First Approach to ZrB2 Thin Films Alloyed with Silver Prepared by Magnetron Co-Sputtering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Calculation Methods
2.2. Experimental Materials and Methods
3. Results and Discussion
3.1. Calculation Results
3.2. Experimental Results
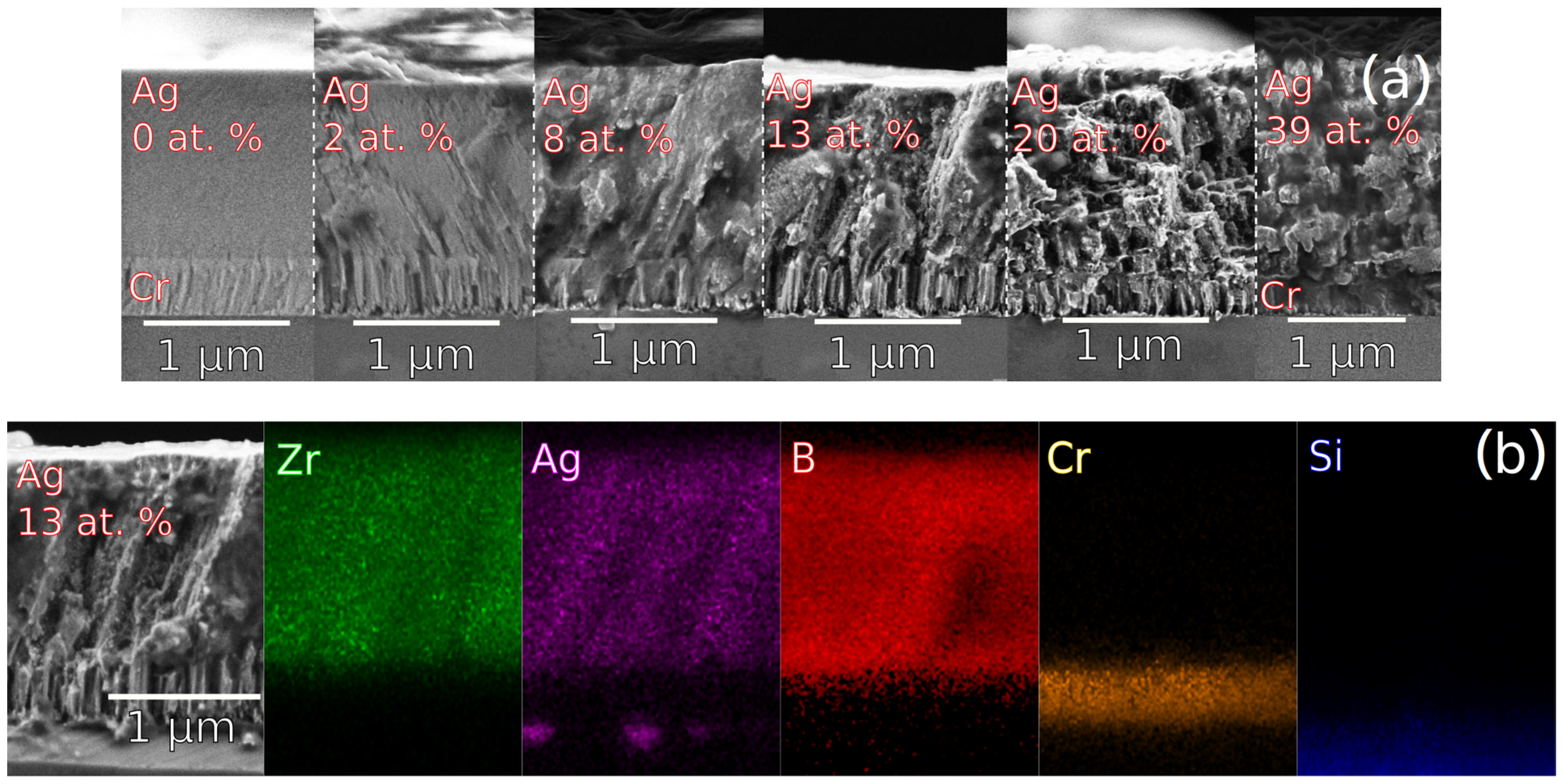
3.2.1. Chemical Composition Analysis
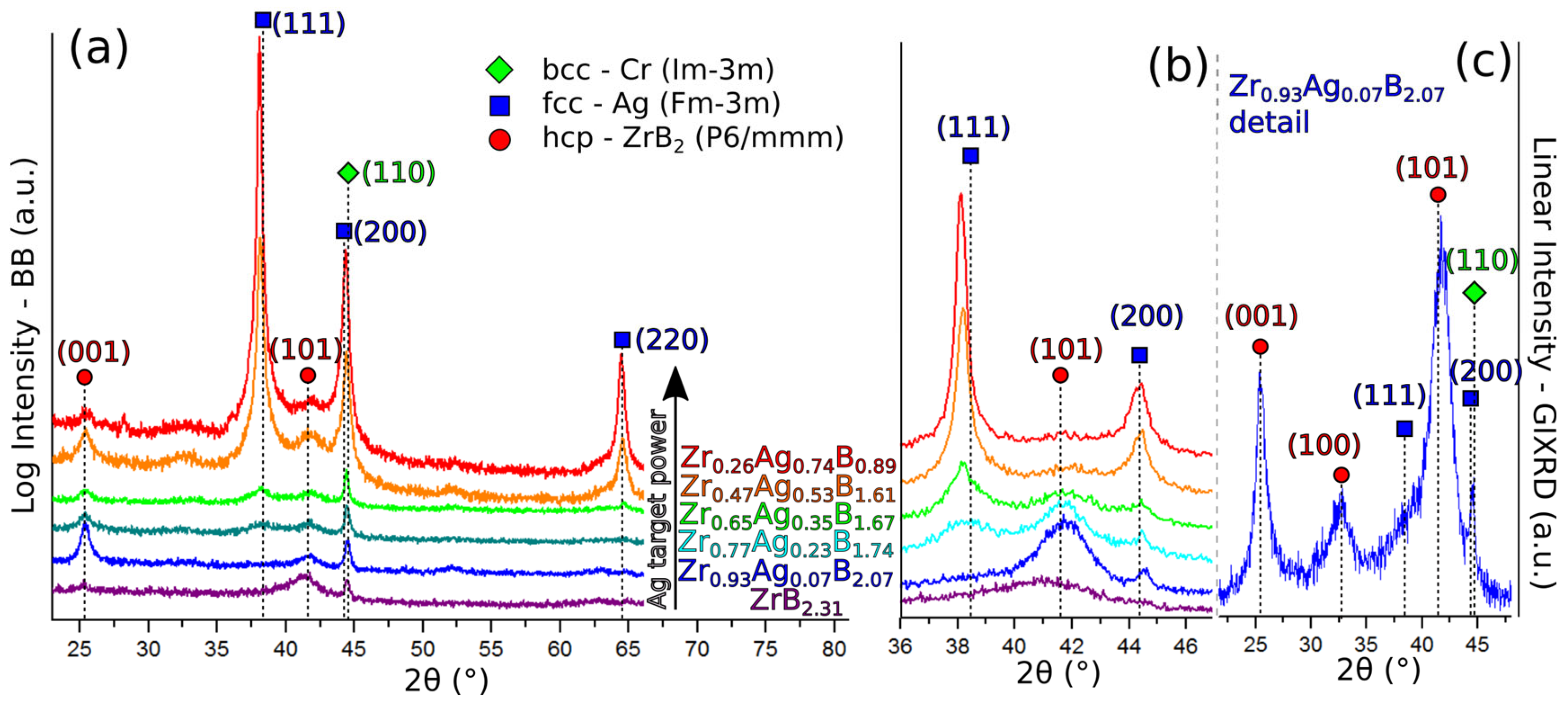
3.2.2. Structure and Morphology Analysis
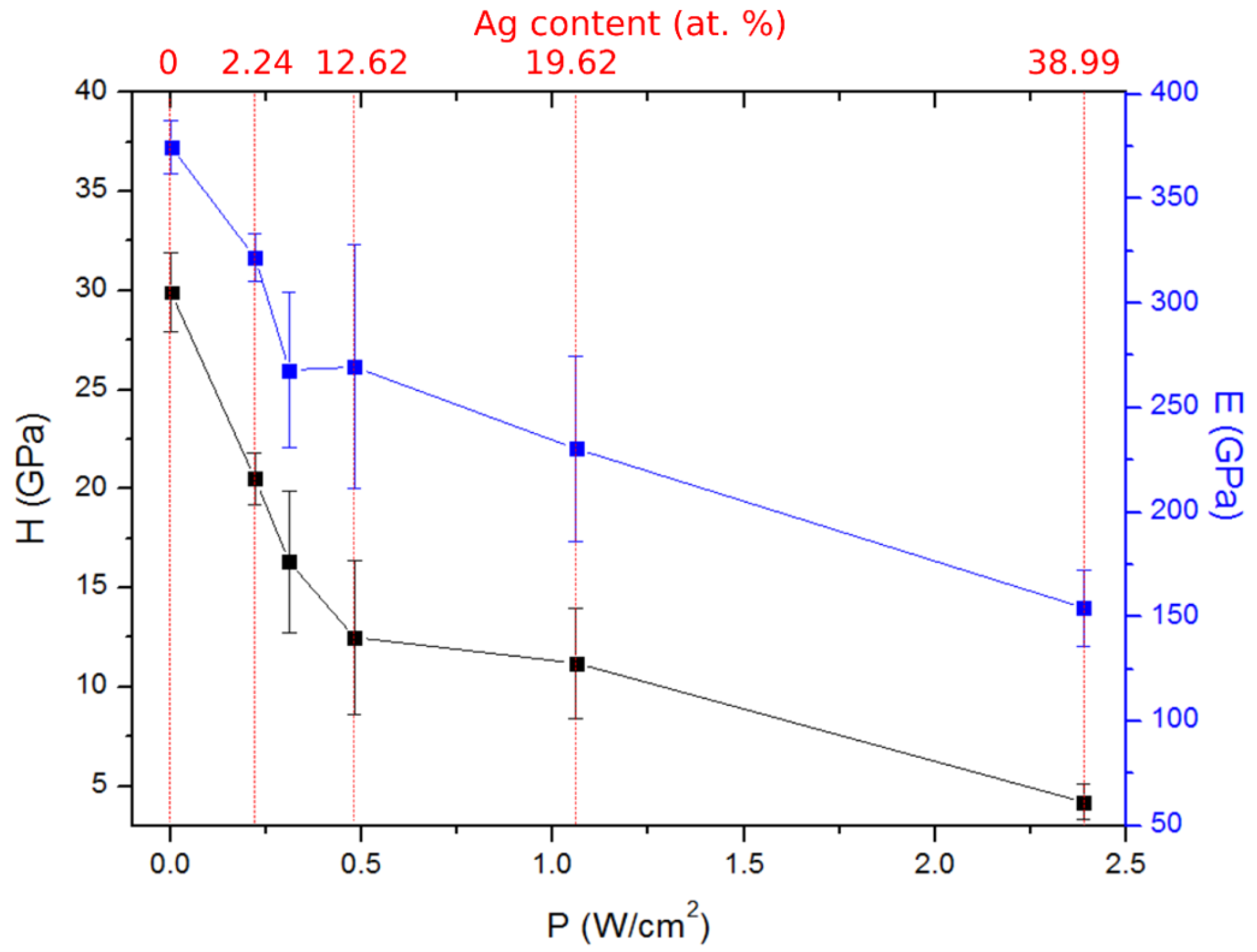
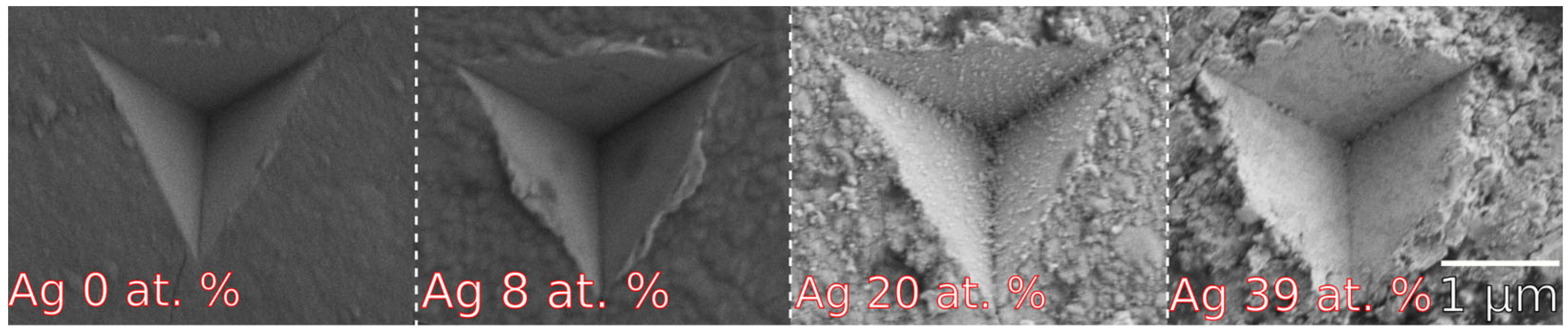
3.2.3. Mechanical and Tribological Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Magnuson, M.; Hultman, L.; Högberg, H. Review of Transition-Metal Diboride Thin Films. Vacuum 2022, 196, 110567. [Google Scholar] [CrossRef]
- Fahrenholtz, W.G.; Hilmas, G.E.; Talmy, I.G.; Zaykoski, J.A. Refractory Diborides of Zirconium and Hafnium. J. Am. Ceram. Soc. 2007, 90, 1347–1364. [Google Scholar] [CrossRef]
- Kurbatkina, V.V.; Patsera, E.I.; Levashov, E.A.; Timofeev, A.N. Self-Propagating High-Temperature Synthesis of Refractory Boride Ceramics (Zr,Ta)B2 with Superior Properties. J. Eur. Ceram. Soc. 2018, 38, 1118–1127. [Google Scholar] [CrossRef]
- Gild, J.; Zhang, Y.; Harrington, T.; Jiang, S.; Hu, T.; Quinn, M.C.; Mellor, W.M.; Zhou, N.; Vecchio, K.; Luo, J. High-Entropy Metal Diborides: A New Class of High-Entropy Materials and a New Type of Ultrahigh Temperature Ceramics. Sci. Rep. 2016, 6, 37946. [Google Scholar] [CrossRef] [Green Version]
- Mayrhofer, P.H.; Mitterer, C.; Clemens, H. Self-Organized Nanostructures in Hard Ceramic Coatings. Adv. Eng. Mater. 2005, 7, 1071–1082. [Google Scholar] [CrossRef]
- Mikula, M.; Grančič, B.; Roch, T.; Plecenik, T.; Vávra, I.; Dobročka, E.; Šatka, A.; Buršíková, V.; Držík, M.; Zahoran, M.; et al. The Influence of Low-Energy Ion Bombardment on the Microstructure Development and Mechanical Properties of TiBx Coatings. Vacuum 2011, 85, 866–870. [Google Scholar] [CrossRef]
- Neidhardt, J.; Mráz, S.; Schneider, J.M.; Strub, E.; Bohne, W.; Liedke, B.; Möller, W.; Mitterer, C. Experiment and Simulation of the Compositional Evolution of Ti–B Thin Films Deposited by Sputtering of a Compound Target. J. Appl. Phys. 2008, 104, 063304. [Google Scholar] [CrossRef]
- Šroba, V.; Fiantok, T.; Truchlý, M.; Roch, T.; Zahoran, M.; Grančič, B.; Švec, P.; Nagy, Š.; Izai, V.; Kúš, P.; et al. Structure Evolution and Mechanical Properties of Hard Tantalum Diboride Films. J. Vac. Sci. Technol. A 2020, 38, 033408. [Google Scholar] [CrossRef] [Green Version]
- Fiantok, T.; Šroba, V.; Koutná, N.; Izai, V.; Roch, T.; Truchlý, M.; Vidiš, M.; Satrapinskyy, L.; Nagy, Š.; Grančič, B.; et al. Structure Evolution and Mechanical Properties of Co-Sputtered Zr-Al-B2 Thin Films. J. Vac. Sci. Technol. A 2022, 40, 033414. [Google Scholar] [CrossRef]
- Fuger, C.; Hahn, R.; Zauner, L.; Wojcik, T.; Weiss, M.; Limbeck, A.; Hunold, O.; Polcik, P.; Riedl, H. Anisotropic Super-Hardness of Hexagonal WB2± z Thin Films. Mater. Res. Lett. 2022, 10, 70–77. [Google Scholar] [CrossRef]
- Nedfors, N.; Tengstrand, O.; Lu, J.; Eklund, P.; Persson, P.O.Å.; Hultman, L.; Jansson, U. Superhard NbB2− Thin Films Deposited by Dc Magnetron Sputtering. Surf. Coat. Technol. 2014, 257, 295–300. [Google Scholar] [CrossRef]
- Yalamanchili, K.; Schramm, I.C.; Jiménez-Piqué, E.; Rogström, L.; Mücklich, F.; Odén, M.; Ghafoor, N. Tuning Hardness and Fracture Resistance of ZrN/Zr0.63Al0.37N Nanoscale Multilayers by Stress-Induced Transformation Toughening. Acta Mater. 2015, 89, 22–31. [Google Scholar] [CrossRef]
- Hahn, R.; Bartosik, M.; Soler, R.; Kirchlechner, C.; Dehm, G.; Mayrhofer, P.H. Superlattice Effect for Enhanced Fracture Toughness of Hard Coatings. Scr. Mater. 2016, 124, 67–70. [Google Scholar] [CrossRef] [Green Version]
- Buchinger, J.; Koutná, N.; Chen, Z.; Zhang, Z.; Mayrhofer, P.H.; Holec, D.; Bartosik, M. Toughness Enhancement in TiN/WN Superlattice Thin Films. Acta Mater. 2019, 172, 18–29. [Google Scholar] [CrossRef]
- Nedfors, N.; Mráz, S.; Palisaitis, J.; Persson, P.O.Å.; Lind, H.; Kolozsvari, S.; Schneider, J.M.; Rosen, J. Influence of the Al Concentration in Ti-Al-B Coatings on Microstructure and Mechanical Properties Using Combinatorial Sputtering from a Segmented TiB2/AlB2 Target. Surf. Coat. Technol. 2019, 364, 89–98. [Google Scholar] [CrossRef]
- Bakhit, B.; Engberg, D.L.J.; Lu, J.; Rosen, J.; Högberg, H.; Hultman, L.; Petrov, I.; Greene, J.E.; Greczynski, G. Strategy for Simultaneously Increasing Both Hardness and Toughness in ZrB2-Rich Zr1−xTaxBy Thin Films. J. Vac. Sci. Technol. A 2019, 37, 031506. [Google Scholar] [CrossRef] [Green Version]
- Umeda, K.; Enomoto, Y.; Mitsui, A.; Mannami, K. Friction and Wear of Boride Ceramics in Air and Water. Wear 1993, 169, 63–68. [Google Scholar] [CrossRef]
- Bhatt, B.; Murthy, T.S.R.C.; Limaye, P.K.; Nagaraj, A.; Singh, K.; Sonber, J.K.; Sairam, K.; Sashanka, A.; Rao, G.V.S.N.; Rao, T.S. Tribological Studies of Monolithic Chromium Diboride against Cemented Tungsten Carbide (WC–Co) under Dry Condition. Ceram. Int. 2016, 42, 15536–15546. [Google Scholar] [CrossRef]
- Lofaj, F.; Moskalewicz, T.; Cempura, G.; Mikula, M.; Dusza, J.; Czyrska-Filemonowicz, A. Nanohardness and Tribological Properties of Nc-TiB2 Coatings. J. Eur. Ceram. Soc. 2013, 33, 2347–2353. [Google Scholar] [CrossRef]
- Sonber, J.K.; Raju, K.; Murthy, T.S.R.C.; Sairam, K.; Nagaraj, A.; Majumdar, S.; Kain, V. Friction and Wear Properties of Zirconium Diboride in Sliding against WC Ball. Int. J. Refract. Met. Hard Mater. 2018, 76, 41–48. [Google Scholar] [CrossRef]
- Medveď, D.; Balko, J.; Sedlák, R.; Kovalčíková, A.; Shepa, I.; Naughton-Duszová, A.; Bączek, E.; Podsiadło, M.; Dusza, J. Wear Resistance of ZrB2 Based Ceramic Composites. Int. J. Refract. Met. Hard Mater. 2019, 81, 214–224. [Google Scholar] [CrossRef]
- Mallik, M.; Mitra, P.; Srivastava, N.; Narain, A.; Dastidar, S.G.; Singh, A.; Paul, T.R. Abrasive Wear Performance of Zirconium Diboride Based Ceramic Composite. Int. J. Refract. Met. Hard Mater. 2019, 79, 224–232. [Google Scholar] [CrossRef]
- Aouadi, S.M.; Bohnhoff, A.; Sodergren, M.; Mihut, D.; Rohde, S.L.; Xu, J.; Mishra, S.R. Tribological Investigation of Zirconium Nitride/Silver Nanocomposite Structures. Surf. Coat. Technol. 2006, 201, 418–422. [Google Scholar] [CrossRef]
- Fenker, M.; Balzer, M.; Kellner, S.; Polcar, T.; Richter, A.; Schmidl, F.; Vitu, T. Formation of Solid Lubricants during High Temperature Tribology of Silver-Doped Molybdenum Nitride Coatings Deposited by DcMS and HIPIMS. Coatings 2021, 11, 1415. [Google Scholar] [CrossRef]
- Sliney, H.E. The Use of Silver in Self-Lubricating Coatings for Extreme Temperatures. ASLE Trans. 1986, 29, 370–376. [Google Scholar] [CrossRef]
- Tengdelius, L.; Broitman, E.; Lu, J.; Eriksson, F.; Birch, J.; Nyberg, T.; Hultman, L.; Högberg, H. Hard and Elastic Epitaxial ZrB2 Thin Films on Al2O3(0001) Substrates Deposited by Magnetron Sputtering from a ZrB2 Compound Target. Acta Mater. 2016, 111, 166–172. [Google Scholar] [CrossRef] [Green Version]
- Blöchl, P.E. Projector Augmented-Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [Green Version]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A Modular and Open-Source Software Project for Quantum Simulations of Materials. J. Phys. Condens. Matter. 2009, 21, 395502. [Google Scholar] [CrossRef]
- Giannozzi, P.; Andreussi, O.; Brumme, T.; Bunau, O.; Nardelli, M.B.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Cococcioni, M.; et al. Advanced Capabilities for Materials Modelling with Quantum ESPRESSO. J. Phys. Condens. Matter. 2017, 29, 465901. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Peng, R.; Yu, Y.; Hu, Z.; Wen, Y.; Song, L. Pressure Effect on Elastic Constants and Related Properties of Ti3Al Intermetallic Compound: A First-Principles Study. Materials 2018, 11, 2015. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.-Q.; Niu, H.; Li, D.; Li, Y. Modeling Hardness of Polycrystalline Materials and Bulk Metallic Glasses. Intermetallics 2011, 19, 1275–1281. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Li, C.; Hu, M.; Hou, X.; Li, C.; Chen, Z. Deformation Modes and Anisotropy of Anti-Perovskite Ti3AN (A = Al, In and Tl) from First-Principle Calculations. Materials 2017, 10, 362. [Google Scholar] [CrossRef] [Green Version]
- Oliver, W.C.; Pharr, G.M. An Improved Technique for Determining Hardness and Elastic Modulus Using Load and Displacement Sensing Indentation Experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Mouhat, F.; Coudert, F.-X. Necessary and Sufficient Elastic Stability Conditions in Various Crystal Systems. Phys. Rev. B 2014, 90, 224104. [Google Scholar] [CrossRef] [Green Version]
- Chakrabarti, U.K.; Barz, H.; Dautremont-Smith, W.C.; Lee, J.W.; Kometani, T.Y. Deposition of Zirconium Boride Thin Films by Direct Current Triode Sputtering. J. Vac. Sci. Technol. A Vac. Surf. Film. 1987, 5, 196–201. [Google Scholar] [CrossRef]
- Guziewicz, M.; Piotrowska, A.; Turos, A.; Mizera, E.; Winiarski, A.; Szade, J. Characteristics of Sputter-Deposited TiN, ZrB2 and W2B Diffusion Barriers for Advanced Metallizations to GaAs. Solid-State Electron. 1999, 43, 1055–1061. [Google Scholar] [CrossRef]
- Shappirio, J.R.; Finnegan, J.J. Synthesis and Properties of Some Refractory Transition Metal Diboride Thin Films. Thin Solid Films 1983, 107, 81–87. [Google Scholar] [CrossRef]
- Bakhit, B.; Primetzhofer, D.; Pitthan, E.; Sortica, M.A.; Ntemou, E.; Rosen, J.; Hultman, L.; Petrov, I.; Greczynski, G. Systematic Compositional Analysis of Sputter-Deposited Boron-Containing Thin Films. J. Vac. Sci. Technol. A 2021, 39, 063408. [Google Scholar] [CrossRef]
- Tengdelius, L.; Samuelsson, M.; Jensen, J.; Lu, J.; Hultman, L.; Forsberg, U.; Janzén, E.; Högberg, H. Direct Current Magnetron Sputtered ZrB2 Thin Films on 4H-SiC(0001) and Si(100). Thin Solid Films 2014, 550, 285–290. [Google Scholar] [CrossRef] [Green Version]
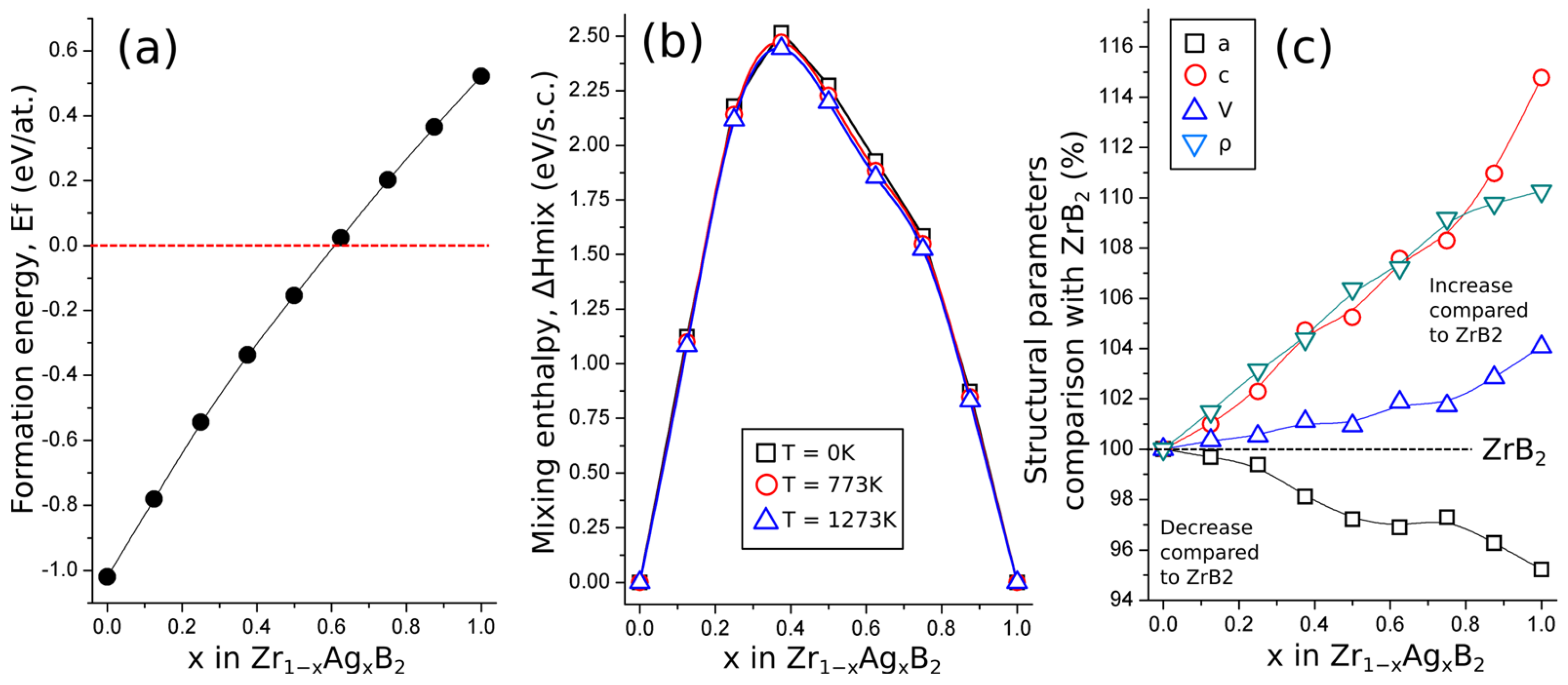


| System | E (GPa) | G (GPa) | B (GPa) | ν | H (GPa) | µm | C12-C66 (GPa) | C13-C44 (GPa) | G/B |
|---|---|---|---|---|---|---|---|---|---|
| ZrB2 | 494 | 216 | 230 | 0.14 | 40 | 0.98 | −184 | −113 | 0.94 |
| Zr0.875Ag0.125B2 | 456 | 196 | 226 | 0.16 | 34 | 1.19 | −156 | −78 | 0.87 |
| Zr0.75Ag0.25B2 | 387 | 162 | 214 | 0.20 | 25 | 1.59 | −129 | −12 | 0.76 |
| Zr0.625Ag0.375B2 | 321 | 130 | 203 | 0.24 | 17 | 2.18 | −114 | 29 | 0.64 |
| Zr0.5Ag0.5B2 | 283 | 112 | 197 | 0.26 | 13 | 3.11 | −90 | 43 | 0.57 |
| Zr0.375Ag0.625B2 | 210 | 81 | 183 | 0.30 | 7 | 6.35 | −60 | 75 | 0.44 |
| Zr0.25Ag0.75B2 | 179 | 68 | 176 | 0.32 | 5 | 4.39 | −33 | 46 | 0.39 |
| Zr0.125Ag0.875B2 | 86 | 33 | 159 | 0.32 | −0.6 | −1304 | 10 | 78 | 0.21 |
| AgB2 | −9 | −3 | 147 | 0.58 | - | −33 | 297 | 72 | −0.02 |
| I (A) | U (V) | P (W/cm2) | Zr (at.%) | Ag (at.%) | B (at.%) | B/(Zr + Ag) |
| 0 | 0 | 0 | 29.6 ± 1 | 0 | 68.4 ± 1 | 2.31 |
| 0.05 | 338 | 0.22 | 29.5 ± 1 | 2.2 ± 1 | 65.8 ± 1 | 2.07 |
| 0.07 | 350 | 0.31 | 27.4 ± 1 | 8.0 ± 1 | 61.4 ± 1 | 1.74 |
| 0.1 | 374 | 0.48 | 23.9 ± 1 | 12.6 ± 1 | 61.0 ± 1 | 1.67 |
| 0.2 | 415 | 1.06 | 17.5 ± 1 | 19.8 ± 1 | 59.9 ± 1 | 1.61 |
| 0.4 | 470 | 2.39 | 13.9 ± 1 | 39.0 ± 1 | 47.2 ± 1 | 0.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiantok, T.; Truchlý, M.; Šroba, V.; Roch, T.; Izai, V.; Vidiš, M.; Haršáni, M.; Satrapinskyy, L.; Mikula, M. First Approach to ZrB2 Thin Films Alloyed with Silver Prepared by Magnetron Co-Sputtering. Coatings 2023, 13, 663. https://doi.org/10.3390/coatings13030663
Fiantok T, Truchlý M, Šroba V, Roch T, Izai V, Vidiš M, Haršáni M, Satrapinskyy L, Mikula M. First Approach to ZrB2 Thin Films Alloyed with Silver Prepared by Magnetron Co-Sputtering. Coatings. 2023; 13(3):663. https://doi.org/10.3390/coatings13030663
Chicago/Turabian StyleFiantok, Tomáš, Martin Truchlý, Viktor Šroba, Tomáš Roch, Vitalii Izai, Marek Vidiš, Marián Haršáni, Leonid Satrapinskyy, and Marián Mikula. 2023. "First Approach to ZrB2 Thin Films Alloyed with Silver Prepared by Magnetron Co-Sputtering" Coatings 13, no. 3: 663. https://doi.org/10.3390/coatings13030663
APA StyleFiantok, T., Truchlý, M., Šroba, V., Roch, T., Izai, V., Vidiš, M., Haršáni, M., Satrapinskyy, L., & Mikula, M. (2023). First Approach to ZrB2 Thin Films Alloyed with Silver Prepared by Magnetron Co-Sputtering. Coatings, 13(3), 663. https://doi.org/10.3390/coatings13030663






