Eco-Friendly Sustainable Dyeing of Cotton Fabric Using Reactive Violet 05 and Direct Violet 09 Dyes
Abstract
:1. Introduction
- To improve the color characteristics of cellulosic fabric dyed with Reactive Violet 5 and Direct Violet 9 dye by using microwave radiation;
- To study the physical and chemical characteristics of fabrics before and after treatment;
- To analyze the dyeing conditions using central composite design through response surface methodology
- To assess the color fastness properties of cotton fabric dyed with Reactive Violet 5 and Direct Violet 9 dye.
2. Experimental Section
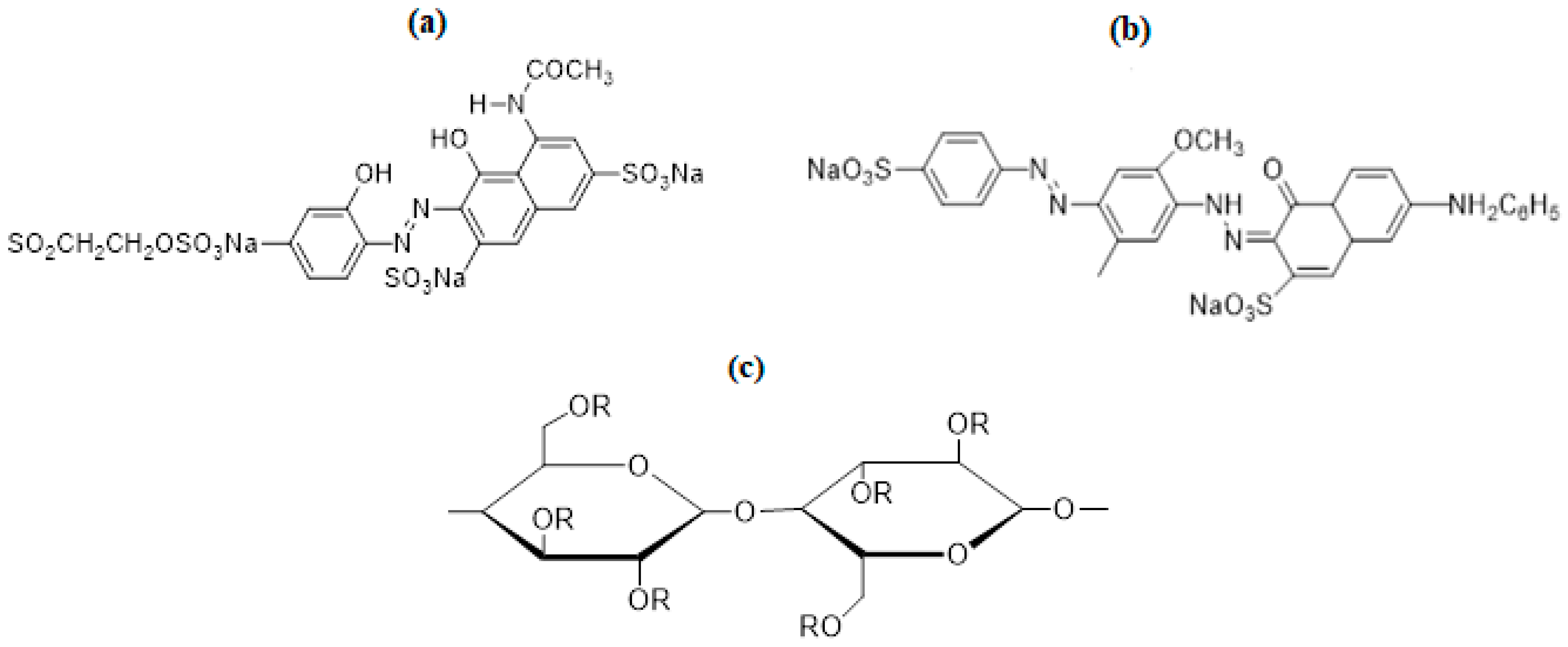
2.1. Materials
2.2. Irradiation Process and Dyeing
2.3. Selection of Dyeing Conditions
2.4. Effect of Different Inorganic Salts
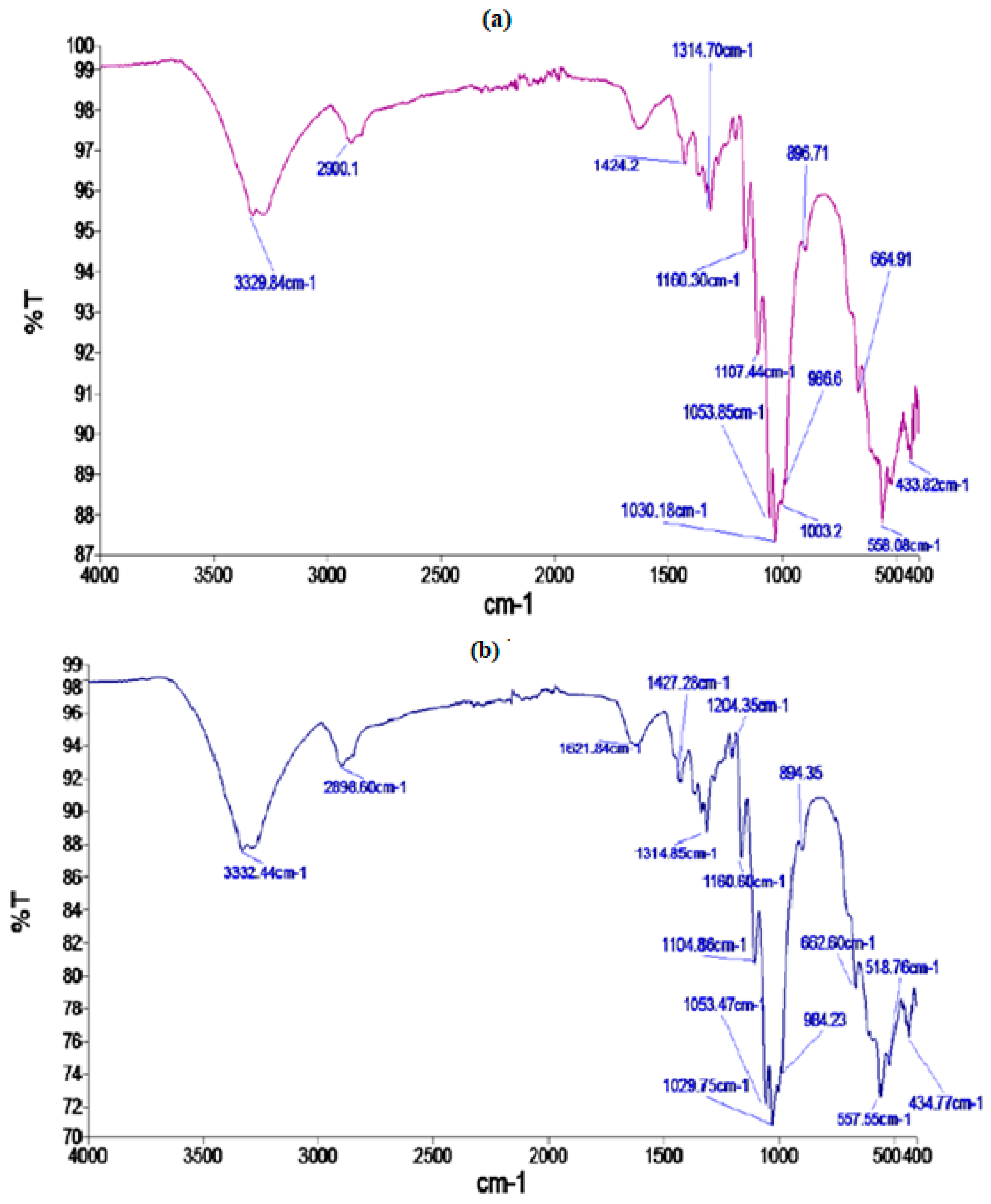
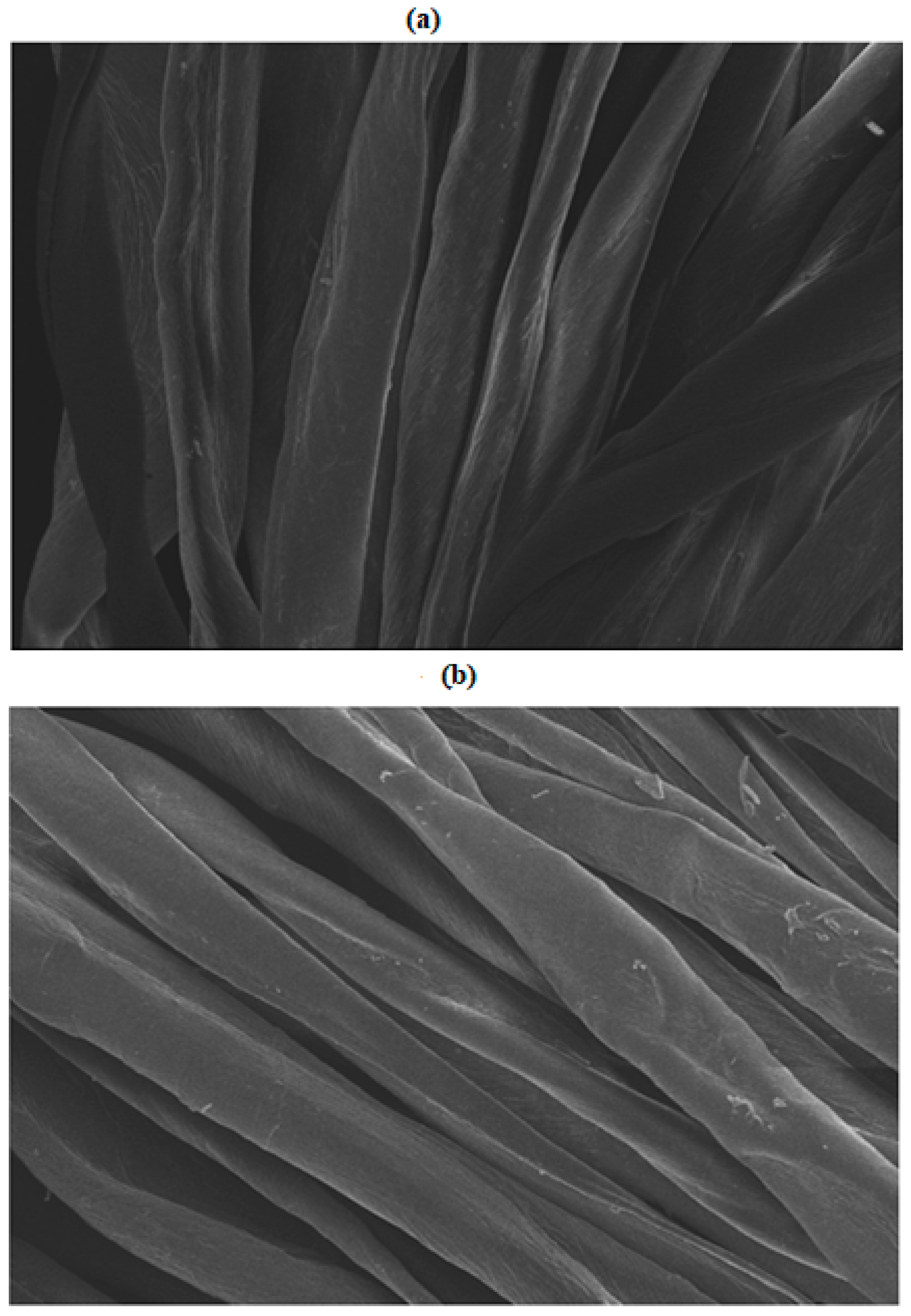
2.5. FTIR and SEM Analysis
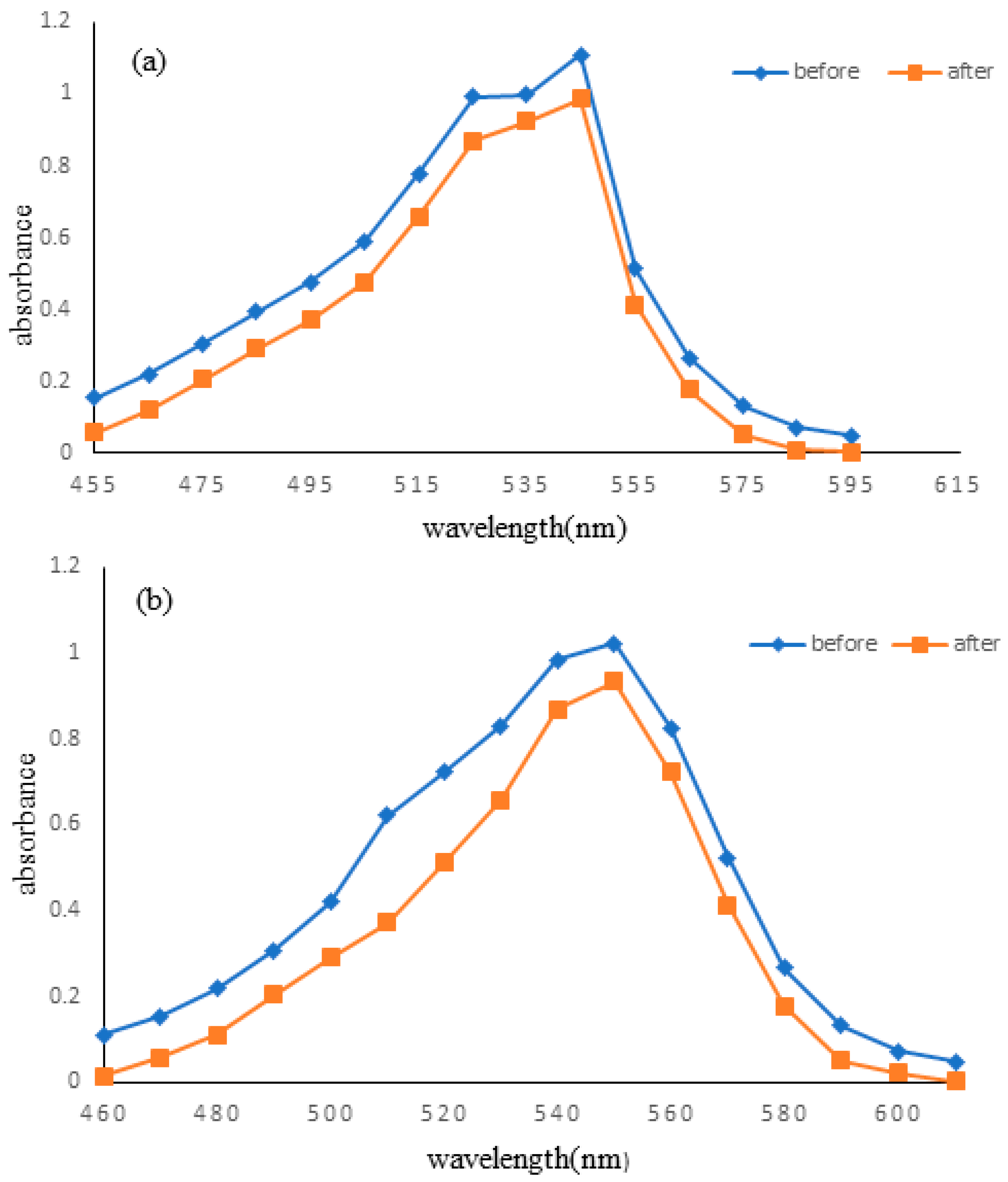
2.6. Determination of Maximum Wavelength
2.7. Evaluation of Quality of Dyed Fabrics
3. Results and Discussion
3.1. FTIR and SEM Analysis
3.2. Determination of λmax of Reactive Violet 05 and Direct Violet 09
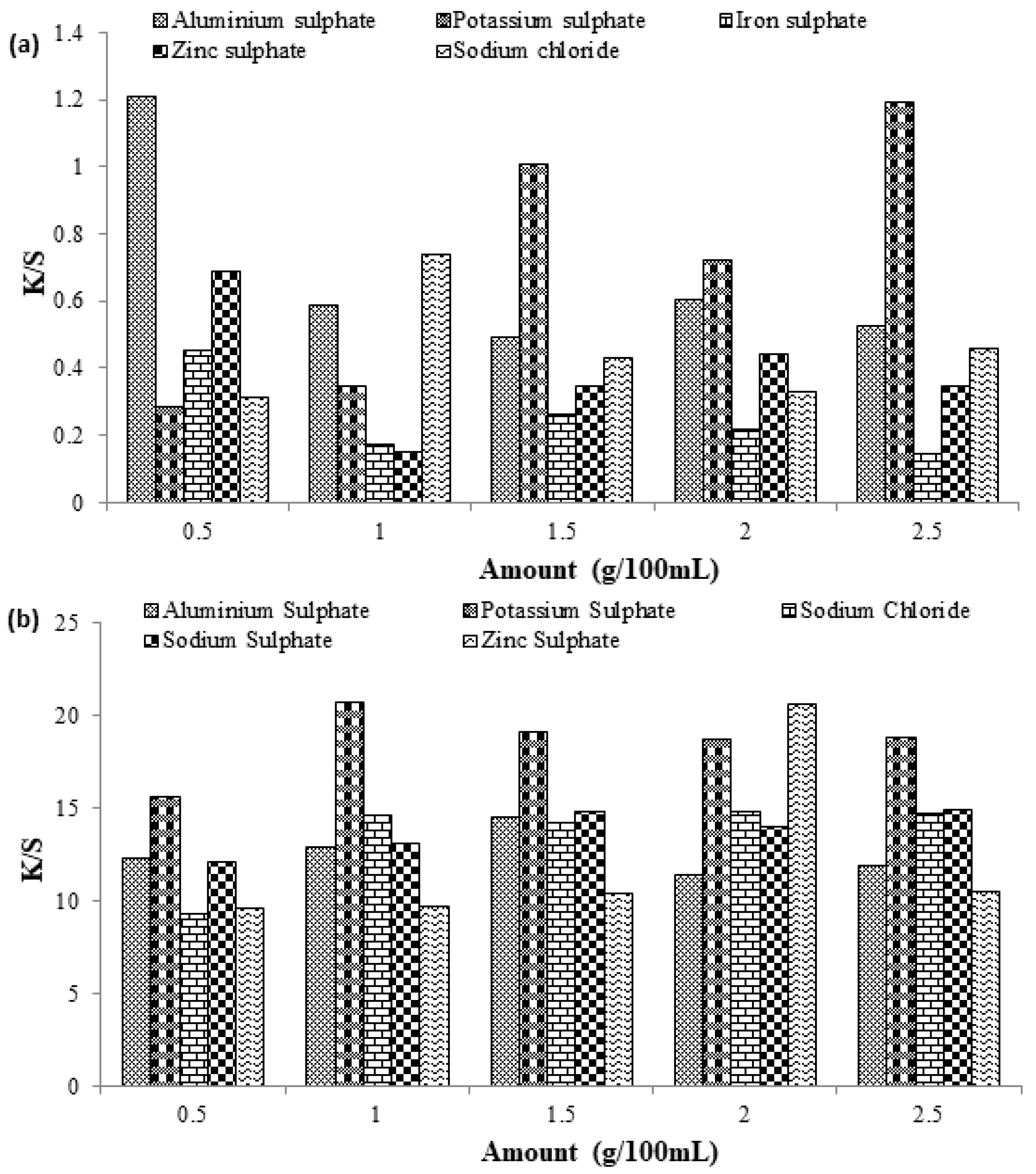
3.3. Effect of Inorganic Salts on the Dyeing of Cotton Fabric
3.4. Statistical Selection of Dyeing Parameters
3.5. Colorfastness Rating
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Slama, H.B.; Chenari Bouket, A.; Pourhassan, Z.; Alenezi, F.N.; Silini, A.; Cherif-Silini, H.; Oszako, T.; Luptakova, L.; Golinska, P.; Belbahri, L. Diversity of synthetic dyes from textile industries, discharge impacts and treatment methods. Appl. Sci. 2021, 11, 6255. [Google Scholar] [CrossRef]
- Abdussalam-Mohammed, W.; Ali, A.Q.; Errayes, A.O. Green chemistry: Principles, applications, and disadvantages. Chem. Methodol. 2020, 4, 408–423. [Google Scholar]
- Gulzar, T.; Farooq, T.; Kiran, S.; Ahmad, I.; Hameed, A. The Impact and Prospects of Green Chemistry for Textile Technology; Elsevier: Alpharetta, GA, USA, 2019. [Google Scholar]
- Adane, T.; Adugna, A.T.; Alemayehu, E. Textile industry effluent treatment techniques. J. Chem. 2021, 2021, 5314404. [Google Scholar] [CrossRef]
- Ahmed, B.; Sultana, S. A Critical Review on PLA-Algae Composite: Chemistry, Mechanical, and Thermal Properties. J. Text. Sci. Eng. 2020, 10. [Google Scholar]
- Adeel, S.; Saeed, M.; Abdullah, A.; Khan, S.G. Ultrasonic assisted improved dyeing of cellulosic fabric using Vat Blue 4. J. Nat. Fibers 2020, 17, 745–758. [Google Scholar] [CrossRef]
- Shabbir, M.U.; Adeel, S.; Bokhari, T.H.; Usman, M.; Khosa, M.K.; Ahmad, T.; Inayat, A. Eco-friendly acid dyeing of silk and wool fabrics using acid violet 49 dye. Environ. Sci. Pollut. 2022, 30, 9808–9819. [Google Scholar] [CrossRef]
- Haji, A.; Naebe, M. Cleaner dyeing of textiles using plasma treatment and natural dyes: A review. J. Clean. Prod. 2020, 265, 121866. [Google Scholar] [CrossRef]
- Ahmed, B.; Gwon, J.; Thapaliya, M.; Adhikari, A.; Ren, S.; Wu, Q. Combined effects of deep eutectic solvent and microwave energy treatments on cellulose fiber extraction from hemp bast. Cellulose 2023, 30, 2895–2911. [Google Scholar] [CrossRef]
- Ahmed, B.; Wu, Q.; Lin, H.; Gwon, J.; Negulescu, I.; Cameron, B. Degumming of hemp fibers using combined microwave energy and deep eutectic solvent treatment. Ind. Crops Prod. 2022, 184, 115046. [Google Scholar] [CrossRef]
- Kiran, S.; Adeel, S.; Rehman, F.U.; Gulzar, T. Ecofriendly dyeing of microwave treated cotton fabric using reactive violet H3R. Glob. Nest J. 2019, 21, 43–47. [Google Scholar]
- Sundhu, M.; Khosa, M.K.; Adeel, S.; Ahmad, T. Microwave-assisted eco-friendly acid dyeing of proteinous fabrics using acid violet 3b dye. J. Nat. Fibers 2022, 19, 8065–8074. [Google Scholar] [CrossRef]
- Benkhaya, S.; M’rabet, S.; El-Harfi, A. A review on classifications, recent synthesis and applications of textile dyes. Inorg. Chem. Commun. 2020, 115, 107891. [Google Scholar] [CrossRef]
- Zolriasatein, A.A. Thermodynamics, Kinetics and Isotherms Studies for Sorption of Direct Dye onto the Pectinase Pre-treated Jute Yarn. Recent Innov. Chem. Eng. 2019, 12, 160–171. [Google Scholar] [CrossRef]
- Burkinshaw, S.M.; Salihu, G. The role of auxiliaries in the immersion dyeing of textile fibres: Part 5 practical aspects of the role of inorganic electrolytes in dyeing cellulosic fibres with direct dyes. Dye. Pigment. 2019, 161, 581–594. [Google Scholar] [CrossRef] [Green Version]
- Irfan, M.; Zhang, H.; Syed, U.; Hou, A. Low liquor dyeing of cotton fabric with reactive dye by an eco-friendly technique. J. Clean. Prod. 2018, 197, 1480–1487. [Google Scholar] [CrossRef]
- Gao, D.; Li, X.; Li, Y.; Lyu, B.; Ren, J.; Ma, J. Long-acting antibacterial activity on the cotton fabric. Cellulose 2021, 28, 1221–1240. [Google Scholar] [CrossRef]
- El-Sayed, E.; Othman, H.; Hassabo, A.G. A short observation on the printing cotton fabric using some technique. J. Text. Color. Polym. 2022, 19, 17–24. [Google Scholar] [CrossRef]
- Shahid, M.J.; Arslan, M.; Ali, S.; Siddique, M.; Afzal, M. Floating wetlands: A sustainable tool for wastewater treatment. Clean–Soil Air Water 2018, 46, 1800120. [Google Scholar] [CrossRef]
- Glavič, P. Evolution and current challenges of sustainable consumption and production. Sustainability 2021, 13, 9379. [Google Scholar] [CrossRef]
- Gbolarumi, F.T.; Wong, K.Y.; Olohunde, S.T. Sustainability assessment in the textile and apparel industry: A review of recent studies. IOP Conf. Ser. Mater. Sci. Eng. 2021, 151, 012099. [Google Scholar] [CrossRef]
- Adeel, S.; Liaqat, S.; Hussaan, M.; Mia, R.; Ahmed, B.; Wafa, H. Environmental friendly bio-dyeing of silk using Alkanna tinctoria based Alkannin natural dye. Ind. Crops Prod. 2020, 186, 115301. [Google Scholar]
- Burkinshaw, S.M. The role of inorganic electrolyte (salt) in cellulosic fibre dyeing: Part 2 theories of how inorganic electrolyte promotes dye uptake. Color. Technol. 2021, 137, 547–586. [Google Scholar] [CrossRef]

| Dye Used | Sample Code | Microwave Treatment Time | Dyeing Conditions |
|---|---|---|---|
| RV 05 | NRS/NRF | 2–10 min | Volume used = 25–65 mL |
| Salt used = 0.5–2.5 g/100 mL | |||
| Temperature = 50–90 °C | |||
| Time = 20–80 min pH = 6–10 | |||
| DV 09 | NRS/NRF | 2–10 min | Volume used = 25–65 mL |
| Salt used = 0.5–2.5 g/100 mL | |||
| Temperature = 50–90 °C | |||
| Time = 20–80 min pH = 6–10 |
| Microwave Treatment Time (min) | Sample Code | K/S | L* | a* | b* | C* |
|---|---|---|---|---|---|---|
| Reactive Violet 05 dye | ||||||
| Control (without radiation) | NRS/NRCF | 0.8709 | 65.97 | 12.44 | 15.11 | 19.57 |
| 2 | NRS/RCF | 0.7552 | 68.05 | 14.09 | 18.82 | 23.51 |
| 4 | RS/NRCF | 0.9258 | 65.02 | 13.44 | 15.38 | 20.42 |
| 6 | RS/NRCF | 1.1807 | 62.41 | 14.99 | 17.08 | 22.73 |
| 8 | RS/NRCF | 1.4974 | 62.00 | 18.71 | 20.43 | 27.71 |
| 10 | MAD | 1.1674 | 23.06 | 21.61 | 14.24 | 25.88 |
| Direct Violet 09 dye | ||||||
| Control (without radiation) | NRS/NRSF | 6.9945 | 35.52 | 21.06 | −29.41 | 36.18 |
| 2 | NRS/RCF | 11.683 | 27.75 | 18.11 | −25.12 | 30.97 |
| 4 | NRS/RCF | 11.180 | 28.39 | 17.64 | −25.34 | 30.88 |
| 6 | NRS/RCF | 13.938 | 25.61 | 18.75 | −24.65 | 30.97 |
| 8 | NRS/RCF | 12.896 | 26.51 | 18.44 | −23.97 | 30.24 |
| 10 | NRS/RCF | 10.933 | 30.21 | 22.96 | −29.92 | 37.71 |
| Reactive Violet 05 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sr. No. | A | B | C | D | E | F | Sr. No. | A | B | C | D | E | F |
| 1 | 7 | 35 | 1 | 30 | 80 | 1.1443 | 17 | 6 | 45 | 1.5 | 40 | 70 | 0.8288 |
| 2 | 9 | 35 | 1 | 30 | 60 | 1.0401 | 18 | 10 | 45 | 1.5 | 40 | 70 | 0.5713 |
| 3 | 7 | 55 | 1 | 30 | 60 | 0.961 | 19 | 8 | 25 | 1.5 | 40 | 70 | 0.6788 |
| 4 | 9 | 55 | 1 | 30 | 80 | 0.9835 | 20 | 8 | 65 | 1.5 | 40 | 70 | 0.6605 |
| 5 | 7 | 35 | 2 | 30 | 60 | 1.3372 | 21 | 8 | 45 | 0.5 | 40 | 70 | 0.522 |
| 6 | 9 | 35 | 2 | 30 | 80 | 0.8456 | 22 | 8 | 45 | 2.5 | 40 | 70 | 0.7775 |
| 7 | 7 | 55 | 2 | 30 | 80 | 0.7885 | 23 | 8 | 45 | 1.5 | 20 | 70 | 0.7869 |
| 8 | 9 | 55 | 2 | 30 | 60 | 0.5299 | 24 | 8 | 45 | 1.5 | 60 | 70 | 0.851 |
| 9 | 7 | 35 | 1 | 50 | 60 | 0.4153 | 25 | 8 | 45 | 1.5 | 40 | 50 | 0.3788 |
| 10 | 9 | 35 | 1 | 50 | 80 | 0.5103 | 26 | 8 | 45 | 1.5 | 40 | 90 | 0.4066 |
| 11 | 7 | 55 | 1 | 50 | 80 | 0.3037 | 27 | 8 | 45 | 1.5 | 40 | 70 | 0.3571 |
| 12 | 9 | 55 | 1 | 50 | 60 | 0.4794 | 28 | 8 | 45 | 1.5 | 40 | 70 | 0.4081 |
| 13 | 7 | 35 | 2 | 50 | 80 | 0.5656 | 29 | 8 | 45 | 1.5 | 40 | 70 | 0.3947 |
| 14 | 9 | 35 | 2 | 50 | 60 | 0.3043 | 30 | 8 | 45 | 1.5 | 40 | 70 | 0.5638 |
| 15 | 7 | 55 | 2 | 50 | 60 | 0.5398 | 31 | 8 | 45 | 1.5 | 40 | 70 | 0.4633 |
| 16 | 9 | 55 | 2 | 50 | 80 | 0.4722 | 32 | 8 | 45 | 1.5 | 40 | 70 | 0.4621 |
| Direct Violet 09 | |||||||||||||
| 1 | 7 | 35 | 1 | 30 | 80 | 16.3982 | 17 | 6 | 45 | 1.5 | 40 | 70 | 18.0419 |
| 2 | 9 | 35 | 1 | 30 | 60 | 14.6886 | 18 | 10 | 45 | 1.5 | 40 | 70 | 17.5838 |
| 3 | 7 | 55 | 1 | 30 | 60 | 18.2292 | 19 | 8 | 25 | 1.5 | 40 | 70 | 17.0857 |
| 4 | 9 | 55 | 1 | 30 | 80 | 17.2958 | 20 | 8 | 65 | 1.5 | 40 | 70 | 18.4175 |
| 5 | 7 | 35 | 2 | 30 | 60 | 17.4534 | 21 | 8 | 45 | 0.5 | 40 | 70 | 15.8018 |
| 6 | 9 | 35 | 2 | 30 | 80 | 16.1935 | 22 | 8 | 45 | 2.5 | 40 | 70 | 18.1445 |
| 7 | 7 | 55 | 2 | 30 | 80 | 18.5447 | 23 | 8 | 45 | 1.5 | 20 | 70 | 17.6709 |
| 8 | 9 | 55 | 2 | 30 | 60 | 18.1901 | 24 | 8 | 45 | 1.5 | 60 | 70 | 18.6072 |
| 9 | 7 | 35 | 1 | 50 | 60 | 17.905 | 25 | 8 | 45 | 1.5 | 40 | 50 | 18.1681 |
| 10 | 9 | 35 | 1 | 50 | 80 | 16.6567 | 26 | 8 | 45 | 1.5 | 40 | 90 | 16.7722 |
| 11 | 7 | 55 | 1 | 50 | 80 | 17.233 | 27 | 8 | 45 | 1.5 | 40 | 70 | 15.7843 |
| 12 | 9 | 55 | 1 | 50 | 60 | 17.9117 | 28 | 8 | 45 | 1.5 | 40 | 70 | 18.1672 |
| 13 | 7 | 35 | 2 | 50 | 80 | 19.0676 | 29 | 8 | 45 | 1.5 | 40 | 70 | 16.4993 |
| 14 | 9 | 35 | 2 | 50 | 60 | 17.3948 | 30 | 8 | 45 | 1.5 | 40 | 70 | 16.7428 |
| 15 | 7 | 55 | 2 | 50 | 60 | 17.905 | 31 | 8 | 45 | 1.5 | 40 | 70 | 18.6072 |
| 16 | 9 | 55 | 2 | 50 | 80 | 18.1549 | 32 | 8 | 45 | 1.5 | 40 | 70 | 17.0915 |
| Source | DF | Adj SS | ADJ MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 20 | 1.98807 | 0.099903 | 27.27 | 0.000 |
| Linear | 5 | 0.58948 | 0.117896 | 32.18 | 0.000 |
| pH | 1 | 0.04217 | 0.042174 | 11.51 | 0.009 |
| Volume | 1 | 0.00078 | 0.000785 | 0.21 | 0.656 |
| Salt | 1 | 0.04988 | 0.049881 | 13.62 | 0.006 |
| Time | 1 | 0.30085 | 0.300850 | 82.12 | 0.000 |
| Temperature | 1 | 0.00004 | 0.000041 | 0.01 | 0.918 |
| Square | 5 | 0.85701 | 0.171402 | 46.79 | 0.000 |
| pH * pH | 1 | 0.10596 | 0.105960 | 28.92 | 0.001 |
| Volume * Volume | 1 | 0.08213 | 0.082128 | 22.42 | 0.001 |
| Salt * Salt | 1 | 0.06817 | 0.068169 | 18.61 | 0.003 |
| Temperature * Temperature | 1 | 0.00464 | 0.004641 | 1.27 | 0.293 |
| Two-Way interaction | 10 | 0.45707 | 0.045707 | 12.48 | 0.001 |
| pH * Volume | 1 | 0.00950 | 0.009499 | 2.59 | 0.146 |
| pH * Salt | 1 | 0.04133 | 0.041332 | 11.28 | 0.010 |
| pH * Time | 1 | 0.01429 | 0.014288 | 3.90 | 0.084 |
| Salt * Temperature | 1 | 0.00027 | 0.000265 | 0.07 | 0.795 |
| Volume * Salt | 1 | 0.03199 | 0.031991 | 8.73 | 0.018 |
| Volume * Time | 1 | 0.19733 | 0.197329 | 53.87 | 0.000 |
| Volume * Temperature | 1 | 0.00007 | 0.000066 | 0.02 | 0.897 |
| Salt * Time | 1 | 0.15095 | 0.150955 | 41.21 | 0.000 |
| Error | 8 | 0.02931 | 0.003663 | - | - |
| Lack-of-Fit | 3 | 0.00302 | 0.001007 | 0.19 | 0.898 |
| Pure Error | 5 | 0.2629 | 0.005257 | - | - |
| Total | 28 | 2.02738 | - | - | - |
| Source | DF | Adj SS | ADJ MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 17 | 22.7476 | 1.33809 | 4.73 | 0.005 |
| Linear | 5 | 14.3755 | 2.87511 | 10.16 | 0.001 |
| pH | 1 | 2.1398 | 2.13977 | 7.56 | 0.018 |
| Volume | 1 | 4.4809 | 4.48088 | 15.83 | 0.002 |
| Salt | 1 | 5.2933 | 5.29333 | 18.70 | 0.001 |
| Time | 1 | 2.1050 | 2.10503 | 7.44 | 0.018 |
| Temperature | 1 | 0.3565 | 0.35653 | 1.26 | 0.284 |
| Square | 4 | 1.8519 | 0.46298 | 1.64 | 0.229 |
| pH * pH | 1 | 0.3133 | 0.31327 | 1.11 | 0.314 |
| Volume * Volume | 1 | 0.2299 | 0.22993 | 0.81 | 0.385 |
| Salt * Salt | 1 | 0.2613 | 0.29130 | 1.03 | 0.330 |
| Time * Time | 1 | 0.9738 | 0.97378 | 3.44 | 0.088 |
| Two-Way interaction | 8 | 6.5201 | 0.81501 | 2.88 | 0.048 |
| pH * Volume | 1 | 1.9121 | 1.91214 | 6.75 | 0.023 |
| pH * Salt | 1 | 0.0019 | 0.00192 | 0.01 | 0.936 |
| pH * Time | 1 | 0.3106 | 0.32064 | 1.3 | 0.308 |
| Salt * Temperature | 1 | 0.2938 | 0.29382 | 1.04 | 0.328 |
| Volume * Salt | 1 | 0.3410 | 0.34100 | 1.20 | 0.029 |
| Volume * Time | 1 | 3.3724 | 3.37236 | 11.91 | 0.005 |
| Volume * Temperature | 1 | 0.2213 | 0.22132 | 0.78 | 0.394 |
| Salt * Time | 1 | 0.0569 | 0.05688 | 0.20 | 0.662 |
| Error | 12 | 3.3970 | 0.28308 | - | - |
| Lack-of-Fit | 9 | 1.7721 | 0.19690 | 0.36 | 0.895 |
| Pure Error | 3 | 1.6249 | 0.54163 | - | - |
| Total | 29 | 26.1445 | - | - | - |
| Reactive Violet 05 Dye | |||||
|---|---|---|---|---|---|
| Shade (%) | Rating 1 | Rating 2 | Rating 3 | ||
| c.c | c.s | Dry | Wet | ||
| 0.5 | 5 | 4/5 | 4/5 | 5 | 4/5 |
| 1.0 | 5 | 4/5 | 4/5 | 5 | 4/5 |
| 1.5 | 5 | 4/5 | 4/5 | 5 | 4/5 |
| 2.0 | 5 | 4/5 | 4/5 | 5 | 4/5 |
| 2.5 | 5 | 4/5 | 4/5 | 5 | 4/5 |
| Direct Violet 09 dye | |||||
| 0.5 | 5 | 4/5 | 4/5 | 5 | 4/5 |
| 1.0 | 5 | 4/5 | 4/5 | 5 | 4/5 |
| 1.5 | 5 | 4/5 | 4/5 | 5 | 4/5 |
| 2.0 | 5 | 4/5 | 4/5 | 5 | 4/5 |
| 2.5 | 5 | 4/5 | 4/5 | 5 | 4/5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bokhari, T.H.; Bano, S.; Adeel, S.; Fazal-ur-Rehman; Ahmed, B.; Al Mahmud, M.A.; Qayyum, M.A.; Khattak, S.P. Eco-Friendly Sustainable Dyeing of Cotton Fabric Using Reactive Violet 05 and Direct Violet 09 Dyes. Coatings 2023, 13, 677. https://doi.org/10.3390/coatings13040677
Bokhari TH, Bano S, Adeel S, Fazal-ur-Rehman, Ahmed B, Al Mahmud MA, Qayyum MA, Khattak SP. Eco-Friendly Sustainable Dyeing of Cotton Fabric Using Reactive Violet 05 and Direct Violet 09 Dyes. Coatings. 2023; 13(4):677. https://doi.org/10.3390/coatings13040677
Chicago/Turabian StyleBokhari, Tanveer Hussain, Sumaira Bano, Shahid Adeel, Fazal-ur-Rehman, Bulbul Ahmed, Md. Abdullah Al Mahmud, Muhammad Abdul Qayyum, and Shahnaz Parveen Khattak. 2023. "Eco-Friendly Sustainable Dyeing of Cotton Fabric Using Reactive Violet 05 and Direct Violet 09 Dyes" Coatings 13, no. 4: 677. https://doi.org/10.3390/coatings13040677
APA StyleBokhari, T. H., Bano, S., Adeel, S., Fazal-ur-Rehman, Ahmed, B., Al Mahmud, M. A., Qayyum, M. A., & Khattak, S. P. (2023). Eco-Friendly Sustainable Dyeing of Cotton Fabric Using Reactive Violet 05 and Direct Violet 09 Dyes. Coatings, 13(4), 677. https://doi.org/10.3390/coatings13040677






