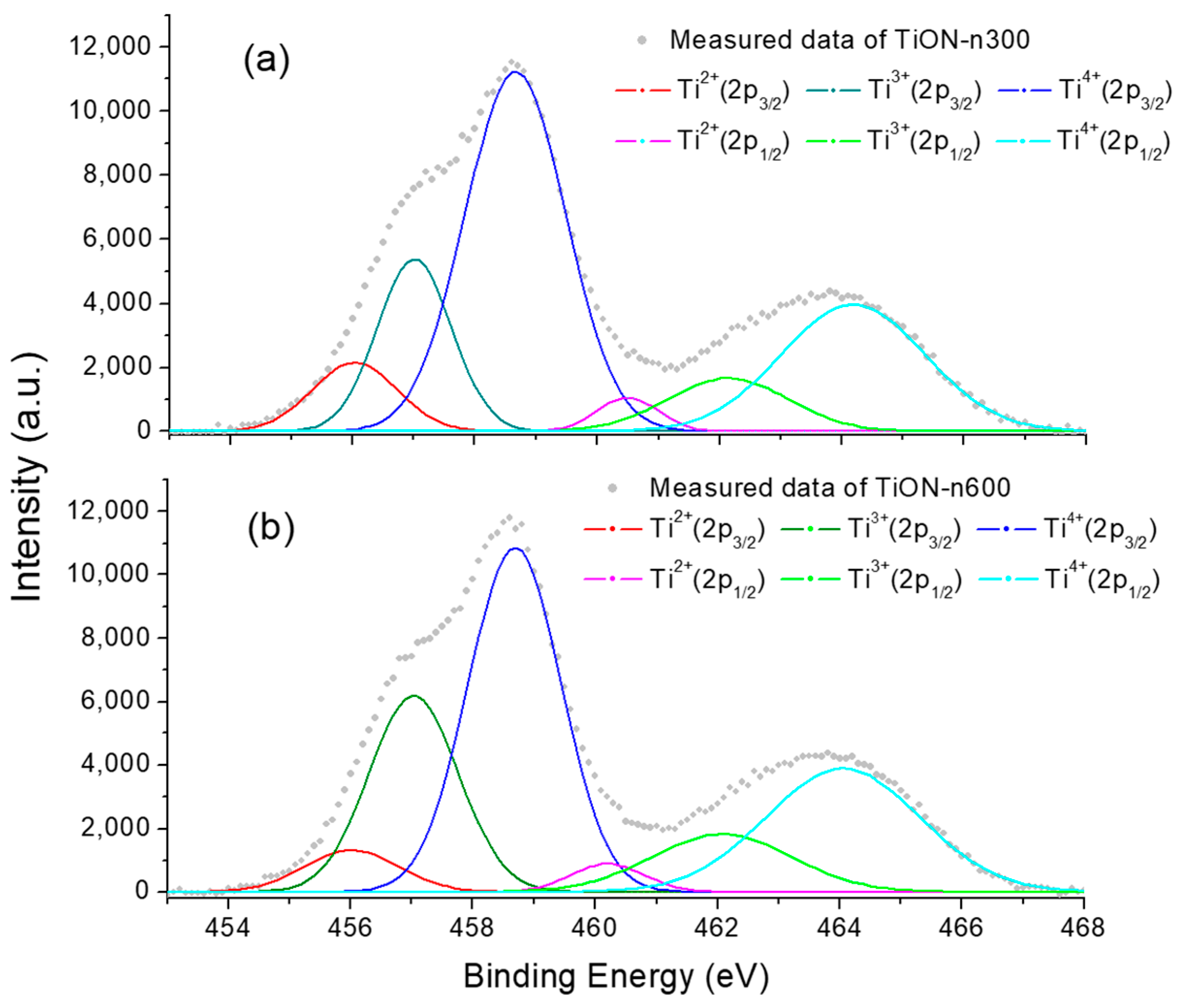
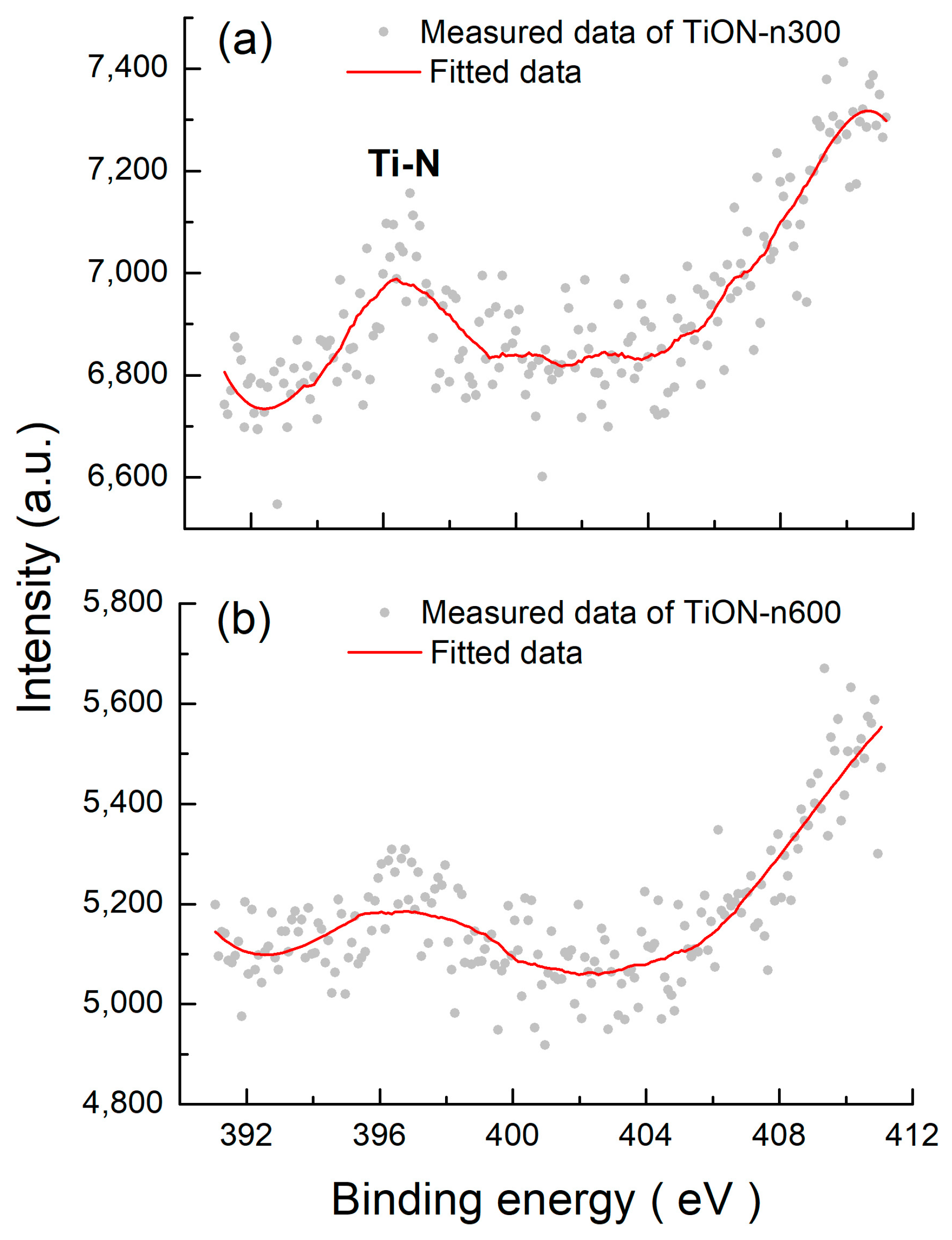
3.6. XPS of N-Doped TiO2 Films
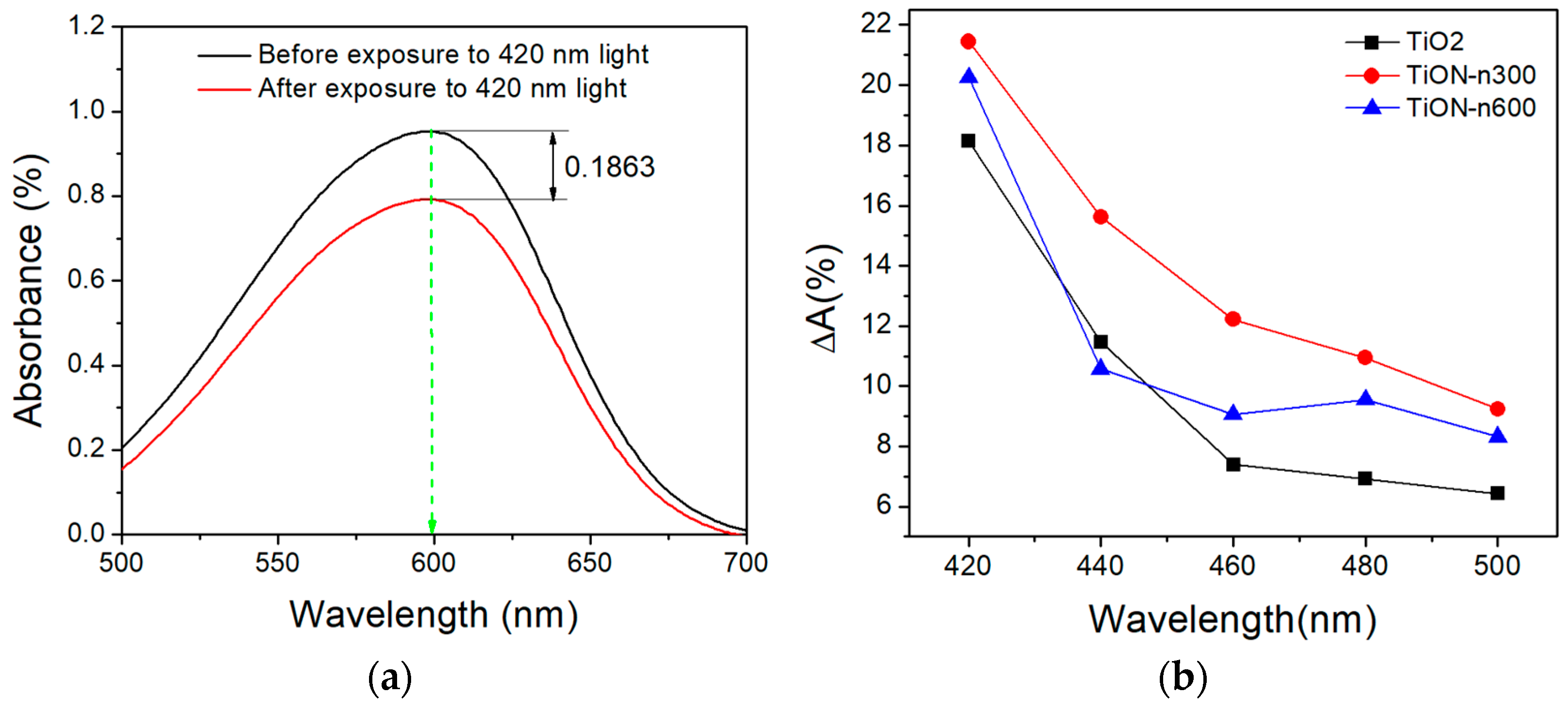
3.7. Photocatalytic Performance of N-Doped TiO2 Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Yang, X.G.; Wang, D.W. Photocatalysis: From fundamental principles to materials and applications. Acs Appl. Energ. Mater. 2018, 1, 6657–6693. [Google Scholar] [CrossRef]
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous photocatalysis: Recent advances and applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef]
- Wenderich, K.; Mul, G. Methods, mechanism, and applications of photo deposition in photocatalysis: A review. Chem. Rev. 2016, 116, 14587–14619. [Google Scholar] [PubMed]
- Sun, Y.; O’Connell, D.W. Application of visible light active photocatalysis for water contaminants: A review. Water Environ. Res. 2022, 94, e10781. [Google Scholar] [CrossRef] [PubMed]
- Elgohary, E.A.; Mohamed, Y.M.A.; El Nazer, H.A.; Baaloudj, O.; Alyami, M.S.S.; El Jery, A.; Assadi, A.A.; Amrane, A. A review of the use of semiconductors as catalysts in the photocatalytic inactivation of microorganisms. Catalysts 2021, 11, 1498. [Google Scholar] [CrossRef]
- Zhang, L.P.; Ran, J.R.; Qiao, S.Z.; Jaroniec, M. Characterization of semiconductor photocatalysts. Chem. Soc. Rev. 2019, 48, 5184–5206. [Google Scholar] [CrossRef] [PubMed]
- Serpone, N.; Emeline, A.V. Semiconductor photocatalysis–past, present, and future outlook. J. Phys. Chem. Lett. 2012, 3, 673–677. [Google Scholar] [CrossRef]
- Areeshi, M.Y. A review on biological synthesis of ZnO nanostructures and its application in photocatalysis mediated dye degradation: An overview. Luminescence 2022. [Google Scholar] [CrossRef]
- Arun, J.; Nachiappan, S.; Rangarajan, G.; Alagappan, R.P.; Gopinath, K.P.; Lichtfouse, E. Synthesis and application of titanium dioxide photocatalysis for energy, decontamination and viral disinfection: A review. Environ. Chem. Lett. 2023, 21, 339–362. [Google Scholar] [CrossRef]
- Yan, H.; Wang, X.D.; Yao, M.; Yao, X.J. Band structure design of semiconductors for enhanced photocatalytic activity: The case of TiO2. Prog. Nat. Sci. Mater. 2013, 23, 402–407. [Google Scholar] [CrossRef]
- Nagaveni, K.; Hegde, M.S.; Ravishankar, N.; Subbanna, G.N.; Madras, G. Synthesis and structure of nano-crystalline TiO2 with lower band gap showing high photocatalytic activity. Langmuir 2004, 20, 2900–2907. [Google Scholar] [CrossRef]
- Pawar, M.; Sendogdular, S.T.; Gouma, P. A brief overview of TiO2 photocatalyst for organic dye remediation: Case study of reaction mechanisms involved in Ce-TiO2 photocatalysts system. J. Nanomater. 2018, 2018, 5953609. [Google Scholar] [CrossRef]
- Wu, H.Y.; Lin, H.C.; Liu, Y.H.; Chen, K.L.; Wang, Y.H.; Sun, Y.S.; Hsu, J.C. Highly sensitive, robust, and recyclable TiO2/AgNP substrate for SERS detection. Molecules 2022, 27, 6755. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, X.J.; Jiang, Q.; Lian, J.S. Enhanced optical absorption and photocatalytic activity of Cu/N-codoped TiO2 nanocrystals. Mat. Sci. Semicon. Proc. 2014, 24, 247–253. [Google Scholar] [CrossRef]
- Ansari, S.A.; Cho, M.H. Highly visible light responsive, narrow band gap TiO2 nanoparticles modified by elemental red phosphorus for photocatalysis and photoelectrochemical applications. Sci. Rep. 2016, 6, 25405. [Google Scholar] [CrossRef] [PubMed]
- Pitre, S.P.; Yoon, T.P.; Scaiano, J.C. Titanium dioxide visible light photocatalysis: Surface association enables photocatalysis with visible light irradiation. Chem. Commun. 2017, 53, 4335–4338. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.J. Recent advances in TiO2 films prepared by sol-gel methods for photocatalytic degradation of organic pollutants and antibacterial activities. Coatings 2019, 9, 613. [Google Scholar] [CrossRef]
- Wang, Y.H.; Rahman, K.H.; Wu, C.C.; Chen, K.C. A review on the pathways of the improved structural characteristics and photocatalytic performance of titanium dioxide (TiO2) thin films fabricated by the magnetron-sputtering technique. Catalysts 2020, 10, 598. [Google Scholar] [CrossRef]
- Patil, M.K.; Shaikh, S.; Ganesh, I. Recent advances on TiO2 thin film based photocatalytic applications (a review). Curr. Nanosci. 2015, 11, 271–285. [Google Scholar] [CrossRef]
- Hao, B.F.; Guo, J.P.; Zhang, L.; Ma, H.Z. Magnetron sputtered TiO2/CuO heterojunction thin films for efficient photocatalysis of rhodamine B. J. Alloys Compd. 2022, 903, 163851. [Google Scholar] [CrossRef]
- Becker, M.; Yezerska, O. Review-recent progress in low temperature synthesis of crystalline TiO2 photocatalytic films by highly controllable electrodeposition. J. Electrochem. Soc. 2022, 169, 052507. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Akhter, P.; Arshad, A.; Saleem, A.; Hussain, M. Recent development in non-metal-doped titanium dioxide photocatalysts for different dyes degradation and the study of their strategic factors: A review. Catalysts 2022, 12, 1331. [Google Scholar] [CrossRef]
- Temam, E.G.; Djani, F.; Rahmane, S.; Ben Temam, H.; Gasmi, B. Photocatalytic activity of Al/Ni doped TiO2 films synthesized by sol-gel method: Dependence on thickness and crystal growth of photocatalysts. Surf. Interfaces 2022, 31, 102077. [Google Scholar] [CrossRef]
- Tang, W.M.; Hu, C.C.; Tsao, C.C.; Chen, C.S.; Roselin, L.S.; Kuo, C.G.; Hsu, C.Y. Photocatalytic activity and mechanical performance of o and n co-doped tio2 thin films. J. Electron. Mater. 2022, 51, 6145–6159. [Google Scholar] [CrossRef]
- Hsu, J.C.; Lin, Y.H.; Wang, P.W. X-ray photoelectron spectroscopy analysis of nitrogen-doped TiO2 films prepared by reactive-ion-beam sputtering with various NH3/O2 gas mixture ratios. Coatings 2020, 10, 47. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, Q. Mechanism of higher photocatalytic activity of anatase TiO2 doped with nitrogen under visible-light irradiation from density functional theory calculation. J. Phys. D Appl. Phys. 2008, 41, 025105. [Google Scholar] [CrossRef]
- Irie, H.; Watanabe, Y.; Hashimoto, K. Nitrogen-concentration dependence on photocatalytic activity of TiO2-xNx powders. J. Phys. Chem. B 2003, 107, 5483–5486. [Google Scholar] [CrossRef]
- Ihara, T.; Miyoshi, M.; Iriyama, Y.; Matsumoto, O.; Sugihara, S. Visible-light-active titanium oxide photocatalyst realized by an oxygen-deficient structure and by nitrogen doping. Appl. Catal. B Environ 2003, 42, 403–409. [Google Scholar] [CrossRef]
- Lindgren, T.; Mwabora, J.M.; Avendano, E.; Jonsson, J.; Hoel, A.; Granqvist, C.G.; Lindquist, S.E. Photoelectrochemical and optical properties of nitrogen doped titanium dioxide films prepared by reactive dc magnetron sputtering. J. Phys. Chem. B 2003, 107, 5709–5716. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, X.W.; Weng, W.H.; Han, G.R. Structure and properties of nitrogen-doped titanium dioxide thin films grown by atmospheric pressure chemical vapor deposition. Thin Solid Films 2007, 515, 7117–7121. [Google Scholar] [CrossRef]
- Powell, M.J.; Dunnill, C.W.; Parkin, I.P. N-doped TiO2 visible light photocatalyst films via a sol-gel route using TMEDA as the nitrogen source. J. Photochem. Photobiol. A 2014, 281, 27–34. [Google Scholar] [CrossRef]
- Chang, J.T.; Su, C.W.; He, J.L. Photocatalytic TiO2 film prepared using arc ion plating. Surf. Coat. Technol. 2006, 200, 3027–3034. [Google Scholar] [CrossRef]
- Chang, J.T.; Lai, Y.F.; He, J.L. Photocatalytic performance of chromium or nitrogen doped arc ion plated-TiO2 films. Surf. Coat. Technol. 2005, 200, 1640–1644. [Google Scholar]
- Zhang, M.; Lin, G.Q.; Dong, C.; Kim, K.H. Mechanical and optical properties of composite TiOxNy films prepared by pulsed bias arc ion plating. Curr. Appl. Phys. 2009, 9, S174–S178. [Google Scholar]
- Lehmann, D.; Seidel, F.; Zahn, D.R.T. Thin films with high surface roughness: Thickness and dielectric function analysis using spectroscopic ellipsometry. Springerplus 2014, 3, 82. [Google Scholar] [CrossRef] [PubMed]
- Eiamchai, P.; Chindaudom, P.; Pokaipisit, A.; Limsuwan, P. A spectroscopic ellipsometry study of TiO2 thin films prepared by ion-assisted electron-beam evaporation. Curr. Appl. Phys. 2009, 9, 707–712. [Google Scholar] [CrossRef]
- Dariani, R.S.; Esmaeili, A.; Mortezaali, A.; Dehghanpour, S. Photocatalytic reaction and degradation of methylene blue on TiO2 nano-sized particles. Optik 2016, 127, 7143–7154. [Google Scholar]
- Hou, C.T.; Hu, B.; Zhu, J.M. Photocatalytic degradation of methylene blue over TiO2 pretreated with varying concentrations of NaOH. Catalysts 2018, 8, 575. [Google Scholar] [CrossRef]
- Lin, F.Y.; Lin, J.Y.; Lo, K.Y.; Sun, Y.S. Use microfluidic chips to study the phototaxis of lung cancer cells. Int. J. Mol. Sci. 2019, 20, 4515. [Google Scholar] [CrossRef]
- Huang, C.H.; Hou, H.S.; Lo, K.Y.; Cheng, J.Y.; Sun, Y.S. Use microfluidic chips to study the effects of ultraviolet lights on human fibroblasts. Microfluid. Nanofluid. 2017, 21, 79. [Google Scholar] [CrossRef]
- Karunagaran, B.; Kumar, R.T.R.; Mangalaraj, D.; Narayandass, S.K.; Rao, G.M. Influence of thermal annealing on the composition and structural parameters of dc magnetron sputtered titanium dioxide thin films. Cryst. Res. Technol. 2002, 37, 1285–1292. [Google Scholar] [CrossRef]
- Nakano, Y.; Morikawa, T.; Ohwaki, T.; Taga, Y. Deep-level optical spectroscopy investigation of n-doped TiO2 films. Appl. Phys. Lett. 2005, 86, 132104. [Google Scholar] [CrossRef]
- Jellison, G.E., Jr.; Modine, F.A. Parameterization of the optical functions of amorphous materials in the interband region. Appl. Phys. Lett. 1996, 69, 371–373. [Google Scholar] [CrossRef]
- Yang, K.S.; Dai, Y.; Huang, B.B. Study of the nitrogen concentration influence on n-doped TiO2 anatase from first-principles calculations. J. Phys. Chem. C 2007, 111, 12086–12090. [Google Scholar] [CrossRef]
- Cheng, X.W.; Yu, X.J.; Xing, Z.P.; Yang, L.S. Synthesis and characterization of n-doped TiO2 and its enhanced visible-light photocatalytic activity. Arab. J. Chem. 2016, 9, S1706–S1711. [Google Scholar] [CrossRef]
- Shao, G.S. Red shift in manganese- and iron-doped TiO2: A DFT+U analysis. J. Phys. Chem. C 2009, 113, 6800–6808. [Google Scholar] [CrossRef]
- Zhou, W.F.; Liu, Q.J.; Zhu, Z.Q.; Zhang, J. Preparation and properties of vanadium-doped TiO2 photocatalysts. J. Phys. D Appl. Phys. 2010, 43, 035301. [Google Scholar] [CrossRef]
- Deng, Q.R.; Han, X.P.; Gao, Y.; Shao, G.S. Remarkable optical red shift and extremely high optical absorption coefficient of V-Ga co-doped TiO2. J. Appl. Phys. 2012, 112, 013523. [Google Scholar] [CrossRef]
- Trenczek-Zajac, A.; Radecka, M.; Zakrzewska, K.; Brudnik, A.; Kusior, E.; Bourgeois, S.; de Lucas, M.C.M.; Imhoff, L. Structural and electrical properties of magnetron sputtered Ti(ON) thin films: The case of TiN doped in situ with oxygen. J. Power Sources 2009, 194, 93–103. [Google Scholar] [CrossRef]
- Hsu, J.C.; Lee, C.C.; Chen, H.L.; Kuo, C.C.; Wang, P.W. Investigation of thin TiO2 films cosputtered with Si species. Appl. Surf. Sci. 2009, 255, 4852–4858. [Google Scholar] [CrossRef]
- Braic, M.; Balaceanu, M.; Vladescu, A.; Kiss, A.; Braic, V.; Epurescu, G.; Dinescu, G.; Moldovan, A.; Birjega, R.; Dinescu, M. Preparation and characterization of titanium oxy-nitride thin films. Appl. Surf. Sci. 2007, 253, 8210–8214. [Google Scholar] [CrossRef]
- Yu, Y.P.; Xing, X.J.; Xu, L.M.; Wu, S.X.; Li, S.W. N-derived signals in the x-ray photoelectron spectra of n-doped anatase TiO2. J. Appl. Phys. 2009, 105, 12353. [Google Scholar] [CrossRef]
- Zhang, S.J.; Li, M.S.; Chen, Z.Q.; Hu, J.X. Effects of N-doped quantity and current density on properties of TiO2 films by arc ion plating. Adv. Mater. Res. 2011, 345, 87–92. [Google Scholar] [CrossRef]
- Zhang, S.J.; Li, M.S.; Chen, Z.Q.; Fan, Y.Z. Influence of annealing treatment on N-doped TiO2 films deposited by pulsed negative bias arc ion plating. Adv. Mater. Res. 2012, 482–484, 1105–1110. [Google Scholar]
- Zhang, S.J.; Li, M.S.; Chen, Z.Q.; Fan, Y.Z. Effects of pulsed negative bias and current density on properties of N-doped TiO2 films deposited by arc ion plating. Adv. Mater. Res. 2012, 476–478, 2357–2362. [Google Scholar] [CrossRef]

| Sample | Substrate | N2 Partial Pressure, PN2 (×10−3 torr) | O2 partial pressure, PO2 (×10−3 torr) | PN2/PO2 |
|---|---|---|---|---|
| TiO2 | Silicon | 0 | 15.0 | 0 |
| TiON-n150 | Silicon | 1.5 | 13.5 | 1/9 |
| TiON-n220 | Silicon | 2.2 | 12.8 | 1/5.8 |
| TiON-n300 | Silicon | 3.0 | 12.0 | 1/4 |
| TiON-n440 | Silicon | 4.4 | 10.6 | 1/2.4 |
| TiON-n600 | Silicon | 6.0 | 9.0 | 1/1.5 |
| Substrate Conditions | Working Pressures | Main Findings | References |
|---|---|---|---|
| 1. No substrate bias 2. Tsub not specified (No substrate heating) | 1. Ptotal = 0.8 Pa 2. PN2/Ptotal = 5%~50% | 1. Amount of TiO2 anatase phase decreased with N doping. 2. N doping generally reduced photocatalytic efficacy, but the performance increased at PN2/Ptotal = 5%~10% | [34] |
| 1. Substrate bias from 0 to −700 V 2. Tsub not specified | 1. Ptotal = 0.2 Pa 2. PN2/PO2 = 1/2 | 1. At a bias of −100 V, these films exhibited a mixture of anatase and rutile phases. | [35] |
| 2. No substrate bias 1. Tsub = 200 °C | 1. Ptotal = 1~1.3 Pa 2. PN2/PO2 = 0, 2/1~5/1 | 1. N doping inhibited the anatase growth and promoted rutile formation. 2. N-doped TiO2 film not only reduced the UV catalytic performance but also extended the catalytic properties to the sunlight. | [54] |
| 1. Substrate bias from 0 to −600 V 2. Tsub = 200~300 °C | 1. Ptotal = 1~1.3 Pa 2. PN2/PO2 = 13/4 | 1. Absorption edges of the as-deposited films are increased with the rising of the negative bias, and a maximum of 550 nm is achieved under −600 V bias. | [55] |
| 1. Substrate bias from 0 to −600 V @20 kHz 2. Tsub = 150~300 °C | 1. Ptotal = 1~1.8 Pa 2. PN2/PO2 = 13/4 | 1. Applying the negative pulse bias extended the absorption edge 5–40 nm towards the visible region. | [56] |
| 1. No substrate bias 2. No substrate heating | 1. Ptotal = 2 Pa 2. PN2/PO2 = 0, 1/9~1/1.5 | 1. All samples exhibited amorphous structures. 2. Sample with PN2/PO2 = 1/4 showed the best photocatalytic performance. | The present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.-Y.; Huang, W.-C.; Wang, J.-L.; Yu, G.-Y.; Sun, Y.-S.; Hsu, J.-C. Characterization of Nitrogen-Doped TiO2 Films Prepared by Arc Ion Plating without Substrate Heating in Various N2/O2 Gas Mixture Ratios. Coatings 2023, 13, 654. https://doi.org/10.3390/coatings13030654
Wu H-Y, Huang W-C, Wang J-L, Yu G-Y, Sun Y-S, Hsu J-C. Characterization of Nitrogen-Doped TiO2 Films Prepared by Arc Ion Plating without Substrate Heating in Various N2/O2 Gas Mixture Ratios. Coatings. 2023; 13(3):654. https://doi.org/10.3390/coatings13030654
Chicago/Turabian StyleWu, Hsing-Yu, Wen-Chun Huang, Jyh-Liang Wang, Guo-Yu Yu, Yung-Shin Sun, and Jin-Cherng Hsu. 2023. "Characterization of Nitrogen-Doped TiO2 Films Prepared by Arc Ion Plating without Substrate Heating in Various N2/O2 Gas Mixture Ratios" Coatings 13, no. 3: 654. https://doi.org/10.3390/coatings13030654
APA StyleWu, H.-Y., Huang, W.-C., Wang, J.-L., Yu, G.-Y., Sun, Y.-S., & Hsu, J.-C. (2023). Characterization of Nitrogen-Doped TiO2 Films Prepared by Arc Ion Plating without Substrate Heating in Various N2/O2 Gas Mixture Ratios. Coatings, 13(3), 654. https://doi.org/10.3390/coatings13030654









